Abstract
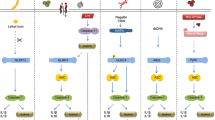
The innate immune response represents the first line of host defense, and it is able to detect pathogen- and damage-associated molecular patterns (PAMPs and DAMPs, respectively) through a variety of pattern recognition receptors (PRRs). Among these PRRs, certain cytosolic receptors of the NLRs family (specifically NLRP1, NLRP3, NLRC4, and NAIP) or those containing at least a pyrin domain (PYD) such as pyrin and AIM2, activate the multimeric complex known as inflammasome, and its effector enzyme caspase-1. The caspase-1 induces the proteolytic maturation of the pro-inflammatory cytokines IL-1ß and IL-18, as well as the pore-forming protein gasdermin D (GSDMD). GSDMD is responsible for the release of the two cytokines and the induction of lytic and inflammatory cell death known as pyroptosis. Each inflammasome receptor detects specific stimuli, either directly or indirectly, thereby enhancing the cell’s ability to sense infections or homeostatic disturbances. In this chapter, we present the activation mechanism of the so-called “canonical” inflammasomes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Christgen S, Place DE, Kanneganti T-D (2020) Toward targeting inflammasomes: insights into their regulation and activation. Cell Res 30(4):315–327. https://doi.org/10.1038/s41422-020-0295-8
Lu A, Magupalli Venkat G, Ruan J, Yin Q, Atianand Maninjay K, Vos MR et al (2014) Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156(6):1193–1206. https://doi.org/10.1016/j.cell.2014.02.008
Stutz A, Horvath GL, Monks BG, Latz E (2013) ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 1040:91 –101. https://doi.org/10.1007/978-1-62703-523-1_8
Chan AH, Schroder K (2019) Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med 217(1):e20190314. https://doi.org/10.1084/jem.20190314
Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426
Taabazuing CY, Griswold AR, Bachovchin DA (2020) The NLRP1 and CARD8 inflammasomes. Immunol Rev 297(1):13–25. https://doi.org/10.1111/imr.12884
Yu CH, Moecking J, Geyer M, Masters SL (2018) Mechanisms of NLRP1-mediated autoinflammatory disease in humans and mice. J Mol Biol 430(2):142–152. https://doi.org/10.1016/j.jmb.2017.07.012
Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K et al (2016) Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via Inflammasome activation. Cell 167(1):187–202.e17. https://doi.org/10.1016/j.cell.2016.09.001
Chavarría-Smith J, Mitchell PS, Ho AM, Daugherty MD, Vance RE (2016) Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 Inflammasome activation. PLoS Pathog 12(12):e1006052. https://doi.org/10.1371/journal.ppat.1006052
Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C et al (2012) Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem 287(30):25030–25037. https://doi.org/10.1074/jbc.M112.378323
Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE (2019) Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science (New York, NY) 364(6435). https://doi.org/10.1126/science.aau1330
Lopes Fischer N, Naseer N, Shin S, Brodsky IE (2020) Effector-triggered immunity and pathogen sensing in metazoans. Nat Microbiol 5(1):14–26. https://doi.org/10.1038/s41564-019-0623-2
Robinson KS, Teo DET, Tan KS, Toh GA, Ong HH, Lim CK et al (2020) Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science (New York, NY) 370(6521). https://doi.org/10.1126/science.aay2002
Tsu BV, Beierschmitt C, Ryan AP, Agarwal R, Mitchell PS, Daugherty MD (2021) Diverse viral proteases activate the NLRP1 inflammasome. elife:10. https://doi.org/10.7554/eLife.60609
Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V (2021) Human NLRP1 is a sensor for double-stranded RNA. Science (New York, NY) 371(6528). https://doi.org/10.1126/science.abd0811
Zhong FL, Robinson K, Teo DET, Tan KY, Lim C, Harapas CR et al (2018) Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding. 293(49):18864–18878. https://doi.org/10.1074/jbc.RA118.004350
Griswold AR, Ball DP, Bhattacharjee A, Chui AJ, Rao SD, Taabazuing CY et al (2019) DPP9’s enzymatic activity and not its binding to CARD8 inhibits Inflammasome activation. ACS Chem Biol 14(11):2424–2429. https://doi.org/10.1021/acschembio.9b00462
Gai K, Okondo MC, Rao SD, Chui AJ, Ball DP, Johnson DC et al (2019) DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death Dis 10(8):587. https://doi.org/10.1038/s41419-019-1817-5
Costa FRC, Leite JA, Rassi DM, da Silva JF, Elias-Oliveira J, Guimarães JB et al (2021) NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes. Cell Rep 35(8). https://doi.org/10.1016/j.celrep.2021.109176
Ting JP, Duncan JA, Lei Y (2010) How the noninflammasome NLRs function in the innate immune system. Science (New York, NY) 327(5963):286–290. https://doi.org/10.1126/science.1184004
Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F et al (2007) Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55(5):443–452. https://doi.org/10.1369/jhc.6A7101.2006
Okondo MC, Rao SD, Taabazuing CY, Chui AJ, Poplawski SE, Johnson DC et al (2018) Inhibition of Dpp8/9 activates the Nlrp1b Inflammasome. Cell Chem Biol 25(3):262–7.e5. https://doi.org/10.1016/j.chembiol.2017.12.013
Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD (2007) The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 17(13):1140–1145. https://doi.org/10.1016/j.cub.2007.05.074
Yap JKY, Pickard BS, Chan EWL, Gan SY (2019) The role of neuronal NLRP1 Inflammasome in Alzheimer’s disease: bringing neurons into the Neuroinflammation game. Mol Neurobiol 56(11):7741–7753. https://doi.org/10.1007/s12035-019-1638-7
Sand J, Haertel E, Biedermann T, Contassot E, Reichmann E, French LE et al (2018) Expression of inflammasome proteins and inflammasome activation occurs in human, but not in murine keratinocytes. Cell Death Dis 9(2):24. https://doi.org/10.1038/s41419-017-0009-4
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 Inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20(13):3328. https://doi.org/10.3390/ijms20133328
Hayward JA, Mathur A, Ngo C, Man SM (2018) Cytosolic recognition of microbes and pathogens: Inflammasomes in action. Microbiol Mol Biol Rev 82(4). https://doi.org/10.1128/mmbr.00015-18
Sharma M, de Alba E (2021) Structure, activation and regulation of NLRP3 and AIM2 inflammasomes. Int J Mol Sci 22(2). https://doi.org/10.3390/ijms22020872
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q et al (2019) Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570(7761):338–343. https://doi.org/10.1038/s41586-019-1295-z
Aksentijevich I, Putnam CD, Remmers EF, Mueller JL, Le J, Kolodner RD et al (2007) The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in north American patients and a new cryopyrin model. Arthritis Rheum 56(4):1273–1285. https://doi.org/10.1002/art.22491
Hafner-Bratkovič I, Sušjan P, Lainšček D, Tapia-Abellán A, Cerović K, Kadunc L et al (2018) NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat Commun 9(1):5182. https://doi.org/10.1038/s41467-018-07573-4
Mayor A, Martinon F, De Smedt T, Pétrilli V, Tschopp J (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol 8(5):497–503. https://doi.org/10.1038/ni1459
Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P, Alice in caspase land. (2002) A phylogenetic analysis of caspases from worm to man. Cell Death Differ 9(4):358–361. https://doi.org/10.1038/sj.cdd.4400989
Lopez-Castejon G (2020) Control of the inflammasome by the ubiquitin system. FEBS J 287(1):11–26. https://doi.org/10.1111/febs.15118
He Y, Zeng MY, Yang D, Motro B, Núñez G (2016) NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530(7590):354–357. https://doi.org/10.1038/nature16959
Niu T, De Rosny C, Chautard S, Rey A, Patoli D, Groslambert M et al (2021) NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly. Nat Commun 12(1):5862. https://doi.org/10.1038/s41467-021-26142-w
Weber ANR, Bittner ZA, Shankar S, Liu X, Chang TH, Jin T et al (2020) Recent insights into the regulatory networks of NLRP3 inflammasome activation. J Cell Sci 133(23). https://doi.org/10.1242/jcs.248344
Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 receptor in infection and inflammation. Immunity 47(1):15–31. https://doi.org/10.1016/j.immuni.2017.06.020
Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M et al (2018) The TWIK2 potassium Efflux Channel in macrophages mediates NLRP3 Inflammasome-induced inflammation. Immunity 49(1):56–65.e4. https://doi.org/10.1016/j.immuni.2018.04.032
Mayes-Hopfinger L, Enache A, Xie J, Huang C-L, Köchl R, Tybulewicz VLJ et al (2021) Chloride sensing by WNK1 regulates NLRP3 inflammasome activation and pyroptosis. Nat Commun 12(1):4546. https://doi.org/10.1038/s41467-021-24784-4
Wang L, Negro R, Wu H (2020) TRPM2, linking oxidative stress and ca(2+) permeation to NLRP3 inflammasome activation. Curr Opin Immunol 62:131 –135. https://doi.org/10.1016/j.coi.2020.01.005
Zewinger S, Reiser J, Jankowski V, Alansary D, Hahm E, Triem S et al (2020) Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol 21(1):30–41. https://doi.org/10.1038/s41590-019-0548-1
Green JP, Yu S, Martín-Sánchez F, Pelegrin P, Lopez-Castejon G, Lawrence CB et al (2018) Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc Natl Acad Sci U S A 115(40):E9371–E9e80. https://doi.org/10.1073/pnas.1812744115
Mohanty A, Tiwari-Pandey R, Pandey NR (2019) Mitochondria: the indispensable players in innate immunity and guardians of the inflammatory response. J Cell Commun Signal 13(3):303–318. https://doi.org/10.1007/s12079-019-00507-9
Meyers AK, Zhu X (2020) The NLRP3 Inflammasome: metabolic regulation and contribution to Inflammaging. Cell 9(8):1808. https://doi.org/10.3390/cells9081808
Hughes MM, O’Neill LAJ (2018) Metabolic regulation of NLRP3. Immunol Rev 281(1):88–98. https://doi.org/10.1111/imr.12608
Chevriaux A, Pilot T, Derangère V, Simonin H, Martine P, Chalmin F et al (2020) Cathepsin B is required for NLRP3 Inflammasome activation in macrophages, through NLRP3. Interaction:8. https://doi.org/10.3389/fcell.2020.00167
Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S et al (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459(7245):433–436. https://doi.org/10.1038/nature07965
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479(7371):117–121. https://doi.org/10.1038/nature10558
Viganò E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A (2015) Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun 6(1):8761. https://doi.org/10.1038/ncomms9761
Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F et al (2016) Human monocytes engage an alternative Inflammasome pathway. Immunity 44(4):833–846. https://doi.org/10.1016/j.immuni.2016.01.012
Tyrkalska SD, Candel S, Mulero V (2021) The neutrophil inflammasome. Dev Comp Immunol 115:103874 . https://doi.org/10.1016/j.dci.2020.103874
Nakamura Y, Kambe N, Saito M, Nishikomori R, Kim Y-G, Murakami M et al (2009) Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med 206(5):1037–1046. https://doi.org/10.1084/jem.20082179
Bonnekoh H, Scheffel J, Kambe N, Krause K (2018) The role of mast cells in autoinflammation. Immunol Rev 282(1):265–275. https://doi.org/10.1111/imr.12633
Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z et al (2009) Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol (Baltimore, Md: 1950) 183(12):8061–8067. https://doi.org/10.4049/jimmunol.0902477
Ali MF, Dasari H, Van Keulen VP, Carmona EM (2017) Canonical stimulation of the NLRP3 Inflammasome by fungal antigens links innate and adaptive B-lymphocyte responses by modulating IL-1β and IgM production. Front Immunol 8:1504 . https://doi.org/10.3389/fimmu.2017.01504
Arbore G, West EE, Spolski R, Robertson AAB, Klos A, Rheinheimer C et al (2016) T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science (New York, NY) 352(6292):aad1210. https://doi.org/10.1126/science.aad1210
Arbore G, West EE, Rahman J, Le Friec G, Niyonzima N, Pirooznia M (2018) Complement receptor CD46 co-stimulates optimal human CD8(+) T cell effector function via fatty acid metabolism. 9(1):4186. https://doi.org/10.1038/s41467-018-06706-z
Fong JJ, Tsai CM, Saha S, Nizet V, Varki A, Bui JD (2018) Siglec-7 engagement by GBS β-protein suppresses pyroptotic cell death of natural killer cells. Proc Natl Acad Sci U S A 115(41):10410–10415. https://doi.org/10.1073/pnas.1804108115
Hottz ED, Monteiro APT, Bozza FA, Bozza PT (2015) Inflammasome in platelets: allying coagulation and inflammation in infectious and sterile diseases? Mediat Inflamm 2015:435783 . https://doi.org/10.1155/2015/435783
He Y, Franchi L, Núñez G (2013) TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. (Baltimore, Md: 1950) 190(1):334–339. https://doi.org/10.4049/jimmunol.1202737
Tourneur L, Witko-Sarsat V (2019) Inflammasome activation: neutrophils go their own way. 105(3):433–436. https://doi.org/10.1002/JLB.3CE1118-433R
Bruchard M, Rebe C, Derangere V, Togbe D, Ryffel B, Boidot R et al (2015) The receptor NLRP3 is a transcriptional regulator of TH2 differentiation 16(8):859–870. https://doi.org/10.1038/ni.3202
Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E et al (2020) Defining trained immunity and its role in health and disease. Nat Rev Immunol 20(6):375–388. https://doi.org/10.1038/s41577-020-0285-6
Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P et al (2014) Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512(7512):69–73. https://doi.org/10.1038/nature13322
Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V (2012) NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. (Baltimore, Md: 1950) 189(8):4175–4181. https://doi.org/10.4049/jimmunol.1201516
Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC (2003) The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem J 373(Pt 1):101–113. https://doi.org/10.1042/bj20030304
Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC et al (2007) Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect Immun 75(3):1484–1492. https://doi.org/10.1128/iai.01315-06
Eren E, Berber M, Özören N (2017) NLRC3 protein inhibits inflammation by disrupting NALP3 inflammasome assembly via competition with the adaptor protein ASC for pro-caspase-1 binding. J Biol Chem 292(30):12691–12701. https://doi.org/10.1074/jbc.M116.769695
Seok JK, Kang HC, Cho Y-Y, Lee HS, Lee JY (2021) Regulation of the NLRP3 Inflammasome by post-translational modifications and. Small Molecules 11. https://doi.org/10.3389/fimmu.2020.618231
Jackson JT, Mulazzani E, Nutt SL, Masters SL (2021) The role of PLCγ2 in immunological disorders, cancer, and neurodegeneration. J Biol Chem 297(2). https://doi.org/10.1016/j.jbc.2021.100905
Tang J, Tu S, Lin G, Guo H, Yan C, Liu Q et al (2020) Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J Exp Med 217(4). https://doi.org/10.1084/jem.20182091
Wan P, Zhang Q, Liu W, Jia Y, Ai S, Wang T et al (2019) Cullin1 binds and promotes NLRP3 ubiquitination to repress systematic inflammasome activation. FASEB J 33(4):5793–5807. https://doi.org/10.1096/fj.201801681R
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z et al (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 Inflammasome. Cell 160(1):62–73. https://doi.org/10.1016/j.cell.2014.11.047
Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA et al (2013) Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat Immunol 14(1):52–60. https://doi.org/10.1038/ni.2474
Sokolowska M, Chen LY, Liu Y, Martinez-Anton A, Qi HY, Logun C et al (2015) Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. J Immunol (Baltimore, Md: 1950) 194(11):5472–5487. https://doi.org/10.4049/jimmunol.1401343
Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R (2017) Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science (New York, NY) 356(6337):513–519. https://doi.org/10.1126/science.aal3535
Salina A, Brandt S, Medeiros AI, Serezani H (2019) Leukotriene B4 is required for inflammasome activation. J Immunol, Suppl 1 202:183 .17
Segovia M, Russo S, Jeldres M, Mahmoud YD, Perez V, Duhalde M et al (2019) Targeting TMEM176B enhances antitumor immunity and augments the efficacy of immune checkpoint blockers by unleashing Inflammasome activation. Cancer Cell 35(5):767–81.e6. https://doi.org/10.1016/j.ccell.2019.04.003
Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, Jagirdar R et al (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7(6):576–582. https://doi.org/10.1038/ni1346
Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP et al (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430(6996):213–218. https://doi.org/10.1038/nature02664
Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI et al (2006) Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7(6):569–575. https://doi.org/10.1038/ni1344
Kofoed EM, Vance RE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477(7366):592–595. https://doi.org/10.1038/nature10394
Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, Sauer JD et al (2011) Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun 79(4):1606–1614. https://doi.org/10.1128/iai.01187-10
Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H et al (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477(7366):596–600. https://doi.org/10.1038/nature10510
Diebolder CA, Halff EF, Koster AJ, Huizinga EG, Koning RI (2015) Cryoelectron tomography of the NAIP5/NLRC4 Inflammasome: implications for NLR activation. Structure (London, England: 1993) 23(12):2349–2357. https://doi.org/10.1016/j.str.2015.10.001
Hu Z, Zhou Q, Zhang C, Fan S, Cheng W, Zhao Y et al (2015) Structural and biochemical basis for induced self-propagation of NLRC4. Science (New York, NY) 350(6259):399–404. https://doi.org/10.1126/science.aac5489
Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q et al (2015) Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science (New York, NY) 350(6259):404–409. https://doi.org/10.1126/science.aac5789
Yang J, Zhao Y, Shi J, Shao F (2013) Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci 110(35):14408. https://doi.org/10.1073/pnas.1306376110
Kortmann J, Brubaker SW, Monack DM (2015) Cutting edge: Inflammasome activation in primary human macrophages is dependent on Flagellin. J Immunol:1403100. https://doi.org/10.4049/jimmunol.1403100
Wang SB, Narendran S, Hirahara S, Varshney A, Pereira F, Apicella I et al (2021) DDX17 is an essential mediator of sterile NLRC4 inflammasome activation by retrotransposon RNAs. Science Immunol 6(66):eabi4493. https://doi.org/10.1126/sciimmunol.abi4493
Man SM, Hopkins LJ, Nugent E, Cox S, Glück IM, Tourlomousis P et al (2014) Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci 111(20):7403. https://doi.org/10.1073/pnas.1402911111
Haloupek N, Grob P, Tenthorey J, Vance RE, Nogales E (2019) Cryo-EM studies of NAIP-NLRC4 inflammasomes. Methods Enzymol 625:177 –204. https://doi.org/10.1016/bs.mie.2019.04.030
Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M et al (2012) Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490(7421):539–542. https://doi.org/10.1038/nature11429
Liu W, Liu X, Li Y, Zhao J, Liu Z, Hu Z et al (2017) LRRK2 promotes the activation of NLRC4 inflammasome during salmonella Typhimurium infection. J Exp Med 214(10):3051–3066. https://doi.org/10.1084/jem.20170014
Li Y, Fu T-M, Lu A, Witt K, Ruan J, Shen C et al (2018) Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc Natl Acad Sci 115(43):10845. https://doi.org/10.1073/pnas.1810524115
Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG et al (2011) Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab 13(5):540–549. https://doi.org/10.1016/j.cmet.2011.04.001
von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N et al (2012) Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490(7418):107–111. https://doi.org/10.1038/nature11351
Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE (2013) Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. (Baltimore, Md: 1950) 191(10):5239–5246. https://doi.org/10.4049/jimmunol.1301581
Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ et al (2017) Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog 13(8):e1006502. https://doi.org/10.1371/journal.ppat.1006502
Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY et al (2017) NAIP-NLRC4 Inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of Caspase-1 and -8. Immunity 46(4):649–659. https://doi.org/10.1016/j.immuni.2017.03.016
Semper RP, Vieth M, Gerhard M, Mejías-Luque R (2019) Helicobacter pylori exploits the NLRC4 inflammasome to dampen host defenses. J Immunol 203(8):2183. https://doi.org/10.4049/jimmunol.1900351
Thomas CJ, Schroder K (2013) Pattern recognition receptor function in neutrophils. Trends Immunol 34(7):317–328. https://doi.org/10.1016/j.it.2013.02.008
Akkaya I, Oylumlu E, Ozel I, Uzel G, Durmus L, Ciraci C (2021) NLRC4 Inflammasome-mediated regulation of eosinophilic functions. Immune Netw 21(6):e42-e. https://doi.org/10.4110/in.2021.21.e42
Gutierrez O, Pipaon C, Fernandez-Luna JL (2004) Ipaf is upregulated by tumor necrosis factor-alpha in human leukemia cells. FEBS Lett 568(1–3):79–82. https://doi.org/10.1016/j.febslet.2004.04.095
Sadasivam S, Gupta S, Radha V, Batta K, Kundu TK, Swarup G (2005) Caspase-1 activator Ipaf is a p53-inducible gene involved in apoptosis. Oncogene 24(4):627–636. https://doi.org/10.1038/sj.onc.1208201
Nordlander S, Pott J, Maloy KJ (2014) NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol 7(4):775–785. https://doi.org/10.1038/mi.2013.95
Hausmann A, Böck D, Geiser P, Berthold DL, Fattinger SA, Furter M et al (2020) Intestinal epithelial NAIP/NLRC4 restricts systemic dissemination of the adapted pathogen salmonella Typhimurium due to site-specific bacterial PAMP expression. Mucosal Immunol 13(3):530–544. https://doi.org/10.1038/s41385-019-0247-0
Janowski AM, Colegio OR, Hornick EE, McNiff JM, Martin MD, Badovinac VP et al (2016) NLRC4 suppresses melanoma tumor progression independently of inflammasome activation. J Clin Invest 126(10):3917–3928. https://doi.org/10.1172/JCI86953
DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA et al (1997) Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15(4):453–457. https://doi.org/10.1038/sj.onc.1201206
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR et al (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458(7237):514–518. https://doi.org/10.1038/nature07725
Fernandes-Alnemri T, Yu J-W, Juliana C, Solorzano L, Kang S, Wu J et al (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11(5):385–393. https://doi.org/10.1038/ni.1859
Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H et al (2009) An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10(3):266–272. https://doi.org/10.1038/ni.1702
Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S et al (2009) HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science (New York, NY) 323(5917):1057–1060. https://doi.org/10.1126/science.1169841
Lugrin J, Martinon F (2018) The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. 281(1):99–114. https://doi.org/10.1111/imr.12618
Sharma BR, Karki R, Kanneganti T-D (2019) Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol 49(11):1998–2011. https://doi.org/10.1002/eji.201848070
Kumari P, Russo AJ, Shivcharan S, Rathinam VA (2020) AIM2 in health and disease: Inflammasome and beyond. Immunol Rev 297(1):83–95. https://doi.org/10.1111/imr.12903
Rathinam VAK, Chan FK (2018) Inflammasome, inflammation, and tissue homeostasis. Trends Mol Med 24(3):304–318. https://doi.org/10.1016/j.molmed.2018.01.004
Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M et al (2012) Critical role of AIM2 in mycobacterium tuberculosis infection. Int Immunol 24(10):637–644. https://doi.org/10.1093/intimm/dxs062
Hanamsagar R, Aldrich A, Kielian T (2014) Critical role for the AIM2 inflammasome during acute CNS bacterial infection. 129(4):704–711. https://doi.org/10.1111/jnc.12669
Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S et al (2011) Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol 187(9):4890. https://doi.org/10.4049/jimmunol.1100381
Kalantari P, DeOliveira RB, Chan J, Corbett Y, Rathinam V, Stutz A et al (2014) Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep 6(1):196–210. https://doi.org/10.1016/j.celrep.2013.12.014
Fisch D, Bando H, Clough B, Hornung V, Yamamoto M, Shenoy AR et al (2019) Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J 38(13):e100926. https://doi.org/10.15252/embj.2018100926
Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S et al (2013) HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res 305(8):723–732. https://doi.org/10.1007/s00403-013-1375-0
Yogarajah T, Ong KC, Perera D, Wong KT (2017) AIM2 Inflammasome-mediated Pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication. Sci Rep 7(1):5845. https://doi.org/10.1038/s41598-017-05589-2
Ekchariyawat P, Hamel R, Bernard E, Wichit S, Surasombatpattana P, Talignani L et al (2015) Inflammasome signaling pathways exert antiviral effect against chikungunya virus in human dermal fibroblasts. Infect Genet Evol 32:401 –408. https://doi.org/10.1016/j.meegid.2015.03.025
Man SM, Karki R, Kanneganti TD (2016) AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 46(2):269–280. https://doi.org/10.1002/eji.201545839
Wang B, Tian Y, Yin Q (2019) AIM2 Inflammasome assembly and Signaling. Adv Exp Med Biol 1172:143 –155. https://doi.org/10.1007/978-981-13-9367-9_7
Wang B, Yin Q (2017) AIM2 inflammasome activation and regulation: a structural perspective. J Struct Biol 200(3):279–282. https://doi.org/10.1016/j.jsb.2017.08.001
Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS et al (2012) AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19(10):1709–1721. https://doi.org/10.1038/cdd.2012.51
El-Zaatari M, Bishu S, Zhang M, Grasberger H, Hou G, Haley H et al (2020) Aim2-mediated/IFN-β-independent regulation of gastric metaplastic lesions via CD8+ T cells. JCI Insight 5(5). https://doi.org/10.1172/jci.insight.94035
Svensson A, Patzi Churqui M, Schlüter K, Lind L, Eriksson K (2017) Maturation-dependent expression of AIM2 in human B-cells. PLoS One 12(8):e0183268-e. https://doi.org/10.1371/journal.pone.0183268
Harris J, Lang T, Thomas JPW, Sukkar MB, Nabar NR, Kehrl JH (2017) Autophagy and inflammasomes. Mol Immunol 86:10 –15. https://doi.org/10.1016/j.molimm.2017.02.013
Liu T, Tang Q, Liu K, Xie W, Liu X, Wang H et al (2016) TRIM11 suppresses AIM2 Inflammasome by degrading AIM2 via p62-dependent selective autophagy. Cell Rep 16(7):1988–2002. https://doi.org/10.1016/j.celrep.2016.07.019
Rodrigue-Gervais IG, Doiron K, Champagne C, Mayes L, Leiva-Torres GA, Vanié P et al (2018) The mitochondrial protease HtrA2 restricts the NLRP3 and AIM2 inflammasomes. Sci Rep 8(1):8446. https://doi.org/10.1038/s41598-018-26603-1
Schnappauf O, Chae JJ, Kastner DL, Aksentijevich I (2019) The pyrin Inflammasome in health and disease. Front Immunol 10:1745 . https://doi.org/10.3389/fimmu.2019.01745
Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ et al (2006) The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. J Proc Natl Acad Sci 103(26):9982–9987. https://doi.org/10.1073/pnas.0602081103
Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer HD et al (2007) The SPRY domain of pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ 14(8):1457–1466. https://doi.org/10.1038/sj.cdd.4402142
Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP et al (2003) Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell 11(3):591–604. https://doi.org/10.1016/s1097-2765(03)00056-x
Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S et al (2006) Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ 13(2):236–249. https://doi.org/10.1038/sj.cdd.4401734
Xu H, Yang J, Gao W, Li L, Li P, Zhang L et al (2014) Innate immune sensing of bacterial modifications of rho GTPases by the pyrin inflammasome. Nature 513(7517):237–241. https://doi.org/10.1038/nature13449
Park YH, Wood G, Kastner DL, Chae JJ (2016) Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17(8):914–921. https://doi.org/10.1038/ni.3457
Yu J-W, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L et al (2007) Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell 28(2):214–227. https://doi.org/10.1016/j.molcel.2007.08.029
Starnes TW, Bennin DA, Bing X, Eickhoff JC, Grahf DC, Bellak JM et al (2014) The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood 123(17):2703–2714. https://doi.org/10.1182/blood-2013-07-516948
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Leal, V., Pontillo, A. (2023). Canonical Inflammasomes. In: Pelegrín, P., Di Virgilio, F. (eds) NLR Proteins. Methods in Molecular Biology, vol 2696. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3350-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3350-2_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3349-6
Online ISBN: 978-1-0716-3350-2
eBook Packages: Springer Protocols