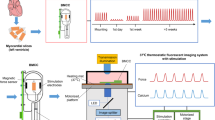
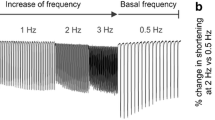
Abstract
Calcium Ca2+ regulation is a key component of numerous cellular functions. In cardiomyocytes, Ca2+ regulates excitation-contraction coupling and influences signaling cascades involved in cell metabolism and cell survival. Prolonged dysregulation of mitochondrial Ca2+ leads to dysfunctional cardiomyocytes, apoptosis and ultimately heart failure. VEGF promotes cardiomyocyte contractility by increasing calcium transients to control the strength of the heartbeat. Here, we describe a method to measure mitochondrial Ca2+ fluxes in human ventricular cardiomocytes after inducing stretch-mediated hypertrophy in vitro.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Walsh C, Barrow S, Voronina S et al (2009) Modulation of calcium signalling by mitochondria. Biochim Biophys Acta Bioenerg 1787:1374–1382. https://doi.org/10.1016/j.bbabio.2009.01.007
Frederick RL, Shaw JM (2007) Moving mitochondria: establishing distribution of an essential organelle. Traffic 8:1668–1675. https://doi.org/10.1111/j.1600-0854.2007.00644.x
Balaban RS, Bose S, French SA et al (2003) Role of calcium in metabolic signaling between cardiac sarcoplasmic reticulum and mitochondria in vitro. Am J Physiol Cell Physiol 284:C285–C293. https://doi.org/10.1152/ajpcell.00129.2002
Ramaccini D, Montoya-Uribe V, Aan F et al (2021) Mitochondrial function and dysfunction in cardiomyopathy. Front Cell Dev Biol 8:624216 https://doi.org/10.3389/fcell.2020.624216
Sadoshima J, Izumo S (1993) Mechanotransduction in stretch-induced hypertrophy of cardiac myocytes. J Recept Res 13(1–4):777–794. https://doi.org/10.3109/107998993090736
Leychenko A, Konorev E, Jijiwa M et al (2011) Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One 6(12):e29055. https://doi.org/10.1371/journal.pone.0029055
Matter ML (2015) Induction of VEGF secretion in cardiomyoctes by mechanical stretch. Methods Mol Biol 1332:67–74. https://doi.org/10.1007/978-1-4939-2917-7_5
Rottbauer W, Just S, Wessels G et al (2005) VEGF–PLCγ1 pathway controls cardiac contractility in the embryonic heart. Genes Dev 19:1624–1634. https://doi.org/10.1101/gad.1319405
Kim HK, Kang YG, Jeong SH et al (2018) Cyclic stretch increases mitochondrial biogenesis in a cardiac cell line. Biochem Biophys Res Commun 505(3):768–774. https://doi.org/10.1016/j.bbrc.2018.10.003
Bonora M, Giorgi C, Bononi A et al (2013) Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat Protoc 8:2015–2118. https://doi.org/10.1038/nprot.2013.127
Acknowledgments
M.L. Matter is supported by a grant from the National Institutes of Health (R01HD091162). C. Giorgi is supported by the Italian Association for Cancer Research (IG-19803) and the Progetti di Rilevante Interesse Nazionale (PRIN20177E9EPY). C. Giorgi is grateful for local funds from University of Ferrara and A-ROSE.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Ramaccini, D., Giorgi, C., Matter, M.L. (2022). Measuring Mitochondrial Calcium Fluxes in Cardiomyocytes upon Mechanical Stretch-Induced Hypertrophy. In: Fiedler, L.R., Pellet-Many, C. (eds) VEGF Signaling. Methods in Molecular Biology, vol 2475. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2217-9_15
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2217-9_15
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2216-2
Online ISBN: 978-1-0716-2217-9
eBook Packages: Springer Protocols