Abstract
pH measurement and color evaluation are two important parameters for determining meat quality. There are the characteristics that after slaughter until the installation of rigor mortis should be assessed to define the quality of a meat and its ability to be processed into quality products. The aim of this chapter is to give a clear and concise view of the techniques used to assess pH and color both physical (CIELAB) and chemical (heme pigment content) in meat and meat products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key words
1 Introduction
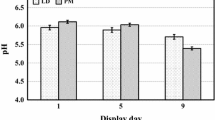
The pH and color are probably the most important physical-chemical characteristics of meat quality evaluation. Both are important in the detection of meat quality deviations once they are related with the post-mortem chemical and biochemical reaction occurring to conversion of muscle to meat [1] and particularly the color is the only characteristic that consumers could assess at the time of purchase and decide this option to buy or not since consumers relate the color of meat with its sensory properties. On the other hand, the discoloration is one of the first indicator of meat spoilage. The pH value of meat affects the color and its quality in terms of water-holding capacity and the sensory quality namely its tenderness and flavor [2]. At slaughter the meat pH is neutral (around 7.0) and after slaughter the muscle under anaerobic metabolism conditions the pH decreases as result the conversion of glycogen to lactate [1] and the pH value at rigor mortis is named the ultimate pH and depending on animal species and muscle type the ultimate pH of 5.3–5.8 is reached at different times [2]. As a result of the effect of the pH in the physicochemical characteristics of the meat, a wrong kinetics of pH during the installation of rigor mortis can affect the suitability of meat to technological processing.
Therefore, pH measurement gives important information on the quality of meat and particularly in the pork the pH value 24 h after slaughter is a fundamental data to detect PSE (pale, soft, exudative) and DFD (dark, firm, dry) meat. Also, the pH measurement is essential to detect DFD beef and sheep meats. So, to monitor the ultimate pH kinetics the method and process of measurement should be defined.
In addition, color deviations in the abnormal course of rigor mortis and meat aging conditions could occur [3]. Color deviations assume a color perceived as normal and in meat quality a normal color is not universally consensual. Anyway, globally is expected that beef and mutton are red, pork, veal, and lamb pink and poultry white meats. Besides white, or red, terms such as shine, pale, or dark are also usually used in meat color characterization. Thus, depending on what is intended to characterize or classify it is important to set the color evaluation procedure method. Color measurement in association with pH is useful for detection of quality deviations such as PSE and DFD in pork or DFD in beef and sheep. Beyond the DFD and PSE meats the National Pork Producers Council (NPPC) [4], based in the pH and color assessment, defines three more categories: RFN (red, firm, non-exudative), RSE (red, soft exudative), and PFN (pale, firm, non-exudative). Color is also used by NPPC to analyze and define the marbling scores corresponding to intramuscular lipid content (Fig. 1). Normal color deviations can result in abnormal kinetics of muscle talk in meat, compromising their quality and adequacy for industry processing or influencing consumer purchase decision. Thus, the color measurement during slaughter and rigor process as well as the values of pH measured at 45–60 min after slaughter and the ultimate pH are essential to assess and define the quality of meat.
Pork Quality Standards defined as Color—Texture—Exudation, Color Standards, and Marbling standards as described [4]
Until the middle of twentieth century, pH measurement was made using as pH indicators a wide diversity of color substances in solution or paper strips or subsequently using glass electrodes that incorporate reference electrodes. Nowadays pH was measured using pH-meter, with large portability and easy handling, with different devices and producers. In the recent years, non-invasive hyperspectral methodologies [5] for rapid pH monitoring for meat quality assessment have been developed. The Raman spectroscopy, a portable device shows considerable potential for some applications in the meat sector, including color measurements and pH [6]. In any case, in the slaughterhouse in the system most currently used to measure the pH is the pH-meter directly on carcass or meat using a probe or an electrode provided with a penetration blade.
The color of the meat can be analyzed using visual or instrumental methods. The visual evaluation of the color consists basically in the use of color pattern cards or photographic scales, resulting in a subjective evaluation regarding the conditions of assessment, lighting, description of the different color tones, training of assessors, and the difficulty to find corrected matching between the patterns and the samples analyzed. Instrumental evaluation using the CIELAB system [7] specifies the color in a space of three coordinates defined as L*, a*, b*. L* is the lightness and varies from 0 (black) to 1 (white). Each series of L *, a*, and b* values correspond a point defined as an exact color in the three-dimensional color sphere (Fig. 2). The a* and b* are chromatic coordinates and measure de red-greenness and yellow-blueness, respectively and are used to calculate saturation or chroma (C*) and hue (h°) [1]. Color coordinates are measured using portable color analyzers. These devices also automatically compute the C* and h°.
The CIELAB color space defined by the coordinates L*, a*, and b* and the color attributes C* and h° [8]. Free available from Konica Minolta webpage (https://www5.konicaminolta.eu/en/measuring-instruments/media-centre/poster-download.html)
Measuring the concentration of heme pigments is another way to analyze the color of meat. Color is determined by the chemical form and the concentration of the heme pigment myoglobin and hemoglobin: oxymyoglobin (MbO2), deoxymyoglobin (Mb), and metmyoglobin (MMb). The MbO2 is responsible for the bright red color of fresh meat and the deoxymyoglobin or reduced myoglobin (Mb) shows a purple-red meat color while the MMb exhibits a brown color as a result of oxidation of myoglobin pigment [1]. The quantification of total three states of pigment in meat samples could be assessed by reflectance spectrophotometry [9].
Therefore, the present chapter of the book aims to describe the procedures to assess pH and color characteristics as important factors to evaluate meat and meat products quality.
2 Materials
In this section, we will describe the procedures for the determination of pH, color by the CIELAB system, and color measurement of total heme pigment concentration.
2.1 pH Determination
The pH determination can be carried out on the carcass or on meat samples or meat products, depending on what is to be analyzed or evaluated (see Note 1). Currently, the most common method uses the following material:
-
1.
A portable pH-meter provided with a penetration electrode (see Note 2).
-
2.
Buffer solutions with pH of 7.0 and 4.0.
2.2 Physical Color (Cielab System)
The model and make of the device used can be varied. The most usual are the chromameters Konica Minolta from the series CR200, CR300, CR400, CR410 (see Note 2).
2.3 Chemical Color (Heme Pigments)
The chemical color determination needs a meat sample preparation that requires the use of some solutions.
-
1.
Distilled water.
-
2.
Acetone.
-
3.
HCl 35%.
-
4.
Filter paper Ø 150 mm and a maximum of 31 μm of pore.
-
5.
A spectrophotometer (see Note 3).
For the measurement of pigments deoxymyoglobin (Mb), oxymyoglobin (MbO2), and metmyoglobin (MMb):
-
1.
PVC film with very low oxygen permeability.
-
2.
10% of sodium dithionite solution (Na2S2O4)
-
3.
1% ferricyanide and potassium cyanide solution [K4Fe(CN)6].
3 Methods
3.1 pH Measurement
Before taking a measurement the pH-meter should be calibrated using buffer solutions with a known pH of 4.0 and 7.0. It is very important to stabilize the temperature of the electrode to the temperature of the muscle (see Note 4). There are metal and glass electrodes, equipped with a piercing tip that allows the measurement of pH directly in the carcass muscles or in pieces of meat or processed meat products (Fig. 3) (see Note 5). Measurements made directly on the carcass are usually taken at m. Longissimus thoracis et lumborum (also known as longissimus dorsi) and in m. Semimembranosus (see Note 6). The equipment is also able to measure in homogenized meat or in a meat sample homogenized in water.
3.2 Color Measurement (CIELAB System)
The instruments take measurements at 400–700 nm and give readings at intervals of 10 nm, allowing for thorough color data analysis and come with software to automatically compute the h* and C* (Fig. 4). Some practical considerations in relation to its use must be considered:
-
1.
A record of the specifications regarding the animal (species, breed, food, age, sex) conditions of transport and slaughter especially the conditions until reaching the rigor with record of pH (see Note 7).
-
2.
Time of sampling and location of measurement should be previously defined (see Note 8).
-
3.
If the samples were stored, the conditions of storage (temperature, moisture, light, and overwrap) should be specified.
-
4.
Fresh muscle samples need at least 15 min of blooming time to the pigments at surface to oxygenate.
-
5.
Three repetitions of each measurement are recommended (see Note 9).
-
6.
A calibration based on the black standard as L * = 0 and white as L * = 100 provided with each chromometer must be performed.
Finally, whenever color measurements are made on samples of meat or fat, all information on the conditions under which they were carried out, as described here, from 1 to 6 must be provided, including the make and model of the equipment used.
3.3 Color Measurement (Heme Pigments Content)
The chemical analysis consists of measuring the amount of pigments present in the muscle. The Hornsey method [10] is a simple and rapid indirect method that allows the determination of the myoglobin hierarchy and hemoglobin. As the residual hemoglobin content is very low, the estimation of the myoglobin obtained in this way is very close to the real value. The procedure consists in (Fig. 5):
-
1.
Take 5 g of meat sample of m. Longissimus thoracis et lumborum (longissimus dorsi) (see Note 10).
-
2.
Mince the sample in meat grinder type Moulinex.
-
3.
A duplicate of each sample will be performed with an extraction volume of 25 mL.
-
4.
In a pyrex tube with screw cap, place 1 mL of distilled water and 5 g of ground beef, 20 mL of acetone. Shake with a glass rod until the meat is a uniform whitish color. Then add 0.5 mL of HCl (35%) (see Note 11).
-
5.
The solution should stand for 24 h in a refrigerator in the dark.
-
6.
The solution is filtered with double filter paper (Ø 150 mm and a maximum of 31 μm of pore).
-
7.
After pigment extraction, measure optical density (OD) with a spectrophotometer at 512 or 640 nm (see Note 12).
-
8.
The results are expressed in percent (see Note 13):
-
At 640 nm: OD × 17.75 = mg myoglobin/g fresh muscle.
-
At 512 nm: OD × 8.82 = mg myoglobin/g fresh muscle.
-
Determination of meat pigment content [10]
The pigment percentages of the meat, deoxymyoglobin (Mb), oxymyoglobin (MbO2), and metmyoglobin (MMb) could be obtained by means of the reflectance on the surface of the meat by the method developed by Hunt et al. [11] (see Note 14).
-
1.
The reflectance spectrum obtained for a sample of meat (3 × 3 × 2 cm) without visible fat or connective tissue after cutting is 100% of Mb.
-
2.
After the time of blooming, the meat sample should remain in contact with the air in a tray cover with a film permeable to the oxygen. The reflectance spectrum is 100% MbO2.
-
3.
Finally, the meat piece becomes 100% (MMb) when the meat is introduced in a 0.5% ferricyanide and potassium cyanide solution.
-
4.
To quantify the amounts of myoglobin redox forms we must have the reflectance values of each one of the pigments (Mb, MMb, MbO2). The process involves the use of surface reflectance to calculate the ratio between K (absorbance coefficient) and S (scattering coefficient) at isobestic wavelengths of each myoglobin redox form, 474, 525, 572, and 610 nm.
-
5.
Once the myoglobin is converted to 100%, the procedure is as follows:
-
Deoxymyoglobin: Place the samples in a 10% sodium dithionite (Na2S2O4) for 1 min. Remove and clean with absorbent paper. Pack the samples in a vacuum and leave them for 2 h at room temperature. At the end, take the measurements immediately after opening the package.
-
Metmyoglobin: Place the samples in a 1% ferricyanide and potassium cyanide solution [K4Fe(CN)6] for 1 min. Remove and clean with absorbent paper. Pack the samples in a vacuum and leave them for 12 h at 2 °C refrigeration. At the end, take the measurements immediately after opening the package.
-
Oxymyoglobin: Place the samples in an atmosphere with a high proportion of oxygen with a temperature between 0 and 2 °C. Oxygenation can be carried out by means of a flow of 100% oxygen for 10 min. After performing the measurements, place the samples in an.
-
Having obtained the measurements of the K/S ratios, calculate the percentages of the pigments using the formulas [11] (see Note 15):
4 Notes
-
1.
Although the procedure is the same, the determination of the pH in the carcasses fundamentally depends on the animal species, the time that elapses after slaughter until installation of rigor mortis, the anatomical point, and muscle of the carcass in which we intend to measure.
-
2.
There are different devices of different equipment producers. Before the pH measurement, the pH-meter should be calibrated using buffer solutions.
-
3.
There are several devices from different brands. In meat science, the spectrophotometer commonly used are the HunterLab series Miniscan and Ultra scan, some of them are portable; Konica Minolta series CM-3600A, CM-3700A, CM-36dg.
-
4.
The pH-meter is provided by an automatic system to adjust the temperature to the temperature of the carcass or muscle to the temperature of the carcass and muscle.
-
5.
A scalpel blade or a knife can be used to make a little incision into the muscle to enable the contact of the electrode with the muscle tissue. After each measurement, the electrode must be washed with distilled water. Over time, they may suffer deterioration, delay in response time or with instability in reading. For better conservation, the electrodes should be stored in a 3 M KCl solution.
-
6.
As there are differences in the pH measured in different muscles, the definition of the anatomical location is of great importance, to provide a series of works with great repeatability and that provide comparisons between different studies and laboratories.
-
7.
Due to the close relationship between color and pH, it is recommendable in carcasses to record the pH at the beginning of chilling and the ultimate pH.
-
8.
At least 24 h post-mortem, the measurement must be made. The anatomical point on the carcass must be defined. Usually, color is assessed in a cross section of m. Longissimus thoracis et lumborum (Longissimus dorsi) and a minimum of 1–3 cm of thickness is recommended to prevent light passing though it.
-
9.
Different colors in the same muscle can occur and muscle infiltrations of marbling and connective tissue can generate some color variability.
-
10.
The sample must be homogeneous, without fascia and fat, blood vessels, nerves, and tendons. If the meat sample is frozen, the liquid exudated during defrosting should be recovered.
-
11.
A meatless solution (white reading) must be prepared, which will be used for the calibration of the spectrophotometer between sample readings.
-
12.
The points where the absorbances or reflectances are equal for the three forms of myoglobin pigment is an isobestic points (512 or 640 nm).
-
13.
The factors 8.82 and 17.75 correspond to the calculation of the final concentration of pigments calculated by the formula [10]:
$$ \mathrm{Factor}\times \mathrm{OD}=\frac{\mathrm{volume}\kern0.17em \mathrm{of}\kern0.17em \mathrm{extract}\times 652\times 1.000\mathrm{g}/\mathrm{kg}}{\varepsilon_{\uplambda}^{\mathrm{mM}}\times {10}^3\;\mathrm{g}\;\mathrm{of}\kern0.17em \mathrm{sample}}\kern1em \mathrm{and}\kern1em {\varepsilon}_{\uplambda}^{\mathrm{mM}}=4.8\kern1em \mathrm{at}\ 640\ \mathrm{nm}\kern1em \mathrm{or}=9.52\kern1em \mathrm{at}\ 512\ \mathrm{nm}. $$ -
14.
The K/S coefficients used are:
-
(a)
K/S474/K/S525 to estimation of Mb.
-
(b)
K/S572/K/S525 to estimation of MMb.
-
(c)
K/S610/K/S525 to estimation of MbO2.
-
(a)
-
15.
Once the myoglobin is converted to 100% and the calculations of % Metmyoglobin and % Deoxymyoglobin were processed, the % Oxymyoglobin could easily be calculated as:
$$ \%\mathrm{Oxymyoglobin}=100-\left(\%\mathrm{Deoxymyoglobin}+\%\mathrm{Metmyoglobin}\right). $$
References
Warriss PD (2010) Measuring the composition and physical characteristics of meat. In: Meat science: an introductory text, 2nd edn. CBI Publishing, Wallingford, pp 229–251
Honikel KO (2004) pH measurement. In: Encyclopedia of meat sciences, 1st edn. Elsevier Academic Press, London, pp 238–242
Monin G (2004) Colour and texture deviations. In: Encyclopedia of meat sciences, 1st edn. Elsevier Academic Press, London, pp 323–330
National Pork Producers Council (2000) Pork composition and quality assessment procedures. NPPC, Des Moines, IA
Yao X, Cai F, Zhu P et al (2019) Non-invasive and rapid pH monitoring for meat quality assessment using alow-cost portable hyperspectral scanner. Meat Sci 152:73–80
Beganović A, Hawthorme LM et al (2019) Critical review on the utilization of handheld and portable Raman spectrometry in meat Science. Foods 8:49
CIE (1976) Recommendations on uniform color spaces – color difference equations. Psychometric color terms. Supplement No. 2 to CIE Publication No. 15 (E-1.3.1) 19078, 1971/(TC-1-3). Commission International de l’Eclairage, Paris, France
Konica Minolta (2021) Konica Minolta colorimetric technology. Giving shape to ideas. https://www5.konicaminolta.eu/en/measuring-instruments/media-centre/poster-download.html
Franke WC, Solberg M (1971) Quantitative determination of metmyoglobin and total pigment in an intact meat sample using reflectance spectrophotometry. J Food Sci 36:515–519
Hornsey KO (1956) The colour of cooked curded pork estimation of the nitric-oxid heam pigments. J Sci Food Agric 7:534–540
Hunt MC, King DA et al (2012) Meat color measurement guidelines. Champaign, IL, American Meat Science Association, p 124
Acknowledgments
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support by national funds FCT/MCTES to CIMO (UIDB/00690/2020) and to Laboratory of Carcass and Meat Quality of Agriculture School of Polytechnic Institute of Bragança “Cantinho do Alfredo.” The authors are members of the Healthy Meat network, funded by CYTED (ref. 119RT0568).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Teixeira, A., Domínguez, R., Rey, J.F., Aleu, G., Pateiro, M., Lorenzo, J.M. (2022). pH and Color. In: Lorenzo, J.M., Domínguez, R., Pateiro, M., Munekata, P.E. (eds) Methods to Assess the Quality of Meat Products. Methods and Protocols in Food Science . Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2002-1_2
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2002-1_2
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2001-4
Online ISBN: 978-1-0716-2002-1
eBook Packages: Springer Protocols