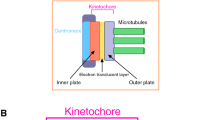
Abstract
The separation of duplicated chromosomes during mitosis is a pivotal step in the process of cellular division. Therefore, the orchestrated events that take place to ensure proper attachment and stabilization of kMTs are keen areas of interest in the mitosis field. Here we describe the methods used to study kMT attachments via in vitro biochemical methods and in vivo cell biological approaches.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore–microtubule interface. Nat Rev Mol Cell Biol 9:33–46. https://doi.org/10.1038/nrm2310
Varma D, Salmon ED (2012) The KMN protein network - chief conductors of the kinetochore orchestra. J Cell Sci 125:5927–5936. https://doi.org/10.1242/jcs.093724
Varma D, Chandrasekaran S, Sundin LJR et al (2012) Recruitment of the human Cdt1 replication licensing protein by the loop domain of Hec1 is required for stable kinetochore–microtubule attachment. Nat Cell Biol 14:593–603. https://doi.org/10.1038/ncb2489
Agarwal S, Smith KP, Zhou Y et al (2018) Cdt1 stabilizes kinetochore–microtubule attachments via an Aurora B kinase–dependent mechanism. J Cell Biol 217:3446–3463. https://doi.org/10.1083/jcb.201705127
Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The |conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997. https://doi.org/10.1016/j.cell.2006.09.039
Castoldi M, Popov AV (2003) Purification of brain tubulin through two cycles of polymerization–depolymerization in a high-molarity buffer. Protein Expr Purif 32:83–88. https://doi.org/10.1016/s1046-5928(03)00218-3
Mckenney RJ, Huynh W, Tanenbaum ME et al (2014) Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345:337–341. https://doi.org/10.1126/science.1254198
Tan R, Foster PJ, Needleman DJ, Mckenney RJ (2018) Cooperative accumulation of dynein-dynactin at microtubule minus-ends drives microtubule network reorganization. Dev Cell 44(2):233–247. https://doi.org/10.1016/j.devcel.2017.12.023
Edelstein AD, Tsuchida MA, Amodaj N et al (2014) Advanced methods of microscope control using μManager software. J Biol Methods 1:10. https://doi.org/10.14440/jbm.2014.36
Helenius J, Brouhard G, Kalaidzidis Y et al (2006) The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 441:115–119. https://doi.org/10.1038/nature04736
Guimaraes GJ, Dong Y, Mcewen BF, Deluca JG (2008) Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol 18:1778–1784. https://doi.org/10.1016/j.cub.2008.08.012
He B, Cimini D (2016) Using photoactivatable GFP to study microtubule dynamics and chromosome segregation. Methods Mol Biol 1413:15–31. https://doi.org/10.1007/978-1-4939-3542-0_2
Bakhoum SF, Genovese G, Compton DA (2009) Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol 19:1937–1942. https://doi.org/10.1016/j.cub.2009.09.055
Ganem NJ, Upton K, Compton DA (2005) Efficient mitosis in human cells lacking poleward microtubule flux. Curr Biol 15:1827–1832. https://doi.org/10.1016/j.cub.2005.08.065
Holubcova Z, Blayney M, Elder K, Schuh M (2015) Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science 348:1143–1147. https://doi.org/10.1126/science.aaa9529
Mogessie B, Schuh M (2017) Actin protects mammalian eggs against chromosome segregation errors. Science 357(6353):eaal1647. https://doi.org/10.1126/science.aal1647
Acknowledgments
We would like to acknowledge the National Institute of Health (NIH) Grant R01GM135391 to D.V. from the National Institute of General Medical Sciences (NIGMS). Sana Afreen and Amit Rahi contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Afreen, S., Rahi, A., Landeros, A.G., Chakraborty, M., McKenney, R.J., Varma, D. (2022). In Vitro and In Vivo Approaches to Study Kinetochore-Microtubule Attachments During Mitosis. In: Hinchcliffe, E.H. (eds) Mitosis. Methods in Molecular Biology, vol 2415. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1904-9_9
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1904-9_9
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1903-2
Online ISBN: 978-1-0716-1904-9
eBook Packages: Springer Protocols