Abstract
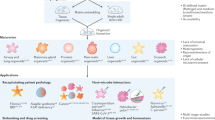
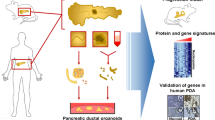
Organoids have emerged as a promising advancement of the two-dimensional (2D) culture systems to improve studies in organogenesis, drug discovery, precision medicine, and regenerative medicine applications. Organoids can self-organize as three-dimensional (3D) tissues derived from stem cells and patient tissues to resemble organs. This chapter presents growth strategies, molecular screening methods, and emerging issues of the organoid platforms. Single-cell and spatial analysis resolve organoid heterogeneity to obtain information about the structural and molecular cellular states. Culture media diversity and varying lab-to-lab practices have resulted in organoid-to-organoid variability in morphology and cell compositions. An essential resource is an organoid atlas that can catalog protocols and standardize data analysis for different organoid types. Molecular profiling of individual cells in organoids and data organization of the organoid landscape will impact biomedical applications from basic science to translational use.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597
Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6(3):331–343
Muthuswamy SK (2017) Bringing together the organoid field: from early beginnings to the road ahead. Development 144:963–967
Rossi G, Manfrin A, Lutolf MP (2018) Progress and potential in organoid research. Nat Rev Genet 19(11):671–687
Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A 89(19):9064–9068
Eiraku M, Takata N, Ishibashi H et al (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472:51–56
Sato T, Vries RG, Snippert HJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265
Betge J, Rindtorff N, Sauer J et al (2019) Multiparametric phenotyping of compound effects on patient derived organoids. bioRxiv. https://doi.org/10.1101/660993
Driehuis E, Kretzschmar K, Clevers H (2020) Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc 15(10):3380–3409
Ooft SN, Weeber F, Dijkstra KK et al (2019) Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med 11. https://doi.org/10.1126/scitranslmed.aay2574
Chen J, Lau BT, Andor N et al (2019) Single-cell transcriptome analysis identifies distinct cell types and niche signaling in a primary gastric organoid model. Sci Rep 9(1):4536
Cattaneo CM, Dijkstra KK, Fanchi LF et al (2020) Tumor organoid-T-cell coculture systems. Nat Protoc 15(1):15–39
Nozaki K, Mochizuki W, Matsumoto Y et al (2016) Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 51(3):206–213
Kim J, Koo BK, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21:571–584
Urbischek M, Rannikmae H, Foets T et al (2019) Organoid culture media formulated with growth factors of defined cellular activity. Sci Rep 9:6193
Karzbrun E, Reiner O (2019) Brain organoids-A bottom-up approach for studying human neurodevelopment. Bioengineering (Basel) 6(1):9
Qin X, Sufi J, Vlckova P et al (2020) Cell-type-specific signaling networks in heterocellular organoids. Nat Methods 17(3):335–342
Lancaster MA, Huch M (2019) Disease modelling in human organoids. Dis Model Mech 12(7):dmm039347
Camp JG, Treutlein B (2017) Human organomics: a fresh approach to understanding human development using single-cell transcriptomics. Development 144:1584–1587
Fiorini E, Veghini L, Corbo V (2020) Modeling cell communication in cancer with organoids: making the complex simple. Front Cell Dev Biol 8. https://doi.org/10.3389/fcell.2020.00166
Huch M, Koo BK (2015) Modeling mouse and human development using organoid cultures. Development 142(18):3113–3125
de Souza N (2018) Organoids. Nat Methods 15:23
Drost J, Clevers H (2017) Translational applications of adult stem cell-derived organoids. Development 144(6):968–975
Fujii M, Sato T (2021) Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater 20:156–169
Qian X, Nguyen HN, Song MM et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165(5):1238–1254
Chugh RM, Bhanja P, Norris A, Saha S (2021) Experimental models to study COVID-19 effect in stem cells. Cell 10:91. https://doi.org/10.3390/cells10010091
Quiroz EJ, Ryan AL (Firth) (2019) In: Stem Cell-Based Ther Lung Dis (Eds: JK Burgess, IH Heijink), Springer International Publishing, Cham, pp. 153–178
Longmire TA, Ikonomou L, Hawkins F et al (2012) Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10(4):398–411
Liang P, Lan F, Lee AS et al (2013) Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 127(16):1677–1691
Bartfeld S, Clevers H (2017) Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl) 95(7):729–738
Naglea PW, Plukkerc JTM, Muijsb CT, van Luijkb P, Coppes RP (2018) Patient-derived tumor organoids for prediction of cancer treatment response. Semin Cancer Biol 53:258–264
Jabs J, Zickgraf FM, Park J et al (2017) Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol Syst Biol 13(11):955
Costales-Carrera A, Fernández-Barral A, Bustamante-Madrid P et al (2020) Comparative study of organoids from patient-derived normal and tumor colon and rectal tissue. Cancers (Basel) 12(8):2302
Driehuis E, Spelier S, Beltrán Hernández I et al (2019) Patient-derived head and neck cancer organoids recapitulate EGFR expression levels of respective tissues and are responsive to EGFR-targeted photodynamic therapy. J Clin Med 8:1880
Eiraku M, Watanabe K, Matsuo-Takasaki M et al (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3(5):519–532
Nakano T, Ando S, Takata N et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10(6):771–785
Sinagoga KL, Schumacher M, Rockich BE et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516(7531):400–404
Wong AP, Bear CE, Chin S et al (2012) Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30(9):876–882
Takebe T, Sekine K, Enomura M et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499(7459):481–484
Takasato M, Er PX, Becroft M et al (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16(1):118–126
Takasato M, Er PX, Chiu HS et al (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526(7574):564–568
Maimets M, Rocchi C, Bron R et al (2016) Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Reports 6(1):150–162
Octavian SA, Pei WY (2018) Organoids as reliable breast cancer study models: an update. Int J Oncol Res 1. https://doi.org/10.23937/ijor-2017/1710008
Gleave AM, Ci X, Lin D, Wang Y (2020) A synopsis of prostate organoid methodologies, applications, and limitations. Prostate 80(6):518–526
Wallach TE, Bayrer JR (2017) Intestinal organoids: new frontiers in the study of intestinal disease and physiology. J Pediatr Gastroenterol Nutr 64(2):180–185
Giese C, Lubitz A, Demmler CD et al (2010) Immunological substance testing on human lymphatic micro-organoids in vitro. J Biotechnol 148(1):38–45
Lancaster MA, Renner M, Martin CA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501(7467):373–379
Dedhia PH, Bertaux-Skeirik N, Zavros Y et al (2016) Organoid models of human gastrointestinal development and disease. Gastroenterology 150(5):1098–1112
Duval K, Grover H, Han LH et al (2017) Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 32(4):266–277
Yin X, Mead BE, Safaee H et al (2016) Engineering stem cell organoids. Cell Stem Cell 18(1):25–38
Shah SB, Singh A (2017) Cellular self-assembly and biomaterials-based organoid models of development and diseases. Acta Biomater 53:29–45
Pham MT, Pollock KM, Rose MD et al (2018) Generation of human vascularized brain organoids. Neuroreport 29(7):588–593
Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA (2017) Fused cerebral organoids model interactions between brain regions. Nat Methods 14(7):743–751
Wang Y, Wang L, Zhu Y, Qin J (2018) Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18:851
Czerniecki SM, Cruz NM, Harder JL et al (2018) High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22(6):929–940
Lancaster MA, Corsini NS, Wolfinger S et al (2017) Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol 35(7):659–666
Broutier L, Andersson-Rolf A, Hindley CJ et al (2016) Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 11(9):1724–1743
Broutier L, Mastrogiovanni G, Verstegen MM et al (2017) Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 23(12):1424–1435
Low JH, Li P, Chew EGY et al (2019) Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25(3):373–387
Morizane R, Lam AQ, Freedman BS et al (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33(11):1193–1200
van den Berg CW, Ritsma L, Avramut MC et al (2018) Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10(3):751–765
Asai A, Aihara E, Watson C et al (2017) Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 144(6):1056–1064
Rennert K, Steinborn S, Gröger M et al (2015) A microfluidically perfused three dimensional human liver model. Biomaterials 71:119–131
Leite SB, Roosens T, El Taghdouini A et al (2016) Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 78:1–10
Koehler K, Hashino E (2014) 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc 9:1229–1244
DeJonge RE, Liu XP, Deig CR (2016) Modulation of Wnt signaling enhances inner ear organoid development in 3D culture. PLOS ONE 11(9):e0162508
Koehler KR, Nie J, Longworth-Mills E et al (2017) Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol 35(6):583–589
Liu XP, Koehler K, Mikosz A et al (2016) Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat Commun 7:11508
Jeong M, O’Reilly M, Kirkwood NK et al (2018) Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell Death Dis 9:1
Seino T, Kawasaki S, Shimokawa M et al (2018) Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22(3):454–467
Boj SF, Hwang CI, Baker LA et al (2015) Organoid models of human and mouse ductal pancreatic cancer. Cell 160:324–338
Huang L, Holtzinger A, Jagan I et al (2015) Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 21(11):1364–1371
Greggio C, De Franceschi F, Figueiredo-Larsen M et al (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140(21):4452–4462
Chen HY, Kaya KD, Dong L, Swaroop A (2016) Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation. Mol Vis 22:1077–1094
DiStefano T, Chen HY, Panebianco C et al (2018) Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports 10(1):300–313
Völkner M, Zschätzsch M, Rostovskaya M et al (2016) Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Reports 6(4):525–538
Deng WL, Gao ML, Lei XL et al (2018) Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Reports 10(4):1267–1281
Reichman S, Slembrouck A, Gagliardi G et al (2017) Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells 35(5):1176–1188
Gracz AD, Ramalingam S, Magness ST (2010) Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol 298(5):G590–G600
Petersen N, Reimann F, Bartfeld S et al (2014) Generation of L-cells in mouse and human small intestine organoids. Diabetes 63:410–420
Dekkers JF, Wiegerinck CL, de Jonge HR et al (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19(7):939–945
Wilson SS, Tocchi A, Holly MK, Parks WC, Smith JG (2015) A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol 8(2):352–361
Tsuruta T, Saito S, Osaki Y et al (2016) Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem Biophys Res Commun 474(1):161–167
Sachs N, de Ligt J, Kopper O et al (2018) A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172(1–2):373–386
Srivastava V, Huycke TR, Phong KT, Gartner ZJ (2020) Organoid models for mammary gland dynamics and breast cancer. Curr Opin Cell Biol 66:51–58
Chandhoke AS, Chanda A, Karve K et al (2017) The PIAS3-Smurf2 sumoylation pathway suppresses breast cancer organoid invasiveness. Oncotarget 8(13):21001–21014
Walsh AJ, Cook RS, Sanders ME et al (2014) Quantitative optical imaging of primary tumor organoid metabolism predicts drug response in breast cancer. Cancer Res 74(18):5184–5194
Goldhammer N, Kim J, Timmermans-Wielenga V, Petersen OW (2019) Characterization of organoid cultured human breast cancer. Breast Cancer Res 21(1):141
Gao D, Vela I, Sboner A et al (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159(1):176–187
Karthaus WR, Iaquinta PJ, Drost J et al (2014) Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159(1):163–175
Chua CW, Shibata M, Lei M et al (2014) Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol 16(10):951–954
Calderon-Gierszal EL, Prins GS (2015) Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PLoS One 10(7):e0133238
Richards Z, McCray T, Marsili J et al (2019) Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience 12:304–317
Saito Y, Onishi N, Takami H et al (2018) Development of a functional thyroid model based on an organoid culture system. Biochem Biophy Res Comm 497:783–789
Bartfeld S, Clevers H (2015) Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter Pylori. J Vis Exp 105:53359
Yan HHN, Siu HC, Law S et al (2018) A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23(6):882–897
McCracken KW, Catá EM, Crawford CM et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516(7531):400–404
Bouchi R, Foo KS, Hua H et al (2014) FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun 5:4242
Steele NG, Chakrabarti J, Wang J et al (2019) An organoid-based preclinical model of human gastric cancer. Cell Mol Gastroenterol Hepatol 7(1):161–184
Sato T, Stange DE, Ferrante M et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5):1762–1772
Lukovac S, Roeselers G (2015) In Impact food bioact health vitro ex vivo models (Eds: K Verhoeckx P, Cotter I et al), Springer International Publishing, Cham, pp. 245–253
Spence JR, Mayhew CN, Rankin SA et al (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470(7332):105–109
DeWard AD, Cramer J, Lagasse E (2014) Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep 9(2):701–711
Trisno SL, Philo KED, McCracken KW et al (2018) Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell 23(4):501–515
Dye BR, Hill DR, Ferguson MAH et al (2015) In vitro generation of human pluripotent stem cell derived lung organoids. elife 4:e05098
Zhang Y, Yang Y, Jiang M et al (2018) 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell 23(4):516–529
Shacham-Silverberg V, Wells JM (2020) In Methods Cell Biol (Ed JR Spence), Academic Press, pp. 1–22
Miller AJ, Dye BR, Ferrer-Torres D et al (2019) Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 14(2):518–540
Gjorevski N, Sachs N, Manfrin A et al (2016) Designer matrices for intestinal stem cell and organoid culture. Nature 539(7630):560–564
Chen YW, Huang SX, de Carvalho ALRT et al (2017) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19(5):542–549
Kim M, Mun H, Sung CO et al (2019) Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun 10(1):3991
Lehmann R, Lee CM, Shugart EC et al (2019) Human organoids: a new dimension in cell biology. Mol Biol Cell 30(10):1129–1137
Angus HCK, Butt AG, Schultz M, Kemp RA (2020) Intestinal organoids as a tool for inflammatory bowel disease research. Front Med 6. https://doi.org/10.3389/fmed.2019.00334
Takebe T, Wells JM, Helmrath MA, Zorn AM (2018) Organoid center strategies for accelerating clinical translation. Cell Stem Cell 22(6):806–809
Ma R, Morshed SA, Latif R, Davies TF (2015) Thyroid cell differentiation from murine induced pluripotent stem cells. Front Endocrinol 6. https://doi.org/10.3389/fendo.2015.00056
Mullenders J, de Jongh E, Brousali A et al (2019) Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci USA 116(10):4567–4574
Lõhmussaar K, Kopper O, Korving J et al (2020) Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids. Nat Commun 11:2660
Nanki Y, Chiyoda T, Hirasawa A et al (2020) Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep 10(1):12581
Maenhoudt N, Defraye C, Boretto M et al (2020) Developing organoids from ovarian cancer as experimental and preclinical models. Stem Cell Reports 14(4):717–729
Rothschild D, Srinivasan T, Aponte-Santiago L et al (2016) The ex vivo culture and pattern recognition receptor stimulation of mouse intestinal organoids. J Vis Exp. https://doi.org/10.3791/54033
Lee SH, Hu W, Matulay JT et al (2018) Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173(2):515–528
Eldred KC, Hadyniak S E, Hussey KA et al (2018) Thyroid hormone signaling specifies cone subtypes in human retinal organoids. bioRxiv. https://doi.org/10.1101/359950
Drost J, Karthaus WR, Gao D et al (2016) Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc 11(2):347–358
Yakoub AM (2019) Cerebral organoids exhibit mature neurons and astrocytes and recapitulate electrophysiological activity of the human brain. Neural Regen Res 14(5):757–761
Rosenbluth JM, Schackmann RCJ, Gray GK et al (2020) Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat Commun 11(1):1711
Roccio M, Edge ASB (2019) Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Development 146(17):dev177188
Subramanian A, Sidhom EH, Emani M et al (2019) Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation. Nat Commun 10:5462
Yoshida S, Miwa H, Kawachi T et al (2020) Generation of intestinal organoids derived from human pluripotent stem cells for drug testing. Sci Rep 10(1):5989
Akbari S, Arslan N, Senturk S, Erdal E (2019) Next-generation liver medicine using organoid models. Front Cell Dev Biol 7. https://doi.org/10.3389/fcell.2019.00345
Barkauskas CE, Chung MI, Fioret B et al (2017) Lung organoids: current uses and future promise. Development 144(6):986–997
Whelan KA, Muir AB, Nakagawa H (2018) Esophageal 3D culture systems as modeling tools in esophageal epithelial pathobiology and personalized medicine. Cell Mol Gastroenterol Hepatol 5(4):461–478
Cowan CS, Renner M, De Gennaro M et al (2020) Cell types of the human retina and its organoids at single-cell resolution. Cell 182(6):1623–1640
Hohwieler M, Illing A, Hermann PC et al (2017) Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66(3):473–486
Moreira L, Bakir B, Chatterji P et al (2017) Pancreas 3D organoids: current and future aspects as a research platform for personalized medicine in pancreatic cancer. Cell Mol Gastroenterol Hepatol 5(3):289–298
Elbadawy M, Abugomaa A, Yamawaki H et al (2020) Development of prostate cancer organoid culture models in basic medicine and translational research. Cancers 12:777
McCray T, Richards Z, Marsili J et al (2019) Handling and assessment of human primary prostate organoid culture. Vis Exp JoVE. https://doi.org/10.3791/59051
Seidlitz T, Merker SR, Rothe A et al (2019) Human gastric cancer modelling using organoids. Gut 68(2):207–217
Kurmann AA, Serra M, Hawkins F et al (2015) Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17(5):527–542
Dekkers JF, Alieva M, Wellens LM et al (2019) High-resolution 3D imaging of fixed and cleared organoids. Nat Protoc 14(6):1756–1771
Glaser AK, Reder NP, Chen Y et al (2019) Multi-immersion open-top light-sheet microscope for high-throughput imaging of cleared tissues. Nat Commun 10(1):2781
Brazovskaja A, Treutlein B, Camp JG (2019) High-throughput single-cell transcriptomics on organoids. Curr Opin Biotechnol 55:167–171
Zanotelli VR, Leutenegger M, Lun XK et al (2020) A quantitative analysis of the interplay of environment, neighborhood, and cell state in 3D spheroids. Mol Syst Biol 16(12):e9798
Tanaka Y, Cakir B, Xiang Y, Sullivan GJ, Park IH (2020) Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep 30(6):1682–1689
Andilla J, Jorand R, Olarte O et al (2017) Imaging tissue-mimic with light sheet microscopy: a comparative guideline. Sci Rep 7:44939
Yang B, Chen X, Wang Y et al (2019) Epi-illumination SPIM for volumetric imaging with high spatial-temporal resolution. Nat Methods 16(6):501–504
Hof L, Moreth T, Koch M et al (2020) Long-term live imaging of epithelial organoids and corresponding multiscale analysis reveal high heterogeneity and identifies core regulatory principles. bioRxiv 2020.07.12.199463
Bolhaqueiro ACF, van Jaarsveld RH, Ponsioen B et al (2018) In Methods Cell Biol (Eds: H Maiato, M Schuh), Academic Press, pp. 91–106
Kim S, Choung S, Sun RX et al (2020) Comparison of cell and organoid-level analysis of patient-derived 3D organoids to evaluate tumor cell growth dynamics and drug response. SLAS Discov Adv Sci Drug Discov 25:744
Kanton S, Boyle MJ, He Z et al (2019) Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574(7778):418–422
Smits LM, Magni S, Kinugawa K et al (2020) Single-cell transcriptomics reveals multiple neuronal cell types in human midbrain-specific organoids. Cell Tissue Res 382(3):463–476
Zhou J, Li C, Sachs N et al (2018) Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci U S A 115:6822
Buzzelli JN, Ouaret D, Brown G et al (2018) Colorectal cancer liver metastases organoids retain characteristics of original tumor and acquire chemotherapy resistance. Stem Cell Res 27:109–120
Velasco S, Kedaigle AJ, Simmons SK et al (2019) Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570:523–527
Alladin A, Chaible L, Reither S et al (2019) Tracking the cells of tumor origin in breast organoids by light sheet microscopy. bioRxiv. https://doi.org/10.1101/617837
Fujii E, Yamazaki M, Kawai S et al (2018) A simple method for histopathological evaluation of organoids. J Toxicol Pathol 31(1):81–85
Xie Y, Park ES, Xiang D, Li Z (2018) Long-term organoid culture reveals enrichment of organoid-forming epithelial cells in the fimbrial portion of mouse fallopian tube. Stem Cell Res 32:51–60
Pastuła A, Middelhoff M, Brandtner A et al (2016) Three-dimensional gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the gastrointestinal stem cell niche. Stem Cells Int:3710836
Lu W, Rettenmeier E, Paszek M et al (2017) Crypt organoid culture as an in vitro model in drug metabolism and cytotoxicity studies. Drug Metab Dispos 45(7):748–754
Yakoub AM, Sadek M (2018) Development and characterization of human cerebral organoids: an optimized protocol. Cell Transplant 27(3):393–406
Xia C, Fana J, Emanuel G et al (2019) Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc Natl Acad Sci 116:19490
Shah S, Lubeck E, Zhou W, Cai L (2016) In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron 92(2):342–357
Mayr U, Serra D, Liberali P (2019) Exploring single cells in space and time during tissue development, homeostasis and regeneration. Development 146(12):dev176727
Schürch CM, Bhate SS, Barlow GL et al (2020) Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182(5):1341–1359
Lin JR, Fallahi-Sichani M, Chen JY, Sorger PK (2016) Cyclic Immunofluorescence (CycIF), A highly multiplexed method for single-cell imaging. Curr Protoc Chem Biol 8(4):251–264
Lin JR, Izar B, Wang C et al (2018) Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. elife 7:e31657
Dueñas ME, Essner JJ, Lee YJ (2017) 3D MALDI Mass spectrometry imaging of a single cell: spatial mapping of lipids in the embryonic development of zebrafish. Sci Rep 7:14946
Fouquet T, Mertz G, Desbenoit N et al (2014) TOF-SIMS/MALDI-TOF combination for the molecular weight depth profiling of polymeric bilayer. Mater Lett 128:23–26
Kaitin KI (2010) Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther 87(3):356–361
Brehm-Stecher BF (2014) In Encycl Food Microbiol Second Ed. (Eds: CA Batt, ML Tortorello), Academic Press, Oxford, pp. 943–953.
Allam M, Cai S, Coskun AF (2020) Multiplex bioimaging of single-cell spatial profiles for precision cancer diagnostics and therapeutics. NPJ Precis Oncol 4:11
Saglam-Metiner P, Gulce-Iz S, Biray-Avci C (2019) Bioengineering-inspired three-dimensional culture systems: organoids to create tumor microenvironment. Gene 686:203–212
Yu F, Hunziker W, Choudhury D (2019) Engineering microfluidic organoid-on-a-chip platforms. Micromachines 10:165
Wong CH, Siah KW, Lo AW (2019) Estimation of clinical trial success rates and related parameters. Biostatistics 20(2):273–286
Zhou Y, Othus M, Araki D et al (2016) Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 30(7):1456–1464
Garcez PP, Loiola EC, Madeiro da Costa R et al (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352(6287):816–818
Quadrato G, Nguyen T, Macosko EZ et al (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545(7652):48–53
Öhlund D, Handly-Santana A, Biffi G et al (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214(3):579–596
Bartucci M, Ferrari AC, Kim IY et al (2016) Personalized medicine approaches in prostate cancer employing patient derived 3D organoids and humanized mice. Front Cell Dev Biol 4. https://doi.org/10.3389/fcell.2016.00064
Al-Lamki RS, Bradley JR, Pober JS (2017) Human organ culture: updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front Med 4. https://doi.org/10.3389/fmed.2017.00148
Willemse J, Lieshout R, van der Laan LJW, Verstegen MMA (2017) From organoids to organs: bioengineering liver grafts from hepatic stem cells and matrix. Best Pract Res Clin Gastroenterol 31(2):151–159
Herron LA, Hansen CS, Abaci HE (2019) Engineering tissue-specific blood vessels. Bioeng Transl Med 4:e10139. https://doi.org/10.1002/btm2.10139
Kolesky DB, Truby RL, Gladman AS et al (2014) 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26(19):3124–3130
Zhang B, Montgomery M, Chamberlain MD et al (2016) Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 15(6):669–678
Akhtar A (2015) The flaws and human harms of animal experimentation. Camb Q Healthc Ethics 24(4):407–419
Acknowledgements
A.F.C. holds a Career Award at the Scientific Interface from Burroughs Wellcome Fund and a Bernie-Marcus Early-Career Professorship. A. F. C. was supported by start-up funds from the Georgia Institute of Technology and Emory University. A.K., S.C., and M.A. contributed equally to work and are equal first co-authors on this study. Figures were created with BioRender.com.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Kumar, A. et al. (2023). Single-Cell and Spatial Analysis of Emergent Organoid Platforms. In: Kasid, U.N., Clarke, R. (eds) Cancer Systems and Integrative Biology. Methods in Molecular Biology, vol 2660. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3163-8_22
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3163-8_22
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3162-1
Online ISBN: 978-1-0716-3163-8
eBook Packages: Springer Protocols