Abstract
Pain is a common symptom and still undertreated in children despite better knowledge. Safety has improved with the development of new drugs and fuller understanding of their pharmacokinetics and dynamics in children. For successful treatment, the mechanism of pain has to be analyzed. A multimodal analgesic treatment strategy is suggested, and analgesics should be used with appropriate complementary methods. Where complex analgesia is needed, consulting an acute pain treatment team with pediatric expertise is the most helpful approach.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Overview of the Ontogeny and Neurobiology of Pain
Over the past 40 years, the practice of pediatric pain management has advanced from a stage of anecdotal report to a research-supported standard of care in developed and many developing countries. Statements, such as the one delivered by Swafford and Allan in a textbook of pediatrics from 1968, are now considered blatantly false: “Pediatric patients seldom need medication for the relief of pain. They tolerate discomfort well. The child will say he does not feel well, or that he is uncomfortable or that he wants his parents but often will not relate this unhappiness to pain.” However, challenges in pediatric pain assessment, cognitive and behavioral changes in a developing patient population, and limitations of controlled and randomized investigations in vulnerable subjects still influence the field. Fortunately, clinicians no longer assume that “neurologic immaturity” limits an infant or child’s appreciation and experience of pain. Anatomic, neurochemical, and neuroimaging studies describe a functional pain transmission system present during the fetal period and maturing throughout childhood. Neuromodulatory systems present predominantly after birth and still demonstrate robust neuroplasticity into late adolescence.
More specifically, sensory nerve terminals for cutaneous nociception are present in the fetal perioral areas at 7 weeks GA (gestational age), with spread to the entire body by 20 weeks GA. At the level of the dorsal horn of the spinal cord, A-fibers enter prior to C-fibers at 8–12 weeks GA. A and C fiber territory overlaps at birth in the developing substantia gelatinosa. Ascending pain pathways are completely myelinated in the spine and brainstem between 22 and 30 weeks GA. Myelination extends to the thalamus at 30 weeks and to the cortex at 37 weeks to term. Descending inhibitory pathways develop post-term.
Excitatory neurotransmitters and neuromodulators develop early in the fetus, at 8–10 weeks GA, including CGRP (calcitonin gene-related polypeptide), SP (substance P), somatostatin, and NMDA (N-methyl-d-aspartate) systems. VIP (vasoactive intestinal peptide) and enkephalin appear at 10–14 weeks. Catecholamines are present in late gestation, and serotonin systems develop 6 weeks post-term. Receptors for excitatory neurotransmitters are numerous and widely distributed in the neonate, regressing to a more adult distribution during the postnatal months. In the late fetus, GABA (gamma aminobutyric acid) and glycine may act as excitatory neurotransmitters. NMDA and NK-1 (neurokinin-1) receptor density are maximal in the late fetal period.
At the cortical level, near-infrared spectroscopy shows hemodynamic and oxygenation changes in the neonatal brain following touch and noxious stimulation of a limb that may relate to cortical processing of tactile and painful stimuli, such as venipuncture. Stress responses are present in the neonate and may generate morbidity through stress-induced hyperglycemia, lactic acidosis, catecholamine release, and substrate mobilization. Neuroendocrine pathways between the hypothalamus and pituitary function at 21 weeks gestation; cortisol and beta-endorphin have been assayed following intrauterine sampling for exchange transfusion. Norepinephrine is present in paravertebral ganglia and adrenal chromaffin cells at 10 weeks gestation and is released with intrauterine stress (asphyxia). In a landmark paper by Anand and Hickey in 1987, the metabolic effects of pain in the fetus and neonate were delineated, providing a scientific basis for the treatment of neonatal pain.
The influence of early pain responses on later pain behaviors is provocative in many animal studies and increasingly replicated, with some controversy, in human reports. One study by Grunau compared 18 preterm infants, subject to repeated painful procedures in the NICU (neonatal intensive care unit), with matched full-term infants regarding their somatic complaints at 18 months. Twenty-five percent of mothers of preterm infants with prolonged NICU stays noted a significantly increased number of somatic complaints in their toddlers as compared to mothers of full-term infants who had brief normal nursery stays.
In summary, the neonatal pain transmission system is anatomically present, readily excitable, with the potential for central sensitization. Neonates and infants feel pain, but the cortical modulation of noxious stimuli is still under investigation.
2 Presenting Symptoms and Recommended Assessment of Pain
There is no objective, generic measurement of pain. It is a complex and multidimensional sensation that is modulated by previous pain experience, affect, attention, family beliefs, and environment. Pain is best assessed by questioning the patient directly, a challenge for infants and nonverbal children who must rely on their caregivers to interpret and report signs of pain and distress.
For verbal and school-aged patients, questions about pain quality, location, duration, and severity are possible, as well as a past history of relieving and exacerbating factors. Severity may be most reliably determined using a numeric rating scale, choosing numbers from 1 to 10, or VAS (visual analog scale), making a mark along a 10 cm line from “no pain” to “worst pain imaginable.” Patients with less appreciation of numeric ranking may substitute a faces scale. Location may be colored onto a figure.
Additional measures for more comprehensive assessment may include an inventory of symptoms, the CDI (Children’s Depression Inventory; 1982 by Maria Kovacs PhD), the Revised Children’s Manifest Anxiety Scale (RCMAS-2; 2008 by Western Psychological Services), and the Pain Response Inventory [1].
Pain assessment methods for infants and preverbal children often combine behavioral and physiologic indices. Scales are based on behavioral features such as facial expression, crying, and posture and behavioral variables such as heart and respiratory rate, blood pressure, and sweating (Table 19.1). Limitations in the use of these measures include distinguishing between pain and distress, such as hunger or fear. Physiologic changes may represent sepsis, hypoxia, or medication effects rather than pain. However, regular use of these scales is indicated in this patient population.
For children with developmental delay, nurses have recently developed a modification of behavioral and physiologic observation, with the assistance of the primary caretakers, the INRS (individualized numeric rating scale).
In pediatric care, cognitive development influences communication of pain as well as coping strategies. Children as young as 18 months may indicate the location of pain, but pain intensity is difficult to elicit before 3 years. At 3 years, children can grossly estimate “no pain; a little pain; and a lot of pain.” Concrete measures, such as “poker chips” and “pieces of hurt” may also be used to convey intensity. The use of more abstract self-report measures, such as the faces scales, is not valid for most patients under 5 years of age. Simple self-report measures are recommended for children older than 6 years, such as the NRS and VAS. Many adult scales are appropriate for adolescents.
Coping strategies are also determined by cognitive levels. Children at 18 months may indicate an awareness of ways to eliminate pain through structured play sessions. They may seek hugs and kisses and ask for medicine. Children who are 3–4 years of age may spontaneously use distraction and also report that play makes them feel better. Deliberate distraction and self-initiated cognitive strategies present at approximately age 5 years. Cognitive and behavioral strategies, such as relaxation and biofeedback, are usually most effective after age 6–7 years.
2.1 Pain Classification
Pain is often described in relation to time (acute vs. long term) or underlying etiology as:
-
Nociceptive
-
Inflammatory
-
Neuropathic
-
Functional (psychogenic)
-
Idiopathic (without known origin)
Pain is often a mixture of different types, such as nociceptive and neuropathic. It is of major importance to analyze what kind of pain the patient is suffering from in order to prescribe the most beneficial treatment and analgesics. Nociceptive pain and neuropathic pain are the two most common pain types in infants, children, and adolescents. Psychogenic pain is now best described as neuropathic central pain. Idiopathic pain usually has a physiologic origin, yet to be defined.
2.2 Acute and Chronic Pain
Pain, as defined by IASP (International Association for the Study of Pain), is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.” Clinically, patients present four different types of pain, primarily based on the time course of symptoms but also reflective of underlying nociceptive mechanisms (nociceptors, primary afferent fibers, ascending nociceptive tracts) and the ultimate distribution and memory of neural activation and modulation throughout the pain transmission system (cortical and subcortical centers, descending modulatory systems). These four patterns are:
-
Transient pain – activation of nociceptors in the absence of tissue damage (venipuncture, lumbar puncture)
-
Acute pain – tissue injury, regional activation of nociceptors and their dorsal horn and central connections, and restoration of normal nociceptive function with tissue healing (trauma, surgery)
-
Chronic pain, cancer related – ongoing tissue injury due to disease and/or repetitive treatments with sustained nociceptive activation/hyperalgesia and central modulation of the pain signals by cortical regions involving discrimination, attention, affect, emotion, memory, and motor control (surgery, radiation, chemotherapy)
-
Chronic pain, nonmalignant disease – persistent pain due to complex central and peripheral neuronal changes that outlast the original nociceptive stimuli, such as injury and disease, or are generated directly by nervous system dysfunction (peripheral neuropathy, spinal cord injury, somatoform disorder)
Pain treatment is guided by recognition of these pain categories, and all temporal patterns may overlap in a single patient, such as a pediatric cancer patient with metastatic osteosarcoma, post-amputation, undergoing radiation and chemotherapy, as well as multiple transient procedures.
Regarding procedural pain, noninvasive options include distraction, parental involvement, choice, cognitive techniques in children developmentally older than 4–5 years, sucrose in infants less than 6 months, and topical anesthetics.
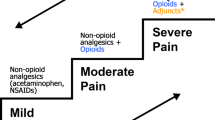
For acute pain, acetaminophen (paracetamol) and nonsteroidal anti-inflammatories, opioids, and possible regional anesthesia will likely be effective until resolution of tissue injury. Opioids are the standard of care for severe acute pain.
For chronic pain, malignant and nonmalignant, recognition of the complex network of pain transmission and modulation is essential, as well as any pain contribution from nerve injury, persistent inflammation, and environmental stress. Treatment may include use of short-acting symptomatic opioids and nonsteroidals, but generally emphasizes long-acting preparations; prophylactic neuropathic pain medications, such as tricyclic antidepressants and antiseizure agents; management of expected side effects; and functional restoration techniques, such as biobehavioral treatment, physical and occupational therapy, reconditioning, and complementary adjuvants. Chronic pain is a multidimensional experience and requires multimodal therapy. Recent brain imaging techniques suggest an extremely distributed system of pain transmission in patients with chronic pain, such as complex regional pain syndrome (Fig. 19.1).
fMRI brain regions of activation after noxious stimulation. (Left) Sensory regions; (right) emotional regions (From Becerra et al. [3])
2.3 Nociceptive Pain
Activity in pain fibers is evoked by stimulation of pain receptors at the nerve endings, nociceptors. The pain receptors may be sensitive to temperature, mechanical, or chemical stimuli. Pain signaling generates two separate experiences depending upon the type of nerve fiber (A-delta or C-fibers) which is transmitting the input. A-delta fibers, which are myelinated, transmit the impulses faster and are projected to a high degree on the sensory cortex. The effect is a sharp, distinct, and well-localized pain. In contrast, activity in the slower transmitting C-fibers reaches the thalamus and from there more diffusely spreads to multiple brain structures, generating a sensation of a more general pain experience (Fig. 19.2).
Brain areas active in pain processing as seen with fMRI. Discrimination: thalamus; SSC (I and II, both nociceptive and allodynic), Motivation/affect: anterior cingulate; amygdala, hippocampus (anxiety), Motor control: cingulate; pre-central gyrus; cerebellum, Memory of pain: insula, Modulation PAG, Pain/temperature DM nucleus of thalamus, Reward/emotional salience: nucleus accumbens PAG
From a clinical point of view, nociceptive pain is the most common origin of pain in pediatric patients. An example of nociceptive pain is postoperative pain which is directly related to surgery. Pain develops as a result of tissue damage and the arising inflammatory process. Within minutes after the surgical damage, secondary hyperalgesia develops and pain is amplified via segmental reflexes within the spinal cord. Postoperative pain diminishes because of the healing process, and pain treatment is usually needed for 3 or 4 days. Another example of an acute pain condition is a tendon or a muscle strain due to a trauma. Pain is usually caused by mechanical pressure from bleeding in the area. Ischemia develops secondary to the damage and may prolong pain, suffering, and the healing process. Nociceptive pain conditions usually respond to analgesic treatment.
2.4 Neuropathic Pain
Neuropathic pain is generated as a direct effect of a lesion or a disease in the somatosensory nervous system. The somatosensory system includes the central nervous system and peripheral nerves. The pain experience is many times not in proportion to the damage. Neuropathic pain can develop at the time of an injury but also weeks to months after a trauma. Interestingly, infants and younger children seldom develop neuropathic pain due to a nerve lesion, such as brachial plexus trauma following birth trauma. Clinical evidence suggests that pain caused by nerve lesion is more likely appreciated after 8–10 years of age.
Pain can be spontaneous (continuous or intermittent) or induced by stimuli and is often described as very intense, sharp, and burning by the patient. On examination, the patient can show signs of hyper- or hyposensitivity to one or several types of stimulation. Pain induced by a non-nociceptive stimulus as touch is called allodynia.
Visceral tissue and organs have complex neurogenic innervations. Conditions and diseases within the visceral system can create severe and complex pain situations. Visceral neuropathic pain is not specifically localized and is often projected to the somatic areas (referred pain).
Treatment with analgesics often has a limited effect on neuropathic pain. Pain reduction can be achieved but seldom total pain relief.
3 Recommended Treatment
3.1 Strategies in the Treatment of Pain
The aim of pain management is to achieve acceptable levels of pain for each individual child with the least side effects possible. Realistic goals are to recognize pain in children with clinical feasible assessment tools, to minimize moderate and severe pain safely, and to prevent pain where it is predictable. Prophylactic treatment in elective surgery and before planned procedures limits the total amount of analgesics needed. A safety web is needed for rapid control of breakthrough pain and to handle undesirable side effects. Analgesics, such as opioids, cause negative side effects, and it is important to treat side effects promptly when they are problematic (Table 19.2).
The underlying mechanism causing pain should be determined as management of nociceptive and neuropathic pain differs to a large extent. A multimodal analgesia approach is often recommended, especially for neuropathic pain. A combination of analgesics often reduces the need of high doses and related side effects (Table 19.3). Functional pain is seldom reactive to analgesic treatment alone.
Analgesic treatment may be combined with complementary physical and psychological approaches when possible. The importance of involving the child and parents in the treatment plan is essential. Reducing fear, discomfort, and creating patient control are major features for treatment success.
3.2 Treatment of Nociceptive Pain Conditions
Many pediatric pain services use techniques of parallel or co-analgesia based on up to six groups of analgesics, namely, local anesthetics, acetaminophen (paracetamol), NSAIDs (nonsteroidal anti-inflammatory drugs), opioids, α(alpha)2-adrenergic receptor agonists, and, to some extent, NMDA blockers, such as ketamine. In particular regional analgesic methods should be used in all cases unless there is a specific contraindication. Analgesics, such as acetaminophen and NSAIDs, should be given on a regular basis and not only when pain becomes troublesome. Analgesic combinations from different classes of drugs are important to achieve a successful pain management.
Regional/local anesthesia is discussed later in this chapter.
3.2.1 Acetaminophen (Paracetamol)
Several mechanisms in the central nervous system are responsible for the analgesic effect. The analgesic potency is relatively low and a ceiling effect has been shown in studies. The i.v. formulation of mannitol-solubilized acetaminophen (paracetamol) has shown to be clinically useful, and it is speculated that the higher effect site concentration achieved in the brain may result in higher analgesic potency. Acetaminophen (paracetamol) is absorbed from the small bowel, and the oral route should therefore only be used when the g.i. (gastrointestinal) function is normal. Of note, both pain and postoperative conditions reduce g.i. motility. Rectal administration is slow and incomplete and therefore the least effective route of acetaminophen (paracetamol) therapy. Time to peak analgesia after i.v. administration is about 1 h and, after oral administration, 2 h.
Dosing regimens for acetaminophen (paracetamol) have been revised in the last years on basis of age, route of delivery, loading dose, maintenance dose, duration of therapy, and liver function to ensure a balance between efficacy and safety. It is recommended to use i.v. administration during the first 24 h to optimize the analgesic effect and, thereafter, switch to oral formulations if the g.i. motility is normalized. If the oral route is used from the beginning of therapy, a loading dose is often used. This is not necessary when using i.v. acetaminophen (paracetamol). Normal dosing in healthy pediatric patients is 60–80 mg kg/day i.v. and 80–100 mg kg/day p.o. divided on four doses. Dose reduction should be performed after 2–3 days of therapy and in younger infants, preterm neonates, and sick children. For more detailed dosing, please consult textbooks of pain pharmacology or the Internet.
3.2.2 NSAIDs
NSAIDs are important in the treatment and prevention of mild and moderate nociceptive pain. The site of action is within both the peripheral and central nervous system. In studies in adults, the combination of NSAIDs and opioids results in an opioid-sparing effect of about 30–40 %, and from a clinical point of view, similar findings are observed in pediatric patients. NSAIDs in combination with acetaminophen (paracetamol) produce better analgesia than either alone. NSAIDs are especially effective in pain emerging from fractures and bone processes. Concerns have been raised about the effect of NSAIDs on bone healing after orthopedic surgery in children, but the benefits of short-term use outweigh the risks. NSAIDs can be given to a majority of children with asthma. Only about 2 % of children react negatively with increased obstructive symptoms when receiving NSAIDs. COX-2 inhibitors are alternatives to nonselective NSAIDs if the patient has a bleeding disorder or thrombocytopenia.
Most commonly used NSAIDs are ibuprofen, diclofenac, ketolorac, and celecoxib (selective COX-2 inhibitor). Recommended dose of ibuprofen is 4–10 mg/kg 4 times daily and diclofenac 1 mg/kg 3 times daily in children aged older than 3 months. Intravenous ketolorac dosage is 0.3 mg/kg 4 times daily. The use of ketolorac should not exceed 5 days.
3.2.3 Opioids
There are several opioids used for the treatment of nociceptive pain. The idea that some opioids are weak (i.e., codeine) and others are strong (i.e., morphine) is not accurate. Most opioids are capable of treating pain in spite of its intensity if the dose is adjusted correctly. At equipotent doses, most opioids have comparable effect and side effects. The individual response to an opioid can differ. Opioid rotation is sometimes a useful tool when the patient is troubled by nausea and vomiting. Therefore, it is reasonable to have the option of several opioids to choose from in management of pain. Codeine is metabolized into morphine which is responsible for most of codeine’s analgesic effect. Children, especially under the age of 6, are to large extent poor metabolizers of codeine and, as a result, lack analgesic effect upon administration. Further, a small proportion of people are “ultra-rapid metabolizers” who convert an appropriate dose of codeine into potentially fatal level of morphine in the blood. Codeine is not usually recommended for pain treatment in children.
In acute pain conditions, opioids should be administered by the intravenous route for most rapid effect and for the opportunity of easy titration. If repeated doses are required, it is advantageous to use an opioid infusion. Among opioids, morphine is the most common choice. Morphine infusions of between 10 and 30 μg/kg/h provide sufficient analgesia with an acceptable level of side effects with the appropriate level of monitoring. Oxycodone, hydromorphone, methadone, and buprenorphine may be offered as alternatives to morphine (Table 19.4). Pethidine (meperidine) is not recommended in children because of the adverse effect of its main metabolite, norpethidine, which may increase cortical excitability.
In appropriately selective cases, the subcutaneous route of administration is a useful technique to the i.v. route. Other methods of opioid delivery are oral, transdermal, sublingual, and intranasal routes.
When pain is under control, opioids may be switched to the oral route. The oral dose is often considerably higher because of limited bioavailability compared to the i.v. dose. For example, the morphine dose given orally has to be about three times higher than the i.v. dose for equivalency. In opioid naïve patients, an initial oral dose of morphine is approximately 0.2–0.3 mg/kg. In neonates and young infants, the dose should be reduced to 0.05–0.1 mg/kg. Time to peak analgesic effect of oral morphine administration is about 30–40 min after intake.
Opioid agonists do present undesirable side effects such as nausea and vomiting, constipation, pruritus, urinary retention, sedation, and, most significantly, respiratory depression. Sedation and respiratory function must be monitored carefully when opioids are administered i.v. and during the initial phase of opioid treatment. Safety protocols and management protocols for side effects are necessary. Most side effects diminish with time, except for constipation. Constipation by itself creates stomach pain and increases the risk of nausea and vomiting. Laxatives and oral mixture of naloxone (opioid antagonist) may be recommended when opioid treatment is used. Naloxone administered orally has a local effect on the opioid gastrointestinal receptors without a central antagonist effect because of an almost first passage effect in the liver. An alternative for severe cases of constipation is subcutaneous injection with methylnaltrexone. The treatment should begin with a limited dosage because of the prompt effect on intestinal motility which can cause intestinal colic.
Withdrawal of opioids is discussed later in this chapter.
3.2.4 Alpha2-Adrenergic Receptor Agonists
Clonidine and dexmedetomidine are the two most clinically used alpha2-agonists. Clonidine can be administered by several routes, as oral, i.v., and as an additive to local anesthetics. Dexmedetomidine is most frequent used as an i.v. drug for sedation and to a lesser extent as an analgesic. The intranasal application of dexmedetomidine, on the other hand, has promising possibilities both for procedural pain and sedation.
Alpha2-agonists reduce pain transmission in the spinal cord and have effect on both nociceptive and neuropathic pain. In supraspinal areas alpha2-agonists produce sedation and affect the vasomotor center. The vasomotor effect is vasodilatation and increased bradycardia in smaller children. Historically, clonidine was earlier used for treatment of hypertension. From a clinical point of view, hypotension and bradycardia are seldom problems in infants and children. There is no ventilatory depression or major constipation effects of alpha2-agonists as compared to opioids, an important advantage in respect to safety issues.
The effect on pain and sedation is dose dependent, with greater effect when higher dosage is used. Several pediatric pain treatment services have been using clonidine as part of the multimodal treatment approach with analgesic benefit. Clonidine administered i.v. has an immediate effect, but time to peak effect after oral administration is about 60–90 min. Oral bioavailability is fairly high, about 80 % but with individual variation. A normal analgesic dose is 1 μg/kg i.v. and 1–2 μg/kg p.o. three times daily. Higher dose is often required in more severe pain conditions.
3.2.5 NMDA Blockers
Ketamine or the isomer S-ketamine (more potent with less side effects) is a possible drug to use for decreasing windup in and following complex surgery. It is used as a continuous infusion and should be started before surgical incision and continued postoperatively for a couple of days. In higher doses there is a risk for developing unpleasant dreams and hallucinations. Close patient monitoring is essential to identify these side effects. Acute neuropathic pain is another area for the use of NMDA blockers.
3.3 Treatment of Neuropathic Pain
The treatment of pain that is initiated or caused by a primary lesion or dysfunction within the nervous system itself presents significant challenges. When the peripheral and central somatosensory and cortical and spinal pain modulatory regions are the symptom generators, medications such as opioids and nonsteroidal anti-inflammatory agents are often ineffective, even in significant increased dosage. More appropriate pharmacologic choices directly target neuromodulatory neurotransmitters (tricyclic antidepressants, TCAs), neuronal membrane channels (antiseizure medications, local anesthetics, and receptors responsible for central nervous system excitation, such as N-methyl-d-aspartate receptors (NMDA antagonists)). For complex and chronic neuropathic disorders, such as intractable headache, CRPS (complex regional pain syndrome), and visceral hyperalgesia, a multidisciplinary approach is warranted, combining neuropathic pharmacology with physical, cognitive, and complementary therapies.
In pediatric practice, studies supporting interventions for neuropathic pain are more limited than for nociceptive pain and are predominantly case studies and clinical series with rare subject control. Placebo effects are not often reported due to ethical concerns, and medication effect is likely altered by central pain-processing changes throughout pediatric development. Interestingly, some common neuropathic pain disorders in the adult population, such as diabetic peripheral neuropathy, brachial plexus neuralgia, radiculopathy, trigeminal neuralgia, and post-herpetic neuralgia, are rare in children. However, there are disorders somewhat unique to pediatrics, such as hereditary and metabolic neuropathies (Fabry’s disease, mitochondrial disorders, lead exposure) and primary erythromelalgia. Adult pain conditions present in children may have a more favorable prognosis, such as CRPS, trauma, and postoperative neuropathic pain.
Medication choice is frequently based on adult studies, despite developmental limitations, and on the safety and efficacy information for medication used for non-painful disorders, such as epilepsy and depression. However, a specific safety concern, suicidal ideation, has been highlighted by the FDA regarding the use of all antiseizure and antidepressant agents in children and adolescents, necessitating appropriate discussion with families when initiating treatment. Fortunately, current data (please see References) supports the thoughtful use of these agents in pediatric patients with and without depression. Parents may appropriately be referred to the websites of the European and American Psychological and Psychiatric Associations for additional information.
Regarding antidepressant agents most frequently used for pediatric pain, amitriptyline is often the initial choice, as this medication has a relatively long record of safety and efficacy in patients with headache and chronic abdominal pain. In general, the starting dose is low, 0.3 mg/kg, with an increase to 1–2 mg/kg/day. A baseline ECG may be useful in patients who are at risk of cardiac arrhythmia or a prolonged QTc. Adverse effects also include sedation, constipation, weight gain, and anticholinergic autonomic effects. Nortriptyline may have less associated orthostatic hypotension and sedation but greater risk of palpitations. Efficacy is attributed to blockade of NE (norepinephrine) and 5-HT (serotonin) reuptake at synapses in the endorphin-mediated pain modulation pathways at central (descending) and spinal cord levels. Newer agents with similar effects, such a duloxetine and venlafaxine, have not been well studied in children. Serotonin reuptake inhibitors (SSRIs) have not shown reliable relief of neuropathic pain.
Dosing of amitriptyline/nortriptyline. Initial 0.05–2 mg/kg at bedtime p.o.; may increase dose over 2–3 weeks, to effect; max 2 mg/kg/day
Antiseizure medications provide a clinical level of comfort for prescribers, due to information available through pediatric epilepsy trials. However, the use of these agents for pediatric neuropathic pain is limited, with gabapentin as the most studied medication in reports of children and adolescents with CRPS, phantom pain, and postoperative trauma. Dosage is weight based, and serum levels do not correlate well with pain response. Adverse effects and potential toxicity guide periodic evaluation of the serum level. Possible side effects are multiple and vary according to the selected agent. Unacceptable symptoms include rash, sedation/mental clouding, renal calculi formation, and severe gastrointestinal distress. Hematologic and hepatic changes seen with some of the older antiseizure medications are less frequent with newer agents (Table 19.5). These newer formulations also potentially bind to a greater variety of receptors within the pain transmission system, expanding from sodium and calcium channel binding to include NMDA and GABA receptors.
3.3.1 Dosing of Antiseizure Medications
Gabapentin. Initial, 5 mg/kg/dose at bedtime; day 2, increase to 5 mg/kg/dose, twice daily; day 3, increase to 5 mg/kg/dose 3 times/day; titrate to effect; usual dosage range, 8–35 mg/kg/day divided into 3 doses/day.
Pregabalin. Initial, 150 mg/day in divided doses (50 mg 3 times/day); may be increased within 1 week based on tolerability and effect; maximum dose, 300 mg/day (dosages up to 600 mg/day were evaluated with no significant additional benefit and an increase in adverse effects).
Topiramate. Children <12 years: initial, 1–3 mg//kg/day (maximum, 25 mg) given nightly for 1 week; increase at 1- to 2-week intervals by 1–3 mg/kg/day given in 2 divided doses; titrate dose to response; usual maintenance, 5–9 mg/kg/day given in 2 divided doses. Adolescents ≥17 years and adults: initial, 25–50 mg/day given daily for 1 week; increase at weekly intervals by 25–50 mg/day; titrate dose to response; usual maintenance, 200 mg twice daily; maximum dose, 1,600 mg/day.
Zonisamide. Initial, 1–2 mg/kg/day given in two divided doses/day; increase dose in increments of 0.5–1 mg/kg/day every 2 weeks; usual dose, 5–8 mg/kg/day. Adolescents >16 years and adults: adjunctive treatment of partial seizures, initial 100 mg/day; dose may be increased to 200 mg/day after 2 weeks. Further dosage increases to 300 mg/day, and 400 mg/day can then be made with a minimum of 2 weeks between adjustments, in order to reach steady state at each dosage level. Doses of up to 600 mg/day have been studied; however, there is no evidence of increased response with doses above 400 mg/day.
Additional pharmacologic considerations for treatment of neuropathic pain include agents that bind to the NMDA receptor, such as ketamine, and alpha2-agonists, such as clonidine, described in the previous section on treatment of nociceptive pain. Local anesthetics will be presented in the next section. Representative dosing for ketamine is as follows: p.o. 6–10 mg/kg per dose, i.m. 3–7 mg/kg per dose, and i.v. 0.5–2 mg/kg per dose. Ketamine is most frequently used for sedation during procedures or anesthesia induction. Short-term infusions are currently being studied for the treatment of severe neuropathic pain. Adverse effects include increased salivation, g.i. distress, sedation and short-term memory loss, increased intracranial pressure, cardiac dysrhythmia, and respiratory depression. Clonidine dosing is 2–4 μg/kg 4 times daily, with use limited by potential hypotension and sedation. If clonidine is used for greater than 2 weeks, a sudden discontinuation may be associated with rebound hypertension.
Of note, for some pediatric patients with pain resistant to pharmacology, such as those with limb pain due to CRPS, physical and cognitive interventions, which activate the diffuse pain-processing system, may be more effective than pharmacologic choices. In a study by Lee in 2002, 28 patients with CRPS of the lower limb participated in physical therapy once vs. three times per week, with cognitive-biobehavioral therapy once per week, for a total of 6 weeks. Outcome measures included pain scores, gait testing, stair climbing, psychological inventories, regional and systemic autonomic examination, and quantitative sensory testing. Results showed that both groups had a greater than 50 % improvement in pain scores (VAS), improved gait and stair climbing, and no need of assistive devices by 6 weeks.
3.4 Local Anesthesia
Local anesthetics reversibly block voltage-gated sodium channels and, therefore, initiation and propagation of neural impulses along peripheral and central nerve pathways. They are generally used for regional anesthesia for procedures, surgery, and as adjuvant therapy for some chronic neuropathic pain disorders (CRPS, cancer pain). Amide agents lidocaine and bupivacaine are most commonly used. An oral analog of lidocaine, mexiletine, may be used for neuropathic pain, but little data is available regarding efficacy in children. The dose is 2–3 mg/kg/day, divided bid-tid. Intravenous lidocaine may be administered in bolus dosing or brief continuous infusion for intractable nerve pain and headache, maintaining a serum level less than 5 μg/mL to avoid cardiac and central nervous system toxicity. The i.v. dosage is 20 μg/kg/min to achieve an analgesic level of 102 μg/mL.
For regional anesthesia, such as epidural and brachial or lumbar plexus blockade, dosing is decreased due to application of drug in proximity to the target receptor (Table 19.6). This technique may, therefore, overcome the limitations of local anesthesia, such as low potency, toxicity near therapeutic dose, and other local actions with spread non-sodium nerve channels, mitochondria, and cellular membrane components. Binding, potency, and potential neurotoxicity of the drug are enhanced by acidosis, previous nerve injury or neuropathy, hypotension, and local vasoconstriction. Acute cardiac toxicity may be reversed by intravenous administration of a lipid emulsion, such as Intralipid 20 %, at 0.25 mL/kg/min. Local anesthetic effectiveness may be decreased by technically poor delivery, tachyphylaxis, infection, and genetic variation. Patients with chronic neuropathic pain show the greatest resistance, possibly due to the distributed nature of pain in these patients. Recent research is focusing on developing sensory selective blocking agents derived from biologic nerve toxins, such as tetrodotoxin and saxitoxin.
Of note, some neuropathic medications have local anesthetic effects, such as tricyclic antidepressants, ketamine, clonidine, and methadone (d-isomer is an NMDA antagonist).
The pharmacology of local anesthetics in neonates and infants may precipitate overdosage in these patients, warranting careful titration due to delayed hepatic degradation and prolonged elimination half-live of amide anesthetics. Neonates and infants also have reduced serum levels of albumin and alpha1-acid glycoproteins that bind local anesthetics, leading to increased levels of free and unbound drug. However, neonatal volumes of distribution are also increased compared to older children, offsetting potential systemic toxicity. Ester local anesthetics, such as chloroprocaine 1.5 %, are often used for epidural analgesia in neonates due to its rapid metabolism by plasma cholinesterase.
Topical anesthetics have clearly advanced the treatment of pediatric procedural pain, with many dosage forms available, including gels, sprays, creams, ointments, and patches. Depending upon the preparation, absorption through the skin for dermal afferent binding is enhanced by eutectic mixture, liposomal preparation, and iontophoresis (EMLA®, LMX-4®, Synera®). Direct placement of a combination of lidocaine, epinephrine, and tetracaine (LET) into open lacerations/wounds may obviate the need for local injection (lidocaine 20 %, racemic epinephrine 2.25 %, and tetracaine 2 %) (Table 19.7).
3.5 Procedural Pain Treatment
Procedural sedation and analgesia is the use of sedative, analgesic, and dissociative drugs to provide analgesia, anxiolysis, and sedation during painful or unpleasant diagnostic and therapeutic procedures (Table 19.8). Progression from minimum sedation to general anesthesia does not lend itself to random partition. Low doses of opioids or sedative-hypnotics induce mild analgesia or sedation respectively, with little danger of adverse events. Higher doses provide progressively deeper sedation, increasing the risk of respiratory and airway compromise. Procedural guidelines are intended to regulate the procedure and most importantly enhance patients’ safety. Continuous observation of patients by a health-care provider capable of recognizing adverse sedation events is essential. This person must be able to continuously observe the patient’s degree of sedation and vital organ functions. Procedural sedation and analgesia personnel should be proficient at maintaining airway patency and assisting ventilation if needed when sedatives are used.
A multimodal analgesic-sedative approach is recommended for best efficacy. Analgesic drugs should always be combined with distraction techniques and the highest degree of patient self-control. The environment should be relaxing and calming, and adding music is a useful option.
Knowledge of pharmacodynamics is essential. Time to peak analgesic effect of acetaminophen (paracetamol) and NSAIDs is about 2 h after oral intake. For clonidine, peak effect is about 60–90 min and for morphine 30–40 min. If the i.v. route is used, the effect is often faster and more prominent. Nasal application, preferably as an aerosol, of analgesics and sedatives is a novel and an increasingly popular technique when the patient lacks an i.v. line. Drugs used in awake children should not give rise to a painful or discomforting experience. It is therefore important to use buffered solutions of local anesthetics and avoid mixtures which taste bitter or create unpleasant sensations upon administration, i.e., midazolam.
Generally safe sedatives that also produce analgesics include acetaminophen (paracetamol), NSAIDs and local anesthetics, alpha2-agonists, S-ketamine (ketamine), and opioids. As mentioned previously, higher doses produce an elevated degree of sedation, requiring adjustment by the attending staff. Inhaled nitrous oxide is another attractive alternative for procedural pain, in doses up to 50 % mixture in oxygen. The onset is fast and the effect is short-lasting when the gas is turned off. It is often an advantage to use systems that offer the possibility to titrate nitrous oxide mixture up to 50 %. In teenagers, loss of self-control can be especially troublesome, and in children experiencing nausea, the nitrous mixture could be lowered, resulting in fewer side effects.
Diazepam, midazolam, chloral hydrate, barbiturates, and propofol are pure sedative-hypnotic drugs without analgesic properties. These drugs should never be used without analgesics when performing a painful procedure. The use of sedatives alone is sadly still quite common at many hospitals and institutions taking care of children. Midazolam has gained a widespread use because of its fast-acting and relatively short-acting effect. Midazolam is also considered to have amnesic properties and is for that reason popular among clinicians. This is partly true and only applicable for the explicit memory (conscious memory). The implicit memory (unconscious memory) which registers discomfort and unpleasant episodes is unchanged or even enhanced when midazolam is used. The effect could lead to distressing behavior for the child, especially when used at repeated procedures. However, some degree of amnesia may be beneficial during procedures. Of note, several children’s hospitals have recently eliminated the use of midazolam for painful procedures.
3.6 Complementary Methods
3.6.1 Physiotherapy
The aim of physiotherapy is to restore the patient’s best possible motor function. Simultaneously, with increased activity, the endogenous opioid system is activated, leading to reduced pain.
3.6.2 Sensory Stimulation
TENS (transcutaneous electric nerve stimulation), acupressure, and acupuncture often create effective pain relief in children. It is of importance that these techniques are introduced cautiously in children and adapted to the age of the child. Dorsal column electric stimulation is occasionally needed in complex pain conditions.
Other forms of sensory stimulation as cold and heat therapy as well as massage have been increasingly used in children during the last decades. Cold therapy is used regularly at the time of joint surgery to reduce pain and decrease inflammation.
3.6.3 Distraction and Self-Control
There are several beneficial distraction techniques to be used, including guided imagery, individual muscle relaxation, and self-hypnosis. Effective distraction reduces significantly pain perception. Distraction is especially important to use in painful procedures. High level of patient self-control is further more an important tool to reduce fear and increase compliance with the treatment.
3.7 Withdrawal of Opioids and Benzodiazepines (Midazolam)
After prolonged exposure to opioids and midazolam, children develop both tolerance and withdrawal. These issues are especially seen in younger age groups and develop commonly during critical illness in the pediatric intensive care unit. A problem with withdrawal often arises when the patient is transferred to a regular ward and the previous drug doses are quickly reduced. It is important to monitor the patient closely to discover early signs of withdrawal and plan a slow reduction of opioids and midazolam. Early signs of withdrawal are changes in sleep pattern, such as short periods of sleep and easily disturbed sleep, and feeding problems. Late signs are the more classic features of withdrawal and for the child include the distressing autonomic symptoms of sweating, jitteriness, and tachycardia. Several scales designed to measure withdrawal problems rarely contain the early signs of withdrawal.
The strategy for management of withdrawal is to prevent oversedation and the development of the need of high dose and prolonged use of opioids and midazolam. The reality is still often that the patient is referred to a regular ward with high doses with opioids and midazolam administered i.v. Close monitoring of withdrawal symptoms and slow reduction of doses are necessary to achieve a successful weaning. As a rule, it takes about as long or double the time to wean a patient compared to how long the drugs have been used. Switching to oral route as soon as possible and using long-acting opioids (i.e., methadone, buprenorphine) are suggested. The use of clonidine, often higher doses than for pain treatment, can help to more rapidly decrease opioids and midazolam.
3.8 Pain Emergencies
The most common emergent problem in pediatric pain is severe breakthrough pain. Symptomatic treatment may include rapid-acting opioids and NSAIDs, especially in an i.v. formulation, as well as adjuvant medications for sedation, anxiolysis, and muscle spasm. Intravenous methadone is an alternative for patients who do not respond to the first-line opioids. (For drugs and doses, see treatment of nociceptive pain.)
For sedation and anxiety:
-
Midazolam: infusion 0.05–0.02 mg/kg/h i.v.; oral single dose 5–10 mg/kg
-
Lorazepam: i.v. 0.05 mg/kg q 3–4 times daily; oral 0.05 mg/kg 4 times daily
-
Ketamine, s-ketamine: i.v. 0.5–2 mg/kg; oral single dose 3–7 mg/kg
-
Hydroxyzine: i.v./oral 0.5–1 mg/kg 4 times daily
-
Diphenhydramine: i.v/oral 1–2 mg/kg 4–6 times daily
For muscle spasm – oral antispasmotics (see also Chap. 17):
-
Diazepam: GABA A agonist at spinal cord; sedation due to reticular activating formation binding
-
Dantrolene: inhibits calcium ion release in muscle fibers; decreases muscle contraction
-
Baclofen: GABA B agonist; inhibits presynaptic release of excitatory transmitters
-
Tizanidine: alpha2-agonist; inhibits release of aspartate and glutamate
Additional emergent conditions related to pain include:
Hypotension:
-
1.
Administer supplemental oxygen.
-
2.
Establish i.v. access for bolus of 250–500 mL of lactated Ringer’s solution (weight >40 kg).
-
3.
Monitor vital signs and verbal communication.
-
4.
Elevate lower extremities or place patient in Trendelenburg.
-
5.
Administer vasopressors, only if necessary: ephedrine:
-
(a)
Children <12 years: i.m., i.v., s.c.: 3 mg/kg/day in 4–6 divided doses
-
(b)
Children ≥12 years and adults: i.m., s.c.: 25–50 mg (range: 10–50 mg); may repeat with a second dose of 50 mg; not to exceed 150 mg/24 h. Intravenous: 10–25 mg; may repeat with a second dose in 5–10 min of 25 mg; not to exceed 150 mg/24 h
-
(a)
Anaphylaxis:
-
1.
Assess airway and administer oxygen.
-
2.
Establish i.v. access for NS or LR bolus of 20 mL/kg.
-
3.
Primary treatment is epinephrine: < 10 kg 1:1,000, 0.01 mg/kg i.m.; 10–25 kg, 0.15 mg i.m.; 26–100 kg, 0.3 mg i.m.
-
4.
Secondary treatment includes diphenhydramine 1 mg/kg po/IV, max 50 mg; methylprednisolone 1 mg/kg i.v., max 50 mg; and possible ranitidine 2 mg/kg i.v., max 50 mg.
Opioid overdose:
-
1.
Support ventilation.
-
2.
Administer incremental naloxone IV; start at 0.05 mg with 0.4 mg as total dose.
-
3.
Consider naloxone infusion if long-acting opioid given; 0.25–0.5 μg/kg/h.
Opioid withdrawal:
-
1.
Resume opioid treatment.
-
2.
Consider clonidine.
-
3.
Treat associated nausea with p.o. prochlorperazine, 0.4 mg/kg/day divide in 4 doses, or i.v. ondansetron. Children <10 kg, 0.5 mg every 8 h as needed; children ≥10 kg to <30 kg, 1 mg every 8 h as needed; children >30 kg and adults, 2 mg every 8 h as needed.
-
4.
Treat muscle pain with NSAIDs.
-
5.
Fluid replacement for diarrhea.
4 Conclusion
Children in all ages can experience pain. Pain conditions are most often nociceptive or neuropathic. It is essential to analyze the cause of pain in order to achieve an effective treatment. The aim of pain management is to reach acceptable levels of pain for each individual child, and a multimodal treatment approach is suggested.
References
Walker LS, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137(2):266–75.
Merkel S, Voepel-Lewis T, Shayevitz J, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7.
Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–46.
Suggested Further Reading
Anand KJS, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37.
Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM, Nicholson C. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5):1208–25.
Anand KJS, Stevens B, McGrath PJ, editors. Pain in neonates. 3rd ed. Edinburgh: Elsevier; 2007.
Anderson BJ, Palmer GM. Recent pharmacological advances in paediatric analgesics. Biomed Pharmacother. 2006;60(7):303–9.
Anderson B. Comparing the efficacy of NSAIDs and paracetamol in children. Paediatr Anaesth. 2004;14:201–17.
Anderson BJ. Paracetamol (acetaminophen): mechanisms of action. Paediatr Anaesth. 2008;18(10):915–21. Review.
Arana A, Morton N, Hansen T. Treatment with paracetamol in infants. Acta Anaesthesiol Scand. 2001;45:20–9.
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84.
Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(1–2):109–17.
Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment. JAMA. 2007;297(15):1683–96.
Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, Nivoche Y, Constant I, Murat I. Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies. Acta Anaesthesiol Scand. 2010;54(4):397–402.
Fitzgerald M, Anand KJS. The neurobiologic basis of pediatric pain. In: Schechter N, Berde C, editors. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 19–42.
Gaffney A, McGrath PJ, Dick B. Measuring pain in children: development and instrument issues. In: Schechter N, Berde C, editors. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 128–41.
Goubet N, Clifton RK, Shah B. Learning about pain in preterm newborns. J Dev Behav Pediatr. 2001;22(6):418–24.
Greco CD, Aner MM, LeBel AA. Acute pain management in infants and children. In: Warfield CA, Bajwa ZH, editors. Principles and practice of pain medicine. 2nd ed. New York: McGraw-Hill; 2004. p. 541–52.
Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11(4):268–75.
Hermann C, Hohmeister J, Demirakça S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school aged children with early pain experiences. Pain. 2006;125(3):278–85.
Howard R, Carter B, Curry J, Morton N, Rivett K, Rose M, Tyrrell J, Walker S, Williams G. Good practice in postoperative and procedural pain management. Paediatr Anaesth. 2008;18 Suppl 1:1–80.
Kaizar EE, Greenhouse JB, Setman H, Kelleher K. Do antidepressants cause suicidality in children? A Bayesian meta-analysis. Clin Trials. 2006;3(2):73–90.
Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 1 – Pharmacokinetics. Paediatr Anaesth. 1997;7:5–11.
Kart T, Christrup L, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 2 – Clinical use. Paediatr Anaesth. 1997;7:93–101.
Krauss B, Green S. Procedural sedation and analgesia in children. Lancet. 2006;367:766–80.
Kristensen AD, Pedersen TA, Hjortdal VE, Jensen TS, Nikolajsen L. Chronic pain in adults after thoracotomy in child or youth. Br J Anaesth. 2010;104(1):75–9.
Lee BH, Scharff L, Sethna NF, McCarthy CF, Scott-Sutherland J, Shea AM, Sullivan P, Meier P, Zurakowski D, Masek BJ, Berde CB. Physical therapy and cognitive-behavioral treatment for complex regional pain syndromes. J Pediatr. 2002;141(1):135–40.
Lönnqvist PA, Morton N. Postoperative analgesia in infants and children. Br J Anaesth. 2005;95:59–68.
Morton NS. Ketamine for procedural sedation and analgesia in pediatric emergency medicine: a UK perspective. Paediatr Anaesth. 2008;18(1):25–9. Review.
Sagie I, Kohane DS. Prolonged sensory–selective nerve blockade. Proc Natl Acad Sci U S A. 2010;107(8):3740–5.
Schechter NL, Zempsky WT, Cohen LL, McGrath PJ, McMurtry CM, Bright NS. Pain reduction during pediatric immunizations: evidence-based review and recommendations. Pediatrics. 2007;119(5):1184–98.
Solodiuk J, Curley MA. Pain assessment in nonverbal children with severe cognitive impairments: the Individualized Numeric Rating Scale (INRS). J Pediatr Nurs. 2003;18(4):295–9.
Stewart SH, Buffett-Jerrott SE, Finley GA, Wright KD, Valois GT. Effects of midazolam on explicit vs. implicit memory in a pediatric surgery setting. Psychopharmacology (Berl). 2006;188(4):489–97.
Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603.
Walco GA, Dworkin RH, Krane EJ, LeBel AA, Treede RD. Neuropathic pain in children: special considerations. Mayo Clin Proc. 2010;85(3 suppl):S33–41.
Weinburg GL. Lipid infusion therapy: translation to clinical practice. Anesth Analg. 2008;106(5):1340–2.
Yaster M, Krane EJ, Kaplan RF, Cote CJ, Lappe DG. Pediatric pain management and sedation handbook. St. Louis: Mosby-Year Book; 1997.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Lundeberg, S., LeBel, A.A. (2014). Acute Pain. In: Sejersen, T., Wang, C. (eds) Acute Pediatric Neurology. Springer, London. https://doi.org/10.1007/978-0-85729-491-3_19
Download citation
DOI: https://doi.org/10.1007/978-0-85729-491-3_19
Published:
Publisher Name: Springer, London
Print ISBN: 978-0-85729-490-6
Online ISBN: 978-0-85729-491-3
eBook Packages: MedicineMedicine (R0)