Abstract
The extraction of compounds from plant and animal sources that have medicinal properties are known as bioactive compounds, which can either improve health or can be hazardous. As novel natural compounds from various plants are uncovered, we are discovering more biologically active compounds. Enzymes can be employed to extract such bioactive compounds by rupturing plant cells. The extraction of a bioactive compound such as stevioside from a stevia plant with the assistance of enzymes is a better example of processing with potential utility for the food sector. The extraction of bioactive compounds from plant sources with the help of environment-friendly enzymes, especially for food, nutraceutical, or pharmaceutical uses, is a cutting-edge technology used in these industries. This study explains the overall idea about bioactive compounds, their extraction processes, importance, and uses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key words
- Bioactive
- Stevioside
- Stevia
- Enzymes
- Enzyme-assisted extraction
- Nutraceutical
- Biotechnological applications
1 Introduction
The chemical compounds that are produced in the plant, which are not involved in the metabolic activity of the plant, are mostly secondary metabolites. These are biologically active compounds [1]. These are produced by the subsidiary pathways. These bioactive compounds have antioxidant, anti-inflammatory, and antimicrobial properties and greater immunomodulatory potential properties. Detailed information about secondary metabolites and their application is mentioned in Table 1. These compounds possess additional nutritional value to the food that is generally found in less amount, which provides health-related benefits over the product’s basic nutritional value [2]. These compounds include pharmaceuticals, flavors, fragrances, cosmetics, food additives, feedstocks, and antimicrobials. These compounds are produced by the plant cell through metabolic pathways such as the malonic acid pathway, shikimic acid pathway, methylerythritol phosphate pathway, and mevalonic acid pathway.
The extraction of bioactive compounds is becoming much easier nowadays, and these extracted compounds are utilized in the drug industries for the production of novel drugs against life-threatening diseases and also utilized in food industries to enhance the quality of the food. Other potential properties of bioactive compounds, for example, antimicrobial, anti-inflammatory, anticancer, antimicrobial, and antidiabetic activities are utilized for various applications. For the extraction of these compounds, the traditional method of extraction gives less yield, and also there is no proper method for extracting the bioactive compounds.
Biotechnologists and other chemists started to find a way to extract bioactive compounds with a greater yield on an industrial scale, which can also be helpful commercially. After so many attempts of research, enzyme-assisted extraction came out to be an efficient way to extract bioactive compounds [3]. The enzymes will disrupt the cell wall and make the cell wall permeable that can be easier to get more yield of bioactive compounds such as flavonoids, terpenoids, and lectins, which are having applications in various types of industries. This method of using specific enzymes for the extraction of compounds seems to be very efficient as compared to the other extraction techniques.
2 Examples of Plant-Based Bioactives
The major classes of bioactive compounds are alkaloids, terpenoids, and phenolic compounds. Other basic examples include bioactive compounds that are flavonoids, terpenes, polyphenols, lycopene, resveratrol, lignan, tannins, and indoles [4]. These types of compounds are mainly used in food industries, for example, marigold flowers are the most significant source of carotenoids. Nevertheless, the efficacy of the extraction depends on the circumstances for collection, drying, and the solvent extraction procedure, which results in 50% losses of the carotenoids. Macerating enzymes have been used before with good results to increase the extraction yield of effective chemicals from natural materials. In this study, a different extraction technique for carotenoids was discovered that involved both a simultaneous enzymatic procedure and solvent extraction [5]. By skipping the ineffective matter and drying procedures, the suggested method uses freshly milled flowers as raw material. A carotenoid recovery yield of 98% was achieved using the described technique at the 80 L scale, under typical testing conditions [6]. Lycopene, a carotenoid found in high concentration in tomatoes and tomato-derived products, is receiving a lot of attention these days because epidemiological evidence suggests that it may protect against cancer and some degenerative diseases that are impacted by free radical reactions. Recent epidemiological studies have shown that consumption of tomatoes and blood lycopene levels are inversely related to the risk of getting cancer at several anatomical sites, including the prostate gland, stomach, and lungs. By successfully delocalizing trapped free radical species, conjugated carbon-carbon double bonds endow lycopene with their antioxidant characteristics. Hence, food industries, pharmaceutical industries, and cosmetic industries all have a significant demand for lycopene [7].
Salvia officinalis, which is communally called sage, is the most commonly known herb used in the kitchen, and it belongs to the family Labiatae. Sage has a long history of helping people stay healthy and alleviate illnesses. Sage essential oil has shown efficiency in the treatment of Alzheimer’s disease and efficiency to enhance memory, according to current research and scientific studies. Alzheimer’s is a neurological disease. Sage is one of the kitchen plants with an aromatic nature and contains an oil-producing capacity. Sage has been the subject of much research for its phenolic antioxidant components since its early days, roughly 20 years ago. Sage has several powerful antioxidants, according to numerous types of research [8]. Sage is a plant of commercial relevance for scientific research because of its essential oil and antioxidant components. All food, cosmetic, and pharmaceutical products start with sage essential oil and flavorings as their primary ingredient [9]. Sage antioxidants can be utilized as a different approach to the popular rosemary antioxidants to protect and preserve specific foods and nutraceutical items to lengthen their shelf life [8]. Other different types of bioactive compounds and their respective plant source and their uses are mentioned in Table 2.
Bioactive compounds can also be extracted from other sources such as microbes, fungi, and animals. Fishes are one of the sources for bioactive compounds. Different types of bioactives extracted from different fish species and their application are listed in Table 3.
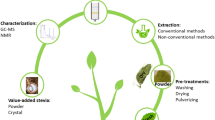
2.1 Extraction Process
The process of extraction involves the separation of our desired natural constituents from the available raw materials. There are different types of extraction methods commonly used in industries for the extraction of compounds from plant and animal sources, for example, distillation extraction, solvent-based extraction, pressing method, and sublimation process, which are categorized based on their working principle. The reason behind the process of extraction of bioactive material is to get the therapeutically valuable molecule and the inert part is eliminated with the help of a selective solvent treatment known as the menstruum. Menstruum is the solvent used in the extraction. The materials that remain after the process of extraction are called “marc.” For the extraction process, we need to prepare concentrations of raw materials using various solvents. The solutions such as alcohol and hydro alcohol sols are prepared from raw materials or chemical substances such as belladonna [11].
Using various solvents with different polarities is the key feature of this process. However, to date, there is no single extraction method for extracting all the metabolites at a given time. Some of the normal methods are listed as follows:
-
1.
Maceration: In this method of extraction, the plant material is crushed or chopped into small pieces. Sometimes the dried powder of plants is used to increase the efficiency of extraction. This method is performed in the closed system of the menstruum, which is the solvent used in the extraction. The plant material and this solvent are placed together; then it is incubated for some days with shaking at certain time intervals. After 5–7 days, the fluidics strained off and we can see the two phases in the system, namely, one supernatant and the residue part. Then the residue part is pressed to get the more fluidic content out and further followed by the filtration. Once the filtration is done, the solution is taken and solidified by evaporation method for ease of analysis of the compound followed by concentrating it and sent for further analysis and preparation of drugs in the pharmaceutical industries.
-
2.
Percolation: This method is preferable for the production of vegetable drugs in the food industry. In this method, the uniform moistening of drugs with solvent for a period of 3–4 h is done in a separable vessel, which is performed in the closed system. A piece of filter paper is placed on the surface, then picked up so that the top layers of drugs are not disrupted. The solvent is poured on the sample slowly at some specific time interval, allowing the bottom area to percolate easily and which is collected in the bottom of containers. This process followed for one complete day and fluid percolated through the plant sample is collected and followed by the evaporation of the sample to get the powder form to quantify the sample then is concentrated and sent for analysis which is further sent for production industries for the production of compounds at an industrial scale.
-
3.
Soxhlet apparatus: This apparatus is used in the small-scale extraction of bioactive compounds in laboratory conditions. These steps are involved in bioactive extraction. The plant sample of holanerrhea pubescus, terminalia elliptica, or any other plant species such as mulberry which have high medicinal properties is first dried in the microwave and then the powdered, dry powder of the plant sample is then placed in the pocket made up of a blotting paper like a pouch. This pouch is loaded in the main chamber of Soxhlet apparatus, the chamber that is attached to the condenser on the top, for the cooling effect, and in the bottom heating mantle for heating the sample. The solvent used here is ethanol or methanol. The phytochemicals in the sample are drawn into the solution and collected at the bottom, and after boiling the sample is cooled by the condenser. The process is continued until the drug or the phytochemicals are extracted and the extracted compounds in the flask are processed by evaporating and followed by the concentration method and sent for analysis [3].
2.2 Solvents Used in Extraction
In the process of extraction of bioactive compounds, different solvents are used. There are two types of solvents, namely, nonpolar solvents and polar solvents. The nonpolar solvents used in the extraction are cyclohexane, toluene, hexane, benzene, ether, chloroform, and ethyl acetate; hence, the products extracted from the nonpolar solvents are alkaloids, terpenoids, coumarins, fatty acids, flavonoids, and terpenoids [12]. The polar solvents used in the extraction are acetone, acetonitrile, butanol, propanol, ethanol, and methane. The products that are extracted by using polar solvents are flavonols, lectins, alkaloids, quassinoids, flavones, polyphenols, tannins, and saponins [12].
3 Process Development
The production of bioactive compounds has a commercial demand in the market; hence, there demand toward the plant tissue culture is at higher rates. The bioactive compounds in plants are obtained in the lower quantity with high-value pharmaceutical properties and food-beneficial properties. Hence, to get the bioactive compounds in the greater quantity for commercial use, biotechnologists go for the method called extraction of bioactive compounds facilitated by enzymes [13]. In an enzymatic method, there will be efficient extraction and a greater yield of bioactive compounds. In this method, specific lytic enzymes are used to damage the outer membrane of the plant cells to improve the productivity of the extraction [6].
The use of enzymes for the extraction of various bioactive compounds, for example, extracting vanillin from vanilla grape seed, extracting carotenoids either from tomato peel or marigold flower, extracting polysaccharides from Sterculia foetida, extracting oil from grape seeds, etc. has shown significantly good results. This method of extraction has greater potential and is commercially attractive [13]. Prior to the adoption of traditional extraction techniques, enzymes were utilized specifically to treat plant material [7]. It is frequently necessary to use a variety of enzymes, such as pectinases, cellulases, also hemicelluloses, to break the structure [14]. Improving the extraction of bioactives from plants while maintaining the rigidity of the plant cell wall is challenging. These enzymes enhance cell wall permeability by hydrolyzing cell wall constituents, leading to increased bioactive extraction yields [13]. Extracts of fungi, bacteria, vegetables, or animal organs, also fruits can all be used to make enzymes. Understanding an enzyme’s catalytic features and mode of action, ideal performing circumstances, and the enzyme or combination of the enzyme to utilize in an extraction application will help you use enzymes more effectively [11].
3.1 The Disintegration of the Cell Wall by the Action of Enzymes
It is the crucial step in the process of getting the bioactive molecules out of cells. Cells are the basic unit of life; cells store valuable bioactive compounds such as flavonoids, anthocyanin, carotene, and many more which has significant value in both food and nutraceutical industries; there is a variety of other classes of bioactive compounds from medicinal plants, which have pharmacological importance. Hence, it is important to extract them without denaturation and also to increase the extraction yield and productivity. Therefore, as the science is developing, there are various techniques that have been introduced for the easy extraction of phytoconstituents from various sources. The enzyme-assisted extraction is one such method that is commonly used nowadays to better intracellular compound extraction methods.
During the process of extraction of intracellular compounds from the cell, it is important to break open the cell so that it allows the intracellular compounds to come out of the cell. The capacity of the enzymes to degrade the cell wall component and also to disintegrate the systematic integrity of the plant cell wall forms the basis for enzyme-assisted extraction. When enzymes and substrates bind to one another, the shape of the enzyme molecule adapts as better as possible with the substrate to improve the interaction. The change in form could put the substrate under strain and stress, which could lead to the bonds breaking and speed up the reaction. When the substrate concentration is high, the enzyme can accelerate the process until the substrate concentration becomes limiting. The operational characteristics of each investigation of enzyme-assisted extraction are taken into consideration, including system pH, enzyme extraction time, substrate particle size, concentration, and reaction temperature. All study findings demonstrate that enzyme-assisted extraction leads to a decrease in extraction time and solvent volume in addition to an increase in yield and product quality (Fig. 1).
Bioactive compounds in plants are secondary metabolites of plants that have therapeutic or harmful effects on living things. Secondary metabolites are created in plants in addition to the basic biosynthetic and metabolic pathways for the substances related to plant growth and development. These by-products of the plant cell are not necessary for the regular operation of the plant, but some of them have been identified to play crucial roles in the survival of living plants, such as signaling and protection. It appears that most plant species can synthesize these compounds. But some chemical groups that make up a plant’s bioactive compounds show toxicological and pharmacological effects on both animals and humans.
Bioactive substances can be used for a variety of purposes, including enhancing the nutritional, sensory, and technological qualities of conventional food, creating functional foods that have proven physiological benefits, creating nutraceuticals from food or agro-industrial waste, key nutritional components, and creating films for use as active, smart, and/or bioactive food packaging.
3.2 Steps Involved in the Extraction of Bioactive Compounds

The purified bioactive compounds can be sent for structural elucidation by NMR, FTIR, and LC-MS/GC-MS and biochemical characterization such as in vitro toxicity, in vitro evaluation, and clinical research.
3.3 Other Different Techniques Combined with EAE to Enhance the Extraction Process
Nowadays, the importance and commercial demands for the enzyme-assisted extracted bioactive compounds from plants are at a high rate. To fulfill the demands of the market, researchers are finding novel techniques, which are going to solve the problem with high commercial value and usage. Some of the novel techniques are microwave-assisted extraction, supercritical fluid extraction, and ultrasound-assisted enzymatic extraction. These techniques are combined with enzyme-assisted extraction to increase the efficiency of extraction to get more yield. These techniques are eco-friendly and efficient with a high yield for the commercial purpose to fulfill the demands of the market.
-
1.
UAEE (Ultrasound-assisted enzymatic extraction):
Ultrasonic irradiation was utilized to expedite the treatment of enzymes due to the increased effectiveness of enzyme-used reactions and the convenience of practical work. As innovative approaches for extracting bioactive compounds, enzyme-assisted extraction methods linked with ultrasonography have been devised [15]. Acoustic cavitation occurs when ultrasound waves flow through a solvent solution, resulting in increased extraction yield [16]. When ultrasonic waves are exposed to a solvent media, tiny vapor-filled bubbles form. It is named cavitation. Whenever the bubbles reach a particular structure, they explode forming in localized high temperatures and pressures. Whenever these bubbles break at the plant cells’ surface, the high temperature and pressure released create liquid jets with shear forces that also are directed toward the plant cells’ surface. The liquid jets and shear pressures that occur during this procedure lead to physical harm to the cell’s wall or cell membrane’s integrity. Ultrasound enhances cell wall penetration, allowing more solvent to penetrate the cell wall and leach compounds into the liquid phase. Furthermore, under ultrasonic treatment optimal conditions (proper frequencies and intensities) can result in increased enzyme work. This is owing to favorable arrangement shifts and structural rigidity, to facilitate bimolecular production [17].
Ultrasound-assisted enzymatic extraction is found to be one of the easiest and quick methods of isolation. An indirect sonication (ultrasonic wash) and also direct sonication, or ultrasound horn can be used. The extraction yield in UAEE of bioactives is related to factors such as ultrasound power enzyme concentration. This technique will minimize the time taken for extraction and can be inculcated in commercial use. The researchers discuss the source of bimolecular extraction, as well as the different aspects that influence ultrasonic extraction, including ultrasound strength, frequency, irradiation period, and extraction yield [15]. Researchers used an enzymolysis-ultrasound-aided extraction (EUAE) technique to extract polysaccharides from corn silk. The ultrasonic treatment will change the chemical makeup and morphological aspects of maize-derived silk polysaccharide. Moreover, it modifies the molecular weight distribution. This is proved based on the previous studies. The polysaccharide output improved from 4.56% to 7.10% under optimal extraction conditions. In addition, polysaccharides isolated using EUAE showed modifications in morphological aspects in the cell wall as well as an increased anticancer and antioxidant activity when observed side by side with polysaccharides isolated through boiling water extraction. Carotenoids were isolated using carrot sap utilizing ultrasound in conjunction with therapy using enzymes by intermittent radiation. This technique increases productivity without causing heat denaturation of carotenoids.
-
2.
Enzyme-assisted high-pressure extraction:
The high-pressure extract (HPE) technique comprises processing the plant matter with solvent, then treating the combination using isostatic ultra-high hydrostatic pressure and purifying the mixture to remove the solids [18]. To acquire the biomolecule of interest, the extracted extract is concentrated, dried, or filtered further. Plants undergo structural alterations as a result of high-pressure treatment due to physical damage to cell membranes, which increases cell wall permeability and phytochemical diffusion through solvents [19]. This results in a higher extraction rate as well as efficiency. In contrast to traditional extraction, HPE can easily separate heat-sensitive chemicals [20]. With this approach, volatile chemicals can be removed quickly and easily without degradation. Most biological biomolecules become more soluble under high pressure [21]. HPE operates at pressures ranging from 100 to 1000 MPa. When compared to other removal technologies, HPE operates at the maximum pressure, allowing it to extract greater phytoconstituent [22]. To investigate the extraction of chemicals from plants using natural sources, researchers combined the benefits of biochemical extraction at high pressures [23].
-
3.
Enzyme-assisted ionic liquid extraction:
Ionic liquids (ILs) are liquid at room temperature and are composed of bulky organic ligands and inorganic anions [24]. Because of their outstanding qualities of low vapor pressure, the flexibility of recycling, miscibility using common organic solvents, thermal and chemical stabilities, and high solubility and extraction rate in organic compounds, they have recently gained prominence in a variety of sectors. Because of the pressing need for environmental preservation, ionic liquids are seen as safer alternatives to standard solvents in the extraction of biomolecules from plants. ILs have previously been used to extract bioactive substances including alkaloids, lignans, and polyphenols [25]. The viscosity of ILs is also important in their application for separating bioactive chemicals from plants. At high temperatures, the fluidity of ILs decreases. ILs can interact with both polar and nonpolar compounds and are helpful when paired with a variety of extraction methods [25]. Chemical composition, concentrations, moisture level, loading rate, pH, column temperature, and enzymes to target ratio are all crucial parameters to monitor during IL extraction. IL-assisted enzymatic extracting has been utilized successfully to extract a wide range of chemicals, including curcumins and phenolic compounds [26]. It has been demonstrated that ILs have a higher viscosity than standard organic solvents. The fluidity of ILs affects the stability and activity of enzymes. The researchers and the scientific community recently confirmed how enzyme conformation changes are delayed in very viscous IL solvents. This maintains enzyme stabilities for long periods. In general, high solvent viscosities lead to effective enzyme stability. The remarkable thermal stability of enzymes in viscoelastic ILs has opened the door to the extraction of pharmaceuticals from plants [26]. The durability of a broad range of enzymes, including thermolyzing, lysozyme, and chymotrypsin, was shown to be much higher in this solvent than in typical organic solvents. From a critical standpoint, enzymes in ILs exhibit good functional and temperature stability in many circumstances [27].
3.4 Types of Bioactives That Can Be Extracted Using Enzyme-Assisted Extraction Method
-
1.
Polyphenols:
Plants with a phenolic composition one and phenolic ring (e.g., polyphenolic compounds or phenolic alcohols) include a diverse range of phenolics. The extraction of phenolic chemicals was done either traditionally using mixed solvents (acetone/water, dioxane/ethanol, biofuel, and methanol/water) or alkaline/acidic techniques. Antioxidants are a large class of phenolics that are among the most coveted bioactive chemicals [28]. They are quite interesting and also serve a variety of biological purposes. They disrupt oxidative cycles to prevent or delay oxidative damage to macromolecules. Many studies have shown that fruit peel, seeds, pomace, and leaves are high in polyphenolic chemicals [29]. Because of the possible toxicity of some chemical preservatives, researchers have made greater attempts to uncover and use naturally produced antioxidant properties from agricultural residues. The early extraction processes are critical during the separation of bioactive compounds from vegetables and fruits. Among these procedures, dehydration and grinding have the greatest impact on removal capacity and mass transfer to obtain polyphenols. Drying, in general, entails the elimination of all bound water from the peel by enhancing the permeability of the cellular matrix, enabling diffusion rate and promoting interaction with enzymes, thereby improving phenol extraction. The extraction efficiency of polyphenols is affected by phenolic component properties, operational drying circumstances, and drying [30].
The citrus industry generates huge amounts of peel and seed wastes (up to 50% of total fruit weight), which could be the main sources of phenolic chemicals in certain peels. Taking this into account, total phenolic extract using food-safe enzyme cellulase, the bioactives from five distinct fruit peels (mandarin, Yen Ben lemons, grapefruit, orange, and Meyer lemon) were extracted. Similarly, Gomez-Garcia et al. performed cellulase- assisted extraction of antioxidative polyphenolic using grape (Vitis vinifera L.) leftovers in an attempt to valorize fruit waste. Not only did protease extraction produce more phenolics (O-coumaric acid as assessed by high-performance liquid chromatography-electrospray mass spectrometry), but it also demonstrated free radical-scavenging ability. These experiments show that enzymatic extraction of phenolics performs far superior to traditional approaches. Various methods are being employed to use agricultural industry trash as a renewable source for high value-added (primarily polyphenolic chemicals) products. The use of enzymes offers an alternate method for producing these beneficial chemicals from agro-industrial waste. Enzyme-assisted extraction has been used to extract antioxidant components from a variety of sources such as lemon balm (Melissa officinalis), red algal leftover (Palmaria palmata), Agaricus blazei Murill (rice bran), and pumpkin peel waste (Cucurbita moschata). Plant cell walls are largely made up of interconnected polysaccharides such as starch, cellulose, hemicellulose (xyloglucans), and pectin, which act as a barrier to the escape of intracellular chemicals [31].
Flavonoids are entrapped in polysaccharide complexes via hydrogen bonding and hydrophobic interactions. A combination of carbohydrate-hydrolyzing enzymes, including cellulose, hemicellulose, and protease, may be a more effective opportunity to sufficiently damage the cell walls, break down complicated interior storage materials, and therefore facilitate the release of free polyphenols. Some polyphenols are always covalently coupled with glucose in the form of glucosides with glycosidic connections. Glucosidase is capable of breaking the -1,4 glucoside bonds in glucosides. Cellulose, hemicellulose, and pectin can be hydrolyzed using the enzymes, cellulase, -glucosidase, and pectinase. To improve the extraction yield of intracellular contents, enzyme-aided treatment was combined with solvent extraction. The authors pretreated Prunus species with various enzymes (cellulase, pectinase, protease, and alpha-amylase) before extracting anthocyanins. Flavonoids are entrapped within polysaccharide complexes via hydrogen bonding and hydrophobic interaction [32]. A combination of carbohydrate-hydrolyzing enzymes including cellulose, hemicellulose, and pectinase may be more effective. By solvent extraction, there is a possibility to adequately damage the wall of the cell and break down complicated inner storage. Cellulase produced the highest anthocyanin content when compared to other enzymes. Furthermore, the quantity of the targeted enzyme has a considerable impact on the extraction. Lower enzyme concentrations result in less enzyme interaction with the peels/pulp, resulting in less extraction of the desirable polyphenols. A larger concentration of enzyme, on the other hand, can successfully extract the necessary polyphenols. However, in some circumstances, using more enzymes for extract may result in final product inhibition. To evaluate the effects of varying cellulase concentrations (ranging from 1% to 10%) on the recovery of lycopene from pumpkin tissues, one researcher observed that as the enzyme concentration increased, lycopene yield increased up to 7% within the enzymatic reaction. In contrast, no notable decrease in anthocyanin extraction yield from saffron tepals was observed as the Pectinex content increased from 1% to 10%. However, the proper enzyme and its quantity are determined by the nature of fruit yield cell walls [33].
The combined impacts of numerous enzymes can result in greater cell wall breakdown, leading to higher removal yields. Several enzyme mixes are commercially available. The synergistic effect of the enzyme combinations Pectinex Ultra Clear and Lallzyme Beta for grapes extraction of juice of Vitis labrusca L. variety Concord was investigated. They found that when two enzymatic preparations were combined, it increased juice yield and the release of bioactive components compared to using individual enzyme preparations. To increase extraction yield, available commercial enzymes are typically mixed with two or more enzymes. Phenolic components and antioxidants were recovered from rice bran in one study by employing available commercial carbohydrates: AMG, Cell clast, Pentopan, Viscozyme, Termamyl, and Ultralow. The ferric-reducing capacity of phenolics released by all carbohydrates increased significantly (1.5–3.3 times). Among the enzyme mixtures listed above, Pentopan (a mixture of Endo-1-4-xylanase as well as feruloyl esterase, caffeoyl esterase, as well as pectinase) emerged to be the most efficient in increasing antioxidant activity, so although Cell clast, Ultralow, as well as Viscozyme appeared to be more effective in increasing phenolic content as well as radical-scavenging activity. Commercial carboxylases successfully boosted phenolic acid extraction yield through enzymatic hydrolysis of cell-wall components as well as the release of free phenolic acids. Bioactive chemicals, particularly anthocyanins as well as other polyphenols, are affected by processing and subsequent storage. As a result, it must be considered while evaluating the possible health advantages of foods and beverages. Using different commercial enzymes, the researchers extracted phytonutrients from bilberry skin and examined the stability in the form of half-life values. Due to enzyme-assisted extraction, the half-life value increased (from 12% to 64%). Because of the electrostatic attraction between anthocyanin and hydrolyzed pectin molecules, this suggested good pigment stability. Other polyphenols (especially flavanols and polyphenols) were also produced after the significant enzymatic degradation of polysaccharide cell walls in bilberry skin. These polyphenols served as co-pigments to enhance anthocyanin retention, complexes (carbohydrates) can transform water-insoluble cell wall and cell membrane substances into water-soluble components (short polysaccharide fragments), resulting in a variety of bioactive characteristics observed. The chemical structure, structural morphology, and physiochemical properties of insoluble and soluble residues are critical in identifying optimal conditions to enhance extraction yield [33].
-
2.
Polysaccharides:
Aside from polyphenols, polysaccharides are indeed the group of metabolites that have been investigated the most. Water-soluble, high-molecular-weight polysaccharides can modify the rheological properties of food and are frequently employed in a variety of food applications as stabilizers, emulsifiers, thickeners, and texture modifiers. Additionally, dietary fibers contain a wide range of bio-functional properties, including immunomodulatory, hematopoiesis-promoting, antioxidant, antibacterial, and anticancer activities, according to several recent studies [34]. The most typical technique for removing polysaccharides is traditional heated reflux extraction. In general, the recovery time and temperature have a significant impact on the yield of this approach. However, prolonged use at high temperatures can weaken polysaccharides, which in turn reduces their biological activity. The enzymatic extraction for bioactive biodegradable polymers has been extensively studied [21]. There are two ways to perform enzyme-assisted carbohydrate extraction. The first is to use enzymes capable of destroying biological membranes such as cell walls and membranes to aid in the isolation of desired polysaccharides. To facilitate extraction, enzymes that partially break down desired polysaccharides to minute fragments are used. Alkaline was used to extract carrageenan from Mastocarpus stellatus (a type of protease). The gelling characteristics of the isolated polysaccharides were excellent [35]. The extraction procedure resulted in a combined extraction of important phytochemicals such as polyphenols adding value to the extract. As a result, enzymes can be used to selectively extract bioactive polysaccharides, potentially allowing for the targeted creation of certain gelation characteristics and desired physical features [22].
Another work used a response surface approach to do cellulase-assisted extraction of water-soluble Malva Sylvester’s carbohydrates (MSP). The maximum MSP yield (10.40%) was obtained at 5.64% cellulase, 55.65 °C temps, 3.4 h, and 5.22 ph. These homogenous polysaccharide fractions were then purified using chromatography. In a dose-dependent pattern, the fractions dramatically improved antioxidant, anticancer (tested on HepG2 and A549), and antibacterial activity. Using enzymes thereby improves not only the removal efficiency but also the medicinal characteristics of polysaccharides. Because of their unique physical and biological qualities, seaweed (algal) disaccharides and polysaccharides are gaining popularity in the functional food and pharmaceutical industries. The researchers conducted enzyme-assisted carbohydrate extraction from the brown cyanobacteria Ecklonia radiata using a combination of six commercial enzyme mixtures: Viscozyme L, Cellclast 1.5 L, Ultraflo L, Alacalase 2.4 L, Neutrase 0.8 L, and Flavourzyme 1000 L. They discovered that total sugar output was unaffected by enzyme type or ph. The abovementioned parameters, however, govern the molecular mass of the isolated polysaccharides. High buffer salt concentrations were discovered to impede polysaccharide extraction. As a result, buffers should not be used in enzyme-aided algal carbohydrate extraction for increased extraction efficiency [22].
It is commonly accepted that the chemical components, structural, molecular mass, and conformation of polysaccharides can influence their bioactivity and other therapeutic qualities. To separate polysaccharides from Epimedium acuminatum, hot water extraction was optimized. Nonenzymatic extracting and heating extraction, on the other hand, resulted in a lower polysaccharide yield (30.2%) as compared to enzyme-aided water extraction (82.4%). Furthermore, unlike previous methods, enzymatic water extraction enhanced time efficiency, reduced solvent usage, and functioned at lower extraction temperatures. SEM images demonstrated that after enzyme-assisted extraction, the cell walls became thin and disordered, facilitating mass transfer among polysaccharides and solvent and therefore increasing yield. The impact of various extraction procedures on the makeup of both acidic and neutral pectic polysaccharides was investigated during bulk pectic extraction from agroindustry leftovers. The most common method for freeing pectin from plant sources is acidic extraction. Previous research has shown that the extraction method can cause significant pectin breakdown, resulting in a low yield and loss of gelling characteristics. Furthermore, they are used in crude form instead of purified pectin to cut production costs. The influence of different extraction procedures (acids, enzymes, and chelators) on the recovery and content of bulk pectic polysaccharides was studied using four distinct wastes as substrates: berry pomace, onions hulk, compressed pumpkin, and sugar beet pulp. Enzymatic hydrolysis employing Cell class, which contains cellulases (endoglucanase), was found to be successful in removing pectin through plant cell walls. Sugar beet pulp or onion hulk was discovered to be appropriate substrates for purifying pectin and manufacturing pectin-based products based on the mechanism of recovering pectic sugars following enzymatic extraction. In addition, the incubation conditions of the different substrates with enzymes were shown to be significantly milder and more time efficient than extraction with acidic and chelators. The enzyme extraction method has demonstrated an advantage over conventional procedures in the sustained extraction of polysaccharides with increased pharmacological of the extract. Complex enzymes have also been shown to increase polysaccharide output and extract quantity. Another benefit of enzyme-aided extraction is that less alcohol is required again for the precipitation of target polysaccharides from the reaction mixture, making the procedure more environmentally friendly.
-
3.
Oils:
Oil extraction via seeds employs traditional procedures including mechanical press by hydrolytic method, solvent extraction, and extruder pressing. In certain circumstances, the oil can be obtained or produced directly from the fruits using a normal mechanical pressing and is taken without further procedures and experimental analysis, such as virgin olive oil. Hexane is commonly used for oil extraction due to its ease of recovery, low boiling point (63–69 °C), and high solubilizing ability. Unfortunately, due to health, safety, and environmental considerations, hexane is not a preferred solvent in the extraction process. Researchers are seeking alternatives to hexane that will not reduce oil yield or quality. As a result, the use of green solvents and fluids may be a viable alternative to oil extraction. Aqueous enzymatic extract (AEE) is an effective method for extracting oils from oil seeds. This approach is straightforward to use, consumes less energy, and is economically viable. In general, oil droplets are surrounded by protein, which is a major component of the cell wall. Proteins and pectin were the main components of cell walls in soybeans and rapeseeds. As a result, degrading these components with proteolytic, cellulase, pectinase, as well as other enzyme mixes increased oil output. The chemical structure, anatomy of the cell wall, and placement of the oil droplet within the seed must all be considered while selecting enzymes [36]. Furthermore, in oil extraction optimization trials, the oil-to-water ratio must be considered in addition to general characteristics such as pH, temperature, and particle size. Enzymes as well as their concentration require moisture content to function, and the presence of low humidity in oilseeds causes the formation of a thick suspension, which inhibits enzyme activity [37].
In the literature survey scientifically, there are various examples and illustrations available in which an enzyme mixture has been proven to function synergistically to enhance and improve the extraction efficiency of required oils including phenolics from bay leaf (Laurus nobilis L.). By disintegrating plant cell walls, the enzyme mixture comprising cellulose, hemicellulose, and xylanase improved the removal efficiency of biomolecules of plant matrix. The extraction rate of essential oils was enhanced by 243%, 227%, 240.54%, and 248% when the substrate was treated using hemicellulose, cellulase, and xylanase individually and their ternary mixture. Further investigation found that essential oils increased the antioxidant activity of enzyme-pretreated substrates. The addition of enzymes altered the proportions of separate components and increased the number of oxygenated monoterpenes. As a result, the essential oils produced from enzyme-treated samples had higher antioxidant activity. Similarly, oil was extracted using bush mango kernel flour by treating it with commercially available enzyme mixtures such as Alkalize, Pectinex, and Viscozyme, yielding oil yields of 35%, 42.2%, and 68.0%, respectively. In addition to boosting oil extraction output, the enzymatic method increased oil sample quality by increasing the number of bioactive components (such as carotenoids and other phenolics) along with their antibacterial activity. The use of tannase increased the total phenolics in the hydrophilic and lipophilic fractions, resulting in the oil having a better antioxidant capacity. Essential oils with enhanced antioxidant capabilities and antibacterial activity have a high potential for usage in the food and pharmaceutical industries. Enzymatic processing was shown to drastically reduce the production of oil left in Chilean hazelnut food in a few situations [38].
Clove essential oil is one of the most important essential oils. Recently, research has concentrated on the pretreatment of clove buds’ powder with enzymes including cellulase, lignocellulose, pectinase, and amylase before extraction. For the recovery of clove oil, the typical procedure of steam distillation yields 10.1%. In recent investigations, enzymes specific for cell wall activity were utilized in preprocessing before extraction to enhance the purity and quantity of the phytochemicals recovered. The extraction of oil from discarded pomegranate seeds using protease (from Aspergillus oryzae) treatment yielded a more than 50% good return than the control samples in terms of quantity of extracted essential oil. SEM images of discarded pomegranate seeds were obtained before and after protease treatment to better understand the extraction mechanism. The micrographs of SEM revealed that even after protease therapy, the cell wall is damaged as a result of the enzymatic treatment, which renders the surface of the seeds very porous, facilitating the recovery of physiologically entrapped oil. Furthermore, the protease-derived oil had 1.4 times the phenolic content and 4% more antioxidant activity than hexane-extracted oil. The preceding example implies that pretreatment with enzymes before resource extraction can boost oil extraction yield. Enzymatic pretreatment before solvent extraction and mechanical pressing softens the cell wall and increases oil extraction [37]. This method reduces the potential of oil production in water emulsion following extraction.
The parameters for enzyme selection are determined by the location of oil inside the cellular structure as well as the chemical composition of the substances surrounding it. The simultaneous assessment of each of these criteria is critical in determining the appropriate enzyme combination for a particular oil-containing substrate. Despite its numerous benefits, the application of AEE is still limited due to the lengthy processing time and difficult drying process following enzymatic treatment. A substantial amount of the enzyme is required (usually more than 1% of the weight of an oilseed ingested), which contributes to the expensive expense. Furthermore, the lack of commercially available enzymes has hampered the development of these methods. An additional issue with AEE is the difficulty in avoiding emulsification of the oil extracted, which necessitates post-extraction demulsification step toward recovering and increasing oil yield. Tabtabaei and Diosady employed aqueous enzymatic emulsion de-emulsion approach to destabilize the oil-in-water emulsions to recover access to the oil before the industrial application [39]. The researchers used enzymatic demulsification therapy by several proteases and phospholipases in their work to test its ability to release free oil. The targeted emulsifiers are hydrolyzed. Protex 6 Land phospholipase therapies proved successful in collecting over 91% of the oil inside the emulsion.
3.4.1 Flavors and Colors
Colors and flavors improve the quality of a food product by influencing its visual appearance and taste. In the food sector, there is an ever-increasing need for natural flavors and colors. Synthetic dyes were suspected of emitting hazardous compounds that are highly polluting, allergenic, carcinogenic, and toxic to humans. Given the health and environmental concerns associated with industrial chemical dyes, researchers redirected their focus away from artificial (synthetic) colorants and toward the excitation of natural colorants derived from plant sources [40]. Colorants taken from roots, bark, foliage, nuts, berries, and flowers include anthocyanins, betalains, chalcones, chlorophyll, carotenoid, and flavones. Traditional natural dye extraction procedures include water and alkali extraction, fermentation, and solvent extraction. If the right enzymes are chosen and the operating conditions are tuned, enzyme-assisted extraction method has a lot of promise for pigment isolation. In the past, commercially accessible enzymes including cellulase, amylase, and pectinase were investigated. This method may hasten the extraction of pigments through tough and compact plant matter such as barks and roots. Conventional means of natural dye extraction, in addition to being time-consuming and ineffective, result in the co-extraction of unwanted compounds such as chlorophyll and waxes [41].
3.5 Enzyme-Enhanced Processes for Plant Materials
-
1.
Extraction of bioactive from by-products.
By enhancing the permeability of plant cell walls, enzyme-assisted extraction (EAE) makes it possible to extract pectin from waste and by-products [42]. Many phenol chemicals, such as flavonoids and anthocyanidins, can be extracted using enzymes [43]. For enzymatic treatment to be as effective as possible, factors such as enzyme activity, treatment time, substrate ratio, and particle size are crucial [44]. Scientists have addressed that ideal condition for extracting pistachio green hull. For the extraction of cellulose, tannases, pectinases, and their mixtures were utilized. Results have revealed that the three enzymes used simultaneously to extract phenolics gave the best result [45].
-
2.
Production of fermented drinks and plant-based drinks from grains.
The production of high-protein food items using grains and cereals, involves a process that requires the degradation of cell walls with the assistance of enzymes to obtain the desired food product. Yearly, dairy-related product consumption is growing by 10%. In the year 2019, America reached to the extent of 1.8 billion dollar spending on dairy products [46]. This is due to the high release of sugars forming the acceptant-sensory organoleptic features [47]. The enzymes which are majorly used to start the earlier steps pectinase-denatured rapeseed fibers and cellulose for the growth of the cultures such as L. johnsonii L63, reuteri L45, Plantarum L47 are some examples for the starter culture and growth of bacteria for high production of nutrients in fermenter tanks [48]. Furthermore, plant-based fermented goods have antibacterial qualities, and their pH is lower than that of typical plant-based drinks, impacting product stability [49].
3.6 A Review of Enzymes and Factors Influencing Bioactive Extraction
Usually, bioactive compounds in natural products exist as either soluble or insoluble conjugates (glycosides). In food industries, for example, the majority of phenolics (24% of overall phenolic content) are found as bounded phenolics [50]. The majority of phenolic compounds are imprisoned inside cell wall polysaccharides such as cellulose, hemicellulose, and pectin, which are connected by chemical bonding and hydrogen bonds. Other phenolic acids create ether links with lignin via their aromatic ring hydroxyl groups and ester links with structural polysaccharides and proteins via their carboxylic groups [51]. Flavonoids are covalently bonded to sugar moieties via glycosidic bonds or carbon-carbon bonds. Tannins have a proclivity to create powerful complexes with proteins. Here, enzymes such as cellulase, hemicellulose, pectinase, and protease are used to solubilize the plant cell wall, consequently speeding the discharge of intracellular biomolecules [45]. Hydrolases, such as lipases, work in the water that is present inside the reaction system. They also act in the presence of additional substrates such as alcohols, amines, and oximes. During the separation of flavonoids in Ginkgo biloba, Penicillium decumbens cellulase outperformed T. reesei cellulase in the condition of maltose as the glycosyl donor. Cellulases and hemicelluloses can be used to separate oils and proteins. These enzymes attack the interior locations of the polysaccharide chains at random. This results in the formation of tiny oligosaccharides of varying lengths, which allow for the easy liberation of entrapped molecules [52].
The extraction efficiency is determined by the solvent system, temperature, enzyme mode of action, substrate availability, extraction length, enzyme loading, and pH condition. Each enzyme has a different optimal pH for enzymatic hydrolysis [53]. Many enzymes have an optimal pH that is close to the neutral pH of proteins. Because proteins are particularly insoluble in this pH range, biomolecule release may be hampered. As a result, pH must be selected in such a manner that not only does it inhibit enzyme action but also does not fall within the range of protein isoelectric point. Temperature, in addition to pH, is an essential aspect to consider during extraction [16].
3.7 Advantages and Disadvantages of Enzyme-Assisted Extraction Method
3.7.1 Advantages of Enzyme-Assisted Extraction Method
The enzyme-assisted extraction method is a highly efficient technique compared to the other extraction techniques as it takes very less time with the high productivity of extracted compounds. It requires the knowledge of the basic and simple methodology for the extraction of compounds. The extracted compounds will retain their structural and physiochemical and configure stability during and after the process of extraction. Consequently, the concept of “green chemistry” is being pursued. People are searching for an effective, ecologically friendly way to increase bioactive recovery rates. Due to its improved extraction capabilities and environmental friendliness, enzymatic extraction has demonstrated many benefits. Through this process, we can exactly figure out our required intracellular compound and can be extracted with high accuracy so that purity of the extracted compound is enhanced. The process required for the extraction of intracellular bioactive chemicals using this unique technology is regarded as lenient, meaning there are no precise requirements to be maintained. The cellular barrier that is the cell wall, which processes cellulose, hemicellulose, and pectin, can efficiently be degraded with enzymes, namely, cellulase, hemicellulose, and pectinase, respectively, without affecting the bioactives [54, 55].
3.7.2 Disadvantages of Enzyme-Assisted Extraction Method
-
Costly equipment is needed; hence, this method is expensive compared to other extraction methods.
-
We establish this technique at a small-scale level and cannot be used at the industrial level.
-
This technique is not suitable for the extraction of by-products such as fibers, phenolic compounds, carotenoids, and anthocyanin.
-
Some plants having different cell wall compositions rather than usual cell wall compositions will bring a difficulty to break open the cell wall so that it allows the intracellular compounds to come out of the cell. There are no such novel enzymes that are available to break open those cell walls with different biochemical compositions.
-
The process of extraction and purification of enzymes for the treatment process is a difficult and crucial step, and it is difficult to store and maintain those enzymes in large quantity, which is the drawback in this method [56].
The industrial importance of bioactives: Bioactive compounds are having more importance in the food industries and pharmaceutical industries.
3.8 Some of the Pharmaceutical Activities of Bioactive Compounds
-
(a)
Antidiabetic activity: Diabetes is concerned with a group of conditions characterized by a high level of blood glucose, commonly known as blood sugar [57]. Too much sugar in the blood causes serious health problems, sometimes even it may lead to death. In diabetics, there are two types: type 1 and type 2. Type 1 diabetes destroys by the immune system by mistake [58]. The reason is insulin binds to its receptor on target cells; hence, less glucose is taken into the cells so that more glucose stays in the blood. Therefore, this type of diabetes is called insulin-dependent. Another type, known as type 2 diabetes, is characterized by insulin resistance. This condition is often associated with factors such as obesity, a sedentary lifestyle, and an unhealthy disposition. Type 2 is related to endocrine metabolism. In such circumstances, plants or natural products containing antidiabetic properties, such as insulinogenic or secretagogue properties, hold significant promise and potential for the development of novel pharmaceuticals. There are so many plants with the antidiabetic properties. Some of them are Acacia arabica, Aegle marmelos, agrimonia eupatoria, allium cepa, Allium sativum, Aloe vera, Azadirachta indica, and Benincasa hispida [59]. The various parts of medicinal plants can treat diabetes in various ways, including insulin secretagogue activity, the insulin release from the pancreas, insulin-like activity, an increase in plasma insulin concentration, an increase in insulin binding to insulin receptors, a decrease in plasma triglyceride levels, insulin-sensitizing activity, and an antihyperglycemic mechanism to stimulate islet insulin release [60]. Additionally, a sizable population worldwide has switched to this complementary method of treating illness because of its varied flora, affordability, and simplicity of use with little negative effects. Studies show that medicinal plants are multitargeting and least likely to fail during treatment, which is supported by the evidence [61].
-
(b)
Anticancer activity: Cancer is the result of uncontrolled, rapid cell division. Numerous types of cancer can be found [62]. Cancer is one the most deadly disease caused due to metabolism. This disease is not completely curable. We can just increase the life span of the patient with chemotherapy and some antibiotics [63]. Presently, ten million people lose their lives every year with this deadly disease, and this may exceed in the future according to the WHO (World Health Organization) [64]. Topoisomerase inhibitors such as irinotecan and doxorubicin and alkylating drugs such as oxaliplatin, carboplatin, and cisplatin are used in chemotherapy. Irinotecan’s adverse effects include neutropenia and sensory neuropathy (side effects include nephron, gastrointestinal, cardiovascular, pulmonary, and hematologic toxicity). Apart from their complexity, expense, and non-eco-friendliness, the aforementioned medications’ main drawbacks are their side effects and toxicity because they also target normal cells [65]. In order to treat cancer naturally and herbally, a group of photochemical known as “vinca alkaloids,” which were extracted from Catharanthus roseus, are used. Vinorelbine, vindesine, vincristine, and vinblastine are the four primary alkaloids found in vinca. Vinblastine and vincristine are particularly effective at stopping the cell cycle in metaphase and interfering with microtubule function. Currently, vinorelbine, vindesine, and vinfosiltine are semisynthetic derivatives of the vinca alkaloids. Consequently, we can predict a bright future for photochemical research because, over the next 10 years, it is anticipated that these compounds will completely change how cancer is treated [66].
-
(c)
Diuretic properties: Today, heart disease, kidney disease, and excessive blood pressure are all fairly frequent. Acute renal failure, edema or an increase in blood calcium or potassium, acute left ventricular failure or heart failure, and acute pulmonary edema are common medical conditions that patients frequently experience. Different medications are used to treat these issues because they assist the body to excrete more electrolytes and urine, which helps to lessen fluid retention. In actuality, many medications have negative long-term effects and are unable to treat the threat of high blood pressure. In contrast, green plants with high flavonoid and polyphenol content have been discovered to have high salt and potassium excretion properties, including Cynodon dactylon, Emblica officinalis, Kalanchoe pinnata, and Bambusa nutans. Hence, many of the plants are shrubs are to be identified and used in the preparation of drugs for the curing of diseases [67].
4 Conclusion
Considering all the extraction methods of the bioactive compounds, enzyme-assisted method seems to be the most efficient method for extracting the bioactive compounds. The enzymes make the cell wall most permeable and which leads to the high yield of metabolites that are having more applications in the food and pharmaceutical industries [28]. This method consists of efforts of food technologists, food chemists, nutritionists, and toxicologists. For improving the level of release of bioactive compounds, synthesis of new enzymes and their purification is important.
Compared to earlier extraction methods, enzymes-assisted extraction is more efficient and reliable. Extracting the terpenoids, polyphenols, and lectins need more research work to uncover the hidden potential, without the release of toxic substances. The extraction methods should use metabolites, which are eco-friendly nature. The approach of genetic engineering also plays an important role in this process to produce on a larger scale. Still, there is a need for finding the available enzymatic processes for further enhancement of the yield of the bioactive compounds.
References
Bernhoft A (2010) Bioactive compounds in plants: benefits and risks for man and animals: proceedings from a symposium held in Norwegian Academy of Science and Letters, Oslo, 13–14 November 2008. Novus Forlag, Oslo
Biesalski H-K et al (2009) Bioactive compounds: definition and assessment of activity. Nutrition 25(11–12):1202–1205. https://doi.org/10.1016/j.nut.2009.04.023
Sasidharan S, Chen Y, Saravanan D, Sundram K, Latha L (2010) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 8(1). https://doi.org/10.4314/ajtcam.v8i1.60483
Patil BS, Jayaprakasha GK, Chidambara Murthy KN, Vikram A (2009) Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem 57(18):8142–8160. https://doi.org/10.1021/jf9000132
Ramadan MF et al (2009) Chromatographic analysis for fatty acids and lipid-soluble bioactives of Derris indica crude seed oil. Chromatographia 70(1–2):103–108. https://doi.org/10.1365/s10337-009-1141-9
Barzana E et al (2002) Enzyme-mediated solvent extraction of carotenoids from Marigold flower (Tagetes erecta). J Agric Food Chem 50(16):4491–4496. https://doi.org/10.1021/jf025550q
Choudhari SM, Ananthanarayan L (2007) Enzyme aided extraction of lycopene from tomato tissues. Food Chem 102(1):77–81. https://doi.org/10.1016/j.foodchem.2006.04.031
Durling N et al (2007) Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem 101(4):1417–1424. https://doi.org/10.1016/j.foodchem.2006.03.050
Garcı́a A, Brenes M, José Moyano M, Alba J, Garcı́a P, Garrido A (2001) Improvement of phenolic compound content in virgin olive oils by using enzymes during malaxation. J Food Eng 48(3):189–194. https://doi.org/10.1016/S0260-8774(00)00157-6
Atef M, Mahdi Ojagh S (2017) Health benefits and food applications of bioactive compounds from fish byproducts: a review. J Funct Foods 35:673–681. https://doi.org/10.1016/j.jff.2017.06.034
Zhi Fu G, Chan A, Minns D (2005) Preliminary assessment of the environmental benefits of enzyme bleaching for pulp and paper making (7 pp). Int J Life Cycle Assess 10(2):136–142. https://doi.org/10.1065/lca2004.06.162
Sun H, Ge X, Lv Y, Wang A (2012) Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J Chromatogr A 1237:1–23. https://doi.org/10.1016/j.chroma.2012.03.003
Sowbhagya HB, Chitra VN (2010) Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit Rev Food Sci Nutr 50(2):146–161. https://doi.org/10.1080/10408390802248775
Wu Y, Cui SW, Tang J, Gu X (2007) Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem 105(4):1599–1605. https://doi.org/10.1016/j.foodchem.2007.03.066
Arvayo-Enríquez H, Mondaca-Fernández I, Gortárez-Moroyoqui P, López-Cervantes J, Rodríguez-Ramírez R (2013) Carotenoids extraction and quantification: a review. Anal Methods 5(12):2916. https://doi.org/10.1039/c3ay26295b
Nadar SS, Rathod VK (2017) Facile synthesis of glucoamylase embedded metal-organic frameworks (glucoamylase-MOF) with enhanced stability. Int J Biol Macromol 95:511–519. https://doi.org/10.1016/j.ijbiomac.2016.11.084
Bansode SR, Rathod VK (2017) An investigation of lipase catalysed sonochemical synthesis: a review. Ultrason Sonochem 38:503–529. https://doi.org/10.1016/j.ultsonch.2017.02.028
Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B (2008) Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci Emerg Technol 9(1):85–91. https://doi.org/10.1016/j.ifset.2007.06.002
Prasad KN, Yang E, Yi C, Zhao M, Jiang Y (2009) Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov Food Sci Emerg Technol 10(2):155–159. https://doi.org/10.1016/j.ifset.2008.11.007
Kim JH, Park Y, Yu KW, Imm J-Y, Suh HJ (2014) Enzyme-assisted extraction of cactus bioactive molecules under high hydrostatic pressure: enzyme-assisted extraction of cactus under HHP. J Sci Food Agric 94(5):850–856. https://doi.org/10.1002/jsfa.6317
del Pilar Sánchez-Camargo A et al (2016) Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem 192:67–74. https://doi.org/10.1016/j.foodchem.2015.06.098
Ezeh O, Gordon MH, Niranjan K (2016) Enhancing the recovery of tiger nut (Cyperus esculentus) oil by mechanical pressing: moisture content, particle size, high pressure and enzymatic pre-treatment effects. Food Chem 194:354–361. https://doi.org/10.1016/j.foodchem.2015.07.151
Bai Y, Liu L, Zhang R, Huang F, Deng Y, Zhang M (2017) Ultrahigh pressure-assisted enzymatic extraction maximizes the yield of longan pulp polysaccharides and their acetylcholinesterase inhibitory activity in vitro. Int J Biol Macromol 96:214–222. https://doi.org/10.1016/j.ijbiomac.2016.11.105
Bogdanov MG (2014) Ionic liquids as alternative solvents for extraction of natural products. In: Chemat F, Vian MA (eds) Alternative solvents for natural products extraction. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 127–166. https://doi.org/10.1007/978-3-662-43628-8_7
Dai Y, van Spronsen J, Witkamp G-J, Verpoorte R, Choi YH (2013) Ionic liquids and deep eutectic solvents in natural products research: mixtures of solids as extraction solvents. J Nat Prod 76(11):2162–2173. https://doi.org/10.1021/np400051w
Mann JP, McCluskey A, Atkin R (2009) Activity and thermal stability of lysozyme in alkylammonium formate ionic liquids—influence of cation modification. Green Chem 11(6):785. https://doi.org/10.1039/b900021f
Correia RT, Borges KC, Medeiros MF, Genovese MI (2012) Bioactive compounds and phenolic-linked functionality of powdered tropical fruit residues. Food Sci Technol Int 18(6):539–547. https://doi.org/10.1177/1082013211433077
Azmir J et al (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117(4):426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldívar SO (2014) Bound phenolics in foods, a review. Food Chem 152:46–55. https://doi.org/10.1016/j.foodchem.2013.11.093
Londoño-Londoño J et al (2010) Clean recovery of antioxidant flavonoids from citrus peel: optimizing an aqueous ultrasound-assisted extraction method. Food Chem 119(1):81–87. https://doi.org/10.1016/j.foodchem.2009.05.075
Li BB, Smith B, Hossain MM (2006) Extraction of phenolics from citrus peels. Sep Purif Technol 48(2):189–196. https://doi.org/10.1016/j.seppur.2005.07.019
Yadav K, Singh MR, Rai VK, Srivastava N, Prasad Yadav N (2020) Commercial aspects and market potential of novel delivery systems for bioactives and biological agents. In: Advances and avenues in the development of novel carriers for bioactives and biological agents. Elsevier, pp 595–620. https://doi.org/10.1016/B978-0-12-819666-3.00020-1
Lotfi L, Kalbasi-Ashtari A, Hamedi M, Ghorbani F (2015) Effects of enzymatic extraction on anthocyanins yield of saffron tepals (Crocos sativus) along with its color properties and structural stability. J Food Drug Anal 23(2):210–218. https://doi.org/10.1016/j.jfda.2014.10.011
Giavasis I (2014) Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr Opin Biotechnol 26:162–173. https://doi.org/10.1016/j.copbio.2014.01.010
Rostami H, Gharibzahedi SMT (2017) Cellulase-assisted extraction of polysaccharides from Malva sylvestris: process optimization and potential functionalities. Int J Biol Macromol 101:196–206. https://doi.org/10.1016/j.ijbiomac.2017.03.078
Chair of Food Plant Chemistry and Processing, Faculty of Food Sciences, University of Warmia and Mazury in Olsztyn, Pl. Cieszyński 1, 10-726 Olsztyn, Poland et al (2016) Optimization of pumpkin oil recovery by using aqueous enzymatic extraction and comparison of the quality of the obtained oil with the quality of cold-pressed oil. Food Technol Biotechnol 54(4). https://doi.org/10.17113/ftb.54.04.16.4623
Mat Yusoff M, Gordon MH, Niranjan K (2015) Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: a review. Trends Food Sci Technol 41(1):60–82. https://doi.org/10.1016/j.tifs.2014.09.003
Womeni HM, Ndjouenkeu R, Kapseu C, Mbiapo FT, Parmentier M, Fanni J (2008) Aqueous enzymatic oil extraction from Irvingia gabonensis seed kernels. Eur J Lipid Sci Technol 110(3):232–238. https://doi.org/10.1002/ejlt.200700172
Soto C, Concha J, Zuniga ME (2008) Antioxidant content of oil and defatted meal obtained from borage seeds by an enzymatic-aided cold pressing process. Process Biochem 43(6):696–699. https://doi.org/10.1016/j.procbio.2008.02.006
Tabtabaei S, Diosady LL (2013) Aqueous and enzymatic extraction processes for the production of food-grade proteins and industrial oil from dehulled yellow mustard flour. Food Res Int 52(2):547–556. https://doi.org/10.1016/j.foodres.2013.03.005
Li Y, Jiang L, Sui X, Wang S (2011) Optimization of the aqueous enzymatic extraction of pine kernel oil by response surface methodology. Procedia Eng 15:4641–4652. https://doi.org/10.1016/j.proeng.2011.08.872
Wilkins MR, Widmer WW, Grohmann K, Cameron RG (2007) Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour Technol 98(8):1596–1601. https://doi.org/10.1016/j.biortech.2006.06.022
Bai J et al (2021) Source of gut microbiota determines oat β-glucan degradation and short chain fatty acid-producing pathway. Food Biosci 41:101010. https://doi.org/10.1016/j.fbio.2021.101010
Catalkaya G, Kahveci D (2019) Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep Purif Technol 219:55–63. https://doi.org/10.1016/j.seppur.2019.03.006
Urbonaviciene D, Viskelis P (2017) The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. LWT – Food Sci Technol 85:517–523. https://doi.org/10.1016/j.lwt.2017.03.034
Tangyu M, Muller J, Bolten CJ, Wittmann C (2019) Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl Microbiol Biotechnol 103(23–24):9263–9275. https://doi.org/10.1007/s00253-019-10175-9
Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK (2016) Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr 56(3):339–349. https://doi.org/10.1080/10408398.2012.761950
Zhu X, Wang L, Zhang Z, Ding L, Hang S (2021) Combination of fiber-degrading enzymatic hydrolysis and lactobacilli fermentation enhances utilization of fiber and protein in rapeseed meal as revealed in simulated pig digestion and fermentation in vitro. Anim Feed Sci Technol 278:115001. https://doi.org/10.1016/j.anifeedsci.2021.115001
Panghal A, Janghu S, Virkar K, Gat Y, Kumar V, Chhikara N (2018) Potential non-dairy probiotic products – a healthy approach. Food Biosci 21:80–89. https://doi.org/10.1016/j.fbio.2017.12.003
Hari Krishna S, Karanth NG (2002) Lipases and lipase-catalyzed esterification reactions in nonaqueous media. Catal Rev 44(4):499–591. https://doi.org/10.1081/CR-120015481
Sharma A, Tewari R, Rana SS, Soni R, Soni SK (2016) Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol 179(8):1346–1380. https://doi.org/10.1007/s12010-016-2070-3
Talley K, Alexov E (2010) On the pH-optimum of activity and stability of proteins. Proteins Struct Funct Bioinforma:n/a–n/a. https://doi.org/10.1002/prot.22786
Peterson ME, Daniel RM, Danson MJ, Eisenthal R (2007) The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem J 402(2):331–337. https://doi.org/10.1042/BJ20061143
Cheng X, Bi L, Zhao Z, Chen Y (2015) Advances in enzyme assisted extraction of natural products. In: Proceedings of the 3rd international conference on material, mechanical and manufacturing engineering, Guangzhou, China. https://doi.org/10.2991/ic3me-15.2015.72
Wang L, Wu Y, Liu Y, Wu Z (2017) Complex enzyme-assisted extraction releases antioxidative phenolic compositions from guava leaves. Molecules 22(10):1648. https://doi.org/10.3390/molecules22101648
Zhang M et al (2021) Optimization of enzyme-assisted extraction and purification of flavonoids from Pinus koraiensis nut-coated film and antioxidant activity evaluation. Molecules 26(7):1950. https://doi.org/10.3390/molecules26071950
Patel D, Prasad S, Kumar R, Hemalatha S (2012) An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2(4):320–330. https://doi.org/10.1016/S2221-1691(12)60032-X
Patel D, Kumar R, Prasad S, Sairam K, Hemalatha S (2011) Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin–induced diabetic rats. Asian Pac J Trop Biomed 1(4):316–322. https://doi.org/10.1016/S2221-1691(11)60051-8
Yang B, Jiang Y, Shi J, Chen F, Ashraf M (2011) Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit – a review. Food Res Int 44(7):1837–1842. https://doi.org/10.1016/j.foodres.2010.10.019
Sy GY, Cissé A, Nongonierma RB, Sarr M, Mbodj NA, Faye B (2005) Hypoglycaemic and antidiabetic activity of acetonic extract of Vernonia colorata leaves in normoglycaemic and alloxan-induced diabetic rats. J Ethnopharmacol 98(1–2):171–175. https://doi.org/10.1016/j.jep.2005.01.024
Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA (2006) Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes 55(4):1043–1050. https://doi.org/10.2337/diabetes.55.04.06.db05-1089
Barzegar E, Fouladdel S, Movahhed TK, Ghahremani MH, Ostad SN, Azizi E (2015) Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines. Iran J Basic Med Sci 18(4):9
Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25(18):2677–2681. https://doi.org/10.1091/mbc.e14-04-0916
Duthie GG, Duthie SJ, Kyle JAM (2000) Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev 13(1):79–106. https://doi.org/10.1079/095442200108729016
Zhaung ZP, McCauley R (1989) Ubiquitin is involved in the in vitro insertion of monoamine oxidase B into mitochondrial outer membranes. J Biol Chem 264(25):14594–14596. https://doi.org/10.1016/S0021-9258(18)63734-2
Almagro L, Fernández-Pérez F, Pedreño M (2015) Indole alkaloids from Catharanthus roseus: bioproduction and their effect on human health. Molecules 20(2):2973–3000. https://doi.org/10.3390/molecules20022973
Sohgaura A, Bigoniya P, Shrivastava B. Diuretic potential of Cynodon dactylon, Emblica officinalis, Kalanchoe pinnata and Bambusa nutans, p 7
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Maled, S.B., Bhat, A.R., Hegde, S., Sivamani, Y., Muthuraman, A., Elayaperumal, S. (2024). Enzyme-Assisted Extraction. In: Sarkar, T., Pati, S. (eds) Bioactive Extraction and Application in Food and Nutraceutical Industries. Methods and Protocols in Food Science . Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3601-5_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3601-5_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3600-8
Online ISBN: 978-1-0716-3601-5
eBook Packages: Springer Protocols