Abstract
When cell death occurs in vivo, cell corpses are not left untreated, but are recognized and engulfed by phagocytes, such as macrophages and dendritic cells. In the past, cell death had been considered the final process of a cell’s life, and cell corpses had been viewed as debris that is simply to be cleared by phagocytes. Recently, however, it has become clearer that various biological responses are induced with dead cells as the starting point. Most of these biological responses followed by cell death are thought to be mediated by macrophages and dendritic cells. In this review, we present the overview of molecular mechanisms and biological significance of dead cell clearance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In the human body, there are 60 trillion cells working in a cooperative manner to sustain life. Cells that have fulfilled their roles in each tissue, or such abnormal cells as cancer cells and virus-infected cells, are swiftly cleared via cell death. The removal of unnecessary or harmful cells via cell death is believed to play a vital role in the maintenance of homeostasis in living organisms. When cell death occurs in vivo regardless of the setting (i.e., whether physiological or pathological), cell corpses are not left untreated, but are recognized and engulfed by phagocytes, such as macrophages and dendritic cells (DC) (Henson et al. 2001; Lauber et al. 2003, 2004; Poon et al. 2014; Ravichandran and Lorenz 2007). In physiological conditions, cell corpses are engulfed by phagocytes so swiftly that they are hardly detected outside the phagocytes (McIlroy et al. 2000). Even in the pathological conditions, massive cell death is often followed by rapid clearance of cell corpses and tissue repair within a short period. For example, injection of dexamethasone results in massive cell death of immature T cells in mouse thymus. Soon after such cell death occurs in thymus, the corpses are rapidly cleared by thymic macrophages, and cellular composition of thymus recovers within 24 h (Scott et al. 2001). Ischemia–reperfusion injury causes necrotic cell death of epithelial cells in cortico-medullary border of kidneys, but swift clearance of injured cells results in tissue regeneration and recovery of kidney functions (Bonventre and Yang 2011). These observations prompt us to consider that rapid clearance of cell corpses is the essential first step for regeneration of injured tissues.
In the past, the engulfment of dead cells by phagocytes was thought to play a role merely in terms of corpse clearance. Recently, however, it has been revealed that macrophages that engulf dead cells, depending on the situation, can elicit a variety of biological responses. In particular, accumulated findings point to the important roles played by macrophages in the phagocytosis of dead cells, including the repair and regeneration of damaged tissue.
In this review, we outline the mechanism of phagocytosis of dead cells by macrophages and discuss the kinds of roles this mechanism plays in biological response following cell death, such as immune responses, inflammation, repair, and regeneration.
2 Phagocytosis of Dead Cells by Macrophages
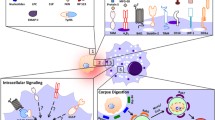
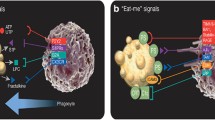
When cell death occurs in a living organism, the corpses are quickly recognized and engulfed by phagocytes, such as macrophages, rather than left alone. It is most likely that the mechanisms of corpse clearance by phagocytes depend on the mode of cell death. But with regard to the mechanism of corpse clearance following the occurrence of cell death, only the analysis of apoptotic cases has seen progress. The molecular mechanisms by which phagocytes recognize and engulf apoptotic cells have been studied intensively since the late 1990s. Previous studies have found that phagocytes recognize and engulf phospholipids called phosphatidylserine (PS), which are exposed on the surface of apoptotic cells (Nagata et al. 2010). As PS serves as a marker when dead cells are subjected to phagocytosis by phagocytes, they are referred to as the “eat-me” signals . In living cells, PS is localized on the inner side of the cell membrane; however, when cells undergo apoptosis, PS is exposed to the extracellular face. Annexin V is well known to have an ability to bind PS specifically and frequently used to detect surface exposure of PS in apoptotic cell corpses by flow cytometry analysis. Early phase of apoptotic cell corpses exhibits Annexin V positive and PI negative, indicating the PS exposure to cell surface without increase in cell membrane permeability.
It was only recently that the underlying molecular mechanism has also become clearer. It has been assumed that the asymmetrical distribution of PS in the cell membrane of living cells involves flippase, which functions to help PS move from the exoplasmic face to the cytoplasmic face of the cell membrane, although the actual molecular state has long been unknown. Recent studies have reported that ATP11c and CDC50A play a substantial role in the asymmetric localization of PS (Segawa et al. 2014). Of these, ATP11c has been found to be cleaved by a caspase during apoptosis. This cleavage is thought to render it inactive as a functional flippase. In addition to the inactivation of flippase activity, it has also been reported that when cells undergo apoptosis, Xkr8 is activated by caspases during apoptosis and plays a critical role in active transportation of PS from the inner surface of the membrane to the outer surface (Suzuki et al. 2013). It is now understood that the inactivation and activation of these two enzymes cooperatively facilitate the exposure of PS on the outer surface of the membrane, allowing for recognition by macrophages.
While PS is the unique molecule as the “eat-me” signals exposed on apoptotic cell corpses, a large number of molecules have been reported as PS-binding molecules expressed by phagocytes. Some of these molecules, such as MFG-E8, Mer, and T-cell immunoglobulin mucin-3 and -4 (Tim-3 and Tim-4) are confirmed to be involved in apoptotic cell clearance in vivo (Fig. 1). Although differences in the roles of these molecules have yet to be clarified in detail, it has been reported that different molecules are used, depending on the types of macrophages in the organism. For instance, macrophages resident in the abdominal cavity engulf apoptotic cells via Mer and Tim-4. Mer has the ability to bind growth arrest-specific gene 6 (Gas6) and protein S, both of which exhibit binding activity of PS on the surface of apoptotic cells (Dransfield et al. 2015; Ishimoto et al. 2000; Nagata et al. 1996; Nakano et al. 1997; Zagorska et al. 2014), whereas Tim-4 can directly bind PS (Miyanishi et al. 2007). These two molecules coordinately play critical roles in efficient engulfment of apoptotic cells in peritoneal resident macrophages (Nishi et al. 2014). On the other hand, inflammatory macrophages induced by the intraperitoneal administration of thioglycollate engulf apoptotic cells in an MFG-E8-dependent manner (Hanayama et al. 2002). Tim-3 is expressed in splenic DCs, and the anti-Tim-3 antibody inhibits phagocytosis of apoptotic cells by CD8 + DCs and subsequently results in a reduced cross-presentation of apoptotic cell-associated antigens (Nakayama et al. 2009).
Consistent with the different expression of these molecules on phagocytes, gene-targeting mice of each molecule exhibit distinct phenotype. MFG-E8 is found mainly in the germinal centers of the spleen and lymph nodes and expressed in tingible body macrophages that engulf lymphocytes undergoing cell death. In MFG-E8-deficient mice, the abnormal phagocytosis of dead cells by these macrophages has been reported (Hanayama et al. 2004). On the other hand, in Mer-deficient mice, the clearance of apoptotic cell in thymus is impaired (Scott et al. 2001).
For cells that have undergone apoptosis to be swiftly engulfed by macrophages , it is imperative that macrophages migrate to the side of apoptotic cells. It has been reported that apoptotic cells release chemo-attractants called “find-me” signals to draw macrophages close. So far, there are reports suggesting that lysophosphatidylcholine and ATP released by apoptotic cells play an important role in accumulating macrophages (Chekeni et al. 2010; Lauber et al. 2003), but how these molecules actually function as find-me signals in vivo remains elusive.
Meanwhile, some studies have reported that molecules that are present on the surface of living cells inhibit phagocytosis by macrophages. These are referred to as the “don’t-eat-me” signals , and CD47 molecules have been reported to possess this function (Oldenborg et al. 2000). However, whether CD47 on the surface of living cells actually inhibits phagocytosis is open to debate.
3 Apoptotic Cell Clearance in Living Organisms
In living organisms, the phagocytosis of apoptotic cells by macrophages occurs very quickly. Therefore, under physiological conditions, it is not usually possible to observe dead cells being left uncleared in any tissues. For instance, a substantial number of TUNEL-positive cells can be observed in thymus, but most of these cells are found to exist inside thymic macrophages in physiological conditions (McIlroy et al. 2000). So why is it that apoptotic cells must be cleared so quickly? Many studies have been carried out to address the significance of apoptotic cell engulfment in living organisms, mainly through the analysis of mice that lack molecules involved in the phagocytosis of dead cells by macrophages. In MFG-E8- and Mer-deficient mice mentioned above, the phagocytosis of apoptotic cells appears impaired, and serum anti-nuclear antibody and anti-DNA antibody titers show abnormal elevations (Hanayama et al. 2004; Scott et al. 2001). From these observations, the phagocytosis of apoptotic cells is believed to play a crucial role in the maintenance of immunological tolerance to self-antigen. To date, this phenomenon has been understood as such that phagocytes, by preventing autoantigens contained in dead cells from flowing out, inhibit the abnormal activation of autoimmune responses. However, another possibility has been pointed out that phagocytes might actually actively induce self-tolerance through the engulfment of dead cells. In other words, phagocytes that engulf dead cells are thought to transmit negative signals (deletion or anergy) to self-reactive T cells by presenting obtained autoantigens on MHC.
In multicellular organisms, a substantial number of tissue-resident cells, which contain tissue-specific self-antigens, undergo apoptosis constantly for turnover, and these apoptotic cells could become sources of tissue-specific self-antigens for antigen-presenting cells in each tissue. In fact, when cells undergoing apoptosis are intravenously administered, T-cell responses to antigens associated with dead cells are reportedly attenuated (Liu et al. 2002; Miyake et al. 2007a; Sun et al. 2004). The intravenously administered dead cells are phagocytosed by dendritic cells (DCs) in the spleen, and antigens associated with dead cells are presented to T cells for immunosuppression. Since the tolerance-inducing effects of apoptotic cells could be overcome when the DCs are stimulated by activation signals, the presentation of cell-associated antigens in the absence of costimulatory signals may lead to deletion or anergy of antigen-specific T cells (Liu et al. 2002).
On the other hand, under certain conditions, the phagocytosis of dead cells could result in the presentation of dead cell antigens by phagocytes, leading to the activation of T-cell responses to these antigens. Immune activation against dead cell-associated antigens has been extensively studied in the field of tumor immunity. It is reported that dead tumor cells, either killed in vivo or in injection of dead tumor cells, could activate tumor antigen-specific T-cell immunity under certain circumstances (Apetoh et al. 2007; Asano et al. 2011; Casares et al. 2005; Tesniere et al. 2008). The efficiency of tumor vaccination by dead tumor cells is largely depended on the nature of cell death in vaccinated tumor cells. It is also reported that calreticulin exposure on the dead tumor cells efficiently elicits anti-tumor immunity (Obeid et al. 2007). In another case, injection of artificial adjuvant vector cells expressing CD1d loaded with a-GalCer and tumor antigens elicits tumor immunity (Fujii et al. 2009; Shimizu et al. 2013). In this system, the vector cells are thought to undergo cell death in vivo and are phagocytosed by DCs. Then, DCs make a cross-presentation of tumor antigens to activate tumor antigen-specific CTLs in cooperation with activated NKT cells. Details of the mechanisms that define the direction of immune response to dead cell-associated antigens are still unknown, but if clarified, those mechanisms might shed light on new ways of controlling immune responses.
4 Subset of Macrophages Responsible for Phagocytosis of Apoptotic Cells
In living organisms, it is likely that macrophages and DCs control immune responses via the phagocytosis of apoptotic cells. So what kinds of cells are macrophages and DCs that actually play this role in living organisms? In each tissue in the living organism, there exist tissue-specific macrophages and DCs, and under physiological conditions, these phagocytes are thought to be responsible for the processing of dead cells. More recently, it has been revealed that these indigenous tissue-specific macrophages not only possess different properties depending on tissue, but also form several subpopulations in each tissue, with each playing a specific role (Gordon et al. 2014). This suggests the possibility that specific subpopulations are responsible for the phagocytosis of dead cells in tissues. Indeed, some subpopulations present in the spleen and lymph nodes have been reported to play a prominent role in the phagocytosis of dead cells, as well in associated immune responses. As described above, the intravenous injection of apoptotic cells induces immune tolerance to dead cell-associated antigens. In this case, marginal metallophilic macrophages and/or marginal zone macrophages are localized in the marginal zone of the spleen, i.e., the region where blood flows into the spleen, and (either one or both) have been shown to take up dead cells in blood (Miyake et al. 2007b). The critical role of these macrophages in the tolerance induction is proved by using the CD169-DTR mice, in which these macrophages can be specifically deleted by DT injection (Miyake et al. 2007b). CD11c-positive and CD103-positive dendritic cells are also localized in the marginal zone of the spleen, and they make cross-presentation of dead cell-associated antigens to CD8 T cells, demonstrating the coordinate immune regulation by macrophages and dendritic cells in the marginal zone (Qiu et al. 2009). Molecular mechanisms of tolerance induction by apoptotic cell infusion are also reported. Intravenous injection of apoptotic cells induces CCL22 expression in splenic metallophilic macrophages, resulting in the accumulation and activation of FoxP3 (+) Tregs (Ravishankar et al. 2014). On the other hand, as described above, massive cell death in tumor can induce immune activation to cell-associated antigens and activates anti-tumor immunity under certain circumstances. The candidate of macrophage/DC subset responsible for the immune activation associated with tumor cell death has been reported. When dead tumor cells are subcutaneously injected into mice, CD169-positive sinus macrophages localized in the lymphatic sinus of the lymph node take up dead cells or cell debris carried by the lymph flow and control immune responses to antigens associated with the dead cells (Asano et al. 2011). CD169-positive sinus macrophages consist of two subpopulations, CD11c-positive and CD11c-negative cells, and CD11c-positive cells, localized in the boundary border between sinus and T-cell zone, make a cross-presentation of dead cell-associated antigens to CD8 T cells. As immune responses to dead cell-associated antigens are closely related to the pathological conditions and the treatment of autoimmune diseases and cancer, the identification and functional analysis of involved macrophage subpopulations are essential research subjects.
5 Dead Cell-Derived Substances and Their Roles in Macrophage Activation and Regeneration
In the past, cell death had been considered the final process of a cell’s life, and cell corpses had been viewed as debris that is simply to be cleared by phagocytes, such as macrophages , i.e., waste. Recently, however, as exemplified by the above-mentioned immune regulation by macrophages, it has become clearer that various biological responses are induced with dead cells as the starting point. Furthermore, we are beginning to understand how cells can actively regulate biological responses after cell death, by releasing physiologically active substances in the process of dying. Among the biological responses initiated by cell death, one of the most analyzed and advanced areas of research is inflammatory response. High mobility group box protein 1 (HMGB1) is one such endogenous stimulator of the immune system released from dead cells. HMGB1 was originally identified as a nuclear protein, but it is also passively released when cells undergo non-apoptotic cell death (Rovere-Querini et al. 2004; Scaffidi et al. 2002). When HMGB1 is once released, this is known to cause inflammation by acting on macrophages and DCs (Dumitriu et al. 2005; Messmer et al. 2004) (Fig. 2a). It is also reported that Mincle, a C-type lectin, is expressed by macrophages and recognizes SAP130 released from dead cells to induce sterile inflammation (Yamasaki et al. 2008). Such inflammation inducers that originate from dead cells are called damage-associated molecular patterns (DAMPs). Meanwhile, dead cell-derived substances have been reported to be involved in tissue repair and regeneration, in addition to inflammation. For example, IL-11, which is released by liver cells that had undergone cell death due to oxidative stress, has been reported to act on surrounding normal cells to promote liver regeneration (Nishina et al. 2012) (Fig. 2b). Similarly, some reports have indicated that semaphorin 3E is expressed in damaged liver cells and controls liver regeneration and fibrogenesis (Yagai et al. 2014). Furthermore, other reports have demonstrated that in chronic liver injury, phagocytosis of dead cells induces the expression of Wnt3a in macrophages , which contributes to regeneration by hepatic progenitors (Boulter et al. 2012) (Fig. 2c). Although cell death and regeneration seem strongly associated, the detailed mechanisms have not been clarified. Thus, in the future, progress of research in this area is highly anticipated.
6 Apoptosis and Non-apoptotic Cell Death in Vivo
In the past, apoptosis was thought to be the main mode of cell death occurring in vivo. Apoptosis is a type of cell death that occurs due to the activation of caspases in cells, resulting in the degradation of many intracellular substrates; it is an active death regulated by molecules. With regard to apoptosis, detection methods such as the TUNEL technique (Gavrieli et al. 1992) and activated caspase assays have already been established, and it is possible to detect apoptotic cells in situ. By using these methods, we clearly find that apoptotic cell death takes place in many organs during development and tissue turnover. Furthermore, a method to observe apoptosis in vivo in real time has also been developed, and the dynamics of apoptosis and its influence on surrounding cells are extensively studied during embryogenesis (Nonomura et al. 2013; Yamaguchi et al. 2011).
In contrast to apoptosis, non-apoptotic cell death, such as one that is caused by heat or other physical stimuli, or pathological cell death observed in various kind of diseases, used to be considered a passive form of cell death, and has been referred to as necrosis based on the morphological characteristics. Originally, it was believed that no special execution mechanisms existed in necrosis; however, in recent years, some modes of necrosis have been identified, which are controlled by molecular regulation. For instance, RIPK1/RIPK3- and MLKL-regulated cell death has been reported, which are referred to as necroptosis (Pasparakis and Vandenabeele 2015). It is also reported that caspase-1-regulated cell death is identified and referred to as pyroptosis (Lamkanfi and Dixit 2014). These non-apoptotic cell deaths exhibit morphological feature of classical necrosis, but especially in various pathological conditions, they appeared to contribute pathology of several diseases. More recently, another mode of cell death called ferroptosis, which requires iron ions, has been reported (Friedmann Angeli et al. 2014; Yang et al. 2014). The mechanisms of these various types of cell death have been clarified through analyses using cultured cells, and subsequent analyses of executing molecules in knockout mice have gradually unraveled their significance in vivo. Yet, when and how each type of cell death occurs in vivo has not been clarified. One of the reasons for the difficulties in analysis is the lack of methods to detect these new cell death events in vivo.
It is most likely that mechanisms of dead cell clearance by phagocytes depend on the mode of cell death. Furthermore, macrophages and dendritic cells could change the response to dead cells, depending on the mode of cell death. In order to explore the physiological and pathological consequence to cell death in vivo, we should carefully examine how macrophages and dendritic cells react to dead cells with different cell death modes.
7 Conclusion
In this review, we overviewed the mechanisms of dead cell clearance and its significance. Whereas studies to date have dramatically advanced the elucidation of molecular mechanisms apoptotic clearance, progress in research has generated new challenges, such as the identification of new cell death modes and the clarification of their significance and the determination of control mechanisms of biological response following cell death. The idea that cell death mechanisms simply exist to ensure cell removal might not fully explain the reason for the diversity in the modes of cell death. The hypothesis that each mode of cell death (purposely) elicits a specific biological response is attractive scientifically, but will require careful verification in the future through detailed analysis of cell death and subsequent biological response mechanisms.
Abbreviations
- DCs:
-
Dendritic cells
- PS:
-
Phosphatidylserine
- MFG-E8:
-
Milk fat globule-EGF 8
- Tim-3:
-
T-cell immunoglobulin mucin-3
- Tim-4:
-
T-cell immunoglobulin mucin-4
- Gas6:
-
Growth arrest-specific gene 6
- TUNEL:
-
TdT-mediated dUTP-biotin nick end labeling
- HMGB1:
-
High mobility group box protein 1
References
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M (2011) CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 34:85–95
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Investig 121:4210–4221
Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ (2012) Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18:572–579
Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G (2005) Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 202:1691–1701
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867
Dransfield I, Zagorska A, Lew ED, Michail K, Lemke G (2015) Mer receptor tyrosine kinase mediates both tethering and phagocytosis of apoptotic cells. Cell Death Dis 6:e1646
Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P (2005) Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174:7506–7515
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16:1180–1191
Fujii S, Goto A, Shimizu K (2009) Antigen mRNA-transfected, allogeneic fibroblasts loaded with NKT-cell ligand confer antitumor immunity. Blood 113:4262–4272
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of cell biology 119:493–501
Gordon S, Pluddemann A, Martinez Estrada F (2014) Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 262:36–55
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187
Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304:1147–1150
Henson PM, Bratton DL, Fadok VA (2001) Apoptotic cell removal. Curr Biol 11:R795–R805
Ishimoto Y, Ohashi K, Mizuno K, Nakano T (2000) Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem (Tokyo) 127:411–417
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157:1013–1022
Lauber K, Blumenthal SG, Waibel M, Wesselborg S (2004) Clearance of apoptotic cells: getting rid of the corpses. Mol Cell 14:277–287
Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S (2003) Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113:717–730
Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM (2002) Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med 196:1091–1097
McIlroy D, Tanaka M, Sakahira H, Fukuyama H, Suzuki M, Yamamura K, Ohsawa Y, Uchiyama Y, Nagata S (2000) An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev 14:549–558
Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N (2004) High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 173:307–313
Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M (2007a) Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Investig 117:2268–2278
Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M (2007b) Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Investig 117:2268–2278
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450:435–439
Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K (1996) Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271:30022–30027
Nagata S, Hanayama R, Kawane K (2010) Autoimmunity and the clearance of dead cells. Cell 140:619–630
Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H (1997) Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem 272:29411–29414
Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K (2009) Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113:3821–3830
Nishi C, Toda S, Segawa K, Nagata S (2014) Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol Cell Biol 34:1512–1520
Nishina T, Komazawa-Sakon S, Yanaka S, Piao X, Zheng DM, Piao JH, Kojima Y, Yamashina S, Sano E, Putoczki T, Doi T, Ueno T, Ezaki J, Ushio H, Ernst M, Tsumoto K, Okumura K, Nakano H (2012) Interleukin-11 links oxidative stress and compensatory proliferation. Sci Signal 5:ra5
Nonomura K, Yamaguchi Y, Hamachi M, Koike M, Uchiyama Y, Nakazato K, Mochizuki A, Sakaue-Sawano A, Miyawaki A, Yoshida H, Kuida K, Miura M (2013) Local apoptosis modulates early mammalian brain development through the elimination of morphogen-producing cells. Dev Cell 27:621–634
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61
Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP (2000) Role of CD47 as a marker of self on red blood cells. Science 288:2051–2054
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517:311–320
Poon IK, Lucas CD, Rossi AG, Ravichandran KS (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14:166–180
Qiu CH, Miyake Y, Kaise H, Kitamura H, Ohara O, Tanaka M (2009) Novel subset of CD8{alpha} + dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol 182:4127–4136
Ravichandran KS, Lorenz U (2007) Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 7:964–974
Ravishankar B, Shinde R, Liu H, Chaudhary K, Bradley J, Lemos HP, Chandler P, Tanaka M, Munn DH, Mellor AL, McGaha TL (2014) Marginal zone CD169 + macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci USA 111:4215–4220
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5:825–830
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211
Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S (2014) Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344:1164–1168
Shimizu K, Mizuno T, Shinga J, Asakura M, Kakimi K, Ishii Y, Masuda K, Maeda T, Sugahara H, Sato Y, Matsushita H, Nishida K, Hanada K, Dorrie J, Schaft N, Bickham K, Koike H, Ando T, Nagai R, Fujii S (2013) Vaccination with antigen-transfected, NKT cell ligand-loaded, human cells elicits robust in situ immune responses by dendritic cells. Cancer Res 73:62–73
Sun E, Gao Y, Chen J, Roberts AI, Wang X, Chen Z, Shi Y (2004) Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ 11:1258–1264
Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341:403–406
Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G (2008) Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 15:3–12
Yagai T, Miyajima A, Tanaka M (2014) Semaphorin 3E secreted by damaged hepatocytes regulates the sinusoidal regeneration and liver fibrosis during liver regeneration. Am J Pathol 184:2250–2259
Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H, Miura M (2011) Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. The Journal of cell biology 195:1047–1060
Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T (2008) Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 9:1179–1188
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331
Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G (2014) Diversification of TAM receptor tyrosine kinase function. Nat Immunol 15:920–928
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (26293089) from Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid for Scientific Research on Innovative Areas (homeostatic regulation by various types of cell death) (26110006) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan, MEXT-Supported Program for the Strategic Research Foundation at Private Universities (2014–2019) in Japan, the Uehara Memorial Foundation, the Takeda Science Foundation, and the Naito Foundation. We thank T. Suito for secretarial assistance.
Competing interests: The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Tanaka, M., Nishitai, G. (2015). Immune Regulation by Dead Cell Clearance. In: Nagata, S., Nakano, H. (eds) Apoptotic and Non-apoptotic Cell Death. Current Topics in Microbiology and Immunology, vol 403. Springer, Cham. https://doi.org/10.1007/82_2015_472
Download citation
DOI: https://doi.org/10.1007/82_2015_472
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23912-5
Online ISBN: 978-3-319-23913-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)