Abstract
We describe a patient with ultra-rare disease, alkaptonuria, who developed tyrosine keratopathy following nitisinone therapy of 2 mg on alternate days. His vision became impaired approximately 7 weeks following the commencement of nitisinone and ophthalmological examination at week nine showed characteristic dendritic keratopathy associated with tyrosinaemia. The corneal lesion as well as his visual symptoms normalized completely following discontinuation of nitisinone. This is the first documented report of keratopathy due to acquired tyrosinaemia due to very low-dose nitisinone.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Alkaptonuria (AKU) is a rare inherited metabolic disorder with severe premature spondyloarthropathy as a major manifestation (Phornphutkul et al. 2002). Although joint disease is a major feature, virtually all connective tissues are affected leading to a variety of clinical features and complications (Helliwell et al. 2008). A promising new agent, called nitisinone, is available for the treatment of AKU (McKiernan 2006). Early nitisinone therapy is likely to prevent morbidity but may only slow or arrest disease progression if started later. Nitisinone is not yet licensed for AKU; therefore, safety is an important issue and potential adverse effects are of interest to those involved in the management of metabolic disorders.
Alkaptonuria is a rare inborn error of metabolism characterized by high circulating homogentisic acid (HGA) due to a genetic defect in the enzyme homogentisate dioxygenase (HGD) (Phornphutkul et al. 2002). The main pathophysiological event is conversion of HGA to a polymeric melanin-like pigment and binding of this pigment to connective tissue, especially cartilage. This process takes many years and is known as ochronosis (Zannoni et al. 1969). The damaging effects of ochronosis include arthritis (especially in the spine and large weight bearing joints), stones (renal, prostatic, gall bladder and salivary), cardiac valve disease especially aortic (Goodfellow et al. 2005), ruptures (muscle, tendons and ligaments), osteopenia and fractures (O’Brien et al. 1963). Virtually all connective tissue is affected.
Nitisinone inhibits p-hydroxyphenyl pyruvate dioxygenase, the enzyme leading to the formation of HGA (Lock et al. 1998). In keeping with the mode of action of nitisinone, circulating tyrosine increases. The tyrosinaemia that occurs during nitisinone treatment resembles hereditary tyrosinaemia type 3. Adverse effects known to be associated with tyrosinaemia include corneal and dermal toxicity (Meissner et al. 2008). Therefore, skin rash and dendritic keratopathy might be expected in some patients with a nitisinone-induced tyrosinaemia.
Nitisinone at a dose of 1–2 mg/kg/day has been widely used for more than 20 years in the treatment of a life-threatening condition called hereditary tyrosinaemia type 1 (HT-1) (Lindstedt et al. 1992). Corneal involvement is not part of the natural history in HT-1. Corneal lesions in medically managed HT-1 are reported as being rare (Masurel-Paulet et al. 2008; Gissen et al. 2003) with less than 7.4% frequency in one large series of 176 HT-1 patients treated with nitisinone (Holme and Linstedt 1998). Another report on 46 HT-1 patients noted 8.7% prevalence of keratitis with nitisinone treatment (Masurel-Paulet et al. 2008). A smaller series reported no eye symptoms or keratopathy in 11 HT-1 patients treated with nitisinone despite seeing high circulating tyrosine concentrations in several patients (Gissen et al. 2003).
In contrast to the quantities of nitisinone employed in HT-1 to prevent hepatic and renal pathology, the dose of nitisinone that decreases homogentisic acid in AKU by greater than 95% is only 2 mg daily, or approximately 5% of the dose used in HT-1. This is based on the experience of using nitisinone in the National Institutes of Health, USA (Phornphutkul et al. 2002; Suwannarat et al. 2005; Introne et al. 2011). However, one patient in the clinical trial of nitisinone in AKU developed corneal keratopathy that resolved fully with discontinuation of nitisinone (Introne et al. 2011).
Nitisinone is not licensed for the management of AKU at present. However, nitisinone continues to be used by physicians in AKU due to its plausible mode of action and efficacy in decreasing serum and urine HGA. Although the dose is much lower than that in HT-1, safety is clearly paramount when using an unlicensed product and we describe one patient with AKU on very low dose of nitisinone who developed symptomatic corneal keratopathy.
Case Report
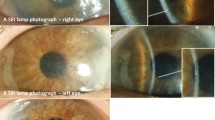
A 21-year-old man (Mr X) was referred to the Liverpool for the management of his AKU. Apart from dark urine he did not have many symptoms of AKU, although he did experience back pain intermittently. The Department of Health National Specialised Services (NSCT) designated the Royal Liverpool University Hospital to host the National Alkaptonuria Service (NAS) in April 2012. The following case report data was collected as part of providing that service. The data from the NAS is being published as a clinical practice article. The NAS has been allowed to administer nitisinone up to 2 mg daily to people with AKU with all appropriate assessments and monitoring in place. Mr X attended the NAS in August 2012 and after baseline assessments that included measurement of visual acuity (6/5 unaided either eye), slit lamp examination of his eyes plus slit lamp photography to demonstrate clarity of the cornea and also document any scleral pigmentation (Fig. 1a), he was commenced on nitisinone 2 mg alternate days and discharged with a dietary and a safety monitoring plan. Approximately 7 weeks after commencing nitisinone 2 mg, he noticed increasing epiphora on alternate evenings while watching television; by the following morning the ocular symptoms had resolved. He did not admit any pain, redness of eye or significant blurring of vision. The symptoms progressed sufficiently for him to contact the NAS at week 9, when his nitisinone was discontinued. At this stage his visual acuity was noticed to be reduced in the right eye (6/9 unaided in the right eye, 6/5 unaided in the left) and slit lamp examination demonstrated a dendritic corneal opacity typical of tyrosine keratopathy (Fig. 1b). A blood sample collected at this time showed that his serum tyrosine was 941 μmol/L (reference range 30–90 μmol/L) (Table 2). Conjunctival swab for Herpes Simplex virus PCR was negative. During his assessments at this time he admitted that he was finding it difficult to modify his diet following nitisinone therapy despite having received dietetic counselling to decrease his protein intake. He was 86.9 kg in weight and 1.74 M in height with a body mass index of 28.7 kg/M2. He also had an itchy urticarial skin rash at the same time his ocular symptoms and with the same diurnal variation (Fig. 2). The rest of the physical examination was within normal limits with a blood pressure of 116/70 mmHg and pulse rate of 70 bpm.
The patient was free-living and his diet was assessed by means of a 7-day food diary at baseline pre-nitisinone when he was consuming 85 g protein per day pre-nitisinone (1 g/kg body weight) and he was asked to reduce this to 0.8 g/kg body weight as he was commencing nitisinone. Once he developed keratopathy, following a washout of nitisinone over a month, he was asked to decrease his protein intake to 0.6 g/kg body weight before he could commence nitisinone. At year 1 review he was estimated to be consuming 0.8 g/kg body weight of protein based on a 7-day food diary analysis.
Following the discontinuation of nitisinone, his ocular symptoms, keratopathy and skin rash all resolved. Figure 1c shows a reduction of corneal opacity at 1 week following cessation of nitisinone and Fig. 1d shows no visible keratopathy at 1 year follow-up (visual acuity returned to 6/5 unaided). Figure 2b shows resolution of the skin rash.
He was trialled on nitisinone 2 mg once a week, which he was able to tolerate fully, with his diet and lifestyle. His liver and renal profiles were monitored and data is shown in Table 1. His transaminases increased to less than three times upper reference intervals and were simply monitored. The metabolic data is shown in Table 2.
Discussion
This is the second published report of tyrosine keratopathy in AKU. The present case is remarkable in that the dose of nitisinone leading to the keratopathy was effectively 1 mg daily, given the very long half-life of nitisinone of 54 h (SPC for Orfadin™ 2 mg capsules: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000555/WC500049195.pdf). This is the lowest dose of nitisinone reported to have resulted in tyrosine keratopathy.
The previous report on tyrosine keratopathy in AKU employed 2 mg daily in the NIH clinical trial (Introne et al. 2011). A 48-year-old male developed corneal irritation 6 weeks after commencing nitisinone 2 mg daily. Slit lamp examination showed branching, sub-epithelial opacities consistent with the classic pattern previously seen with tyrosine crystal deposition. The patient was tried on a protein restricted diet but the ocular findings and symptoms were unchanged. Nitisinone was discontinued and the patient made a complete recovery. Two attempts were made to restart nitisinone; on each subsequent attempt, symptoms recurred. Nitisinone was stopped permanently. In the NIH study dietary protein was not restricted in the adults enrolled. Plasma tyrosine levels averaged 800 μM, with individual levels reaching as high as 1,500 μM; these levels were remarkably well tolerated. The single individual who developed a keratopathy classical for tyrosine toxicity approximately 6 weeks following initiation of oral nitisinone showed plasma tyrosine levels, measured while symptomatic, approximately 200 μM below the average for the treated cohort. Thus, the conclusion of the investigators in the NIH study was that about 5% of the AKU population might experience corneal toxicity as a result of nitisinone-induced tyrosinaemia, regardless of the specific plasma tyrosine level, suggesting that some predisposition to toxicity exists independent of the peak plasma tyrosine concentration.
The data in the present case shows that circulating tyrosine increases rapidly following the commencement of nitisinone counterbalancing the decrease in serum and urine HGA. Despite counselling, this patient was unable to decrease his protein intake sufficiently to minimize the increase in circulating tyrosine. The disappearance of symptoms and the improvement of his keratopathy and skin rash within a week of discontinuing nitisinone suggest that tyrosinaemia was the causal factor. There are published reports of poor compliance with diet leading to an exaggerated tyrosinaemia post-nitisinone when used in the treatment of HT-1 (Ahamad et al. 2002).
An interesting feature of the ocular symptoms was that these occurred in the evenings and also every other day. This may be consistent with the diurnal variation in the circulating tyrosine, known to be higher late in the day and lowest first thing in the morning (Fernstrom et al. 1979). A similar diurnal variation in ocular pain in the evenings has been reported previously (Schauwvlieghe et al. 2012). The symptoms on alternate days could be explained by the nitisinone dosing on alternate days; he could clarify whether his symptoms were on the day of administering nitisinone or the following.
The lack of correlation between serum tyrosine and keratopathy may reflect differences in tyrosine concentrations in different body pools, namely that the ocular anterior chamber tyrosine may be much higher than circulating tyrosine as has been suggested previously (Lock et al. 1996), and local factors may be important in determining the size of each tyrosine pool.
Monitoring of circulating tyrosine is important and the aim is to try to maintain these below 700 μM wherever possible; low protein diet is reinforced to achieve levels of serum tyrosine below 500 μM wherever possible. It is noteworthy that other patients in the National AKU Centre with much higher circulating tyrosine levels do not show any ocular or skin symptoms. The one other report of keratopathy on 2 mg daily when used in AKU describes circulating tyrosine of 200 μM lower than the average values post-nitisinone reported as being 670–826 μM, i.e. 470–626 μM, although the exact value is not stated in the manuscript (Introne et al. 2011). Clearly factors other than just circulating tyrosine concentrations determine which patient on nitisinone develops ocular toxicity.
The important feature of keratopathy post-nitisinone is that it is fully reversible upon discontinuation, when identified and acted upon swiftly. With the potential for greater use of nitisinone in AKU, physicians need to be aware of this easily manageable complication, and further research may be required to identify an alternative treatment for ochronosis in these patients.
Our patient also had an itchy skin rash showing the same temporal relationship with the keratopathy. The close relationship would suggest that the causal factor is similar for both these complications, namely tyrosine. The published literature in this area is unclear on the relationship between the tyrosinaemia and skin lesions. Despite this cutaneous manifestations of tyrosinaemia have been described previously (Meissner et al. 2008).
We thus conclude that even very low doses of nitisinone, an inhibitor of a key enzyme in the tyrosine degradation pathway, can lead to tyrosine crystal keratopathy.
References
Phornphutkul C, Introne WJ, Perry MB, Bernardini I, Murphey MD, Fitzpatrick DL, Anderson PD, Huizing M, Anikster Y, Gerber LH, Gahl WA (2002) Natural history of Alkaptonuria. N Engl J Med 347:2111–2121
Helliwell TR, Gallagher JA, Ranganath L (2008) Alkaptonuria – a review of surgical and autopsy pathology. Histopathology 53:503–512
McKiernan PJ (2006) Nitisinone in the treatment of hereditary tyrosinaemia type I. Drugs 66(6):743–750
Zannoni VG, Lomtevas N, Goldfinger S (1969) Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim Biophys Acta 177:94–105
Goodfellow RJ, Schwartz J, Leya F (2005) Black Aorta: a rare finding at aortic valve replacement. J Invasive Cardiol 17:165–167
O’Brien WM, La Du BN, Bunim JJ (1963) Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: review of world literature (1584–1962). Am J Med 34:813–838
Lock EA, Ellis MK, Gaskin P et al (1998) From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 21:498–506
Meissner T, Betz RC, Pasternak SM, Eigelshoven S, Ruzicka T, Kruse R, Laitenberger G, Mayatepek E (2008) Richner-Hanhart syndrome detected by expanded newborn screening. Paediatr Dermatol 25:378–380
Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B (1992) Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340:813–817
Masurel-Paulet A, Poggi-Bach J, Rolland MO, Bernard O, Guffon N, Dobbelaere D, Sarles J, deBaulny HO, Touati G (2008) NTBC treatment in tyrosinaemia type 1: long-term outcome in French patients. J Inherit Metab Dis 31:81–87
Gissen P, Preece MA, Willshaw HA, McKiernan PJ (2003) Ophthalmic follow-up of patients with tyrosinaemia type 1 on NTBC. J Inherit Metab Dis 26:13–16
Holme E, Linstedt S (1998) Tyrosinaemia type 1 and NTBC 2-(2-nitro-4-trifluromethylbenzoyl)-1,3-cyclohexanedione. J Inherit Metab Dis 21:507–517
Suwannarat P, O'Brien K, Perry MB, Sebring N, Bernardini I, Kaiser-Kupfer MI, Rubin BI, Tsilou E, Gerber LH, Gahl WA (2005) Use of nitisinone in patients with alkaptonuria. Metabolism 54(6):719–728
Introne WJ, Perry MB, Troendle J, Tsilou E, Kayser MA, Suwannarat P, O'Brien KE, Bryant J, Sachdev V, Reynolds JC, Moylan E, Bernardini I (2011) Gahl WA A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 103(4):307–314
Ahamad S, Teckman JH, Lueder GT (2002) Corneal opacities associated with NTBC treatment. Am J Ophthalmol 134:266–268
Fernstrom JD, Wurtman RJ, Hammarstrom-Wiklund B, Rand WM, Munro HN, Davidson CS (1979) Diurnal variations in plasma concentrations of tryptophan, tyrosine and other neutral amino acids: effect of dietary protein intake. Am J Clin Nutr 32:1912–1922
Schauwvlieghe PP, Jacken J, Kestelyn P, Claerhout I (2012) Confocal microscopy of corneal crystals in a patient with hereditary tyrosinaemia type 1, treated with NTBC. Cornea 2:1–4
Lock EA, Gaskin P, Ellis MK et al (1996) Tissue distribution of 2-(2-nitro-4-trifluromethylbenzoyl)-1,3-cyclohexanedione (NTBC): effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol Appl Pharmacol 141:439–447
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Additional information
Communicated by: Pascale de Lonlay
Appendices
Compliance with Ethics Guidelines
This manuscript has not been published elsewhere.
All co-authors are aware and have agreed to this submission.
Stewart RMK, Briggs MC, Jarvis JC, Gallagher JA and Ranganath L declare that there are no conflicts of interest in submission of the paper.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. In addition, the institutional review body (Royal Liverpool University Hospital) explicitly approved the National Alkaptonuria Service from which this data was generated.
Informed consent was obtained from all patients for being included in the study. This is being published as a clinical practice article and standard research ethics process is not therefore appropriate. The data from this patient have been completely anonymised to ensure he is not recognized from the publication of this manuscript. The data obtained were following standard clinical assessments upon referral to the National Alkaptonuria Service in Liverpool. Patients are informed verbally and through being handwritten materials about the activities of the National AKU Service. They are explicitly informed in the Patient information booklet of the National AKU Service that:
We could publish results from the study but if we do, we will make sure you cannot be identified in any way. All data used for publicity or for other research purposes will ensure total anonymity. Please let us know when you are visiting Ward 9 B (where the National AKU Service is located) that you understand this and have no objection to it.
All the ocular photos were acquired during the standard assessments during the patient visit.
The skin rash photos were acquired following specific consent obtained by the medical photography department employing standard hospital guidance.
Contribution from Authors
LR Ranganath: Carried out patient assessments
MC Briggs and RMK Stewart: Performed all the ophthalmology examinations
JC Jarvis and JA Gallagher: Intellectual input and support, editing the manuscript
Rights and permissions
Copyright information
© 2014 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Stewart, R.M.K., Briggs, M.C., Jarvis, J.C., Gallagher, J.A., Ranganath, L. (2014). Reversible Keratopathy Due to Hypertyrosinaemia Following Intermittent Low-Dose Nitisinone in Alkaptonuria: A Case Report. In: Zschocke, J., Gibson, K., Brown, G., Morava, E., Peters, V. (eds) JIMD Reports, Volume 17. JIMD Reports, vol 17. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2014_307
Download citation
DOI: https://doi.org/10.1007/8904_2014_307
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44577-8
Online ISBN: 978-3-662-44578-5
eBook Packages: MedicineMedicine (R0)