Abstract
Bronchiectasis is a significant cause of morbidity and mortality. It is the end point of a pathological process. We should be aiming to identify at risk patients before they develop bronchiectasis and treat them aggressively to prevent disease progression. With improved social conditions and health care, infective causes of bronchiectasis have diminished in the developed world, and genetic causes are therefore relatively more common. The underlying cause of bronchiectasis should always be sought and redressed, for example as discoveries of innate immune defects are made. ‘Idiopathic bronchiectasis’ should be a diagnosis of last resort. This chapter reviews potential genetic causes of bronchiectasis and suggests a plan for investigating the underlying aetiology. Management is discussed but it is important to note that suggested treatment strategies are often extrapolated from evidence in bronchiectasis associated with cystic fibrosis; this is likely to be inappropriate in diseases of differing pathophysiology. Rare lung diseases need to be moved out of the ‘orphan’ category by instigating multi-centre, multi-national clinical trials and producing disease specific evidence based guidelines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Bronchiectasis is a disorder of the segmental and sub-segmental bronchi that is characterised by destruction of the elastic and muscular elements of the bronchial walls resulting in bronchial dilatation and chronic infection. Predisposing conditions are those associated with bronchial obstruction, inefficient pulmonary toilet, and susceptibility to chronic or recurrent infection. In developing countries, bronchiectasis remains a frequent complication of acute infection. Post-infectious bronchiectasis is becoming very rare in western countries due to the availability of antibiotics and immunisation, particularly against pertussis and measles [1]; bronchiectasis in adults who developed disease in early childhood predating these developments is not, however, uncommon [2]. Focal bronchiectasis may occur in the context of isolated bronchial obstruction due, for example, to an inhaled foreign body. In the developed world, diffuse bronchiectasis is typically found in association with underlying disorders of mucociliary clearance, immune defence or bronchial anatomy. A number of genetic disorders are known to predispose to bronchiectasis and it is likely that some less well characterised heritable disorders underlie a proportion of cases currently regarded as diffuse idiopathic bronchiectasis.
Pathophysiology and Presentation
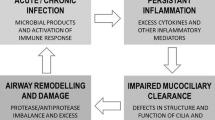
Recurrent or chronic infection results in repeated plugging of the airway, recruitment of inflammatory cells and release of inflammatory mediators and cytokines; this, in turn, can cause permanent structural airway changes [3, 4]. As a consequence of the unopposed traction exerted by the surrounding lung parenchyma, destruction of the elastic and muscular elements of the bronchial walls causes airway dilatation. Accumulation of purulent secretions ensues. In the 1980s Cole proposed the ‘vicious cycle’ mechanism of on-going and self-perpetuating damage in bronchiectasis [5]: once airway dilation occurs, mucociliary clearance is impaired and the airway becomes at greater risk of repeated infection and further damage.
The age of onset of symptoms is variable, and depends on the underlying cause. For example, the majority of infants with primary ciliary dyskinesia have respiratory symptoms from the early neonatal period due to congenital impaired mucociliary clearance. Children with antibody deficiencies may not present until passive maternal immunity subsides. Early investigation of persistent cough is essential if interventions are to be implemented before irreversible bronchiectasis ensues.
Persistent cough is a cardinal feature of bronchiectasis; cough is generally described as wet or loose although in the authors’ experience parents will often incorrectly describe their child’s cough as dry. The sputum is typically purulent during recurrent or persistent infections. Reduced exercise tolerance and increased work of breathing may be present, but these are generally associated with advanced disease or infective exacerbations. Haemoptysis is relatively rare in children [6] but is more common in adults with bronchiectasis. Haemoptysis may occur as a consequence of dilating airways encroaching upon bronchial blood vessels or as a result of bronchial artery proliferation in association with chronic inflammation [7]. The bleeding is generally mild, presenting as streaks of blood in the sputum, but can be massive and fatal. Chronic alveolar hypoxia can cause right-sided heart failure. Other complications of bronchiectasis include empyema and pneumothorax.
In the past bronchiectasis commonly presented with wasting and anaemia, alongside prominent crackles and clubbing [8]. Probably due to earlier recognition of less severe disease, this picture is less often seen today. Examination may be normal or may include crackles, wheeze, or chest deformity. Clubbing is reported to occur in approaching 50 % of patients with bronchiectasis [9], and is an important, but not sensitive, distinguishing feature in patients with symptoms of wheeze or cough otherwise suggestive of asthma [10]. Individuals with bronchiectasis may wheeze, but in contrast to people with asthma, this is almost invariably associated with wet cough.
Epidemiology
The introduction of immunisations against pertussis in the 1950s and measles in the 1960s contributed greatly to the decline of bronchiectasis, as has the decline of pulmonary tuberculosis and the general improvement in social circumstances. The introduction of sensitive high resolution computed tomography (HRCT) techniques in the 1990s, capable of detecting minimally symptomatic disease, makes it difficult to compare historical data with more recent estimates. Prevalence in UK adults from the Bedford region in the 1950s was estimated to be in the region of 1 per 1,000 [2], more recent estimates from the US suggest a prevalence of 4 in 100,000 rising to 3 in 1,000 in older adults [11]. Paediatric population-based calculations from the United Kingdom estimated hospitalisation due to bronchiectasis to be 50 per 10,000 population at the beginning of the 1950s reducing to 10 per 10,000 with the introduction of antibiotic therapy [12]. During the 1960s the incidence of bronchiectasis was estimated to decrease further to 1 case per 10,000 population [13]. Recent data from New Zealand suggest a prevalence in children under the age of 15 years of around 4 per 100,000 [14], whilst data from a tertiary referral centre in the north east of England suggest the prevalence this age group may be as high as 1 per 5,800 [10]. Differences between nations may partly reflect genetic and environmental discrepancies, however, it is likely that the diagnosis of bronchiectasis in children is often delayed or never considered, making true prevalence difficult to establish.
Bronchiectasis is particularly prevalent in children from certain indigenous subpopulations, including Pacific Islanders, Indigenous Australians and the Inuit community in North America [14–16]. Poor access to antibiotics and immunisations may partially explain these differences, although it is likely that genetic propensity may play a role [17, 18]. Certainly, children of consanguineous parents are at a disproportionately high risk of genetic causes of bronchiectasis [19]. Moreover, although environmental, immune or anatomical factors may explain the observation that non-CF bronchiectasis is more common and more severe in females, this too may have a genetic basis [20].
The most common genetic cause of diffuse bronchiectasis is cystic fibrosis (CF), the incidence of which is estimated to be 1 in 2,500 births in white Caucasians. Reports of primary ciliary dyskinesia (PCD) prevalence in European populations have varied greatly from 1 in 10,000 to 1 in 40,000 [21]. As with most orphan diseases this variation is likely to reflect a lack of awareness of the disease amongst clinicians, absence of a gold standard test, and lack of facilities for investigation, leading to considerable under-diagnosis. A recent survey by a European PCD Taskforce suggested that PCD in children is under-diagnosed and diagnosed late, particularly in countries with low health expenditures [22]. The prevalences of other genetic causes of bronchiectasis are very low and are considered individually later it this chapter.
Genetic Causes of Diffuse Bronchiectasis
Bronchiectasis associated with genetic mutations is usually a consequence of recurrent or persistent pulmonary infection caused by disorders of mucociliary clearance or primary immunodeficiency (Table 4.1). More rarely disorders of cartilage or collagen are the underlying cause.
Disorders of Mucociliary Clearance
Ciliated respiratory epithelium lines the airways. The cilia, bathed in perciliary fluid, beat in a coordinated fashion at 11–18 Hz, to propel the overlying mucus along with particles and bacteria to the oropharynx where it can be swallowed or expectorated (Fig. 4.1). Diseases affecting ciliary function, or that change the composition of the periciliary fluid and mucus can impair mucociliary clearance, leading to recurrent infections and inflammation which predispose to bronchiectasis.
In healthy persons, respiratory cilia beat in a coordinated sweeping pattern, which moves mucus and debris, including pathogens towards the oropharynx for swallowing or expectorating. In PCD, immotile or dyskinetic cilia do not beat effectively, and mucus and debris persist in the airways. In CF the inefficient mucociliary clearance is due to an abnormal periciliary fluid layer compromising ciliary beating, and viscous mucus which is resistant to clearance (Image provided by Robert Scott)
Cystic Fibrosis
Cystic fibrosis (CF) is an autosomal recessive disorder, with an estimated incidence of 1 per 2,500 live births in Caucasian populations. It is caused by mutations in the cystic fibrosis trans-membrane conductance regulator (CFTR) gene which is located on chromosome 7 and encodes for the CFTR chloride channel on cell membranes. Mutations affect CFTR function by a variety of routes including complete loss of the protein, rapid breakdown of CFTR and surface expression of a dysfunctional protein. The most common mutation is ΔF508; a deletion of three nucleotides which results in loss of phenylalanine at the 508th position on the protein causing abnormal tertiary structure and rapid degradation with no apical membrane expression. A very large number of mutations (>500) have been identified in the CFTR gene, but a select group are more commonly associated with disease (e.g. G542X, G551D, N1303K). The prevalence of mutations differs between ethnic populations.
Abnormalities in CFTR cause abnormal ion transport regulation across the cell membrane. In the lungs this results in abnormal airway fluid. Dehydrated mucus is characteristically highly viscoelastic, and adheres to the cilia and airway cells, causing airway plugging. The reduced-volume periciliary fluid layer does not adequately support and lubricate the cilia, and results in defects of ciliary function (Fig. 4.1). Adherent mucus and impaired ciliary function both contribute to reduced airway clearance, chronic infection and biofilm formation. Eventually the chronic infection and inflammation lead to bronchiectasis, which can develop very early in life [23]. Bronchiectasis in CF predominantly affects the upper lobes initially, spreading to all lobes over time. The reasons for upper lobe predominance have been suggested to include aspiration, poor clearance by cough and poor lymphatic clearance to this region, although the disparity with PCD which tends to have worse disease in the middle lobe is difficult to explain.
Although respiratory disease accounts for the vast majority of morbidity and mortality [24]. CF is a multisystem disorder, with manifestations including meconium ileus, pancreatic insufficiency, steatorrhea, failure to thrive, liver disease, diabetes, nasal polyposis and sinusitis. Most cases present before the age of 2 years although later presentation, including in adulthood, is not unheard of particularly in individuals carrying mutations other than ΔF508 who are more likely to be pancreatic sufficient and less likely to have diabetes or pseudomonas colonisation [25]. Diagnosis of classical cases of CF is based on an abnormal sweat test (sweat chloride > 60 mmol/L). Supplementary investigations include CFTR mutations and abnormal transepithelial potential difference.
The majority of patients with CF are easy to diagnose early as they have classical lung disease ± pancreatic insufficiency associated with two CF associated CFTR mutations and an abnormal sweat test result (>60 mmol/l). A further group of patients with mild lung disease and pancreatic sufficiency is confidently diagnosed, often in adulthood, on the basis of a diagnostic sweat test and two disease causing mutations. The group that cause particular difficulty are those with clinically milder phenotypes associated with a variety of equivocal outcomes from genotype and functional investigations (sweat test and nasal potential difference). This is a difficult and controversial area for clinicians and recently international consensus guidelines have been proposed to help in the diagnosis of patients with “CFTR dysfunction that does not fulfil diagnostic criteria for CF” [26]. These single organ disease states are becoming recognised as a spectrum of CFTR related disorders. Very many mutations have been identified in CFTR, but not all are associated with disease and it has been suggested that some only cause disease when interacting with certain environmental factors or other genes. Some patients present with two known disease-causing mutations, but an equivocal sweat test result. These patients generally have more severe lung disease than those with an equivocal sweat test and normal genotype, but they have milder disease than a patient with the same genotype and sweat chloride concentration >60 mmol/l. This highlights different phenotypes associated with a spectrum of test results, indicating the need to perform functional tests even in the presence of two mutations. It is probably appropriate to consider patients with mild CF-like disease associated with two mutations and a normal sweat test as an atypical CF variant. Another group that cause diagnostic uncertainty are those with only one CFTR mutation. These patients may have atypical CF. However the diagnosis of mild or atypical CF with equivocal CF results should only be made after all other causes of bronchiectasis have been excluded. It is prudent to keep the diagnosis under review as our understanding of CFTR related disorders evolves.
Primary Ciliary Dyskinesia
After CF, primary ciliary dyskinesia (PCD) is the most prevalent (1:10–40,000) genetically determined cause of impaired mucociliary clearance. PCD is inherited in an autosomal recessive pattern, and is characterised by chronic infection of the upper and lower airway [21, 27]. The impaired mucociliary clearance is a consequence of abnormal ciliary beat function which is usually [27], but not always [28, 29], associated with abnormal ciliary ultrastructure seen by transmission electron microscopy (Fig. 4.2a–d). Motile cilia are important in organ systems besides the respiratory tract, such as the embryonic node, sperm flagella (Fig. 4.2e), the female reproductive tract, and ependyma of the brain and spinal cord. PCD patients therefore often have extra-pulmonary symptoms caused by dysmotile cilia such as serous otitis media, infertility and rarely hydrocephalus. The cilia of the embryonic node, responsible for left-right asymmetry, are similar in structure to respiratory cilia. Embryonic node dysfunction in PCD causes situs inversus (termed Kartagener’s syndrome) in 50 % of cases (Fig. 4.3a, b) and is associated with congenital heart disease in approximately 6 % of cases [30]. Neonates typically present with respiratory distress of unknown cause and rhinitis. As infants, patients have a persistent wet cough, recurrent respiratory tract infections, rhinitis, and frequently glue ear associated with conductive hearing difficulty. Patients frequently develop sinusitis (Fig. 4.3c). These symptoms may not always be recognised as indicative of PCD and although the mean age of diagnosis is approximately 4 years [31], the range is considerable and reflects local expertise and clinical suspicion. Respiratory symptoms continue into later childhood and adulthood, with many patients developing bronchiectasis, commonly affecting the middle and lower, rather than upper lobes as in CF.
(a) Diagram of transverse section of a respiratory cilium as seen by transmission EM. Motile cilia in the respiratory tract and fallopian tubes have a highly organized “9 + 2” arrangement with nine peripheral microtubule doublets surrounding a central pair of single microtubules running the length of the ciliary axoneme. Nexin and radial spokes maintain the organized structure. Attached to the peripheral microtubules are inner and outer dynein arms. Dynein is a mechanochemical ATPase and generates the force for ciliary beating, hence abnormalities of the dynein arms affect ciliary beating. Transmission EM of a respiratory cilia from (b) a healthy individual and patients with PCD due to (c) an outer dynein arm defect and (d) a radial spoke defect. (e) TEM of a sperm demonstrates similar “9 + 2” ultra-structure (Cartoon image provided by Robert Scott; EM images obtained using FEI Tecnai 12 transmission electron microscope (FEI UK Limited, Cambridge, UK) at 80 kV). Scale bars 580 nm. EM images provided by P. Goggin (Primary Ciliary Dyskinesia Group, University Hospitals Southampton NHS Foundation Trust, Southampton, UK)
HRCT of the chest in a 49-year old man with primary ciliary dyskinesia and Kartagener syndrome at the time of first evaluation for lung transplantation, demonstrating numerous bronchiectases and consolidation in the anterior lateral segment of the left lower lobe (a), and bilateral bronchietasis in the lung bases (associated with centrilobular nodules and tree-in-bud pattern suggestive of bronchiolitis). Note the presence of situs inversus (b). Pansinusitis was present in the same patient (c) (Courtesy of Pr V. Cottin, University of Lyon, France)
The diagnosis of PCD requires specialist investigation, and a number of different tests should be available, since no one investigation can be considered confirmatory [27, 32].
Extremely low levels of nasal nitric oxide are supportive of the diagnosis of PCD [33]. However, some patients with PCD have been described with normal nitric oxide levels, and levels can be low in other conditions including CF. Nasal nitric oxide measurement should therefore only be used as a screening test, and if clinical suspicion is high, further diagnostic investigation should be conducted even if the levels are normal [21].
Patients should have their respiratory epithelial cells visualised by high speed video microscopy for evidence of ciliary dysfunction (e.g. static, dyskinetic, or vibratory cilia). Subtle abnormalities may be significant, but difficult to recognise; facilities should therefore be based at centres with expertise and a high throughput of PCD diagnostic patients. Following abnormal high speed video microscopy analysis, confirmation of diagnosis by an additional method is required because ciliary dysfunction can be secondary, for example to infection. Most, patients with PCD have diagnostic abnormalities of ciliary ultrastructure on transmission electron microscopy and this used to be considered the ‘gold standard’ investigation for PCD (Fig. 4.2). However, it is now recognised that at least 15 % of patients with PCD have normal ciliary ultrastructure and this investigation should not be used in isolation. Transmission electron microscopy analysis requires expert interpretation.
Air liquid interface cell culture techniques are particularly helpful where secondary ciliary defects are suspected, for example due to chronic infection. Re-differentiation of basal epithelial cells to ciliated cells is achieved by culturing the cells, allowing a repeat HSV analysis and electron microscopy having reduced environmental factors that compromise ciliary function for example pollution, infection. However, air liquid interface culture is technically demanding and time consuming; it is routinely available in a small number of specialist centres.
Immunofluorescence microscopy analysis, is a promising technique to help in the clinical diagnosis; it is based on the ability to detect and localise intra-ciliary proteins e.g. DNAH5 by immunofluorescence. However, this method is only routinely available at one centre which is based in Germany.
Genetic testing is currently only able to identify perhaps 50 % of PCD cases, but it is an active area of international research with potential for significant advances. Whilst CF is caused by mutations in one gene, PCD is polygenic with over 200 proteins involved in the formation of cilia. Additionally the disease-causing genes are large, making genetic diagnosis of PCD a challenge. Mutations in 24 genes have been associated with PCD to date, mainly encoding for dynein arm components (reviewed in [32, 34], and summarised in Table 4.2). The two commonest mutations, dynein, axonemal, heavy chain 5 (DNAH5) mutation [37] and dynein arm intermediate chain 1 (DNAI1) mutation [35], have a combined prevalence of 17–35 % in patients with PCD and are associated with static cilia on light microscopy, and dynein arm deficiencies on electron microscopy. Other known mutations are all rare. Mutations in the kintoun gene (KTU) [43], which is required for cytoplasmic pre-assembly of axonemal dyneins results in a similar ciliary phenotype to DNAH5. Mutations in the radial spoke head genes RSPH9 and RSPH4A have been reported in PCD patients with abnormalities of the central microtubular pair causing an abnormal rotating movement of the cilia [56]. Patients with mutations in the dynein axonemal heavy chain 11 (DNAH11) have hyperkinetic vibratory cilia, but apparently normal ultrastructure on electron microscopy [39]. There are therefore a variety of ciliary phenotypes seen on microscopy depending on the responsible mutations, but all result in a severe mucociliary clearance impairment, and clinical phenotype is indistinguishable.
In extremely rare cases, PCD has been genetically linked to other ciliopathies. X-linked recessive retinitis pigmentosa, sensory hearing deficits, and PCD have been associated with mutations in the Retinitis Pigmentosa guanosine triphosphatase regulator (RPGR) [63]. A single family has been reported with a novel syndrome that is caused by Oral-facial-digital type 1 syndrome (OFD1) gene mutations characterized by X-linked recessive mental retardation, macrocephaly, and PCD [62].
Young’s Syndrome
Young’s syndrome, or Barry-Perkins-Young syndrome, is a clinical entity characterised by bronchiectasis, chronic sinusitis and impaired fertility. The diagnosis is considered most commonly in middle aged men presenting with infertility. Although Young’s syndrome has been reported in identical twins prompting suggestions of a genetic aetiology, there is some evidence that this syndrome has declined following the removal of mercury from teething powders and worm medicines in the 1950s [64]. Moreover, whilst Young’s syndrome and other disorders of mucociliary clearance have partially overlapping symptomatology, a common causative link has not been identified. Indeed the mechanism of reduced fertility in Young’s appears to be due to functional obstruction of sperm transport down the epididymal tract rather than due to absence of the vas deferens which is seen in CF or to dysmotility seen in PCD. There are reports that mucociliary clearance is impaired in Young’s syndrome [65] but ciliary ultrastructure is largely normal [66]. Similarly, patients with Young’s are not frequently carriers of common CF mutations [67], although possibly there may be an association with atypical CF mutations [68]. Some authors have even questioned whether Young’s syndrome exists as a recognised clinical entity at all [69], and it is quite possible that as diagnoses of rare variants of CF are established, Young’s syndrome will disappear as a diagnosis.
Other Ciliopathies
Non-motile or ‘primary’ cilia are found on the surface of many cells in the body. An increasing number of diseases are attributed to abnormal motile or primary ciliary function, collectively known as ciliopathies (http://www.ciliopathyalliance.org). For example, in the eye and kidney ciliopathies can cause retinitis pigmentosa, autosomal dominant polycystic kidney disease (ADPKD) or nephronophthisis. Primary cilia are similar in structure to the respiratory epithelial cilia (9 + 2), but in cross section, there is no central pair of microtubules (9 + 0). There are several case reports of patients with PCD-like disease in association with retinitis pigmentosa [70, 71]. In some patients it may be that both primary and motile cilia are impaired. In others it may be that the problem is purely of primary cilia, with abnormalities of primary pulmonary cilia causing lung disease. This needs further evaluation.
Autosomal dominant polycystic kidney disease (ADPKD) is an example of renal ciliopathy associated with bronchiectasis. It affects between 1 in 400 and 1 in 1,000 people [72]. The disease most commonly manifests in adulthood and is caused by defective ciliary function in renal epithelial cells. Two genes, PKD1 and PKD2 coding for proteins known as polycystins have been implicated in the pathogenesis of ADPKD. In ADPKD, impaired primary cilial sensing results in abnormal intracellular signalling, cell hyperproliferation, and cyst formation [73]. A predisposition to bronchiectasis is recognised, although the mechanism for this is not fully understood [74].
Alpha-1 Antitrypsin Deficiency
An association between alpha-1 antitrypsin (AAT) deficiency and emphysema is well-known but there are also reported cases of an association with severe PiZ genotypes and bronchiectasis, this is largely seen in adulthood. A study of 74 patients with severe AAT deficiency found high resolution CT scan evidence of bronchiectasis in 70 subjects and judged this to be clinically significant in 20 [75]. AAT deficiency alleles are over represented in patients with bronchiectasis and asthma combined, suggesting that bronchiectasis may occur as a consequence of airway obstruction, in turn reducing airway clearance [76].
Disorders of Immunity
Immunodeficiency accounts for a significant proportion of cases of bronchiectasis in developed countries. Recent series suggest as many as 7 % of adults [77] and 20–30 % of paediatric cases in the developed world may be attributable to primary immunodeficiency [1, 10]. Immunodeficiency should be considered following respiratory infections that are unusually severe, recurrent, unresponsive to conventional treatment, or atypical [78]. Common associated features include failure to thrive, severe atopic disease, and occasionally, auto-immune disease [79]. Primary immunodeficiencies include a heterogeneous group of disorders of immune development or function affecting innate or adaptive immunity. Common variable immunodeficiency (CVID), X-linked agammaglobulinemia (XLA) and chronic granulomatous disease (CGD) are the most common immunodeficiencies found in association with bronchiectasis [1, 10]. Disorders of innate immunity are currently poorly characterised but are likely to be responsible for a proportion of cases of bronchiectasis currently labelled ‘idiopathic’.
Common Variable Immunodeficiency
Common variable immunodeficiency (CVID) has an estimated prevalence of 1 per 25,000 population and is the commonest immunodeficiency. Presentation is usually in young adulthood but diagnosis may be delayed [80]. Patients affected by CVID display a defective antibody response to protein and polysaccharide antigens and low IgG, IgM and IgA levels. This places affected individuals at risk of recurrent bacterial infection as well as autoimmune disease and malignancy [81]. Clinical features vary in CVID, perhaps reflecting heterogeneity of the molecular defects underlying the disease. Susceptibility loci for CVID have been identified within the MHC class II alleles [82], and polymorphisms in the tumour necrosis factor gene have been also been reported in association with CVID [83]. Mutations associated with CVID have been identified within the inducible costimulator (a molecule expressed by T cells) [84], CD19 (a T cell marker important in B cell development, activation and proliferation) [85], and in transmembrane activator and CAML interactor (a receptor believed to be important in antibody responses to type II T-independent antigens) [86].
In individuals with CVID at least one episode of pneumonia has usually occurred before diagnosis of CVID is made [81]. Possibly as a consequence of delayed diagnosis, the risk of bronchiectasis is greater in patients with CVID than in those with X-linked agammaglobulinemia or other immunodeficiency diseases and may approach 70 % [87]. Bronchiectasis tends to be more common in the lingual, middle and lower lobes. The threshold for HRCT imaging should be low in cases of known or suspected immunodeficiency, particularly if there are signs of persisting lung disease [78].
X-Linked Agammaglobuinemia
X-linked agammaglobuinemia (XLA) is characterised by almost complete absence of circulating B lymphocytes and of all immunoglobulin [88]. Mutations of the Bruton’s tyrosine kinase gene cause an incomplete block of B cell development at the pre-B cell stage [89]. This leaves affected patients susceptible to infection by encapsulated bacteria and mycoplasma [90]. More recently autosomal recessive forms of agammaglobulinemia have been identified that are caused by mutations in genes that encode for other components of the pre-B cell receptor and its signalling pathway [91]. Pulmonary infections can occur shortly after birth but generally become noticeable beyond 6 months of age following the disappearance of maternal IgG. The most common age for diagnosis is under a year but presentation as late as 5 years has been known [92]. Although a recent survey has found less than a third of adults with XLA to be severely affected by bronchiectasis [93], there is evidence to suggest that the risk of developing significant lung disease increases over time and can be reduced by early detection and treatment [94]. Chronic lung disease, including bronchiectasis, has also been observed in children with IgA deficiency, particularly when associated with IgG2 deficiency [95].
Chronic Granulomatous Disease and Other Disorders of Neutrophil Function
Chronic granulomatous disease (CGD) results from impaired function of NADPH oxidase. This enzyme is required for effective functioning of the phagocytic respiratory burst and for superoxide production. Impaired NADPH oxidase is generally transmitted by X-linked inheritance but autosomal recessive variants are also recognised [96]. Mean age at presentation in autosomal recessive disease is 10 years, slightly later than X-linked disease where the mean is 5 years suggestive of a more severe phenotype [97]. Sufferers are vulnerable to recurrent and severe bacterial and fungal infections, frequently Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia and Aspergillus spp.
A similar pattern of recurrent pneumonia and lung aspergillosis may also be observed in patients with severe congenital neutropenia [98]. Most commonly this disorder occurs as a consequence of mutations in the gene encoding for neutrophil elastase [99] but can also be attributable to mutations affecting a mitochondrial protein thought to be involved in protecting myeloid cells from apoptosis [100] or an endosomal protein involved in intracellular signalling [101]. The characteristic feature of this disease is low levels of circulating neutrophils and hence vulnerability to bacterial and fungal pathogens.
Bronchiectasis, alongside severe pneumonias, empyemas and pneumatoceles, is common in children with hyper-IgE or Job’s syndrome, in which neutrophil defects occur in combination with a wide variety of lymphocyte and humoral function defects as well as very high levels of serum IgE [102]. Job’s syndrome is inherited in an autosomal dominant pattern. Mutation of the signal transducer and activator of transcription 3 or STAT3 gene is known to cause Job’s syndrome [103], although in some cases the responsible mutation is unknown. STAT3 is a transcription factor which influences the expression of a variety of genes and plays a key role in many cellular processes such as growth and apoptosis. An autosomal recessive form of Job’s syndrome is recognised, this is less common than the autosomal dominant form and less likely to have respiratory complications. Bronchiectasis is also seen in association with Shwachman-Bodian-Diamond syndrome, a rare autosomal recessive disorder characterised by exocrine pancreatic insufficiency, bone marrow dysfunction, leukemia predisposition, and skeletal abnormalities. In most cases, Shwachman-Bodian-Diamond syndrome is associated with mutations in the Shwachman-Bodian-Diamond Syndrome (SBDS) gene located on chromosome 7 [104]. Bone marrow dysfunction results in neutropenia in the majority of patients and may be accompanied by defects in neutrophil mobility, migration, and chemotaxis.
Other Immunodeficiency Diseases Associated with Bronchiectasis
It is currently difficult to identify many causes of innate immunodeficiency, and it is likely that as new defects are discovered, many cases currently labelled as suffering from ‘idiopathic bronchiectasis’ will have an underlying cause. Rare instances of bronchiectasis in association with deficiency of C2, or mannose binding lectin, have been reported and an association between deficiency of l-ficolin and bronchiectasis has been reported in adult patients [105]. Complement deficiency is also known to affect the severity of bronchiectatic disease in CVID [106] and in CF [107].
Ataxia telangiectasia is an autosomal recessive multisystem disorder resulting from mutation of the Ataxia telangiectasia mutated (ATM) gene, characterised by the development of telangiectasia and cerebellar ataxia. It is the most common of the DNA repair disorders and is associated with chromosomal instability and cellular radiosensitivity rendering sufferers susceptible to cancer and to infection. The ATM gene is involved in antibody class switch recombination and defects in this process may underlie the increased susceptibility of ataxia telangiectasia patients to bacterial infections [108]. The most common humoral immunological defects are diminished or absent serum IgA and IgG2, and impaired antibody responses to vaccines [109]. Ataxia telangiectasia leads to thymic hypoplasia and variable T cell deficiency. It is likely that recurrent aspiration due to swallowing impairment also contributes to respiratory disease [110]. Fifty percent of patients die in adolescence from overwhelming bronchopulmonary disease [109].
Collagen Disorders
Although the majority of causes of bronchiectasis are related to defects of mucociliary clearance or immunodeficiency, congenital abnormalities affecting the structure of the bronchial wall can predispose to bronchiectasis. In particular, a number of congenital syndromes of collagen and cartilage abnormalities have been associated with bronchiectasis. This suggests that abnormally compliant or distended bronchi can predispose to bronchiectasis.
Marfan Syndrome
Marfan syndrome is a rare hereditary disorder characterised by skeletal, cardiovascular and ocular abnormalities. Pulmonary abnormalities occur in approximately 10 % of patients, the commonest being spontaneous pneumothorax and emphysema [111]. Although rare, cases of bronchiectasis in adults [112] and children [113] who have Marfan syndrome have been described. Marfan syndrome is autosomal dominantly inherited with variable expression; features of the condition arise as a consequence of a defect in Type 1 collagen. The defect in collagen may be responsible for the reduced tensile strength of the connective tissue leading to bronchiectasis and increased susceptibility to infection.
Other Collagen Disorders
In the 1960s Williams and Campbell first described a series of children with bronchiectasis who had a bronchial cartilage deficiency from the third division with normal trachea and central bronchi [114]. It is hypothesised that the compliant bronchi collapse during coughing, leading to poor airway drainage [115]. The familial pattern and the early onset of symptoms support the possibility that this is a rare congenital syndrome [116]. Williams-Campbell syndrome has most frequently been described in children although adult cases have been reported. CT imaging demonstrates bilateral bronchiectasis affecting segmental and subsegmental bronchi [117]. Cartilage deficiency is not evident outside of the bronchi and the cause of the deficiency is not well understood. It has been hypothesised that children who are born with a bronchial cartilaginous defect develop pathology after suffering a viral respiratory infection in infancy. As a precise genetic defect has not been identified and solitary cases have been reported with greater frequency than familial, the genetic basis of Williams-Campbell syndrome is debateable, indeed secondary cartilaginous damage due to infection and inflammatory change cannot be completely excluded.
A contrasting condition characterised by dilated trachea and large bronchi was first described by Mounier-Kuhn in 1932 [118]. This idiopathic tracheobronchomegaly is associated with varying degrees of bronchiectasis varies in age and severity at presentation but usually presents in mid-adulthood. Males are more frequently affected than females, and a racial predominance has been proposed [119]. The trachea may have a ridged appearance with multiple diverticula. Dynamic collapse of the enlarged airways in expiration, particularly posteriorly, is also likely [120] and pooling of secretions may predispose to lower respiratory tract infections [118]. The aetiology of Mounier-Kuhn syndrome remains uncertain, although histological data suggest that tracheal and bronchial dilatation occur as a consequence of abnormal development of airway connective tissue, with a deficiency of elastic and muscular fibres [121]. Tracheobronchomegaly occurs in association with other congenital connective tissue disorders, including Ehlers Danlos syndrome [122] and cutis laxa [123]. Together with reports of the disorder occurring in siblings [119] this makes an unidentified genetic cause possible, at least in some cases. Generally, there is no curative treatment for the syndrome, although the use of tracheobronchial prostheses may improve symptoms in adults [124].
Other Genetic Predispositions to Bronchiectasis
Bronchiectasis has been reported in association with autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, relapsing polychondritis and inflammatory bowel disease [125]. A genetic association in ulcerative colitis-associated bronchiectasis has recently been reported with functional polymorphisms in the cytokine IFNγ and the neutrophil chemokine, CXCR1 [126]. Yellow nail syndrome is a rare disorder observed in association with a number of systemic diseases including rheumatoid arthritis, and also reported in cases of tuberculosis, immunological disorders and malignancies [127]. This syndrome is characterised clinically by dystrophic yellow nails, lymphoedema and pleural effusion [128]. A significant number of patients also have sinusitis [129], lower respiratory tract infections [130], and bronchiectasis [131]. The syndrome is most often seen in middle-aged individuals although a case report has described bronchiectasis in a 6-year-old [132]. Congenital lymphatic malformation and dysfunction is believed to be responsible for the syndrome [133], although a selective deficiency of humoral immune function has also been suggested [134]. Whilst the exact aetiology is unknown, a genetic component has been proposed [135].
Idiopathic Bronchiectasis
An underlying cause cannot be found in at least 30 % of patients with bronchiectasis, these cases are referred to as idiopathic [1, 10, 14, 77]. Some cases are familial and it is likely that a number of patients have unrecognised impairment of the innate immune system, or have one of the increasingly recognised CFTR mutations associated with milder CF phenotypes causing isolated lung disease. CFTR mutations have been found to be overrepresented in individuals identified as suffering from idiopathic bronchiectasis who do not have a full CF phenotype. The 5 T CFTR mutation in particular has been found at high frequency in this patient group. Recent data suggest that the 5 T polythymidine tract sequence of intron 8 on specific haplotype backgrounds (TG12 and M470V) may underlie low levels of full-length functional CFTR protein and cause CF-like lung disease [136].
Diagnosis
A number of international guidelines exist for the diagnosis and management of bronchiectasis in adults and children [15, 78, 137]. Ideally patients at risk of bronchiectasis should be identified before irreversible damage develops. For example, it is widely accepted that newborn screening for CF, with early introduction of prophylactic treatment, and aggressive management of infections, reduces long-term pulmonary morbidity [138–140]. Similarly in PCD, observational data suggests that lung function decline is stabilised and can even be reversed following diagnosis and instigation of appropriate pulmonary management [141–143].
A diagnostic approach to a patient with symptoms suggestive of bronchiectasis is summarised in Fig. 4.4. HRCT is the gold standard investigation for bronchiectasis, but a number of non-specific investigations assist evaluation of disease severity. A history of recurrent or prolonged wet cough is suggestive of significant endobronchial infection which may progress to bronchiectasis. Individuals in whom bronchiectasis should be suspected include those with a persistent cough for at least 8 weeks [78]. Intermittent hemoptysis is common, particularly in adults. Finger clubbing and persistent wet crackles are indicative of severe disease.
Investigation of patients with prolonged lower respiratory tract symptoms should usually include a standard posterolateral chest x-ray and culture of sputum. Dilated airways with thickened walls are sometimes visible either on chest x-ray as parallel ‘tram tracks’ or ‘ring shadows’. Fluid-filled bronchi may be visible as ‘gloved-finger’ shadows. Situs inversus might direct investigations for PCD (Fig. 4.5). However chest x-ray is not a sensitive test for bronchiectasis, and if the diagnosis is suspected, further investigation is warranted. Patients should have sputum cultured; Staphylococcus aureus, Haemophilus influenzae, Pseudomonas aeruginosa, non-tuberculous mycobacteria or Burkholderia cepacia are suggestive of significant lower respiratory tract pathology [78]. Measures of lung function are non-specific and non-sensitive in bronchiectasis, but contribute to the assessment of disease severity. Forced expiratory volume in 1 s (FEV1) is often normal in early disease although a reduced FEV1 in the presence of normal functional vital capacity (FVC) is common. Standard spirometry is not a good measure of disease decline, and alternative measures are sought. Lung clearance index is a measure that has been suggested to be a good monitor of disease in CF [144] and is being evaluated as an early marker of lung disease in other bronchiectatic diseases such as PCD [145].
High-resolution computed tomography (HRCT) has sensitivity and specificity in excess of 90 % [146] and may detect signs of bronchiectasis not identified by plain film imaging [147]. For this reason HRCT is the gold standard diagnostic investigation (Figs. 4.6a, b and 4.7). Diagnostic radiological criteria were described by Naidich et al. in 1982 [148] and are also summarised in the Fleischner Society: glossary of terms for thoracic imaging [149]. Characteristically enlarged bronchi seen in cross-section are larger than the accompanying arteries giving rise to the ‘signet ring’ sign. Other characteristic features include dilated airways with air-fluid levels that do not taper and remain visible in the extreme lung periphery [150]. Expiratory HRCT images can be useful. Parenchymal hypoattenuation may identify small airways disease due to air-trapping associated with mucous-filled bronchi in bronchiectasis and evidence of bronchial wall or tracheal collapse can also be detected, providing an alternative investigation to bronchoscopy [151]. HRCT should not be performed during acute respiratory exacerbations as bronchial dilation is difficult to assess in the presence of consolidated lung, whilst pulmonary collapse can cause misleading ‘traction bronchiectasis’ by pulling on neighbouring bronchi [152].
Fibreoptic bronchoscopy should be considered in patients with a prolonged wet cough to assess airway structure and calibre and exclude pathology such as severe tracheomalacia, bronchomalacia or tracheal bronchi which may contribute to bronchiectatic change. Bronchoscopic examination can also provide lavage fluid for evidence of chronic aspiration measured by pepsin, amylase or fat-laden macrophages, and for culture and microscopy.
Once a diagnosis of bronchiectasis has been made, investigation of the underlying cause should be sought. The clinical history should direct investigations which will usually include investigation for CF, PCD and immunodeficiency (Table 4.3).
Treatment
The overall aims of management should be to treat inflammation and to maximise lung function, exercise tolerance, quality of life and nutrition [78]. In children, it is estimated that identification of a specific cause of bronchiectasis prompts a management change in over 50 % of cases [157]. Identification and prompt treatment of any underlying cause is an important treatment aim as it can significantly limit lung damage and improve prognosis [157]. Treatment is directed at reducing exacerbation frequency as this has a positive impact upon quality of life and may prevent disease progression [158]. Treatment includes promoting clearance of secretions and use of antibiotic therapies both to prevent and to treat recurrent infection. In addition to routine vaccination schedules, patients should receive pneumococcal and influenza vaccinations and avoid exposure to tobacco smoke. It is also important to achieve and maintain an adequate nutritional status in order to support immune function and tissue repair; iron deficiency, in particular, should be avoided as it is known to increase the risk of pneumonia. Treatment is best delivered within a multidisciplinary team where specialist medical, nursing, pharmacy and dietetic expertise can be maintained and promoted. Input from genetic counsellors may be particularly helpful around the time of diagnosis and when planning future children.
Many management strategies focus upon common mechanisms underlying bronchiectatic disease, however, these strategies are often based upon an extrapolation of treatments proven to be effective in the more common disorders such as CF and may not be appropriate for other forms of diffuse bronchiectasis of genetic origin. Specific randomised controlled trials are required, which by necessity for rare diseases will be multi centre and likely require international collaboration. Underlying pathophysiology is different between diseases and optimal treatment is therefore likely to differ; appropriate outcome measures are similarly likely to differ.
Airway Clearance
A variety of physiotherapy techniques are available to assist airway clearance, including chest percussion, postural drainage, breathing exercises and mechanical interventions such as cough-assist and airway oscillation devices. The aims of physiotherapy include mobilising and aiding clearance of secretions, and maximising ventilation efficiency and exercise tolerance. Although physiotherapy is of proven benefit in CF [159] there are few studies demonstrating the efficacy of physiotherapy in non-CF paediatric bronchiectasis that can guide frequency or choice of therapy. Generally it is believed that all patients should be taught a suitable airway clearance technique for use during exacerbations and those patients with a chronic productive cough should be encouraged to conduct regular chest physiotherapy [78].
The most commonly used airway clearance technique, particularly in adults, is the active cycle of breathing technique; this is based upon deep breaths followed by ‘huffs’ and ‘coughs’ to aid sputum clearance interspersed with periods of relaxed controlled breathing. The active cycle of breathing can be combined with postural drainage and manual techniques. CT scanning can help to identify affected bronchopulmonary segments so that appropriate postural drainage positions can be selected. Postural drainage positions for the mid and basal zones of the lung require a head-down tilt which may be uncomfortable for the breathless patient; modified horizontal positions for postural drainage and the use of non-invasive ventilation during tilt manoeuvres have been suggested as potential solutions to this problem. Positive end expiratory pressure (PEP) techniques provide an alternative to physiotherapy based upon the active cycle of breathing techniques; there is evidence that oscillating PEP devices, such as a Flutter valve or Acapella, when combined with postural drainage and the forced expiration technique are equally as efficacious in terms of sputum clearance as a combination of active cycle of breathing and postural drainage [160]. A further alternative is the technique of autogenic drainage in which a sequence of controlled breaths at low then progressively higher lung volumes is used to collect and expectorate sputum. Whilst autogenic drainage has been demonstrated to be superior to no physiotherapy it is unclear how this technique compares to others in terms of objective outcomes such as sputum clearance or airway resistance [161]. Given the absence of a clear superiority of any one technique, patient acceptability is an important determinant of the techniques employed, the Flutter, for example, often being preferred over active cycle of breathing by patients who perceive it to be less time-consuming [160].
In CF, airway clearance during physiotherapy may be improved by use of humidification or inhaled hyperosmolar agents such as hypertonic saline or mannitol prior to treatment. Hypertonic saline is thought to induce liquid flux from the epithelium into the mucus layer, altering its rheology such that it is cleared more easily. Hypertonic saline should be used with caution in patients with significant reactive airways disease because of the potential for bronchoconstriction. Treatment with hypertonic saline, however, has been shown to improve secretion clearance above that achieved by either nebulised saline, nebulised terbutaline or a combination of both treatments in individuals with stable non-CF bronchiectasis [162]. In contrast, the benefit associated with aerosolised recombinant DNase treatment in non-CF bronchiectasis appears to be limited, possibly because there are fewer airway neutrophils in non-CF bronchiectasis. Nebulised DNase is therefore not generally recommended [78, 163], although anecdotal accounts of successful DNase treatment of PCD patients have been reported [164, 165].
Bronchodilators are likely to be of greatest benefit when bronchial hyperreactivity is demonstrable. There is some evidence that bronchodilator therapy may improve ciliary beat frequency and thereby aid airway clearance [166, 167], but no randomised controlled studies have demonstrated a therapeutic effect in either paediatric or adult bronchiectasis [168–171].
There is little research on the effects of physical exercise in patients with bronchiectasis and less still on those with non-CF bronchiectasis. Data from a limited number of studies suggest that exercise training has a positive effect on exercise capacity, strength and lung function in patients with CF [172], and that pulmonary rehabilitation and inspiratory muscle training may improve endurance and health-related quality of life [173]. It is not, however, possible to make recommendations regarding the particular type of exercise most likely to be beneficial. In the authors’ experience exercise that the patient enjoys is beneficial in maintaining lung health and improving quality of life.
Management of Infections
Antibiotic therapy may be used continuously as prophylaxis or intermittently in response to exacerbations. In infants with cystic fibrosis diagnosed following newborn screening, prophylactic flucloxacillin treatment has been shown to reduce cough and hospital admission in the first 2 years of life [174]. In adults with non-CF bronchiectasis long-term high dose oral amoxicillin has been shown to reduce airway inflammation [175] and long-term macrolide treatment, for example with azithromycin, has also been suggested to be a beneficial approach [176]. Macrolides have additional effects beyond their anti-bacterial actions which may be useful in the context of bronchiectasis. Macrolide therapy can reduce inflammatory cytokine release [177] and neutrophil influx and are also believed to reduce biofilms. [178] There is evidence that long-term prophylactic antibiotic therapy is effective in reducing sputum volume and exacerbation frequency [179, 180]. There is a concern that long-term use of antibiotics may accelerate the development of antibiotic resistance, particularly in the case of long-term quinolone prophylaxis for patients colonised with Pseudomonas [181]; regular testing of sputum for culture and sensitivity is essential. In patients chronically colonised with Pseudomonas aeruginosa nebulised colistin may improve quality of life and slow the rate of lung function decline [182].
Antibiotic choice should reflect the fact that Streptococcus pneumoniae, Moraxella catarrhalis and non-encapsulated Haemophilus influenzae account for the majority of positive isolates but treatment choice should be individualised based upon the patient’s history of infection. Persistent pseudomonas infection is less common in children the United Kingdom and Europe [10] than in adult patients with non-CF bronchiectasis. Inhaled tobramycin has been demonstrated to reduce Pseudomonas aeruginosa load in sputum [183]. In patients with immunodeficiency, antibiotic therapy needs to reflect the pathogens to which a particular disease confers vulnerability [184]. Co-trimoxazole and itraconazole prophylaxis are required in CGD, for example.
Antibiotics are recommended for exacerbations that present with an acute deterioration with worsening cough or increased sputum volume or purulence over several days [78]. there are no randomised placebo-controlled trials of antibiotic use in infective exacerbations of bronchiectasis. During exacerbations, antibiotic choice is usually empirical; initially, selection should be based upon local microbial patterns, sensitivities and cost. Previous culture results may also inform antibiotic choice, although if there is no previous bacteriology amoxicillin is a reasonable first choice [78, 185]. High dosages for relatively prolonged periods may be necessary to achieve bactericidal antibiotic levels in the endobronchial mucus [186]. Generally a 14 day course is recommended. Before starting antibiotics a sputum sample should be sent for culture and antibiotic prescription modified depending upon the result. However, sputum culture is possible only in adults and older children who are able to expectorate. In younger children cough swabs or nasopharyngeal aspirates can be taken for bacterial culture, although upper airway specimens are inferior and bronchoalveolar lavage specimens may be required. Antibiotic cover may be needed even during viral exacerbations to prevent an increase in bacterial load as a consequence of the reduced lysozyme release and bactericidal activity associated with such exacerbations [187].
Anti-inflammatory Management
Inhaled corticosteroids have been employed with the aim of reducing inflammatory damage in the lung and systemic corticosteroids may be used to treat acute exacerbations of reactive airways disease. Randomised controlled trials in adult patients with bronchiectasis have shown extremely high dose inhaled corticosteroids to improve FEV1 but not symptoms or exacerbation frequency; no studies have been conducted in children and corticosteroids are not routinely recommended in bronchiectasis uncomplicated by asthma [78, 188].
Other unproven medical therapies of possible benefit include and leukotriene-receptor antagonists, due to their bronchodilator and anti-inflammatory properties; indomethacin, which may reduce sputum elastase [189] by blocking the cyclooxygenase pathway; and methylxanthines, which are believed to have anti-inflammatory properties and may increase respiratory muscle efficiency even at doses too low for a bronchodilator effect.
Immune Therapy
Patients with CVID benefit from immunoglobulin substitution therapy; this has been proven by a randomised multi-centre trial conducted in the Netherlands [190]. Subcutaneous infusions are better tolerated by patients than intravenous infusion and may achieve more stable trough levels with a lower risk of adverse reactions [191]. Doses may need to be increased in the presence of active lung disease as this increases immunoglobulin turnover [192]. Subcutaneous immunoglobulin infusion has been demonstrated to reduce the frequency of exacerbations and to slow bronchiectasis progression. Efforts to reconstitute the immune system can also be effective. Recombinant IFNγ therapy, for example, is also effective in reducing the number of severe infections [193, 194] and recombinant granulocyte stimulating factor can be used in severe congenital neutropenia [195].
Surgery
Surgical treatments were used more widely prior to the introduction of effective antibiotic therapy [12]. There remains a place for surgical treatment, particularly pulmonary segmental resection when damage is severe but well localised. In such cases it is useful to demonstrate pre-operatively the extent and exact site of any defect in ventilation or perfusion to the affected portion of the lung [196]. Mortality and morbidity associated with early surgical procedures were high but the introduction of effective antibiotic treatment and improvements in surgical techniques have dramatically reduced perioperative risks. Surgery has the advantage of removing infected tissue and thereby preventing spread of disease to other areas of the lung and, in some instances, may be curative [197]. Overall mortality ranges from 0 to 3.5 %, whilst the most common complications of lung resection are atelectasis, bronchopleural fistula, empyema and wound infection [198]. End-stage diffuse bronchiectasis may be treated with bilateral lung transplantation; this is mainly limited to patients with CF. A study of 78 PCD patients from the US reported very severe disease with lung failure in 38 % of adults. All of these severely affected individuals had received a lung transplant, were awaiting one, or were oxygen dependent [199]. Outcomes can be improved by pre-operative nutritional supplementation and aggressive antimicrobial therapy tailored to likely colonising organisms. In appropriately selected patients transplantation has been shown to improve quality of life and to prolong survival [200, 201].
Potential Future Therapies – Gene Therapy
The only curative treatment at present for many immune deficiencies is matched stem cell transplantation; patients must receive antimicrobial cover, particularly for the organisms they are known to be colonised with for the duration of immunosuppression related to this procedure [202, 203]. Gene therapy provides an alternative strategy with which to cure or alleviate select inherited diseases. A corrected copy of a gene is transferred to the somatic cells of affected individuals. This type of therapy is limited to correction of genetic defects in either terminally differentiated, long-lived post-mitotic cells or easily accessible stem cells. To treat stem cells such as those of the haematopoietic system, implicated in primary immunodeficiency, a viral vector capable of integration within the host genome is required for gene delivery. Barriers to the success of this treatment strategy are: (1) low protein expression due to poor gene transfer [204], (2) risk of insertional mutagenesis [205], and (3) immunogenicity of the vector or transgene product [206]. Nevertheless gene-modified autologous bone marrow transplantation represents a promising treatment free from the immunological complications associated with transplantation from a HLA-mismatched donor. Whilst bone marrow transplantation from an HLA-matched sibling donor confers an approximately 80 % cure of primary immunodeficiency and that from a fully HLA-matched unrelated donations confer a 70 % chance of cure, survival following an HLA-mismatched donation is substantially less (37 %) and complicated by graft-versus-host disease, partial immunological reconstitution and attendant infection risk [207].
It appears that in order to replenish the peripheral pool of immune cells with cells containing the transduced gene, the transduced cells should have a selective advantage. The importance of selective advantage is seen in gene therapy of adenosine deaminase deficiency (a primary immunodeficiency associated with pneumocystis and CMV infection leading to interstitial and alveolar infiltrates, although not commonly bronchiectasis). T lymphocyte precursors genetically modified to contain the adenosine deaminase enzyme missing in this disorder are able to metabolise toxic purine products and have a selective advantage over unmodified lymphocytes; the success of the gene-transfer is reduced by treatment with adenosine deaminase enzyme replacement therapy as this reduces the selective advantage of the modified cells [208]. Similarly rare spontaneous partial phenotypic correction of severe T cell immunodeficiencies have been observed in which clonal expansion of one or several T cell precursors carrying a wild-type sequence of the disease-causing gene can differentiate into mature, functional T cells capable of supporting normal immunity [209]. For haematopoietic disorders in which wild-type gene expression is essential to the function of terminally differentiated cells chances of success can be improved by mild myelosuppressive treatment prior to gene-therapy which leads to a higher proportion of transduced progenitor cells due to better engraftment.
Preliminary but promising results for gene therapy have been reported following non-myeloablative conditioning with busulfan and gene-therapy treatment in two adults with X-linked CGD [210]. A retroviral vector containing wild-type gp91phox was used and following transplantation of genetically modified autologous CD34+ cells 10–15 % engraftment of transduced granulocytes was achieved and there was notable clinical improvement in both patients. Clonal expansion of myeloid cells, thought to be due to retroviral vector insertions near cellular proto-oncogenes, complicated this therapy although malignant transformation was not observed during follow-up.
Clinical Vignette
A patient was referred for PCD diagnostic testing at 4 years of age (see PCD details above). She had been born at term and was noted to have nasal congestion and tachypnoea from shortly after birth, but did not require medical intervention. Throughout infancy she had recurrent chest infections and a daily wet productive cough. She also had glue ear treated with grommets which resulted in otorrhea and no improvement in hearing. She had normal cardiac situs and her parents are white Caucasian and non-consanguineous. Her sister has glue ear but there is otherwise no family history of note.
Using HSV analysis it was impossible to obtain an accurate beat frequency on two separate occasions. The cilia demonstrated stiff vibrating movements rather than the usual coordinated sweeping motion. Electron microscopy demonstrated normal ultrastructure (Fig. 4.8) with normal arrangement of microtubules radial spokes and outer dynein arms. In view of the normal transmission electron microscopy, genetic analysis was undertaken, confirming mutations in DNAh11 gene; this gene had previously been reported as a cause of PCD with normal ciliary ultrastructure [29].
TEM of a cilium from the patient described in the Vignette. Despite having PCD, the patient has normal ciliary ultrastructure by EM. EM images obtained using FEI Tecnai 12 transmission electron microscope (FEI UK Limited, Cambridge, UK at 80 kV). Scale bars 580 m. EM images provided by P. Goggin (Primary Ciliary Dyskinesia Group, University Hospitals Southampton NHS Foundation Trust, Southampton, UK)
Since diagnosis she has commenced twice daily airways clearance (physiotherapy) and is aware of the need for prompt treatment of any intercurrent infection. In addition to the usual childhood vaccinations, she has influenza cover annually. She is reviewed by a multidisciplinary team which includes a respiratory pediatrician, ENT consultant, physiotherapist and respiratory nurse 4 monthly. She also has audiology reviews annually to monitor the need for hearing aids.
Learning points from case:
-
PCD often presents in the neonatal period but diagnosis is often delayed until later childhood [22].
-
Diagnostic evaluation is often complicated and requires specialist expertise.
-
Although 50 % of patients have situs inversus, the diagnosis should be suspected in patients with situs solitus if other symptoms are present.
-
Management of non-pulmonary disease necessitates the involvement of a multidisciplinary team, which may include ENT, audiology, cardiology, and fertility specialists.
-
The case patient is under specialist PCD care. However, as with most orphan diseases, the majority of patients are managed by non-specialists. Inappropriate respiratory treatment is more likely to lead to bronchiectasis and a poorer prognosis. Inappropriate ENT care can lead to poor management of hearing impairment and rhino-sinus disease.
-
Unlike many orphan diseases, consensus statements are available to provide guidelines for diagnosis and management of PCD [39, 154]. However there have been no clinical trials in PCD and management guidelines are extrapolated from more prevalent diseases; this is almost certainly inappropriate.
-
The management of hearing impairment caused by glue ear is controversial in PCD (reviewed in [21]) but many PCD specialists report excess complications caused by grommets (for example otorrhea and failure for hearing to improve) and recommend that patients with PCD should be treated with hearing aids as the primary approach.
Summary
It is likely that the true incidence of bronchiectasis with a genetic basis is underestimated. As our understanding of innate immunity and ion-transport disorders are better characterised, a number of cases currently labeled as ‘idiopathic’ will have their aetiology elucidated. As with other Orphan Diseases, the diagnosis and management of patients with bronchiectasis is largely determined by local interests and provision, and many patients find it difficult to access appropriate care. Most doctors have little experience of rarer causes of bronchiectasis and will base management on evidence from CF. Indeed, the evidence base for managing non-CF bronchiectasis is poor. For example there have been no clinical trials in PCD, which is distinctly different from CF in underlying pathophysiology, and is likely to benefit from different management. The CF community has made substantial advances in recent decades, resulting in improved morbidity and mortality. These advances have been beneficial to the care of patients with non-CF bronchiectasis, but more rapid developments are likely if clinical standards and evidence based guidelines are individualized for different diseases.
References
Nikolaizik WH, Warner JO. Aetiology of chronic suppurative lung disease. Arch Dis Child. 1994;70(2):141–2.
Wynn-Williams N. Bronchiectasis: a study centred on Bedford and its environs. Br Med J. 1953;1(4821):1194–9.
Shum DK, Chan SC, Ip MS. Neutrophil-mediated degradation of lung proteoglycans: stimulation by tumor necrosis factor-alpha in sputum of patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162(5):1925–31.
Gaga M, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax. 1998;53(8):685–91.
Cole P. The damaging role of bacteria in chronic lung infection. J Antimicrob Chemother. 1997;40 Suppl A:5–10.
Tsao PC, Lin CY. Clinical spectrum of bronchiectasis in children. Acta Paediatr Taiwan. 2002;43(5):271–5.
Liebow AA, Hales MR, Lindskog GE. Enlargement of the bronchial arteries, and their anastomoses with the pulmonary arteries in bronchiectasis. Am J Pathol. 1949;25(2):211–31.
Williams H. Bronchiectasis in children: its multiple clinical and pathological features. Med J Aust. 1959;46(2):385–90.
Edwards EA, Asher MI, Byrnes CA. Paediatric bronchiectasis in the twenty-first century: experience of a tertiary children’s hospital in New Zealand. J Paediatr Child Health. 2003;39(2):111–7.
Eastham KM, et al. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax. 2004;59(4):324–7.
Weycker D, et al. Prevalence and economic burden of bronchiectasis. Clin Pulmon Med. 2005;12(4):205–9.
Field CE. Bronchiectasis. Third report on a follow-up study of medical and surgical cases from childhood. Arch Dis Child. 1969;44(237):551–61.
Clark NS. Bronchiectasis in childhood. Br Med J. 1963;1(5323):80–8.
Twiss J, et al. New Zealand national incidence of bronchiectasis “too high” for a developed country. Arch Dis Child. 2005;90(7):737–40.
Chang AB, et al. Bronchiectasis in indigenous children in remote Australian communities. Med J Aust. 2002;177(4):200–4.
Singleton R, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol. 2000;29(3):182–7.
Waite DA, et al. Polynesian bronchiectasis. Eur J Respir Dis Suppl. 1983;127:31–6.
Griese EU, et al. Allele and genotype frequencies of polymorphic cytochromes P4502D6, 2C19 and 2E1 in aborigines from western Australia. Pharmacogenetics. 2001;11(1):69–76.
O’Callaghan C, Chetcuti P, Moya E. High prevalence of primary ciliary dyskinesia in a British Asian population. Arch Dis Child. 2010;95(1):51–2.
Morrissey BM, Harper RW. Bronchiectasis: sex and gender considerations. Clin Chest Med. 2004;25(2):361–72.
Lucas JSA, et al. Primary ciliary dyskinesia. Eur Resp Monogr. 2011;54:201–17.
Kuehni CE, et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J. 2010;36(6):1248–58.
Stick SM, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155(5):623–8.e1.
Wood RE, Boat TF, Doershuk CF. Cystic fibrosis. Am Rev Respir Dis. 1976;113(6):833–78.
Rodman DM, et al. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2005;171(6):621–6.
Bombieri C, et al. Recommendations for the classification of diseases as CFTR-related disorders. J Cyst Fibros. 2011;10 Suppl 2:S86–102.
Barbato A, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34(6):1264–76.
Lucas JS, et al. Static respiratory cilia associated with mutations in Dnahc11/DNAH11: a mouse model of PCD. Hum Mutat. 2012;33(3):495–503.
Schwabe GC, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29(2):289–98.
Kennedy MP, et al. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol. 2007;188(5):1232–8.
Coren ME, et al. Primary ciliary dyskinesia: age at diagnosis and symptom history. Acta Paediatr. 2002;91(6):667–9.
Leigh MW, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: improving the diagnostic approach. Curr Opin Pediatr. 2009;21(3):320–5.
Walker WT, et al. Nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2012;40(4):1024–32.
Zariwala MA, Omran H, Ferkol TW. The emerging genetics of primary ciliary dyskinesia. Proc Am Thorac Soc. 2011;8(5):430–3.
Pennarun G, et al. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet. 1999;65(6):1508–19.
Knowles MR, et al. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188(8):913–22.
Olbrich H, et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet. 2002;30(2):143–4.
Knowles MR, et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67(5):433–41.
Bartoloni L, et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2002;99(16):10282–6.
Loges NT, et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet. 2008;83(5):547–58.
Duriez B, et al. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2007;104(9):3336–41.
Mazor M, et al. Primary ciliary dyskinesia caused by homozygous mutation in DNAL1, encoding dynein light chain 1. Am J Hum Genet. 2011;88(5):599–607.
Omran H, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456(7222):611–6.
Loges NT, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85(6):883–9.
Mitchison HM, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44(4):381–9, S1–2.
Horani A, et al. LRRC6 mutation causes primary ciliary dyskinesia with dynein arm defects. PLoS One. 2013;8(3):e59436.
Kott E, et al. Loss-of-function mutations in LRRC6, a gene essential for proper axonemal assembly of inner and outer dynein arms, cause primary ciliary dyskinesia. Am J Hum Genet. 2012;91(5):958–64.
Panizzi JR, et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat Genet. 2012;44(6):714–9.
Knowles MR, et al. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013;92(1):99–106.
Onoufriadis A, et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet. 2013;92(1):88–98.
Wu DH, Singaraja RR. Loss-of-function mutations in CCDC114 cause primary ciliary dyskinesia. Clin Genet. 2013;83(6):526–7.
Horani A, et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2012;91(4):685–93.
Horani A, et al. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS One. 2013;8(8):e72299.
Moore DJ, et al. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am J Hum Genet. 2013;93(2):346–56.
Zariwala MA, et al. ZMYND10 is mutated in primary ciliary dyskinesia and interacts with LRRC6. Am J Hum Genet. 2013;93(2):336–45.
Castleman VH, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84(2):197–209.
Kott E, et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Genet. 2013;93(3):561–70.
Olbrich H, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet. 2012;91(4):672–84.
Merveille AC, et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43(1):72–8.
Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, Wilson R, Taylor-Cox T, Dewar A, Jackson C, Goggin P, Loges NT, Olbrich H, Jaspers M, Jorissen M, Leigh MW, Wolf WE, Daniels ML, Noone PG, Ferkol TW, Sagel SD, Rosenfeld M, Rutman A, Dixit A, O’Callaghan C, Lucas JS, Hogg C, Scambler PJ, Emes RD, Uk10k, Chung EM, Shoemark A, Knowles MR, Omran H, Mitchison HM. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganisation and absent inner dynein arms. Hum Mutat. 2013;34(3):462–72.
Wirschell M, et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet. 2013;45(3):262–8.
Budny B, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120(2):171–8.
Hong DH, et al. A single, abbreviated RPGR-ORF15 variant reconstitutes RPGR function in vivo. Invest Ophthalmol Vis Sci. 2005;46(2):435–41.
Hendry WF, A’Hern RP, Cole PJ. Was Young’s syndrome caused by exposure to mercury in childhood? BMJ. 1993;307(6919):1579–82.
Greenstone MA, et al. Ciliary function in Young’s syndrome. Thorax. 1988;43(2):153–4.
de Iongh R, Ing A, Rutland J. Mucociliary function, ciliary ultrastructure, and ciliary orientation in Young’s syndrome. Thorax. 1992;47(3):184–7.
Le Lannou D, et al. Obstructive azoospermia with agenesis of vas deferens or with bronchiectasia (Young’s syndrome): a genetic approach. Hum Reprod. 1995;10(2):338–41.
Wellesley D, Schwarz M. Cystic fibrosis and Young’s syndrome. Lancet. 1998;352(9133):1065–6.
Arya AK, et al. Does Young’s syndrome exist? J Laryngol Otol. 2009;123(5):477–81.
Bonneau D, et al. Usher syndrome type I associated with bronchiectasis and immotile nasal cilia in two brothers. J Med Genet. 1993;30(3):253–4.
Iannaccone A, et al. Clinical and immunohistochemical evidence for an X linked retinitis pigmentosa syndrome with recurrent infections and hearing loss in association with an RPGR mutation. J Med Genet. 2003;40(11):e118.
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–301.
Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int. 2003;64(4):1157–62.
Driscoll JA, et al. Autosomal dominant polycystic kidney disease is associated with an increased prevalence of radiographic bronchiectasis. Chest. 2008;133(5):1181–8.
Parr DG, et al. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;176(12):1215–21.
Cuvelier A, et al. Distribution of alpha(1)-antitrypsin alleles in patients with bronchiectasis. Chest. 2000;117(2):415–9.
Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respir Med. 2007;101(6):1163–70.
Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1–58.
Woroniecka M, Ballow M. Office evaluation of children with recurrent infection. Pediatr Clin North Am. 2000;47(6):1211–24.
Chapel H, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86.
Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92(1):34–48.
Kralovicova J, et al. Fine-scale mapping at IGAD1 and genome-wide genetic linkage analysis implicate HLA-DQ/DR as a major susceptibility locus in selective IgA deficiency and common variable immunodeficiency. J Immunol. 2003;170(5):2765–75.
Mullighan CG, et al. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159(12):6236–41.
Grimbacher B, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4(3):261–8.
van Zelm MC, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354(18):1901–12.
Castigli E, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829–34.
Thickett KM, et al. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM. 2002;95(10):655–62.
Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9(6):722–8.
Tsukada S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72(2):279–90.
Furr PM, Taylor-Robinson D, Webster AD. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinaemia and their role in arthritis: microbiological observations over twenty years. Ann Rheum Dis. 1994;53(3):183–7.
Conley M. Autosomal recessive agammaglobulinemia. In: Ochs HS, Smith CIE, Puck JM, editors. Primary immunodeficiency diseases: a molecular and genetic approach. New York: Oxford University Press; 2007. p. 304–12.
Winkelstein JA, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine. 2006;85(4):193–202.
Howard V, et al. The health status and quality of life of adults with X-linked agammaglobulinemia. Clin Immunol. 2006;118(2–3):201–8.
Plebani A, et al. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol. 2002;104(3):221–30.
Bjorkander J, et al. Impaired lung function in patients with IgA deficiency and low levels of IgG2 or IgG3. N Engl J Med. 1985;313(12):720–4.
Roos DK, Kuijpers TW, Curnutte JT. Chronic granulomatous disease. In: Ochs HDS, Smith CIE, Puck JM, editors. Primary immunodeficiency disease: a molecular and genetic approach. New York: Oxford University Press; 2007. p. 525–49.
van den Berg JM, et al. Chronic granulomatous disease: the European experience. PLoS One. 2009;4(4):e5234.
Dale DC, et al. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am J Hematol. 2003;72(2):82–93.
Horwitz MS, et al. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. 2007;109(5):1817–24.
Klein C, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet. 2007;39(1):86–92.
Bohn G, et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med. 2007;13(1):38–45.
Grimbacher B, et al. Hyper-IgE syndrome with recurrent infections–an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702.
Levy DE, Loomis CA. STAT3 signaling and the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1655–8.
Boocock GR, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet. 2003;33(1):97–101.
Kilpatrick DC, et al. Stable bronchiectasis is associated with low serum L-ficolin concentrations. Clin Respir J. 2009;3(1):29–33.
Fevang B, et al. Common variable immunodeficiency and the complement system; low mannose-binding lectin levels are associated with bronchiectasis. Clin Exp Immunol. 2005;142(3):576–84.
Garred P, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104(4):431–7.
Pan-Hammarstrom Q, et al. Disparate roles of ATR and ATM in immunoglobulin class switch recombination and somatic hypermutation. J Exp Med. 2006;203(1):99–110.
Canny GJ, et al. A pulmonary infiltrate in a child with ataxia telangiectasia. Ann Allergy. 1988;61(6):422–3, 466–8.
Lefton-Greif MA, et al. Oropharyngeal dysphagia and aspiration in patients with ataxia-telangiectasia. J Pediatr. 2000;136(2):225–31.
Katz HL. Thoracic manifestation in Marfan’s syndrome (arachnodactyly). Q Bull Sea View Hosp. 1952;13(2):95–106.
Dwyer Jr EM, Troncale F. Spontaneous pneumothorax and pulmonary disease in the Marfan syndrome. Report of two cases and review of the literature. Ann Intern Med. 1965;62:1285–92.
Teoh PC. Bronchiectasis and spontaneous pneumothorax in Marfan’s syndrome. Chest. 1977;72(5):672–3.
Williams H, Campbell P. Generalized bronchiectasis associated with deficiency of cartilage in the bronchial tree. Arch Dis Child. 1960;35:182–91.
Williams HE, Landau LI, Phelan PD. Generalized bronchiectasis due to extensive deficiency of bronchial cartilage. Arch Dis Child. 1972;47(253):423–8.
Jones VF, et al. Familial congenital bronchiectasis: Williams-Campbell syndrome. Pediatr Pulmonol. 1993;16(4):263–7.
George J, Jain R, Tariq SM. CT bronchoscopy in the diagnosis of Williams-Campbell syndrome. Respirology. 2006;11(1):117–9.
Mounier-Kuhn P. Dilatation de la trachee; constatations radiographiques et bronchoscopiques. Lyon Med. 1932;150:106–9.
Johnston RF, Green RA. Tracheobronchiomegaly. Report of five cases and demonstration of familial occurrence. Am Rev Respir Dis. 1965;91:35–50.
Doyle AJ. Demonstration on computed tomography of tracheomalacia in tracheobronchomegaly (Mounier-Kuhn syndrome). Br J Radiol. 1989;62(734):176–7.
Van Schoor J, Joos G, Pauwels R. Tracheobronchomegaly – the Mounier-Kuhn syndrome: report of two cases and review of the literature. Eur Respir J. 1991;4(10):1303–6.
Ayres JG, et al. Abnormalities of the lungs and thoracic cage in the Ehlers-Danlos syndrome. Thorax. 1985;40(4):300–5.
Grunebaum M, et al. Tracheomegaly in Brachmann-de Lange syndrome. Pediatr Radiol. 1996;26(3):184–7.
Barakat J, et al. Treatment of tracheobronchomegaly with an Ultraflex prosthesis. A case report. Rev Pneumol Clin. 2002;58(1):19–22.
Cohen M, Sahn SA. Bronchiectasis in systemic diseases. Chest. 1999;116(4):1063–74.
Boyton RJ, et al. IFN gamma and CXCR-1 gene polymorphisms in idiopathic bronchiectasis. Tissue Antigens. 2006;68(4):325–30.
Hershko A, et al. Yellow nail syndrome. Postgrad Med J. 1997;73(862):466–8.
Samman PD, White WF. The “yellow nail” syndrome. Br J Dermatol. 1964;76:153–7.
Varney VA, et al. Rhinitis, sinusitis and the yellow nail syndrome: a review of symptoms and response to treatment in 17 patients. Clin Otolaryngol Allied Sci. 1994;19(3):237–40.
Battaglia A, et al. Pleural effusion and recurrent broncho-pneumonia with lymphedema, yellow nails and protein-losing enteropathy. Eur J Respir Dis. 1985;66(1):65–9.
Bowers D. Unequal breasts, yellow nails, bronchiectasis and lymphedema. Can Med Assoc J. 1969;100(9):437–8.
Cebeci F, Celebi M, Onsun N. Nonclassical yellow nail syndrome in six-year-old girl: a case report. Cases J. 2009;2:165.
Bull RH, Fenton DA, Mortimer PS. Lymphatic function in the yellow nail syndrome. Br J Dermatol. 1996;134(2):307–12.
Bokszczanin A, Levinson AI. Coexistent yellow nail syndrome and selective antibody deficiency. Ann Allergy Asthma Immunol. 2003;91(5):496–500.
Finegold DN, et al. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum Mol Genet. 2001;10(11):1185–9.
Noone PG, et al. Lung disease associated with the IVS8 5 T allele of the CFTR gene. Am J Respir Crit Care Med. 2000;162(5):1919–24.
Rosen MJ. Chronic cough due to tuberculosis and other infections: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):197S–201.
Dankert-Roelse JE, te Meerman GJ. Long term prognosis of patients with cystic fibrosis in relation to early detection by neonatal screening and treatment in a cystic fibrosis centre. Thorax. 1995;50(7):712–8.
Waters DL, et al. Clinical outcomes of newborn screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed. 1999;80(1):F1–7.
Sims EJ, et al. Neonatal screening for cystic fibrosis is beneficial even in the context of modern treatment. J Pediatr. 2005;147(3 Suppl):S42–6.
Corkey CW, Levison H, Turner JA. The immotile cilia syndrome. A longitudinal survey. Am Rev Respir Dis. 1981;124(5):544–8.
Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J. 1997;10(10):2376–9.
Hellinckx J, Demedts M, De Boeck K. Primary ciliary dyskinesia: evolution of pulmonary function. Eur J Pediatr. 1998;157(5):422–6.
Aurora P, et al. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;183(6):752–8.
Green K, et al. Ventilation inhomogeneity in children with primary ciliary dyskinesia. Thorax. 2012;67(1):49–53.
Hansell DM. Bronchiectasis. Radiol Clin North Am. 1998;36(1):107–28.
Fall A, Spratt J, Mitchell L. Plain chest X-Ray vs high resolution CT (HRCT) in non-CF bronchiectasis (NCFB) in children. Thorax. 2001;56 Suppl 3:26.
Naidich DP, et al. Computed tomography of bronchiectasis. J Comput Assist Tomogr. 1982;6(3):437–44.
Hansell DM, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722.
Kang EY, Miller RR, Muller NL. Bronchiectasis: comparison of preoperative thin-section CT and pathologic findings in resected specimens. Radiology. 1995;195(3):649–54.
Coleman LT, et al. Bronchiectasis in children. J Thorac Imaging. 1995;10(4):268–79.
Fall A, Spencer D. Paediatric bronchiectasis in Europe: what now and where next? Paediatr Respir Rev. 2006;7(4):268–74.
Cystic Fibrosis Trust: Standards for the clinical care of children and adults with cystic fibrosis in the UK. Second edition, 2011 accessible from: http://cysticfibrosis.org.uk/about-cf/publications/consensus-documents
Bush A, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92(12):1136–40.
Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190–7.
Loeys BL, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–85.
Li AM, et al. Non-CF bronchiectasis: does knowing the aetiology lead to changes in management? Eur Respir J. 2005;26(1):8–14.
Wilson CB, et al. Validation of the St. George’s Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):536–41.
Konstan MW, Stern RC, Doershuk CF. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. J Pediatr. 1994;124(5 Pt 1):689–93.
Patterson JE, et al. Airway clearance in bronchiectasis: a randomized crossover trial of active cycle of breathing techniques versus Acapella. Respiration. 2005;72(3):239–42.
Schoni MH. Autogenic drainage: a modern approach to physiotherapy in cystic fibrosis. J R Soc Med. 1989;82 Suppl 16:32–7.
Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7 %) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respir Med. 2005;99(1):27–31.
Crockett AJ, et al. Mucolytics for bronchiectasis. Cochrane Database Syst Rev. 2001;(1):CD001289.
ten Berge M, et al. DNase treatment in primary ciliary dyskinesia – assessment by nocturnal pulse oximetry. Pediatr Pulmonol. 1999;27(1):59–61.
Desai M, Weller PH, Spencer DA. Clinical benefit from nebulized human recombinant DNase in Kartagener’s syndrome. Pediatr Pulmonol. 1995;20(5):307–8.
Devalia JL, et al. The effects of salmeterol and salbutamol on ciliary beat frequency of cultured human bronchial epithelial cells, in vitro. Pulm Pharmacol. 1992;5(4):257–63.
Frohock JI, Wijkstrom-Frei C, Salathe M. Effects of albuterol enantiomers on ciliary beat frequency in ovine tracheal epithelial cells. J Appl Physiol. 2002;92(6):2396–402.
Franco F, Sheikh A, Greenstone M. Short acting beta-2 agonists for bronchiectasis. Cochrane Database Syst Rev. 2003;(3):CD003572.
Sheikh A, Nolan D, Greenstone M. Long-acting beta-2-agonists for bronchiectasis. Cochrane Database Syst Rev. 2001;(4):CD002155.
Corless JA, Warburton CJ. Leukotriene receptor antagonists for non-cystic fibrosis bronchiectasis. Cochrane Database Syst Rev. 2000;(4):CD002174.
Steele K, Greenstone M, Lasserson JA. Oral methyl-xanthines for bronchiectasis. Cochrane Database Syst Rev, 2001;(1):CD002734.
Bradley J, Moran F. Physical training for cystic fibrosis. Cochrane Database Syst Rev. 2002;(2):CD002768.
Bradley J, Moran F, Greenstone M. Physical training for bronchiectasis. Cochrane Database Syst Rev. 2002;(3):CD002166.
Smyth AR, Walters S. Prophylactic antibiotics for cystic fibrosis (Cochrane Review). In: Cochrane Library. Oxford: Update software; 2003.
Currie DC, et al. Double-blind randomized study of prolonged higher-dose oral amoxycillin in purulent bronchiectasis. Q J Med. 1990;76(280):799–816.
Anwar GA, et al. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respir Med. 2008;102(10):1494–6.
Gorrini M, et al. Inhibition of human neutrophil elastase by erythromycin and flurythromycin, two macrolide antibiotics. Am J Respir Cell Mol Biol. 2001;25(4):492–9.
Prince AS. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med. 2002;347(14):1110–1.
Davies G, Wilson R. Prophylactic antibiotic treatment of bronchiectasis with azithromycin. Thorax. 2004;59(6):540–1.
Cymbala AA, et al. The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treat Respir Med. 2005;4(2):117–22.
Rayner CF, et al. Efficacy and safety of long-term ciprofloxacin in the management of severe bronchiectasis. J Antimicrob Chemother. 1994;34(1):149–56.
Steinfort DP, Steinfort C. Effect of long-term nebulized colistin on lung function and quality of life in patients with chronic bronchial sepsis. Intern Med J. 2007;37(7):495–8.
Barker AF, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162(2 Pt 1):481–5.
Smith J, Finn A. Antimicrobial prophylaxis. Arch Dis Child. 1999;80(4):388–92.
Rosen MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):122S–31.
Evans DJ, Greenstone M. Long-term antibiotics in the management of non-CF bronchiectasis – do they improve outcome? Respir Med. 2003;97(7):851–8.
Edwards EA, Twiss J, Byrnes CA. Treatment of paediatric non-cystic fibrosis bronchiectasis. Expert Opin Pharmacother. 2004;5(7):1471–84.
Kapur N, et al. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2009;(1):CD000996.
Tamaoki J, et al. Effect of indomethacin on bronchorrhea in patients with chronic bronchitis, diffuse panbronchiolitis, or bronchiectasis. Am Rev Respir Dis. 1992;145(3):548–52.
Eijkhout HW, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135(3):165–74.
Nicolay U, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunodeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26(1):65–72.
Roifman CM, Levison H, Gelfand EW. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987;1(8541):1075–7.
Gallin JI, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348(24):2416–22.
Sechler JM, et al. Recombinant human interferon-gamma reconstitutes defective phagocyte function in patients with chronic granulomatous disease of childhood. Proc Natl Acad Sci U S A. 1988;85(13):4874–8.
Bonilla MA, et al. Long-term safety of treatment with recombinant human granulocyte colony-stimulating factor (r-metHuG-CSF) in patients with severe congenital neutropenias. Br J Haematol. 1994;88(4):723–30.
Otgun I, et al. Surgical treatment of bronchiectasis in children. J Pediatr Surg. 2004;39(10):1532–6.
Agasthian T, et al. Surgical management of bronchiectasis. Ann Thorac Surg. 1996;62(4):976–8; discussion 979–80.
Mauchley DC, Mitchell JD. Surgery for bronchiectasis. Eur Respir Monogr. 2011;52:248–57.
Noone PG, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169(4):459–67.
Courtney JM, et al. Quality of life and inflammation in exacerbations of bronchiectasis. Chron Respir Dis. 2008;5(3):161–8.
Loebinger MR, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J. 2009;34(4):843–9.
Pai SY, et al. Stem cell transplantation for the Wiskott-Aldrich syndrome: a single-center experience confirms efficacy of matched unrelated donor transplantation. Bone Marrow Transplant. 2006;38(10):671–9.
Seger RA, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood. 2002;100(13):4344–50.
Jiang H, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14(3):452–5.
Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–9.
Aubert D, et al. Cytotoxic immune response blunts long-term transgene expression after efficient retroviral-mediated hepatic gene transfer in rat. Mol Ther. 2002;5(4):388–96.
Antoine C, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361(9357):553–60.
Aiuti A, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58.
Wada T, et al. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc Natl Acad Sci U S A. 2001;98(15):8697–702.
Ott MG, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–9.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Lucas, J.S., Pike, K.C. (2015). “Diffuse Bronchiectasis of Genetic Origin”. In: Cottin, V., Cordier, JF., Richeldi, L. (eds) Orphan Lung Diseases. Springer, London. https://doi.org/10.1007/978-1-4471-2401-6_4
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2401-6_4
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2400-9
Online ISBN: 978-1-4471-2401-6
eBook Packages: MedicineMedicine (R0)