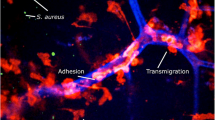
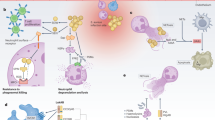
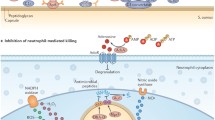
Abstract
A complex interplay between host and bacterial factors allows Staphylococcus aureus to occupy its niche as a human commensal and a major human pathogen. The role of neutrophils as a critical component of the innate immune response against S. aureus, particularly for control of systemic infection, has been established in both animal models and in humans with acquired and congenital neutrophil dysfunction. The role of the adaptive immune system is less clear. Although deficiencies in adaptive immunity do not result in the marked susceptibility to S. aureus infection that neutrophil dysfunction imparts, emerging evidence suggests both T cell- and B cell-mediated adaptive immunity can influence host susceptibility and control of S. aureus. The contribution of adaptive immunity depends on the context and site of infection and can be either beneficial or detrimental to the host. Furthermore, S. aureus has evolved mechanisms to manipulate adaptive immune responses to its advantage. In this chapter, we will review the evidence for the role of adaptive immunity during S. aureus infections. Further elucidation of this role will be important to understand how it influences susceptibility to infection and to appropriately design vaccines that elicit adaptive immune responses to protect against subsequent infections.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Staphylococcus aureus is a major human pathogen. Data from the USA and Europe indicate it is the predominant cause of both cutaneous and invasive infections and is the leading cause of infectious morbidity and mortality in the industrialized world (Tong et al. 2015). Strain-specific virulence strategies and acquisition of resistance against a variety of antibiotics reflect the adaptive capabilities that have shaped its ability to cause continually shifting patterns of disease (Chambers and Deleo 2009; Tong et al. 2015). Despite its clear pathogenic potential, S. aureus has the ability to coexist with its human host as a commensal, with 20–30 % of the population colonized at mucocutaneous surfaces and significantly higher proportions exposed at least intermittently (Verhoeven et al. 2014). The success of S. aureus as a human commensal and pathogen suggests the evolution of a complex and intricate interplay between host and bacterial factors.
S. aureus has a plethora of virulence factors that evade and modulate components of the human innate and adaptive immune system (Nizet 2007; Lowy 1998; Rooijakkers et al. 2005). Much attention has been rightly focused on interactions with the innate immune system, in particular neutrophils, which play a central role in host defense against S. aureus. However, the readily detectable antibody and T cell responses in humans and the extensive mechanisms for staphylococcal evasion of antibody and T cell-mediated host defense suggest an important contribution of adaptive immunity that may influence host susceptibility and will need to be invoked by a successful vaccine. In this chapter, we will highlight the major findings related to adaptive immune responses induced by S. aureus and the evasion mechanisms it uses to escape this aspect of host defense.
2 Immunological Overview
The immune response against S. aureus involves activation of both the innate and the adaptive immune systems. As the first line of defense against infections, the innate immune response is rapidly activated by pathways that detect pathogen-associated molecular patterns. A key result of this is activation of phagocytic cells such as macrophages and neutrophils. Neutrophils are recognized as a key component of the acute response and centrally important against S. aureus, as declared by the susceptibility of humans and mice with inherited and acquired neutrophil defects to deep-seated infections. The adaptive immune response kicks in later during the course of infection, dependent on the presentation of bacterial antigens by antigen-presenting cells (APCs) and influenced by the cytokine milieu generated by the innate response. Through T cell activation and B cell production of antibodies, the adaptive immune response targets specific bacterial antigens and can be recalled during subsequent infections to provide ‘memory’ against that particular pathogen. Antibodies and T cells can have direct activity against bacteria, but also amplify the activity of innate immune cells, e.g., by increasing phagocyte killing and recruitment. The prevalence of recurrent infections with S. aureus suggests the adaptive memory response is not completely effective, although it could be argued that the relative paucity of systemic infections despite the high rate of colonization may be evidence for its protective role. Understanding the contribution of the adaptive immune response in determining S. aureus susceptibility may help identify risk factors and therapeutic strategies, and will be essential to harness for successful vaccine development.
3 Role of B Cells and Antibodies
The major function of B cells is to secrete immunoglobulins (antibodies) that neutralize the function of target proteins (e.g., toxins and other virulence factors) or opsonize pathogens to optimize phagocytosis and clearance. The importance of antibody-mediated protection against infectious agents is clearly demonstrated by patients with X-linked agammaglobulinemia (XLA), in whom lack of appropriate B cell maturation leads to susceptibility to infections with a variety of viruses and encapsulated bacteria that is largely reversed with the periodic administration of pooled donor immunoglobulins (Bruton 1952; Conley and Howard 1993). The apparent lack of increased susceptibility in this patient population to invasive S. aureus infection argues that antibodies are unimportant in protection against S. aureus infection. Although these patients have a recognized susceptibility to cellulitis, this has also not been clearly attributed to S. aureus. The lack of increased susceptibility to S. aureus infection in B cell- or antibody-deficient mice (Gjertsson et al. 2000; Schmaler et al. 2011; Gaidamakova et al. 2012) parallels the observations in patients with XLA. However, recent work has revealed that primary S. aureus cutaneous infection can induce antibody-mediated protection against a subsequent infection in certain mouse strains (Montgomery et al. 2014), and numerous preclinical studies have shown at least partial protection from subsequent infection after induction of antibodies by vaccination (see below). Furthermore, the ubiquitous presence of antibodies after S. aureus exposure in humans and animal models, and the virulence strategies of S. aureus that have evolved to evade antibodies, suggests antibodies may have a role in modulating susceptibility to infection. Evidence for this potential role will be examined in further detail here.
3.1 Preexisting Antibodies as Immunologic Correlates for Protection
The immune correlates of protection from and susceptibility to staphylococcal infections are still not well understood. A few reports have suggested that preexisting antibodies toward certain staphylococcal virulence factors can correlate with clinical outcome in humans. Adhikari et al. (2012a) measured serum antibodies to an array of staphylococcal exotoxins and observed that low antibody titers correlated with a higher risk for the development of sepsis. Another study found that elevated serum titers against S. aureus α-hemolysin (Hla) correlated with the protection from subsequent infection, and invasive infections elicited a more durable antibody response when compared to cutaneous infections (Fritz et al. 2013). This study also reported high titer anti-staphylococcal antibodies in colonized individuals without a history of overt infection (carriers), which may explain the enhanced recovery from infection observed in carriers despite their increased risk of developing infection compared to non-carriers (Wertheim et al. 2005; von Eiff et al. 2001a).
3.2 Role of Antibodies in Vaccine-Mediated Protection
S. aureus has been generally regarded as an extracellular pathogen. Consequently, complement and antibodies with neutralizing and opsonizing qualities were considered major players not only in mediating neutralization of secreted virulence factors, but also in facilitating uptake and clearance of the pathogen by innate immune cells (van Kessel et al. 2014; Verbrugh et al. 1982; Leijh et al. 1981). Because most vaccines in use today are thought to work through the elicitation of protective antibody responses, it is also not surprising that most of the vaccine candidates against S. aureus to date were chosen and evaluated based heavily on their ability to generate opsonizing and neutralizing antibodies (Pozzi et al. 2012; Fattom et al. 1990; Ohlsen and Lorenz 2010).
The conjugate vaccine StaphVax (Nabi Biopharmaceuticals) was the first S. aureus vaccine candidate to enter a phase III clinical trial. It targeted clinically prevalent capsular polysaccharide (CP) serotypes 5 (CP5) and 8 (CP8), emulating the successful strategy of targeting CPs to prevent infections with Streptococcus pneumonia and Haemophilus influenzae. Preclinical studies demonstrated that CP-specific antibodies protected mice from lethal S. aureus challenge and bacterial dissemination (Fattom et al. 1990, 1996), and an initial phase III clinical trial in hemodialysis patients suggested modest reductions in bacteremia early after vaccination (Shinefield et al. 2002). However, a booster dose in a subsequent phase III study failed to prevent bacteremia despite augmenting antibody titers (Fattom et al. 2004; Schaffer and Lee 2008). The reasons for this failure remain incompletely understood, but the outcome highlighted that S. aureus virulence is not solely dependent on CP production, a fact exemplified by the lack of capsule production in some highly virulent strains such as USA300.
A more recent vaccine candidate for which preclinical promise failed to translate into clinical trial success targeted the S. aureus iron-binding surface determinant B (IsdB). This protein was identified as a vaccine candidate by screening patients with high antibody titers against S. aureus surface antigens displayed by an E. coli expression library (Etz et al. 2002). Immunization with this protein in preclinical and phase I clinical studies showed protection in mouse models of sepsis and antibody induction in mice, macaques, and humans (Kuklin et al. 2006; Stranger-Jones et al. 2006; Kim et al. 2010; Harro et al. 2010, 2012). However, a phase IIB/III clinical trial with the IsdB vaccine (Merck V710) in cardiothoracic surgery patients was stopped prematurely when, despite induction of IsdB antibodies, excessive deaths were noted in the vaccine group among subjects who developed postoperative S. aureus infections (Fowler et al. 2013). Subsequent serum cytokine analysis showed that low IL-2 and IL-17 levels post-vaccination correlated with mortality in subjects who later developed S. aureus infections, consistent with a potential T cell-based mechanism although immune analysis after infection to further characterize the associated immune response has not been reported (McNeely et al. 2014).
Instead of targeting cell surface antigens to promote opsonophagocytic clearance of organisms, other vaccine approaches have attempted to generate neutralizing antibodies against secreted S. aureus virulence factors. Active and passive immunization studies have validated this strategy in experimental models. The clearest rationale for this approach has been against toxic shock syndrome, which is driven by superantigen toxins such as staphylococcal enterotoxin A (SEA), SEB, or toxic shock syndrome toxin 1 (TSST-1). Several studies have reported that immunization of mice and rhesus macaques with recombinant superantigen toxoids devoid of their superantigenic activity induces toxin-specific antibodies and protects from lethal shock induced by the targeted wild-type toxins (Bavari et al. 1996; Lowell et al. 1996; Stiles et al. 1995; Boles et al. 2003). Furthermore, active immunization with recombinant SEA and TSST-1 toxoid vaccines, as well as adoptive transfer of immune sera, protected mice from systemic S. aureus infection (Hu et al. 2003; Nilsson et al. 1999).
The option of targeting other secreted toxins that contribute to S. aureus infection became apparent when antibodies generated against HlaH35L, a non-pore-forming mutant of Hla, were shown to protect mice from lethal pneumonia (Bubeck Wardenburg and Schneewind 2008) and from skin and soft tissue infections (Kennedy et al. 2010; Mocca et al. 2014). Similarly, antibodies raised against a recombinant fusion protein (AT-62) designed to mimic key topographic features of the Hla heptamer protected mice from bacteremia and lethal pneumonia (Adhikari et al. 2012b). Antibodies raised against attenuated recombinant LukF-PV and LukS-PV, subunits of the bicomponent Panton–Valentine leukocidin (PVL), showed protective efficacy in a mouse bacteremia model and appeared to have cross-neutralizing activity toward other leukocidins in PVL-deficient strains (Karauzum et al. 2013), a potentially important characteristic given the complex redundant and antagonistic interactions between these bicomponent toxins (Yoong and Torres 2015). Considering the multiple virulence strategies employed by S. aureus, Spaulding et al. (2014) demonstrated that vaccination with a cocktail of 7 secreted virulence factors, consisting of superantigens and cytolysins, induced antibody-mediated protection against lethal pneumonia in a rabbit model. Interestingly, in the same report, vaccination with a cocktail of surface antigens enhanced lethality in a rabbit model of infective endocarditis, an outcome suggested to be due to antibody-mediated bacterial aggregation (Spaulding et al. 2014). This highlighted the potential for deleterious antibody responses that may be elicited depending on the antigenic targets and the model of infection. Based on the role of antibody shown in such active immunization studies, the therapeutic potential of passive immunization has also been demonstrated in mouse models using mouse, human, and/or chimeric monoclonal antibodies targeting secreted or surface-bound virulence factors such as clumping factor A (ClfA) (Domanski et al. 2005), lipoteichoic acid (LTA) (Weisman et al. 2009, 2011), Hla (Ragle and Bubeck Wardenburg 2009; Tkaczyk et al. 2012), and SEB (Larkin et al. 2010; Karauzum et al. 2012; Varshney et al. 2014).
3.3 Evasion Mechanisms from the Humoral Immune Response
S. aureus has developed evasion mechanisms that combat the B cell antibody response. In particular, staphylococcal protein A (SpA) and the second immunoglobulin binding protein (Sbi) (Zhang et al. 1998; Smith et al. 2011) are virulence factors that bind immunoglobulins. SpA is a highly expressed, cell wall-anchored surface protein that binds to the complement-binding Fcγ portion of mammalian IgG. Decoration of the staphylococcal surface with IgG molecules bound in this reverse manner interferes with the complement activation and opsonophagocytosis. In addition, SpA in its secreted form acts as a B cell superantigen, binding the F(ab)2 portion of the B cell receptor to induce B cell proliferation and death (Kobayashi and DeLeo 2013). Beyond its direct effects on opsonophagocytosis and B cell survival, SpA activity has been shown to inhibit the development of antibody responses against other staphylococcal antigens in mouse models and in humans (Kim et al. 2011; Falugi et al. 2013; Pauli et al. 2014). In contrast to SpA, baseline expression of Sbi on the cell surface is low but increases in the presence of IgG, suggesting a highly specific mechanism of immune evasion (Zhang et al. 2000). Like SpA, Sbi can act as both a cell wall-anchored or secreted virulence factor, binding the Fcγ portion of IgG on the cell surface and the soluble complement factor C3, respectively (Smith et al. 2011).
In addition to these specific mechanisms, S. aureus can also abandon its usual niche as an extracellular pathogen and evade the humoral immune response as a facultative intracellular organism. For example, it can resist killing and grow within neutrophils (Voyich et al. 2005), or persist in epithelial cells in the form of small colony variants (SCVs). Persistence as SCVs enables the bacterium to avoid antimicrobial treatment, promote disease pathogenesis, and facilitate recurrent infections (Tuchscherr et al. 2011; Proctor et al. 1995; von Eiff et al. 2001b; Gresham et al. 2000). Furthermore, certain antibody responses generated against S. aureus can promote its virulence. For example, treatment with anti-PVL antibodies increased bacterial loads in mouse skin abscesses and inhibited in vitro killing of S. aureus by human neutrophils (Yoong and Pier 2010). Further highlighting the unpredictable potential for negative effects of antibody responses, the combination of two antibodies against surface polysaccharides (CP and poly-N-acetyl glucosamine) interfered with the beneficial effects of each individually on opsonophagocytic activity and protection in mouse models of bacteremia and skin infection (Skurnik et al. 2010).
In sum, antibody deficiency in mice and humans shows us that antibodies are not necessary for protection against S. aureus infections. However, they may very well contribute to the protective response as suggested by the modulation of antibody responses by S. aureus virulence factors, the ubiquitous presence of anti-staphylococcal serum antibodies, antibody-mediated protection after active and passive immunization in preclinical models, and human data correlating antibody titers with protection. Published data also support the possibility of ineffective or deleterious antibody responses, emphasizing the need to better understand the characteristics of a protective antibody response in order to elucidate contributions to natural immunity and implications for vaccine design.
4 Role of T Cells
T cells are thymic-derived cells that express unique T cell receptors (TCRs) that recognize antigen-derived peptides in the context of major histocompatibility complex (MHC) molecules on APCs. Similar to B cells and antibodies, a case can be made for a role for T cells during S. aureus infection based on the presence of detectable T cell responses in humans (Zielinski et al. 2012; Kolata et al. 2015) and the ability of the bug to modulate T cells as exemplified by its expression of a multitude of T cell superantigens (Spaulding et al. 2013). However, it has been reported that T cells are not essential for protection against S. aureus in mice (Schmaler et al. 2011). Furthermore, S. aureus shows up only occasionally as a cause of infection in evaluations of humans with T cell deficiencies (Stephan et al. 1993), although the severe susceptibility of these patients to other organisms confounds our ability to fully assess the contribution of T cells to staphylococcal immunity in this context. Various subsets of T cells have differing functions, and a more nuanced role for these subsets has become evident in mouse studies and with the recognized susceptibility to staphylococcal infections of patients with HIV and other partial T cell disorders (Hidron et al. 2010; Cook and Tangye 2009). These will be discussed in further detail below.
The majority of T cells are comprised of CD4+ and CD8+ T cells that have long been recognized to be the major cellular arm of adaptive immunity. The major function of CD8+ T cells is to target intracellular pathogens by cytolytic killing of the infected host cell. Consistent with S. aureus being a primarily extracellular pathogen, a clear role for CD8+ T cells has not been reported, although CD8+ T cell activation can be detected during S. aureus infection and staphylococcal superantigen exposure. Naïve CD4+ T cells are polarized toward different effector functions depending on the cytokine milieu in which activation of their TCR occurs. These helper T cell (Th) subsets are functionally characterized by their cytokine expression profiles, which will be detailed below. A percentage of these polarized cells will persist in the host as memory cells awaiting re-activation by subsequent antigen exposure. The role of these different subsets of effector CD4+ T cells in the context of S. aureus infection will be reviewed below. In addition to CD4+ and CD8+ T cells, more recently described subsets of T cells, such as γδ T cells, innate lymphoid cells (ILC), and NK T cells, contribute mainly to the innate immune response at mucosal sites rather than antigen-specific memory, although recent reports have suggested the potential for γδ T cells to contribute to a memory response under certain circumstances (Murphy et al. 2014).
4.1 Th1 Cells
TCR-mediated activation of naïve CD4+ T cells in the presence of IL-12 signaling via STAT4 leads to the generation of Th1 effector cells. Although capable of producing multiple inflammatory cytokines, including IL-2, TNFα, and GM-CSF, Th1 cells are defined by the secretion of their signature cytokine interferon (IFN)-γ and expression of the transcriptional regulator T-bet (O’Shea and Paul 2010; Schmitt and Ueno 2015; Raphael et al. 2014). Th1 cells are not the only source of IFNγ, with various innate immune cells, including NK cells and ILC being notable producers. Among its functions, IFNγ activates phagocytic cells such as macrophages and neutrophils to promote killing of intracellular pathogens. Its role in protection against these organisms is highlighted by the susceptibility of patients with hereditary defects in IFNγ signaling to infections with Mycobacteria, Salmonella, and certain viruses (Rosenzweig and Holland 2005). Unregulated IFNγ production can contribute to immunopathology and autoimmunity (Feldmann et al. 1998).
In the context of S. aureus infections, it appears Th1 cells and IFNγ can have both beneficial and detrimental roles. Guillen et al. reported a protective role of an enhanced Th1 response in a mouse model of septicemia and septic arthritis in mice transgenic for lactoferrin. The enhanced production of IFNγ and TNFα in these mice during infection resulted in higher bacterial clearance and lower mortality compared to their wild-type littermates (Guillen et al. 2002). An overproduction of this cytokine, however, can be associated with immunopathology. An early study evaluating the role of T cells in S. aureus-induced arthritis indicated that Th1 cells, stained positive for the IL2R and intracellular IFNγ, infiltrated the synovium of joints of infected mice, and depletion of CD4+ but not CD8+ T cells in the infected animals ameliorated disease (Abdelnour et al. 1994). However, intravenous inoculation of mice deficient in T-bet, which may have deficiencies beyond a defect in Th1 cell IFNγ production (Lazarevic et al. 2013), had increased severity of septic arthritis that was associated with increased weight loss, mortality, and kidney bacterial burden (Hultgren et al. 2004). Consistent with the potential duality of roles for Th1 cells during S. aureus infection, Th1 cells and IFNγ production were reported to promote chemokine-mediated neutrophil recruitment in a wound infection model, but this resulted in a paradoxical increase of bacterial burden, potentially due to the ability of S. aureus to persist in neutrophils (McLoughlin et al. 2006, 2008).
Th1 cells appear to be able to contribute to vaccination-induced protection against subsequent S. aureus infection. CD4+ T cell IFNγ production was required for the protection against subsequent systemic infection after vaccination with a recombinant protein derived from Als3p, a Candida protein that cross-protected against S. aureus (Lin et al. 2009). Similarly, vaccination with extracellular vesicles released from S. aureus induced a Th1 response, and protection in a pneumonia model was dependent on CD4+ T cells and IFNγ (Choi et al. 2015). However, protection after vaccination against cutaneous infection with a lethally irradiated whole-cell vaccine was not associated with an IFNγ response (Gaidamakova et al. 2012), and increased mortality in similarly vaccinated mice after intravenous challenge was dependent on CD4+ T cell IFNγ production (Karauzum and Datta, unpublished data). Another study also hinted at potential detrimental effects of vaccine-induced Th1 responses by showing that mice vaccinated with heat-killed S. aureus had significant disease burden after intravenous infection despite detectable CD4+ T cell IFNγ production (Schmaler et al. 2011); however, lack of direct comparison to an unvaccinated control group prevents conclusive interpretation of these results.
In sum, it appears Th1 cells can have protective, detrimental, or non-contributory roles against S. aureus infection, likely dependent on factors such as route of infection, organism burden, antigenic targets, level of induction, and balance with other immune mechanisms. Clarification of the conditions under which Th1 cells exert these apparently contradictory effects will better guide approaches to interventions aimed at therapy and prevention.
4.2 Th2 Cells
Activation of naïve CD4 T cells in the presence of IL-4 via STAT6 signaling leads to the priming of Th2 cells. This subset of CD4 T cells is characterized by its signature transcription factor GATA-3, which promotes induction of Th2 cytokines that include IL-4, IL-5, and IL-13. Th2 cells play an important role in host defense against extracellular parasites, driving various aspects of cellular and humoral immunity to promote parasite clearance and tissue repair (Allen and Sutherland 2014). Their dysregulation contributes to allergic and atopic diseases (Raphael et al. 2014; Geginat et al. 2013). Of particular relevance to staphylococcal disease is atopic dermatitis (AD), a prevalent inflammatory skin disorder that is characterized by the overexpression of Th2 cytokines (Hamid et al. 1994), which contribute to barrier permeability issues and other features of AD. Skin colonization and infection with S. aureus is almost a universal feature of AD (Boguniewicz and Leung 2011). The propensity of Th2 cytokines to inhibit antimicrobial gene programs, including induction and mobilization of antimicrobial peptides such as human beta-defensin (HBD)-3, are thought to contribute to this susceptibility (Kisich et al. 2008; Nomura et al. 2003; Howell et al. 2006). Th2 cytokines may not only drive aspects of AD and staphylococcal susceptibility, but S. aureus colonization may further promote this Th2-driven milieu. Staphylococcal cell wall components, such as peptidoglycan (Matsui and Nishikawa 2012) and lipoteichoic acid (Matsui and Nishikawa 2002), were shown to induce Th2 cells and may contribute along with other secreted toxins toward the inflammatory environment in the skin of AD patients (Schlievert et al. 2010; Nakamura et al. 2013; Brauweiler et al. 2014).
The role of Th2 responses during S. aureus infection outside the setting of AD is less clear. S. aureus footpad infection was less severe in the Th2-biased DBA and BALB/c mouse strains than in Th1-biased C57BL/6 mice (Nippe et al. 2011). A protective role for Th2 cells was also suggested in an ocular keratitis model where unexpectedly high Th2 responses in C57BL/6 mice correlated with the protection compared to less robust responses seen in more susceptible BALB/c mice (Hume et al. 2005). However, these correlative observations do not definitively address whether the Th2 response is driving resistance to infection or whether other immunological parameters are responsible. A recent study in a model of persistent biofilm infection did show STAT6-dependent clearance in BALB/c mice that suggested a contribution of Th2 responses to protection (Prabhakara et al. 2011). In the same study, neutrophilic inflammatory responses worsened infection and this effect could be reversed by neutralization of IL-12p40 or IL-6, treatments that would be predicted to dampen Th1 and Th17 responses, respectively, and skew toward Th2 responses (Prabhakara et al. 2011).
The complexity of Th2 responses and their downstream effects may potentially trigger both beneficial and detrimental responses. It seems clear that Th2 responses contribute to a vicious cycle of inflammation and S. aureus susceptibility at the skin in the context of AD. However, Th2 effects may play a role in achieving the appropriate balance between inflammatory and anti-inflammatory responses in other situations, particularly during chronic infection.
4.3 Th17 Cells
Th17 cells are a relatively recently recognized subset of effector CD4+ T cells. They are defined by their expression of Rorγt and secretion of inflammatory cytokines, including IL-17A, IL-17F, and IL-22 (Liang et al. 2006; Chung et al. 2006; Ivanov et al. 2006). These cytokines predominantly act on epithelial cells to enhance barrier function, antimicrobial properties, and neutrophil recruitment (Ouyang et al. 2008). Initially discovered in the context of autoimmunity, they have been shown to play a protective role in mouse models of extracellular bacterial and fungal infections, especially at mucosal sites (Ouyang et al. 2008).
A protective role for Th17 cells against S. aureus was first suggested by a report that mice deficient in both IL-17A and IL-17F spontaneously developed mucocutaneous S. aureus infections (Ishigame et al. 2009). Subsequent work clarified that induction of IL-17A from γδ T cells in the skin played a critical role in controlling S. aureus burden and abscess size after subcutaneous inoculation (Cho et al. 2010). In this context, γδ T cells functioned in their traditional role as a cytokine-activated component of innate immunity, implicating an important role for innate immune cell-derived IL-17A. Hla-dependent Th17 induction (Frank et al. 2012) and influenza-mediated antagonism of Th17-dependent protection (Kudva et al. 2011) indicated a role for Th17 cells in a model of S. aureus pneumonia. Interestingly, CD4+ T cell depletion in the context of this Th17-inducing pneumonia model improved outcomes in another study, hinting at a delicate balance within the CD4+ T cell compartment between protective immunity and immunopathology (Parker et al. 2015). Deficiency in IL-17A also enhanced susceptibility to S. aureus joint infection, although the relevant cellular source was not identified (Henningsson et al. 2010). Deficiency in IL-17A did not increase susceptibility to systemic challenge with S. aureus in multiple studies (Ishigame et al. 2009; Lin et al. 2009; Narita et al. 2010; Henningsson et al. 2010), consistent with a primary role for this cytokine at skin and mucosal sites. However, a protective function for IL-17A from vaccination-induced Th17 cells has been shown against skin (Gaidamakova et al. 2012) and systemic S. aureus infections (Lin et al. 2009; Narita et al. 2010; Joshi et al. 2012). Consistent with this, antibody-dependent protection against multiple models of infection by a four-component vaccine was further enhanced by Th1 and Th17 cell induction with inclusion of a TLR7 agonist adjuvant (Bagnoli et al. 2015). The Merck IsdB vaccine also showed contribution of IL-17A, but not IL-22 or IFNγ, to protection in a mouse model of sepsis (Joshi et al. 2012), and, as mentioned previously, low IL-2 and IL-17 levels post-vaccination correlated with mortality in S. aureus-infected human subjects (McNeely et al. 2014). Of note, the Th17-associated cytokine IL-22 seems to have either no or minimal effects on the course of acute cutaneous infection in mice (Myles et al. 2013; Chan et al. 2015), but can independently contribute to protection against pneumonia (Kudva et al. 2011) and vaccine-induced protection against skin and systemic infection (Yeaman et al. 2014).
Autoantibody- and genetically mediated dysfunction of the IL-17 pathway predisposes to mucocutaneous Candida infections (Burbelo et al. 2010; Kisand et al. 2010; Puel et al. 2010, 2011). Only a striking minority of these patients reported S. aureus infections, making it unclear whether IL-17 is a critical element for human anti-staphylococcal responses. However, patients with HyperIgE (or Job’s) Syndrome, who are susceptible to staphylococcal skin and lung infections, lack normal Th17 generation due to STAT3 dysfunction (Minegishi et al. 2007; Holland et al. 2007; Milner et al. 2008). The role for Th17 cytokines in promoting keratinocyte and epithelial antimicrobial function (Minegishi et al. 2009) is also consistent with an IL-17-dependent basis for their susceptibility specifically to skin and lung infections, although other functions of STAT3, including its direct role in antimicrobial peptide production (Choi et al. 2013), very likely contribute. Consistent with a potential contribution of Th17 cells to human immunity is the Th17 depletion seen in HIV-infected patients early in the course of disease that correlates with their increased likelihood of S. aureus skin and soft tissue infections (Hidron et al. 2010; Prendergast et al. 2010). Patients with AD also have decreased IL-17 pathway cytokines and decreased antimicrobial peptides in lesional skin, potentially contributing to their staphylococcal susceptibility (Guttman-Yassky et al. 2008). The relative induction of these pathways in psoriasis has been postulated to contribute to the relative resistance of these patients to S. aureus (Guttman-Yassky et al. 2008).
In sum, IL-17 from either innate or adaptive sources plays an important role against S. aureus in mouse models of infection at skin and mucosal sites. Induction of Th17 cells by vaccination can enhance protection at these sites and also against bacteremia. Th17 cells appear to be potential key players in immunity against S. aureus; however, their exact contribution to the control of human staphylococcal infection remains to be fully elucidated and their potential for autoimmune inflammation will need to be kept in check if they are to be targeted by clinical vaccines.
4.4 Regulatory T Cells
Regulatory T cells (Treg) display contact-dependent and cytokine-mediated immunosuppressive functions that counteract inflammatory responses and maintain immune homeostasis. S. aureus may exploit these immunosuppressive functions by inducing Treg responses that contribute, along with other immunosuppressive mechanisms, to diminished effector T cell responses during models of persistent infection (Ziegler et al. 2011; Tebartz et al. 2015). Increased Treg numbers may also contribute to the immune dysregulation and S. aureus susceptibility seen in the skin of patients with AD (Ou et al. 2004). However, depletion of Treg exacerbated a model of chronic biofilm infection, suggesting that an appropriate balance between inflammatory and anti-inflammatory responses is needed for optimal bacterial control (Prabhakara et al. 2011). Further studies will be needed to increase our nascent understanding of the role of Treg in modulating the response to S. aureus infection and how this may influence susceptibility.
5 Conclusion
Immune control of acute S. aureus infection is critically dependent on the innate immune system. However, adaptive immunity in the form of B cell and T cell responses may influence this control and is potentially of particular importance in determining the outcomes of chronic persistent infections. The search for a protective vaccine will depend on our ability to induce an effective adaptive immune response. Recent studies suggest that induction of an antibody response alone may not be sufficient, and an appropriate vaccine-induced T cell response will be needed to confer protective immunity. The potential for eliciting deleterious adaptive immune responses has become apparent in both animal models and clinical vaccination trials. This highlights the need for further elucidation of the components of an effective immune response, a task complicated by the multiple virulence strategies and sites of infection employed by this bug that will each likely require targeting by unique strategies for effective prevention and therapy.
References
Abdelnour A, Bremell T, Holmdahl R, Tarkowski A (1994) Role of T lymphocytes in experimental Staphylococcus aureus arthritis. Scand J Immunol 39(4):403–408
Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC (2012a) Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 206(6):915–923. doi:10.1093/infdis/jis462
Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, Warfield KL, Aman MJ (2012b) Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One 7(6):e38567. doi:10.1371/journal.pone.0038567
Allen JE, Sutherland TE (2014) Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol 26(4):329–340. doi:10.1016/j.smim.2014.06.003
Bagnoli F, Fontana MR, Soldaini E, Mishra RP, Fiaschi L, Cartocci E, Nardi-Dei V, Ruggiero P, Nosari S, De Falco MG, Lofano G, Marchi S, Galletti B, Mariotti P, Bacconi M, Torre A, Maccari S, Scarselli M, Rinaudo CD, Inoshima N, Savino S, Mori E, Rossi-Paccani S, Baudner B, Pallaoro M, Swennen E, Petracca R, Brettoni C, Liberatori S, Norais N, Monaci E, Bubeck Wardenburg J, Schneewind O, O’Hagan DT, Valiante NM, Bensi G, Bertholet S, De Gregorio E, Rappuoli R, Grandi G (2015) Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci USA 112(12):3680–3685. doi:10.1073/pnas.1424924112
Bavari S, Dyas B, Ulrich RG (1996) Superantigen vaccines: a comparative study of genetically attenuated receptor-binding mutants of staphylococcal enterotoxin A. J Infect Dis 174(2):338–345
Boguniewicz M, Leung DY (2011) Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 242(1):233–246. doi:10.1111/j.1600-065X.2011.01027.x
Boles JW, Pitt ML, LeClaire RD, Gibbs PH, Torres E, Dyas B, Ulrich RG, Bavari S (2003) Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin Immunol 108(1):51–59
Brauweiler AM, Goleva E, Leung DY (2014) Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol 134(8):2114–2121. doi:10.1038/jid.2014.43
Bruton OC (1952) Agammaglobulinemia. Pediatrics 9(6):722–728
Bubeck Wardenburg J, Schneewind O (2008) Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205(2):287–294. doi:10.1084/jem.20072208
Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, Kristosturyan E, Rajan A, Ding L, Ching KH, Berman A, Oliveira JB, Hsu AP, Klimavicz CM, Iadarola MJ, Holland SM (2010) Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood 116(23):4848–4858. doi:10.1182/blood-2010-05-286161
Chambers HF, Deleo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7(9):629–641. doi:10.1038/nrmicro2200
Chan LC, Chaili S, Filler SG, Barr K, Wang H, Kupferwasser D, Edwards JE Jr, Xiong YQ, Ibrahim AS, Miller LS, Schmidt CS, Hennessey JP Jr, Yeaman MR (2015) Non-redundant roles of IL-17A and IL-22 in murine host defense against cutaneous and hematogenous infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. doi:10.1128/IAI.01061-15
Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120(5):1762–1773. doi:10.1172/JCI40891
Choi SM, McAleer JP, Zheng M, Pociask DA, Kaplan MH, Qin S, Reinhart TA, Kolls JK (2013) Innate Stat3-mediated induction of the antimicrobial protein Reg3gamma is required for host defense against MRSA pneumonia. J Exp Med 210(3):551–561. doi:10.1084/jem.20120260
Choi SJ, Kim MH, Jeon J, Kim OY, Choi Y, Seo J, Hong SW, Lee WH, Jeon SG, Gho YS, Jee YK, Kim YK (2015) Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS ONE 10(9):e0136021. doi:10.1371/journal.pone.0136021
Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C (2006) Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res 16(11):902–907. doi:10.1038/sj.cr.7310106
Conley ME, Howard VC (1993) X-linked agammaglobulinemia. In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews. University of Washington, Seattle
Cook MC, Tangye SG (2009) Primary immune deficiencies affecting lymphocyte differentiation: lessons from the spectrum of resulting infections. Int Immunol 21(9):1003–1011. doi:10.1093/intimm/dxp076
Domanski PJ, Patel PR, Bayer AS, Zhang L, Hall AE, Syribeys PJ, Gorovits EL, Bryant D, Vernachio JH, Hutchins JT, Patti JM (2005) Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infect Immun 73(8):5229–5232. doi:10.1128/IAI.73.8.5229-5232.2005
Etz H, Minh DB, Henics T, Dryla A, Winkler B, Triska C, Boyd AP, Sollner J, Schmidt W, von Ahsen U, Buschle M, Gill SR, Kolonay J, Khalak H, Fraser CM, von Gabain A, Nagy E, Meinke A (2002) Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc Natl Acad Sci USA 99(10):6573–6578. doi:10.1073/pnas.092569199
Falugi F, Kim HK, Missiakas DM, Schneewind O (2013) Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. MBio 4(5):e00575–e00513. doi:10.1128/mBio.00575-13
Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, Robbins JB (1990) Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun 58(7):2367–2374
Fattom AI, Sarwar J, Ortiz A, Naso R (1996) A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun 64(5):1659–1665
Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, Naso R, Horwith G (2004) Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23(5):656–663. doi:10.1016/j.vaccine.2004.06.043
Feldmann M, Brennan FM, Maini R (1998) Cytokines in autoimmune disorders. Int Rev Immunol 17(1–4):217–228
Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A (2013) Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309(13):1368–1378. doi:10.1001/jama.2013.3010
Frank KM, Zhou T, Moreno-Vinasco L, Hollett B, Garcia JG, Bubeck Wardenburg J (2012) Host response signature to Staphylococcus aureus alpha-hemolysin implicates pulmonary Th17 response. Infect Immun 80(9):3161–3169. doi:10.1128/IAI.00191-12
Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA (2013) A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56(11):1554–1561. doi:10.1093/cid/cit123
Gaidamakova EK, Myles IA, McDaniel DP, Fowler CJ, Valdez PA, Naik S, Gayen M, Gupta P, Sharma A, Glass PJ, Maheshwari RK, Datta SK, Daly MJ (2012) Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio-protective Mn2+ -Peptide complex from Deinococcus. Cell Host Microbe 12(1):117–124. doi:10.1016/j.chom.2012.05.011
Geginat J, Paroni M, Facciotti F, Gruarin P, Kastirr I, Caprioli F, Pagani M, Abrignani S (2013) The CD4-centered universe of human T cell subsets. Semin Immunol 25(4):252–262. doi:10.1016/j.smim.2013.10.012
Gjertsson I, Hultgren OH, Stenson M, Holmdahl R, Tarkowski A (2000) Are B lymphocytes of importance in severe Staphylococcus aureus infections? Infect Immun 68(5):2431–2434
Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP (2000) Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164(7):3713–3722
Guillen C, McInnes IB, Vaughan DM, Kommajosyula S, Van Berkel PH, Leung BP, Aguila A, Brock JH (2002) Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J Immunol 168(8):3950–3957
Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, Khatcherian A, Novitskaya I, Carucci JA, Bergman R, Krueger JG (2008) Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol 181(10):7420–7427
Hamid Q, Boguniewicz M, Leung DY (1994) Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest 94(2):870–876. doi:10.1172/JCI117408
Harro C, Betts R, Orenstein W, Kwak EJ, Greenberg HE, Onorato MT, Hartzel J, Lipka J, DiNubile MJ, Kartsonis N (2010) Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from the first study of the vaccine dose range in humans. Clin Vaccine Immunol 17(12):1868–1874. doi:10.1128/CVI.00356-10
Harro CD, Betts RF, Hartzel JS, Onorato MT, Lipka J, Smugar SS, Kartsonis NA (2012) The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two Phase I studies. Vaccine 30(9):1729–1736. doi:10.1016/j.vaccine.2011.12.045
Henningsson L, Jirholt P, Lindholm C, Eneljung T, Silverpil E, Iwakura Y, Linden A, Gjertsson I (2010) Interleukin-17A during local and systemic Staphylococcus aureus-induced arthritis in mice. Infect Immun 78(9):3783–3790. doi:10.1128/IAI.00385-10
Hidron AI, Kempker R, Moanna A, Rimland D (2010) Methicillin-resistant Staphylococcus aureus in HIV-infected patients. Infect Drug Resist 3:73–86
Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schaffer AA, Puck JM, Grimbacher B (2007) STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 357(16):1608–1619. doi:10.1056/NEJMoa073687
Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY (2006) Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 24(3):341–348. doi:10.1016/j.immuni.2006.02.006
Hu DL, Omoe K, Sasaki S, Sashinami H, Sakuraba H, Yokomizo Y, Shinagawa K, Nakane A (2003) Vaccination with nontoxic mutant toxic shock syndrome toxin 1 protects against Staphylococcus aureus infection. J Infect Dis 188(5):743–752. doi:10.1086/377308
Hultgren OH, Verdrengh M, Tarkowski A (2004) T-box transcription-factor-deficient mice display increased joint pathology and failure of infection control during staphylococcal arthritis. Microbes Infect 6(6):529–535. doi:10.1016/j.micinf.2004.02.005
Hume EB, Cole N, Khan S, Garthwaite LL, Aliwarga Y, Schubert TL, Willcox MD (2005) A Staphylococcus aureus mouse keratitis topical infection model: cytokine balance in different strains of mice. Immunol Cell Biol 83(3):294–300. doi:10.1111/j.1440-1711.2005.01326.x
Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y (2009) Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30(1):108–119. doi:10.1016/j.immuni.2008.11.009
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126(6):1121–1133. doi:10.1016/j.cell.2006.07.035
Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, McNeely T (2012) Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccine Immunother 8(3):336–346. doi:10.4161/hv.18946
Karauzum H, Chen G, Abaandou L, Mahmoudieh M, Boroun AR, Shulenin S, Devi VS, Stavale E, Warfield KL, Zeitlin L, Roy CJ, Sidhu SS, Aman MJ (2012) Synthetic human monoclonal antibodies toward staphylococcal enterotoxin B (SEB) protective against toxic shock syndrome. J Biol Chem 287(30):25203–25215. doi:10.1074/jbc.M112.364075
Karauzum H, Adhikari RP, Sarwar J, Devi VS, Abaandou L, Haudenschild C, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Warfield KL, Shulenin S, Aman MJ (2013) Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS ONE 8(6):e65384. doi:10.1371/journal.pone.0065384
Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR (2010) Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202(7):1050–1058. doi:10.1086/656043
Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, Schneewind O (2010) IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 28(38):6382–6392. doi:10.1016/j.vaccine.2010.02.097
Kim HK, Kim HY, Schneewind O, Missiakas D (2011) Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J 25(10):3605–3612. doi:10.1096/fj.11-187963
Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A (2010) Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207(2):299–308. doi:10.1084/jem.20091669
Kisich KO, Carspecken CW, Fieve S, Boguniewicz M, Leung DY (2008) Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol 122(1):62–68. doi:10.1016/j.jaci.2008.04.022
Kobayashi SD, DeLeo FR (2013) Staphylococcus aureus protein A promotes immune suppression. MBio 4(5):e00764–e00713. doi:10.1128/mBio.00764-13
Kolata JB, Kuhbandner I, Link C, Normann N, Vu CH, Steil L, Weidenmaier C, Broker BM (2015) The fall of a dogma? Unexpected high T-cell memory response to Staphylococcus aureus in humans. J Infect Dis 212(5):830–838. doi:10.1093/infdis/jiv128
Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF (2011) Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186(3):1666–1674. doi:10.4049/jimmunol.1002194
Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, Noble L, Brown MJ, Zorman JK, Wang XM, Pancari G, Fan H, Isett K, Burgess B, Bryan J, Brownlow M, George H, Meinz M, Liddell ME, Kelly R, Schultz L, Montgomery D, Onishi J, Losada M, Martin M, Ebert T, Tan CY, Schofield TL, Nagy E, Meineke A, Joyce JG, Kurtz MB, Caulfield MJ, Jansen KU, McClements W, Anderson AS (2006) A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun 74(4):2215–2223. doi:10.1128/IAI.74.4.2215-2223.2006
Larkin EA, Stiles BG, Ulrich RG (2010) Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin B. PLoS ONE 5(10):e13253. doi:10.1371/journal.pone.0013253
Lazarevic V, Glimcher LH, Lord GM (2013) T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol 13(11):777–789. doi:10.1038/nri3536
Leijh PC, van den Barselaar MT, Daha MR, van Furth R (1981) Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect Immun 33(3):714–724
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203(10):2271–2279. doi:10.1084/jem.20061308
Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B (2009) Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5(12):e1000703. doi:10.1371/journal.ppat.1000703
Lowell GH, Colleton C, Frost D, Kaminski RW, Hughes M, Hatch J, Hooper C, Estep J, Pitt L, Topper M, Hunt RE, Baker W, Baze WB (1996) Immunogenicity and efficacy against lethal aerosol staphylococcal enterotoxin B challenge in monkeys by intramuscular and respiratory delivery of proteosome-toxoid vaccines. Infect Immun 64(11):4686–4693
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339(8):520–532. doi:10.1056/NEJM199808203390806
Matsui K, Nishikawa A (2002) Lipoteichoic acid from Staphylococcus aureus induces Th2-prone dermatitis in mice sensitized percutaneously with an allergen. Clin Exp Allergy 32(5):783–788
Matsui K, Nishikawa A (2012) Peptidoglycan from Staphylococcus aureus induces T(H)2 immune response in mice. J Investig Allergol Clin Immunol 22(2):80–86
McLoughlin RM, Solinga RM, Rich J, Zaleski KJ, Cocchiaro JL, Risley A, Tzianabos AO, Lee JC (2006) CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci USA 103(27):10408–10413. doi:10.1073/pnas.0508961103
McLoughlin RM, Lee JC, Kasper DL, Tzianabos AO (2008) IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J Immunol 181(2):1323–1332
McNeely TB, Shah NA, Fridman A, Joshi A, Hartzel JS, Keshari RS, Lupu F, DiNubile MJ (2014) Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccine Immunother 10(12):3513–3516. doi:10.4161/hv.34407
Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452(7188):773–776. doi:10.1038/nature06764
Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H (2007) Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448(7157):1058–1062. doi:10.1038/nature06096
Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, Tsuge I, Karasuyama H (2009) Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 206(6):1291–1301. doi:10.1084/jem.20082767
Mocca CP, Brady RA, Burns DL (2014) Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of Staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol 21(5):622–627. doi:10.1128/CVI.00051-14
Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS (2014) Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun 82(5):2125–2134. doi:10.1128/IAI.01491-14
Murphy AG, O’Keeffe KM, Lalor SJ, Maher BM, Mills KH, McLoughlin RM (2014) Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J Immunol 192(8):3697–3708. doi:10.4049/jimmunol.1303420
Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK (2013) Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol 14(8):804–811. doi:10.1038/ni.2637
Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, Otto M, Inohara N, Nunez G (2013) Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 503(7476):397–401. doi:10.1038/nature12655
Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A (2010) Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun 78(10):4234–4242. doi:10.1128/IAI.00447-10
Nilsson IM, Verdrengh M, Ulrich RG, Bavari S, Tarkowski A (1999) Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J Infect Dis 180(4):1370–1373. doi:10.1086/315023
Nippe N, Varga G, Holzinger D, Loffler B, Medina E, Becker K, Roth J, Ehrchen JM, Sunderkotter C (2011) Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J Invest Dermatol 131(1):125–132. doi:10.1038/jid.2010.282
Nizet V (2007) Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol 120(1):13–22. doi:10.1016/j.jaci.2007.06.005
Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY (2003) Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol 112(6):1195–1202. doi:10.1016/j.jaci.2003.08.049
Ohlsen K, Lorenz U (2010) Immunotherapeutic strategies to combat staphylococcal infections. Int J Med Microbiol 300(6):402–410. doi:10.1016/j.ijmm.2010.04.015
O’Shea JJ, Paul WE (2010) Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327(5969):1098–1102. doi:10.1126/science.1178334
Ou LS, Goleva E, Hall C, Leung DY (2004) T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol 113(4):756–763. doi:10.1016/j.jaci.2004.01.772
Ouyang W, Kolls JK, Zheng Y (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28(4):454–467. doi:10.1016/j.immuni.2008.03.004
Parker D, Ryan CL, Alonzo F 3rd, Torres VJ, Planet PJ, Prince AS (2015) CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia. J Infect Dis 211(5):835–845. doi:10.1093/infdis/jiu525
Pauli NT, Kim HK, Falugi F, Huang M, Dulac J, Henry Dunand C, Zheng NY, Kaur K, Andrews SF, Huang Y, DeDent A, Frank KM, Charnot-Katsikas A, Schneewind O, Wilson PC (2014) Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J Exp Med 211(12):2331–2339. doi:10.1084/jem.20141404
Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB (2012) Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS ONE 7(10):e46648. doi:10.1371/journal.pone.0046648
Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME (2011) Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun 79(12):5010–5018. doi:10.1128/IAI.05571-11
Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, Goulder P, Klenerman P (2010) HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 24(4):491–502. doi:10.1097/QAD.0b013e3283344895
Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD (1995) Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis 20(1):95–102
Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL (2010) Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207(2):291–297. doi:10.1084/jem.20091983
Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL (2011) Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332(6025):65–68. doi:10.1126/science.1200439
Ragle BE, Bubeck Wardenburg J (2009) Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 77(7):2712–2718. doi:10.1128/IAI.00115-09
Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2014) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. doi:10.1016/j.cyto.2014.09.011
Rooijakkers SH, van Kessel KP, van Strijp JA (2005) Staphylococcal innate immune evasion. Trends Microbiol 13(12):596–601. doi:10.1016/j.tim.2005.10.002
Rosenzweig SD, Holland SM (2005) Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev 203:38–47. doi:10.1111/j.0105-2896.2005.00227.x
Schaffer AC, Lee JC (2008) Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents 32(Suppl 1):S71–S78. doi:10.1016/j.ijantimicag.2008.06.009
Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY (2010) Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol 125(1):39–49. doi:10.1016/j.jaci.2009.10.039
Schmaler M, Jann NJ, Ferracin F, Landmann R (2011) T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J Immunol 186(1):443–452. doi:10.4049/jimmunol.1001407
Schmitt N, Ueno H (2015) Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol 34:130–136. doi:10.1016/j.coi.2015.03.007
Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H, Naso R (2002) Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 346(7):491–496. doi:10.1056/NEJMoa011297
Skurnik D, Merighi M, Grout M, Gadjeva M, Maira-Litran T, Ericsson M, Goldmann DA, Huang SS, Datta R, Lee JC, Pier GB (2010) Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest 120(9):3220–3233. doi:10.1172/JCI42748
Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ (2011) The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun 79(9):3801–3809. doi:10.1128/IAI.05075-11
Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM (2013) Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26(3):422–447. doi:10.1128/CMR.00104-12
Spaulding AR, Salgado-Pabon W, Merriman JA, Stach CS, Ji Y, Gillman AN, Peterson ML, Schlievert PM (2014) Vaccination against Staphylococcus aureus pneumonia. J Infect Dis 209(12):1955–1962. doi:10.1093/infdis/jit823
Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, Durandy A, Griscelli C, Fischer A (1993) Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr 123(4):564–572
Stiles BG, Krakauer T, Bonventre PF (1995) Biological activity of toxic shock syndrome toxin 1 and a site-directed mutant, H135A, in a lipopolysaccharide-potentiated mouse lethality model. Infect Immun 63(4):1229–1234
Stranger-Jones YK, Bae T, Schneewind O (2006) Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci USA 103(45):16942–16947. doi:10.1073/pnas.0606863103
Tebartz C, Horst SA, Sparwasser T, Huehn J, Beineke A, Peters G, Medina E (2015) A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J Immunol 194(3):1100–1111. doi:10.4049/jimmunol.1400196
Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR (2012) Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 19(3):377–385. doi:10.1128/CVI.05589-11
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28(3):603–661. doi:10.1128/CMR.00134-14
Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Loffler B (2011) Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3(3):129–141. doi:10.1002/emmm.201000115
van Kessel KP, Bestebroer J, van Strijp JA (2014) Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front Immunol 5:467. doi:10.3389/fimmu.2014.00467
Varshney AK, Wang X, MacIntyre J, Zollner RS, Kelleher K, Kovalenko OV, Pechuan X, Byrne FR, Fries BC (2014) Humanized staphylococcal enterotoxin B (SEB)-specific monoclonal antibodies protect from SEB intoxication and Staphylococcus aureus infections alone or as adjunctive therapy with vancomycin. J Infect Dis 210(6):973–981. doi:10.1093/infdis/jiu198
Verbrugh HA, Peterson PK, Nguyen BY, Sisson SP, Kim Y (1982) Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol 129(4):1681–1687
Verhoeven PO, Gagnaire J, Botelho-Nevers E, Grattard F, Carricajo A, Lucht F, Pozzetto B, Berthelot P (2014) Detection and clinical relevance of Staphylococcus aureus nasal carriage: an update. Expert Rev Anti Infect Ther 12(1):75–89. doi:10.1586/14787210.2014.859985
von Eiff C, Becker K, Machka K, Stammer H, Peters G (2001a) Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344(1):11–16. doi:10.1056/NEJM200101043440102
von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G (2001b) Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with darier’s disease. Clin Infect Dis 32(11):1643–1647. doi:10.1086/320519
Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 175(6):3907–3919
Weisman LE, Fischer GW, Thackray HM, Johnson KE, Schuman RF, Mandy GT, Stratton BE, Adams KM, Kramer WG, Mond JJ (2009) Safety and pharmacokinetics of a chimerized anti-lipoteichoic acid monoclonal antibody in healthy adults. Int Immunopharmacol 9(5):639–644. doi:10.1016/j.intimp.2009.02.008
Weisman LE, Thackray HM, Steinhorn RH, Walsh WF, Lassiter HA, Dhanireddy R, Brozanski BS, Palmer KG, Trautman MS, Escobedo M, Meissner HC, Sasidharan P, Fretz J, Kokai-Kun JF, Kramer WG, Fischer GW, Mond JJ (2011) A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics 128(2):271–279. doi:10.1542/peds.2010-3081
Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL (2005) The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5(12):751–762. doi:10.1016/S1473-3099(05)70295-4
Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, Hennessey JP Jr, Fu Y, Schmidt CS, Edwards JE Jr, Xiong YQ, Ibrahim AS (2014) Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci USA 111(51):E5555–E5563. doi:10.1073/pnas.1415610111
Yoong P, Pier GB (2010) Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci USA 107(5):2241–2246. doi:10.1073/pnas.0910344107
Yoong P, Torres VJ (2015) Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat Commun 6:8125. doi:10.1038/ncomms9125
Zhang L, Jacobsson K, Vasi J, Lindberg M, Frykberg L (1998) A second IgG-binding protein in Staphylococcus aureus. Microbiology 144(Pt 4):985–991
Zhang L, Rosander A, Jacobsson K, Lindberg M, Frykberg L (2000) Expression of staphylococcal protein Sbi is induced by human IgG. FEMS Immunol Med Microbiol 28(3):211–218
Ziegler C, Goldmann O, Hobeika E, Geffers R, Peters G, Medina E (2011) The dynamics of T cells during persistent Staphylococcus aureus infection: from antigen-reactivity to in vivo anergy. EMBO Mol Med 3(11):652–666. doi:10.1002/emmm.201100173
Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F (2012) Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484(7395):514–518. doi:10.1038/nature10957
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, NIAID.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Karauzum, H., Datta, S.K. (2016). Adaptive Immunity Against Staphylococcus aureus . In: Bagnoli, F., Rappuoli, R., Grandi, G. (eds) Staphylococcus aureus. Current Topics in Microbiology and Immunology, vol 409. Springer, Cham. https://doi.org/10.1007/82_2016_1
Download citation
DOI: https://doi.org/10.1007/82_2016_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72061-6
Online ISBN: 978-3-319-72063-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)