Abstract
Cholesterol efflux (ChE) capacity is associated with the incidence of cardiovascular events and has been proposed as an emerging cardiovascular risk factor. ChE has been traditionally assessed by in vitro radioactive methods but these are not appropriate when assessing a large number of samples. Therefore, alternative, reproducible nonradioactive methods have been developed. This chapter describes a robust nonradioactive method using a fluorescent tracer to assess ChE in vitro.
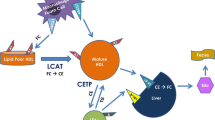
The measurement of ChE in vitro requires three main components: a cholesterol-loaded donor cell, a cholesterol tracer, and a cholesterol acceptor. This method involves labeling of murine macrophage J774A.1 cells using the fluorescent sterol dipyrromethene boron difluoride (BODIPY)-cholesterol. The cholesterol acceptors from humans or animals include lipid-free apolipoprotein (ApoA)-1, high-density lipoprotein (HDL), HDL2 and HDL3 subfractions, serum, plasma or ApoB-depleted serum or plasma. While lipid-free ApoA-1 mediates ChE via only ATP-binding cassette (ABC)A1 transporter, the remaining acceptors mediate ChE via ABCA1 , ABCG1 and scavenger receptor class B type 1 (SRB1) transporters. The reproducibility of this BODIPY-ChE assay is excellent as the intra-assay coefficients of variation (CVs) were <10% (30 replicates on the same day) and the interassay CVs were <14% (10 experiments performed on different days, with 3 replicates each). The fluorescent method therefore represents a reproducible, safe and useful tool to evaluate ChE as an emerging cardiovascular risk factor.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Karathanasis SK, Freeman LA, Gordon SM, Remaley AT (2017) The changing face of HDL and the best way to measure it. Clin Chem 63:196–210. https://doi.org/10.1373/clinchem.2016.257725
Lüscher TF, Landmesser U, Von Eckardstein A, Fogelman AM (2014) High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 114:171–182. https://doi.org/10.1161/CIRCRESAHA.114.300935
Rye K-A, Bursill CA, Lambert G et al (2009) The metabolism and anti-atherogenic properties of HDL. J Lipid Res 50(Suppl):S195–S200. https://doi.org/10.1194/jlr.R800034-JLR200
Favari E, Chroni A, Tietge UJFF et al (2015) Cholesterol efflux and reverse cholesterol transport. Handb Exp Pharmacol 224:181–206. https://doi.org/10.1007/978-3-319-09665-0
Khera AV, Cuchel M, de la Llera-Moya M et al (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364:127–135. https://doi.org/10.1056/NEJMoa1001689
Anastasius M, Kockx M, Jessup W et al (2016) Cholesterol efflux capacity: an introduction for clinicians. Am Heart J 180:1–10. https://doi.org/10.1016/j.ahj.2016.07.005
Saleheen D, Scott R, Javad S et al (2015) Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 3:507–513. https://doi.org/10.1016/S2213-8587(15)00126-6
Litvinov D, Savushkin E, Garaeva E, Dergunov A (2016) Cholesterol efflux and reverse cholesterol transport: experimental approaches. Curr Med Chem 23:3883–3908. https://doi.org/10.2174/0929867323666160809093009
Escolà-Gil JC, Lee-Rueckert M, Santos D et al (2015) Quantification of in vitro macrophage cholesterol efflux and in vivo macrophage-specific reverse cholesterol transport. Methods Mol Biol 1339:211–233. https://doi.org/10.1007/978-1-4939-2929-0
Rohatgi A (2015) High-density lipoprotein function measurement in human studies: focus on cholesterol efflux capacity. Prog Cardiovasc Dis 58:32–40. https://doi.org/10.1016/j.pcad.2015.05.004
Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M et al (2011) A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res 52:2332–2340. https://doi.org/10.1194/jlr.D018051
Adorni MP, Zimetti F, Billheimer JT et al (2007) The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 48:2453–2462. https://doi.org/10.1194/jlr.M700274-JLR200
Sankaranarayanan S, Oram JF, Asztalos BF et al (2009) Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res 50:275–284. https://doi.org/10.1194/jlr.M800362-JLR200
Zhang J, Cai S, Peterson BR et al (2011) Development of a cell-based, high-throughput screening assay for cholesterol efflux using a fluorescent mimic of cholesterol. Assay Drug Dev Technol 9:136–146. https://doi.org/10.1089/adt.2010.0288
Rohatgi A, Khera A, Berry JD et al (2014) HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371:2383–2393. https://doi.org/10.1038/jid.2014.371
de la Llera MM, Atger V, Paul JL et al (1994) A cell culture system for screening human serum for ability to promote cellular cholesterol efflux. Relations between serum components and efflux, esterification, and transfer. Arterioscler Thromb 14:1056–1065. https://doi.org/10.1161/01.ATV.14.7.1056
Rosenson RS, Brewer HB, Ansell BJ et al (2016) Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 13:48–60. https://doi.org/10.1038/nrcardio.2015.124
Miyazaki O, Ogihara J, Fukamachi I, Kasumi T (2014) Evidence for the presence of lipid-free monomolecular apolipoprotein A-1 in plasma. J Lipid Res 55:214–225. https://doi.org/10.1194/jlr.M041038
Hafiane A, Genest J (2015) HDL-mediated cellular cholesterol efflux assay method. Ann Clin Lab Sci 45:659–668
Hölttä-Vuori M, Uronen RL, Repakova J et al (2008) BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic 9:1839–1849. https://doi.org/10.1111/j.1600-0854.2008.00801.x
Liu Z, Thacker SG, Fernandez-Castillejo S et al (2014) Synthesis of cholesterol analogues bearing BODIPY fluorophores by Suzuki or Liebeskind-Srogl cross-coupling and evaluation of their potential for visualization of cholesterol pools. Chembiochem 15:2087–2096. https://doi.org/10.1002/cbic.201402042
Acknowledgments
This work was funded by the AppleCOR Project, which was made possible with the support of the Ministerio de Economía, Indústria y Competitividad, the Agencia Estatal de Investigación, and the European Regional Development Fund. The NFOC-Salut group is a consolidated research group of the Generalitat de Catalunya, Spain (reference no. 2017 SGR 522). The role of the funders was limited to an economic contribution through a competitive call. The funders had no role in the conception, design, performance, or approval of the work.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Fernández-Castillejo, S., Pedret, A., Catalán Santos, Ú., Solà, R. (2022). A Fluorescence-Based In Vitro Method to Assess Cholesterol Efflux. In: Ramji, D. (eds) Atherosclerosis. Methods in Molecular Biology, vol 2419. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1924-7_15
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1924-7_15
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1923-0
Online ISBN: 978-1-0716-1924-7
eBook Packages: Springer Protocols