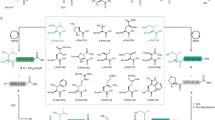
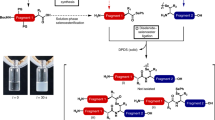
Abstract
Peptide ligation techniques enable the controlled chemical synthesis of native and engineered proteins, including examples that display site-specific post-translational modifications (PTMs) and non-proteinogenic functionality. Diselenide-selenoester ligation (DSL) is a recent addition to the synthetic methodology that offers several advantages over existing strategies. The standard DSL reaction involves the additive-free ligation of a peptide carrying an N-terminal selenocysteine (Sec) residue with a fragment bearing a C-terminal selenoester. This operationally simple ligation proceeds rapidly at sterically hindered junctions and is efficient across a broad pH range. The incorporation of deselenization and oxidative deselenization techniques into the DSL protocol enables conversion of the Sec residue at the ligation site to alanine (Ala) and serine (Ser), respectively, thus enhancing the scope and versatility of the method. In this chapter, we describe the application of DSL to the one-pot chemical synthesis of proteins via both two-component and three-component ligation pathways.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dawson PE, Muir TW, Clark-Lewis I, Kent S (1994) Synthesis of proteins by native chemical ligation. Science 266(5186):776–779
Kent SB (2009) Total chemical synthesis of proteins. Chem Soc Rev 38(2):338–351
Wan Q, Danishefsky SJ (2007) Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew Chem Int Ed 119(48):9408–9412
Lee M, Neukirchen S, Cabrele C, Reiser O (2017) Visible-light photoredox-catalyzed desulfurization of thiol-and disulfide-containing amino acids and small peptides. J Pept Sci 23(7–8):556–562
Gao X-F, Du J-J, Liu Z, Guo J (2016) Visible-light-induced specific desulfurization of cysteinyl peptide and glycopeptide in aqueous solution. Org Lett 18(5):1166–1169
Chisholm TS, Clayton D, Dowman LJ, Sayers J, Payne RJ (2018) Native chemical ligation–photodesulfurization in flow. J Am Chem Soc 140(29):9020–9024
Conibear AC, Watson EE, Payne RJ, Becker CF (2018) Native chemical ligation in protein synthesis and semi-synthesis. Chem Soc Rev 47(24):9046–9068
Kulkarni SS, Sayers J, Premdjee B, Payne RJ (2018) Rapid and efficient protein synthesis through expansion of the native chemical ligation concept. Nat Rev Chem 2(4):1–17
Hondal RJ, Nilsson BL, Raines RT (2001) Selenocysteine in native chemical ligation and expressed protein ligation. J Am Chem Soc 123(21):5140–5141
Gieselman MD, Xie L, van der Donk WA (2001) Synthesis of a selenocysteine-containing peptide by native chemical ligation. Org Lett 3(9):1331–1334
Quaderer R, Sewing A, Hilvert D (2001) Selenocysteine-mediated native chemical ligation. Helv Chim Acta 84(5):1197–1206
Durek T, Alewood PF (2011) Preformed selenoesters enable rapid native chemical ligation at intractable sites. Angew Chem Int Ed 50(50):12042–12045
Mitchell NJ, Malins LR, Liu X, Thompson RE, Chan B, Radom L, Payne RJ (2015) Rapid additive-free selenocystine–selenoester peptide ligation. J Am Chem Soc 137(44):14011–14014
Chisholm TS, Kulkarni SS, Hossain KR, Cornelius F, Clarke RJ, Payne RJ (2019) Peptide ligation at high dilution via reductive diselenide-selenoester ligation. J Am Chem Soc 142(2):1090–1100
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Metanis N, Keinan E, Dawson PE (2010) Traceless ligation of cysteine peptides using selective deselenization. Angew Chem Int Ed 49(39):7049–7053
Mitchell NJ, Kulkarni SS, Malins LR, Wang S, Payne RJ (2017) One-pot ligation–oxidative deselenization at selenocysteine and selenocystine. Chem Eur J 23:946–952
Malins LR, Mitchell NJ, McGowan S, Payne RJ (2015) Oxidative deselenization of selenocysteine: applications for programmed ligation at serine. Angew Chem Int Ed 54:12716–12721
Dery S, Reddy PS, Dery L, Mousa R, Dardashti RN, Metanis N (2015) Insights into the deselenization of selenocysteine into alanine and serine. Chem Sci 6(11):6207–6212
Mitchell NJ, Sayers J, Kulkarni SS, Clayton D, Goldys AM, Ripoll-Rozada J, Pereira PJB, Chan B, Radom L, Payne RJ (2017) Accelerated protein synthesis via one-pot ligation-deselenization chemistry. Chem 2(5):703–715
Malins LR, Payne RJ (2012) Synthesis and utility of β-selenol-phenylalanine for native chemical ligation–deselenization chemistry. Org Lett 14(12):3142–3145
Wang X, Corcilius L, Premdjee B, Payne RJ (2019) Synthesis and utility of β-selenophenylalanine and β-selenoleucine in diselenide-selenoester ligation (DSL). J Org Chem 85:1567–1578
Dardashti RN, Kumar S, Sternisha SM, Reddy PS, Miller BG, Metanis N (2020) Selenolysine: a new tool for traceless isopeptide bond formation. Chem Eur J 26(22):4952
Sayers J, Karpati PM, Mitchell NJ, Goldys AM, Kwong SM, Firth N, Chan B, Payne RJ (2018) Construction of challenging proline–proline junctions via diselenide–selenoester ligation chemistry. J Am Chem Soc 140(41):13327–13334
McDonald DM, Hanna CC, Ashhurst AS, Corcilius L, Byrne SN, Payne RJ (2018) Synthesis of a self-adjuvanting MUC1 vaccine via diselenide-selenoester ligation-deselenization. ACS Chem Biol 13(12):3279–3285
Watson EE, Ripoll-Rozada J, Lee AC, Wu MC, Franck C, Pasch T, Premdjee B, Sayers J, Pinto MF, Martins PM (2019) Rapid assembly and profiling of an anticoagulant sulfoprotein library. Proc Natl Acad Sci U S A 116(28):13873–13878
Kulkarni SS, Watson EE, Premdjee B, Conde-Frieboes KW, Payne RJ (2019) Diselenide–selenoester ligation for chemical protein synthesis. Nat Protoc 14:2229–2257
Kajihara Y, Yoshihara A, Hirano K, Yamamoto N (2006) Convenient synthesis of a sialylglycopeptide-thioester having an intact and homogeneous complex-type disialyl-oligosaccharide. Carbohydr Res 341(10):1333–1340
Hackenberger CP (2006) The reduction of oxidized methionine residues in peptide thioesters with NH4I–Me2S. Org Biomol Chem 4(11):2291–2295
Thompson RE, Liu X, Alonso-García N, Pereira PJB, Jolliffe KA, Payne RJ (2014) Trifluoroethanethiol: an additive for efficient one-pot peptide ligation− desulfurization chemistry. J Am Chem Soc 136(23):8161–8164
Acknowledgments
The authors would like to acknowledge financial support from the EPSRC (EP/S028323/1, EP/S017739/1) and the University of Nottingham (PhD studentship for RG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Griffiths, R.C., Mitchell, N.J. (2021). The Chemical Synthesis of Site-Specifically Modified Proteins Via Diselenide-Selenoester Ligation. In: Hussein, W.M., Stephenson, R.J., Toth, I. (eds) Peptide Conjugation. Methods in Molecular Biology, vol 2355. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1617-8_18
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1617-8_18
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1616-1
Online ISBN: 978-1-0716-1617-8
eBook Packages: Springer Protocols