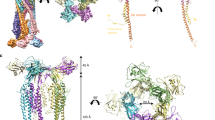
Abstract
The bacterial flagellum is a large assembly of about 30 different proteins and is divided into three parts: the filament that acts as a screw propeller, the hook as a universal joint, and the basal body as a rotary motor. In the case of Salmonella, the filament length is 10–15 μm, which is more than ten times longer than the size of the cell. The filament is composed of only one component protein, flagellin, and is made of 11 protofilaments. The filament can form 12 different supercoiled structures as polymorphic forms. Each protofilament can take either the L (left-handed) or R (right-handed) state, and the number ratio of the protofilaments in these two states determines the shape of the supercoil. Some point mutations in flagellin make the filament straight by making all the protofilaments in one of the two states. The straight filaments enable us to use their helical symmetries for structural analysis by electron cryomicroscopy (cryoEM) and single particle image analysis. Here, we describe the methods for the purification of the flagellar filament and cryoEM data collection and image analysis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others

References
Berg HC (2003) The rotary motor of bacterial flagella. Annu Rev Biochem 72:19–54
Nakamura S, Hanaizumi Y, Morimoto YV et al (2020) Direct observation of speed fluctuations of flagellar motor rotation at extremely low load close to zero. Mol Microbiol 113:755–765
Mimori Y, Yamashita I, Murata K et al (1995) The structure of the R-type straight flagellar filament of Salmonella at 9 Å resolution by electron cryomicroscopy. J Mol Biol 249:69–87
Yonekura K, Maki-Yonekura S, Namba K (2003) Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424:643–650
Samatey FA, Imada K, Nagashima S et al (2001) Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410:331–337
Asakura S (1970) Polymerization of flagellin and polymorphism of flagella. Adv Biophys 1:99–155
Macnab RM, Ornston MK (1977) Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol 112:1–30
Yamaguchi T, Toma S, Terahara N et al (2020) Structural and functional comparison of Salmonella flagellar filaments composed of FljB and FliC. Biomol Ther 10:246
Zheng SQ, Palovcak E, Armache J-P et al (2017) MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14:331–332
Zhang K (2016) Gctf: real-time CTF determination and correction. J Struct Biol 193:1–12
Zivanov J, Nakane T, Forsberg BO et al (2018) New tools for automated high-resolution cryo-EM structure determination in RELION-3. elife 7:e42166
Afonine PV, Poon BK, Read RJ et al (2018) Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol 74:531–544
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera – a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinform 54:5.6.1–5.6.37
Emsley P, Lohkamp B, Scott WG et al (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501
Acknowledgments
This work has been supported by JSPS KAKENHI Grant Number JP25000013 (to K.N.) and JP18K06155 (to T.M.). This work has also been supported by Platform Project for Supporting Drug Discovery and Life Science Research (BINDS) from AMED under Grant Number JP19am0101117 to K.N., by the Cyclic Innovation for Clinical Empowerment (CiCLE) from AMED under Grant Number JP17pc0101020 to K.N. and by JEOL YOKOGUSHI Research Alliance Laboratories of Osaka University to K.N.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Yamaguchi, T., Miyata, T., Makino, F., Namba, K. (2023). Purification and CryoEM Image Analysis of the Bacterial Flagellar Filament. In: Minamino, T., Miyata, M., Namba, K. (eds) Bacterial and Archaeal Motility. Methods in Molecular Biology, vol 2646. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3060-0_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3060-0_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3059-4
Online ISBN: 978-1-0716-3060-0
eBook Packages: Springer Protocols