Abstract
Streptococcal species are a diverse group of bacteria which can be found in animals and humans. Their interactions with host organisms can vary from commensal to pathogenic. Many of the pathogenic species are causative agents of severe, invasive infections in their hosts, accounting for a high burden of morbidity and mortality, associated with high economic costs in industry and health care. Among them, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus pneumoniae, and Streptococcus suis are discussed here. An environmentally stimulated and tightly controlled expression of their virulence factors is of utmost importance for their pathogenic potential. Thus, the most universal and widespread regulators from the classes of stand-alone transcriptional regulators, two-component signal transduction systems (TCS), eukaryotic-like serine/threonine kinases, and small noncoding RNAs are the topic of this chapter. The regulatory levels are reviewed with respect to function, activity, and their role in pathogenesis. Understanding of and interfering with transcriptional regulation mechanisms and networks is a promising basis for the development of novel anti-infective therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Streptococcal species can colonize and live in animals and humans and are present in almost all body compartments. They mostly live in benign and commensal relationship with their hosts. However, several pathogenic species are known, which act as zoonosis pathogens or represent exclusive human pathogens. Infections are multifaceted and occur in all age groups ranging from newborns to adults and the elderly. The pathogenic streptococcal species reviewed in this chapter can cause severe and live-threatening invasive diseases in humans. They are responsible for substantial mortality and morbidity. In the era of spreading antibiotic resistance, these species are studied intensively to understand their pathogenesis mechanisms, including the regulatory machinery in control of virulence factor expression. This knowledge will help to discover novel targets for innovative therapies.
Streptococcus pyogenes is an exclusive human pathogen, which is transmitted from person to person and causes diseases ranging from mild superficial and self-healing to severe invasive diseases. Sepsis, toxic shock like syndrome and necrotizing fasciitis are the most frightening disease characteristics (Cunningham 2000; Carapetis et al. 2005). Antibiotic treatment is still the therapy of choice and is mandatory to prevent post-streptococcal autoimmune sequelae.
Streptococcus pneumoniae, commonly referred to as the pneumococcus, is known to cause a high burden of human disease and death. S. pneumoniae can switch from commensal and asymptomatic stage to infectious stage, thereby infecting the lung, blood, and brain. Otitis media and pneumonia are the most frequent infections caused by these bacteria (Mitchell and Mitchell 2010; Gamez and Hammerschmidt 2012).
Like the other streptococci mentioned above, Streptococcus agalactiae is also a common asymptomatic colonizer of healthy adults. As an opportunistic pathogen, it is able to defend itself against all host immune system functions, and to cause severe invasive diseases and tissue damage in unborn and newborn children as well as adults (Rajagopal 2009).
For a long time, Streptococcus suis was considered a classical swine pathogen responsible for high economic losses in the swine industry worldwide. S. suis associated diseases include meningitis, septicemia and endocarditis (Fittipaldi et al. 2012). Late in the 1960s first cases of human infections were reported and since then S. suis gained increasing importance as zoonosis pathogen also affecting humans.
Common to all these severe and invasive disease causing pathogens is environmentally driven, coordinated, and fine-tuned regulation of expression of their armament of virulence factor genes. The activity of the most prevalent stand-alone transcriptional regulators, two-component regulatory systems (TCS), eukaryotic-like serine/threonine kinases and, a rather just recently appreciated level, small noncoding RNAs (sRNAs) is the topic of this review.
Numerous so-called stand-alone transcriptional regulators influence the coordinated expression of virulence factors in all streptococci discussed here. Some of them have been extensively studied, especially in S. pyogenes (Kreikemeyer et al. 2003; McIver 2009; Fiedler et al. 2010a). In this review, we will focus on those stand-alone regulators that can be found and are involved in virulence regulation in more than one of the pathogenic streptococcal species, such as the members of the RofA-like protein (RALP) family of regulators, Mga and orthologous proteins, as well as several regulators primarily responsible for controlling metabolism.
A common way for bacteria to adapt to environmental conditions is mediated by two-component signal TCS (Hoch 2000; Stock et al. 2000), which are encoded in varying numbers on the chromosomes of these species. In S. pyogenes, the genome sequencing era allowed identification of 11 up to 13 such systems (Kreikemeyer et al. 2003; Fiedler et al. 2010a), in S. pneumoniae 13 systems are present (Paterson et al. 2006), in S. agalactiae up to 20 TCS have been reported, depending on the strain investigated (Glaser et al. 2002; Tettelin et al. 2002), and in S. suis 15 TCS were predicted from comparative genomics analyses (Chen et al. 2007). Common to almost all TCS is the basic composition of two proteins. A membrane-associated sensor histidine kinase (HK) is responsible for receiving external signals and autophosphorylates a conserved histidine residue. The phosphate group is subsequently transferred by the HK to a conserved aspartate residue in the cognate response regulator (RR). The RR undergoes a conformational change enabling DNA binding and regulation of gene expression (Stock et al. 2000; Hoch 2000). There are variations of this common activation theme. In several studies, response regulators were found which are independent of their cognate histidine kinases for phosphorylation. Eukaryotic-like serine/threonine kinase/phosphatase systems have been discovered and studied, which either independently regulate pathogen physiology and virulence, or which are networking with TCS systems (Burnside and Rajagopal 2011). For all stand-alone regulators and TCS discussed in this review, designations and known or putative functions are summarized in Table 1.
In addition to the known protein based transcriptional regulation, the role of sRNAs in the control of bacterial gene expression became increasingly evident. The high number of regulatory RNAs identified in different bacterial species was unexpected (Brantl 2009; Narberhaus and Vogel 2009; Waters and Storz 2009) and the variability in length, structure, and mode of action of the different RNAs is very striking (Gottesman and Storz 2011). Bacterial regulatory RNAs influence the expression of genes involved in processes as diverse as stress response, sugar metabolism, and surface composition (Vanderpool and Gottesman 2005; Gottesman et al. 2006; Heidrich et al. 2006; Gorke and Vogel 2008; Gogol et al. 2011; Sharma et al. 2011). Thus, it is not surprising that pathogens employ regulatory RNAs for the tightly controlled expression of virulence factor genes and in some cases for the fine tuning of conventional, protein-mediated gene regulation (Livny et al. 2006; Papenfort and Vogel 2010). Although the regulatory character is shared by this class of RNAs, the basic properties between subtypes vary immensely. There is an intriguing diversity of regulatory mechanisms. Some cis-regulatory RNAs are located on untranslated regions (UTRs) of a coding transcript and act as RNA-thermometers or riboswitches (Klinkert and Narberhaus 2009; Bastet et al. 2011). Another group consists of small sRNAs, which are transcribed independently and function via cis- and trans-antisense base pairing. Of those, cis-acting sRNAs are encoded on the opposite strand of their respective target gene. Consequently, they show a high sequence complementarity to their target RNA, which guarantees very specific binding. Recently, differential sequencing analysis revealed a high antisense transcriptional activity in several model organisms, including Helicobacter pylori and S. pyogenes, which points to the importance of cis-regulatory elements in bacteria in general with implications for specific involvement in bacterial virulence regulation (Sharma et al. 2010; Deltcheva et al. 2011). Compared to their cis-acting counterparts, trans-acting sRNAs exhibit only a short and imperfect complementarity to their target RNAs, allowing them to control several different target genes. These sRNAs are usually regulated in response to environmental stimuli. Some trans-acting sRNA molecules act as repressors of translation and destabilize mRNA transcripts but others activate and stabilize the target mRNAs (Thomason and Storz 2010; Storz et al. 2011). Although the conventional point of view regarded trans-acting sRNAs as inhibitory antisense regulators, to date a significant number of sRNAs activating bacterial gene expression is known (Frohlich and Vogel 2009). Furthermore, regulatory mechanisms include the stabilization as well as the destabilization of target transcripts (Podkaminski and Vogel 2010). Later in this chapter, we will focus on the structure and function of regulatory RNAs in streptococci. We will give an overview of already characterized sRNAs known to mediate virulence factor regulation in streptococcal diseases, but we will also summarize recent whole-genome screens for sRNAs in pathogenic streptococci.
2 Streptococcal Stand-Alone Transcriptional Regulators
2.1 Multiple Gene Regulators of Group A Streptococci—Mga and Orthologous Regulators
One of the best characterized and probably most important stand-alone virulence regulators in S. pyogenes is Mga. Initially, Mga was identified as positive regulator of the expression of the M-protein encoding emm gene (Caparon and Scott 1987; Okada et al. 1993; Podbielski et al. 1995). Nowadays, it is known that Mga is a global transcriptional activator in the exponential growth phase (Kreikemeyer et al. 2003). The Mga regulon of S. pyogenes comprises multiple genes encoding for virulence factors involved in host cell adhesion (e.g., M- and M-like proteins, fibronectin- and collagen-binding proteins) and immune evasion (e.g., C5a peptidase and other complement inhibitors) (Hondorp and McIver 2007; Fiedler et al. 2010a). Genomic analysis revealed the serotype-dependent presence of two allelic variants of the mga gene in S. pyogenes, mga-1 and mga-2 (Haanes et al. 1992; Hollingshead et al. 1993). The genomic presence of either version is correlated to the S. pyogenes strain’s M-protein class, serum opacity factor production, and especially to the structure of the adjacent emm gene region, i.e., the absence or presence of emm-related genes upstream and downstream of the respective emm gene (Bessen and Hollingshead 1994; Hollingshead and Bessen 1995; Bessen and Lizano 2010). It has been proposed that the allelic mga variants are indicative of tissue tropism of S. pyogenes strains, with mga-1 primarily associated with throat infecting strains and mga-2 associated with skin infecting or “generalist” strains (Bessen et al. 2005; Bessen and Lizano 2010).
Genes directly activated by Mga are referred to as the Mga core regulon and comprise the virulence genes emm, scpA (C5a peptidase), sclA (collagen-like protein), sic (secreted inhibitor of complement), fba (fibronectin binding protein), and sof (serum opacity factor) as well as the mga gene itself (Ribardo and McIver 2006). Furthermore, there are several virulence genes that can be regulated by Mga indirectly, such as the capsule biosynthesis (has-) operon or the cysteine protease gene speB (Ribardo and McIver 2006; Hondorp and McIver 2007). It was also shown that Mga acts as a transcriptional repressor of genes related to sugar metabolism such as the mannose/fructose phosphotransferase system component IIA (Ribardo and McIver 2006; Hondorp and McIver 2007).
For activation of the core regulon genes, a direct binding of Mga in the promoter region of the respective genes is necessary (McIver et al. 1995, 1999; Podbielski et al. 1995; Almengor and McIver 2004). The mechanism of transcriptional activation by Mga binding seems to vary for different genes, depending on the position of the binding sites in relation to the transcriptional start site. Usually, the Mga binding site overlaps at least in part with the -35 region and is thus located in close proximity to the transcriptional start site of the respective genes. Mga bound to these proximal sites probably stabilizes the binding of the RNA polymerase by direct interaction. For some genes (sof-sfbX, sclA), the Mga binding site is located further upstream, indicating that binding of Mga to these distal sites rather activates transcription via DNA binding than via direct interaction of Mga with the RNA polymerase. Finally, upstream of the mga gene there is both, a distal and a proximal Mga binding site combining both activation mechanisms (McIver et al. 1995, 1999; Almengor and McIver 2004; Almengor et al. 2006). Mga activity displays growth phase association with a maximum in the exponential phase (McIver and Scott 1997; Ribardo and McIver 2003). The transcription of the mga gene is not entirely depending on autoregulation but is influenced and fine-tuned by numerous other transcriptional regulators of S. pyogenes, such as the regulators of the RALP-family, the sugar metabolism regulator CcpA, the CovR repressed response regulator TrxR and the sugar metabolism regulator MsmR (Podbielski et al. 1999; Beckert et al. 2001; Almengor et al. 2007; Kreikemeyer et al. 2007; Kratovac et al. 2007; Leday et al. 2008; Fiedler et al. 2010a).
Since Mga is involved in the activation of many of the major S. pyogenes virulence factors, the deletion of the mga gene leads to a dramatic loss of virulence of S. pyogenes in vitro and in animal models. Mga-defective mutants are more sensitive to phagocytosis, exhibit a decreased adherence to human skin tissue sections, an attenuated virulence in intraperitoneal and skin infection mouse models, and show a reduced ability to bind human serum and matrix proteins (Perez-Casal et al. 1993; Kihlberg et al. 1995; Luo et al. 2008; Fiedler et al. 2010b).
Mga orthologous regulators were found in S. pneumoniae (Hemsley et al. 2003; Solano-Collado et al. 2012) and other streptococci, such as S. dysgalactiae, S. equi, S. gordonii, and S. mitis (Vahling and McIver 2006). No Mga-like regulators have been described in S. agalactiae and S. suis. In S. pneumoniae the Mga-like regulator is designated MgrA (TIGR4 strain) or Mga Spn (R6 strain) (Hemsley et al. 2003; Solano-Collado et al. 2012). It has been shown that MgrA is involved in virulence, i.e., by repression of the genes of the pilus-encoding rlrA pathogenicity islet (Hava and Camilli 2002; Hemsley et al. 2003). Since MgrA/Mga Spn can be found in all currently sequenced pneumococcal strains while the rlrA pathogenicity islet is only present in some of them, it is improbable that the rlrA pathogenicity islet genes are the major target of MgrA/Mga Spn regulation (Hoskins et al. 2001; Tettelin et al. 2001; Lanie et al. 2007). Transcriptional activation of the operon downstream of the mga Spn gene in S. pneumoniae R6 by Mga Spn binding to two binding sites in the promoter region of this operon has been shown. The function of the respective operon is not known to date (Solano-Collado et al. 2012). Although the regulatory mechanisms and the role of the S. pneumoniae Mga-like regulators in pathogenesis are not very well understood, it is apparent that they can function as transcriptional activator and repressor as it has also been shown for Mga of S. pyogenes. It is likely that the Mga orthologous regulator(s) play a crucial role in development of full virulence in S. pneumoniae, as it has been shown that the presence of MgrA is required for development of pneumonia in a mouse model (Hava and Camilli 2002; Hemsley et al. 2003).
2.2 LuxS and AI-2 Dependent Quorum Sensing
LuxS is an enzyme of the activated methyl cycle (AMC) and catalyzes the reaction from S-ribosylhomocysteine to homocysteine and 4,5-dihydroxy-2,3-pentanedione which can spontaneously convert into an active furanosyl borate diester designated autoinducer 2 (AI-2) (Schauder et al. 2001; Zhu et al. 2004). AI-2 has originally been described to be involved in cell density dependent gene regulation in Vibrio harveyi (Surette et al. 1999). Nowadays, it is known that AI-2 is produced by numerous gram-negative and gram-positive species and serves the intra- and interspecies quorum sensing (Schauder et al. 2001; Federle and Bassler 2003; Federle 2009). In many pathogenic bacteria, LuxS/AI-2 has been associated with regulatory processes in virulence (Vendeville et al. 2005; Antunes et al. 2010). In S. pyogenes, S. pneumoniae, and S. suis the impact of LuxS/AI-2 on virulence regulation has been investigated to some extent. Also in S. agalactiae the presence of luxS/AI-2 has been described but a detailed analysis is still lacking (Ou et al. 2005; Ouyang et al. 2006).
In S. pyogenes, there is evidence that LuxS/AI-2 is influencing virulence factor expression in a growth phase dependent manner (Lyon et al. 2001). S. pyogenes luxS deletion mutants exhibit, in addition to growth deficiencies, an increased transcription of the streptolysin S precursor gene sagA accompanied by enhanced hemolytic activity (Lyon et al. 2001). Furthermore, a decreased secretion of the immunoglobulin degrading cysteine protease SpeB can be observed. Transcription of speB or secretion of SpeB might depend on LuxS and seems to be S. pyogenes strain specific (Lyon et al. 2001; Marouni and Sela 2003). It has been shown that capsule biosynthesis as well as the transcription of the sRNA gene fasX, the emm3 gene and the immunoglobulin-binding protein-encoding sib gene can be influenced by LuxS in S. pyogenes in a serotype or strain dependent manner (Marouni and Sela 2003; Siller et al. 2008). LuxS deficient mutants are more efficiently internalized into epithelial cells and show better growth in acidic environments or in the presence of human serum (Siller et al. 2008).
LuxS has been proposed to be the key regulator for early biofilm formation in S. pneumoniae (Joyce et al. 2004; Romao et al. 2006; Vidal et al. 2011; Trappetti et al. 2011). Biofilm formation in LuxS deficient mutants is drastically hampered and autolysin and pneumolysin production is decreased. While LuxS activates the transcription of the pneumolysin (ply) and autolysin (lytA) genes (Joyce et al. 2004; Vidal et al. 2011), the com-operon genes involved in the regulation of the genetic competence of S. pneumoniae are repressed by LuxS (Romao et al. 2006; Trappetti et al. 2011). Consequently, LuxS deficient mutants proved to be less virulent in nasopharyngeal mouse models and were outcompeted by the wild-type strain when co-administered in an intraperitoneal mouse model (Stroeher et al. 2003; Joyce et al. 2004).
In S. suis, the deletion of luxS was shown to lead to decreased biofilm formation, capsule biosynthesis, adherence to epithelial cells, hemolytic activity and hydrogen peroxide tolerance (Cao et al. 2011; Wang et al. 2011). In a luxS deficient mutant of a S. suis serotype 2 strain, the transcription of several virulence-associated genes (e.g. genes for fibronectin/fibrinogen-binding protein FbpS, muraminidase released protein Mrp, or extracellular factor EF) was decreased compared to the parental strain (Wang et al. 2011). In zebra fish or piglet models, luxS deficient S. suis mutants were described to be severely attenuated in virulence (Wang et al. 2011; Cao et al. 2011).
In S. pyogenes, S. suis, and S. pneumoniae luxS is transcribed as a monocistronic mRNA. In batch cultures, the highest luxS expression level can be observed during early exponential growth while maximum AI-2 secretion is reached in the transition phase. Hence, the luxS gene expression is apparently not depending on (or correlating with) AI-2 levels (Siller et al. 2008; Han and Lu 2009; Vidal et al. 2011). Although it has been shown that luxS transcription is induced by iron in S. pneumoniae or repressed by the CovR (CsrR) response regulator in S. pyogenes, the regulatory mechanisms controlling luxS expression and AI-2 production in streptococci are not well understood (Marouni and Sela 2003; Trappetti et al. 2011). Anyway, phenotypes caused by the deletion of luxS might not necessarily be associated with the lack of AI-2 production but could be caused by the accumulation of toxic intermediates of the AMC such as S-adenosylhomocystein, as recently described for Streptococcus sanguinis (Redanz et al. 2012). Here, transcriptome analysis revealed that in a luxS deficient mutant 216 genes were differentially expressed in comparison to the wild-type strain. When this strain was complemented with an alternative route of the AMC, preventing the accumulation of S-adenosylhomocystein, only nine genes showed altered transcription in comparison to the wild type (Redanz et al. 2012). This experimental approach dissects the AI-2 effects and the AMC effects and clearly demonstrates that biofilm formation of S. sanguinis is not depending on AI-2 quorum sensing but on an intact AMC. This demonstrates the importance of distinguishing between the quorum sensing and metabolic or toxic effects of luxS deletions. Especially in terms of biofilm formation, an impact of AI-2/LuxS has been described not only for S. pyogenes, S. pneumoniae, and S. suis but also for several streptococci residing in the human oral cavity such as S. mutans, S. anginosus, S. gordonii, or S. intermedius (Blehert et al. 2003; Yoshida et al. 2005; Petersen et al. 2006; Ahmed et al. 2008). Doubtlessly, LuxS is crucial for biofilm formation in streptococci, but it is necessary to reconsider the role of AI-2 dependent quorum sensing in the above-mentioned context.
2.3 Regulators in Control of Metabolism
Streptococcaceae have a relatively small genome but need to be able to quickly adapt to changing nutritional conditions in the course of infection. Consequently, complex regulatory mechanisms are applied to allow efficient use of the nutrients available at the respective site of infection. Transcriptional changes as a consequence of varying nutritional conditions not only affect metabolism but also virulence-related genes. One global regulator responsible for regulation of metabolism and virulence in streptococci CcpA is the central sugar metabolism regulator. CcpA is primarily responsible for C-catabolite repression (CCR), which means repression of genes involved in catabolism of sugars less favorable than glucose. This is achieved by binding of CcpA to C-responsive elements (cre) in the promoter of the respective genes (Price et al. 2011). In S. pyogenes, apart from sugar utilization associated genes also numerous virulence-related genes are repressed by CcpA either directly or indirectly (Kinkel and McIver 2008; Shelburne et al. 2008; Kietzman and Caparon 2011). The impact of CcpA on virulence gene regulation is more pronounced under nutrient limitation (Shelburne et al. 2008). One of the major interfaces between CcpA and virulence in S. pyogenes is the control of mga expression by direct binding of CcpA to at least one cre element in the promoter of the mga gene (Pmga). Binding of CcpA to this cre element increases mga transcription and therefore indirectly induces the expression of Mga-regulated genes (Almengor et al. 2007). Furthermore, CcpA apparently represses the streptolysin S (sag operon) genes (Shelburne et al. 2008; Kinkel and McIver 2008; Kietzman and Caparon 2010). Whether this is due to direct binding to the promoter upstream of the sagA gene or to an indirect regulatory effect is controversially discussed (Shelburne et al. 2008; Kinkel and McIver 2008; Kietzman and Caparon 2010). Furthermore, direct regulation of speB expression by CcpA has been demonstrated (Kietzman and Caparon 2010). Interestingly, data on the contribution of CcpA to virulence in vivo are contradictory. Two studies have been published on the effect of a ccpA deletion in the S. pyogenes M1 strain MGAS5005 on virulence in a CD-1 mouse model. While one of the studies found the mutant to be less virulent (Shelburne et al. 2008) the other group observed a hypervirulent phenotype (Kinkel and McIver 2008).
Much less is known about the contribution of CcpA on virulence gene regulation in other streptococci. In S. suis and S. pneumoniae as well as in S. pyogenes CcpA was shown to be involved in regulation of the capsule biosynthesis (Giammarinaro and Paton 2002; Shelburne et al. 2008; Willenborg et al. 2011). Furthermore, CcpA regulates the transcription of the plasminogen-binding surface enolase (eno) gene and the β-galactosidase gene which is crucial for the colonization of the nasopharynx by S. pneumoniae (Iyer et al. 2005; Kaufman and Yother 2007; Carvalho et al. 2011). A comprehensive transcriptome study on S. pneumoniae wild-type and CcpA deletion strains under several nutritional conditions additionally revealed a CcpA mediated repression of the expression of neuraminidase encoding genes nanA/B, the superoxide dismutase gene sodA and the choline-binding protein gene pcpA (Carvalho et al. 2011). In S. suis, a recent publication described CcpA to be involved in capsule biosynthesis, expression of surface enolase and transcriptional activation of virulence factor encoding genes ofs and cpsA2 (Willenborg et al. 2011). Generally, the role of CcpA in virulence regulation is extremely complex, since CcpA activity is strongly influenced by the availability of sugars in the environment of the bacteria. Hence, the impact of a ccpA deletion on virulence gene expression might be different in the presence of glucose than in the presence of less-favorable sugars in the growth medium.
Another metabolic regulator commonly involved in virulence gene regulation is the branched chain amino acid (BCAA) activated CodY, which is induced under nutritional deprivation conditions and can exclusively be found among the Firmicutes (Sonenshein 2005; Stenz et al. 2011). With a helix-turn-helix motif the dimeric CodY with one BCAA bound to each monomer can bind to conserved palindromic DNA sequences called CodY boxes. Common targets of CodY regulation in streptococci and other bacteria are i.e., oligopeptide permeases (opp) (Malke et al. 2006; Malke and Ferretti 2007; Hendriksen et al. 2008; Stenz et al. 2011) and the codY gene itself (Guedon et al. 2005). Additionally, several virulence factors are directly or indirectly regulated by CodY in streptococci. In S. pyogenes, CodY has been shown to activate the expression of mga, fasX, rofA, and rivR and to repress the expression of covRS and sptRS (Malke et al. 2006; Malke and Ferretti 2007; Kreth et al. 2011). Since all these genes are encoding for important S. pyogenes virulence regulators, the effect of CodY on virulence gene expression, although indirect, is very comprehensive. A deletion of codY in S. pyogenes consequently leads to decreased expression of the genes encoding for M-protein, streptokinase, streptolysin O, NAD-glycohydrolase, C5a peptidase, SpeH and others while an increased transcription of e.g., capsule biosynthesis genes can be observed (Malke et al. 2006; Malke and Ferretti 2007; Kreth et al. 2011). Although CodY binds in the promoter region of its own gene, neither in the promoter regions of the regulator nor of the virulence factor genes, CodY binding motifs have been detected (Malke and Ferretti 2007; Kreth et al. 2011). Hence, the mechanism by which CodY influences the expression of the regulator genes is not known to date. It has been speculated that at least the regulation of CovRS (see Sect. 3.1) could depend on the intracellular potassium level, which is influenced by the impact of CodY on the expression of potassium uptake system genes (Malke and Ferretti 2007). Especially, the interplay between CodY and CovRS has been investigated in more detail by analysis of codY, covR, and codY-covR deletion mutants (Kreth et al. 2011). It has been postulated that the expression of the above-mentioned virulence genes in S. pyogenes are well balanced by the actions of CcpA (integrating information on sugar availability via PTS), CodY (integrating amino acid nutrition information), and CovRS directly acting at the promoter of many virulence factors to adapt to either invasive or noninvasive phenotypes depending on the nutritional conditions. CodY is postulated to counteract the activity of CovRS by stimulating expression of those genes repressed by CovRS (Kreth et al. 2011), leading to a rather invasive phenotype when nutritional conditions are unfavorable.
In S. pneumoniae, the investigation of CodY regulatory effects is complicated by the fact that a deletion of codY is fatal unless an ectopic copy of the gene is present (Caymaris et al. 2010). Originally, it had been published that CodY is activating the expression of pcpA, a choline-binding protein necessary for successful adherence of the bacteria to nasopharyngeal cells (Hendriksen et al. 2008). The S. pneumoniae D39 codY deletion strain used in the study of Hendriksen et al. (2008) has later been sequenced and it has been postulated that mutations in genes for the ferric uptake iron permease (fatC) and an oligopeptide permease (amiC) allowed for tolerance of the codY deletion in that strain (Caymaris et al. 2010). The data indicate that in S. pneumoniae CodY action to certain extent controls the ability of the bacteria to adhere to host cells.
To the best of our knowledge, the role of CodY in S. suis and S. agalactiae has not been investigated yet. For an overview on the involvement of CodY in virulence gene regulation in S. mutans and other gram-positive bacteria, we refer to a recent review published by Stenz et al. (2011).
Next to CcpA and CodY, there are many other metabolic regulators involved in virulence gene regulation in streptococci, such as the regulatory tagatose-1,6-bisphosphate aldolase LacD.1 in S. pyogenes (Loughman and Caparon 2006; 2007), the methionine transport regulator MtaR in S. agalactiae (Shelver et al. 2003; Bryan et al. 2008) or the stringent response amino acid metabolism regulator RelA in S. pyogenes and S. pneumoniae (Malke et al. 2006; Kazmierczak et al. 2009), to name only a few. Apart from the direct or indirect effects of metabolic regulators on virulence gene expression, tight regulation of metabolic processes in response to the changing nutritional conditions encountered by streptococci in the course of infection is crucial for the general fitness of the bacteria and therefore essential for successful infection of the host.
2.4 Transcriptional Regulators of Streptococcal Pilus Genome Regions
Two classes of transcriptional regulators, which are encoded on the chromosomes of several streptococcal species, gained special attention during the last couple of years. The RALP-family of RofA/Nra regulators is present and active in S. pyogenes, S. agalactiae, S. pneumoniae whereas no information is available in S. suis. The second family is the MsmR-like stand-alone transcriptional regulator family, which belongs to the large group of AraC/XylS-type regulators. This family is present and active in S. pyogenes and S. agalactiae. No orthologous genes have been reported in S. pneumoniae and S. suis yet.
As the most intriguing and unique feature of these regulators, presence of the genes of both regulator families in so-called pathogenicity island-like chromosomal regions can be noted. Such regions encode all necessary proteins enabling streptococci to form long pilus-appendages on their surface. Pili are now recognized to play important and pivotal roles in infections caused by streptococci. The genetic makeup of these discrete genomic regions, their size, their composition, aspects of expression, assembly, and function in virulence have recently been reviewed on a comparative basis (Kreikemeyer et al. 2011). Briefly, in S. pyogenes nine different pilus region variants can be found among clinical isolates (FCT1–FCT9), S. pneumoniae isolates encode two pilus variant regions (PI-1 and PI-2), in S. agalactiae, the pilus proteins are encoded in three related genomic regions (island-1, island-2a, and island-2b), and recently S. suis was shown to encode four distinct genomic regions encoding pilus proteins (srtBCD, srtE, srtF, and srtG) (Telford et al. 2006; Takamatsu et al. 2009; Kreikemeyer et al. 2011). However, these important virulence factors are not limited to S. pyogenes, S. agalactiae, S. pneumoniae, and S. suis. There is a growing list of streptococcal species for which pilus genome islands and gene clusters have been identified and surface-localized pilus expression was proven. Recently, Streptococcus gallolyticus, a causative agent of infective endocarditis also associated with colon cancer, was shown to encode three separate pilus loci, named pil1-pil3 (Danne et al. 2011). Also S. oralis, S. mitis, and S. sanguinis, all members of the Mitis group of streptococci, harbor a pilus-encoding genomic region and express variable pili on their surface (Zahner et al. 2011), resembling the genetic organization of the PI-2 islet of S. pneumoniae.
From the virulence point of view, all pili play a similar role in the respective species during the infection process. In case of S. pyogenes, numerous studies have shown their importance for attachment to pharyngeal cells, human tonsillar epithelium, and keratinocytes and suggested a role in autoaggregation and biofilm formation. In S. agalactiae, pili are important for adherence to human brain microvascular endothelial cells and cervical epithelial cells, and have important functions in biofilm formation and resistance against cationic antimicrobial peptides as well as for survival in phagocytes. Mutational analyses revealed a function for S. pneumoniae pili in many pathogenic processes, including adhesion to respiratory cells. Interestingly, mutants in the S. suis srtF pili cluster were not attenuated in host cell adherence and a murine model of S. suis sepsis, suggesting that pili structures are dispensable for critical steps of S. suis pathogenesis and infection (Fittipaldi et al. 2010). The role of pili in the Mitis group of streptococci needs to be elucidated, whereas in S. gallolyticus pili are critical for collagen binding, biofilm formation, and virulence in experimental endocarditis (Zahner et al. 2011; Danne et al. 2011).
Since pilus proteins of many streptococcal species are apparently immunogenic—they might even represent excellent candidates for vaccine development—it is critical for streptococcal pathogens to tightly control expression of the pilus-encoding genes. Within the pilus gene clusters, this is achieved by aforementioned stand-alone transcriptional regulators of the RALP- and XylS/AraC-families. However, also stand-alone regulators and two-component signal TCS encoded outside of the core pilus genomic regions are implicated in pilus gene regulation, but are discussed in other sections of this review.
In S. pyogenes pilus islands, the RALP-family regulator RofA/Nra and the XylS/AraC-family regulator MsmR, both encoded within the FCT regions, adversely control pilus gene transcription (Nakata et al. 2005; Kreikemeyer et al. 2007). Of note, S. pyogenes serotype-dependent regulatory circuits have been reported (Luo et al. 2008), further complicating the attribution of common functions of these regulators. External signals to which pilus expression is responsive include temperature, anaerobic atmosphere, and pH (Granok et al. 2000; Nakata et al. 2009; Manetti et al. 2010). In S. agalactiae, three RALP-family regulators have been described and investigated: (I) RogB, which is present 284 bp upstream of island-2a but is absent from island-2b, has been described to positively control transcription of fbsA, encoding a S. agalactiae fibrinogen-binding protein, negatively regulates capsular polysaccharide gene cluster expression (Gutekunst et al. 2003), and acts as a positive transcriptional regulator of the PI-2a encoded genes (Dramsi et al. 2006), (II) Rga which is located outside of the pilus region and is involved in transcriptional control of the secA2 locus (Mistou et al. 2009) and was recently identified as the major transcriptional activator of the PI-2a island in S. agalactiae (Samen et al. 2011; Dramsi et al. 2012), (III) Gbs1426, which is more distantly related to RogB and Rga. In S. agalactiae, the sag0644 gene, present 380 bp upstream of the island 1, is the respective pilus region AraC/XylS-type regulator with 72 % identity toward the S. pyogenes MsmR regulators present in S. pyogenes FCT regions 3, 4, 7, and 8 (Nakata et al. 2005). Deletion of this gene in S. agalactiae decreased production of pili, suggesting a positive role in pilus expression. Of note, S. agalactiae pilus region PI-2b does not encode any RALP- or AraC/XylS-type regulator.
In S. pneumoniae, almost nothing is known on regulation of genes encoded in PI-1, and PI-2 regulation is only partially studied. Both pilus genomic islands are flanked by regulator-encoding genes (Scott and Zahner 2006; Telford et al. 2006). One gene encodes the transcriptional regulator RlrA, which belongs to the RALP-family, and which was shown to be required for colonization, lung infection, and systemic infection. RlrA is positively autoregulated, and controls expression of six genes within the PI-2 island, acting on four different promoters (Hava et al. 2003; Kreikemeyer et al. 2011). Downstream of both S. pneumoniae pilus islands MgrA is encoded. This transcriptional regulator belongs to the Mga family, best described in S. pyogenes. MgrA activity is also important for development of pneumonia and this regulator acts as transcriptional repressor of the same four promoters controlled by RlrA (Hemsley et al. 2003). MgrA is discussed into more detail elsewhere in this review. It should be noted that S. pneumoniae is the only species, in which MgrA is encoded so close to the pilus genomic regions. In S. pyogenes, the Mga gene is more distantly located to the FCT regions, although there is a clear regulatory circuit connection of Mga and RALP regulators in S. pyogenes, which is absent in S. pneumoniae. Moreover, also metal-dependent regulators were implicated in pilus gene control. For example, the PsaR (Mn2+-dependent) and MerR (metal-dependent) regulator activities converge in the pilus island control (Rosch et al. 2008).
In the S. suis pilus genome islands no transcriptional regulators have been characterized into detail. Only a MerR metal-responsive regulator with homology to S. agalactiae MerR-type regulators has been annotated in the SrtE-S. suis pilus island (Takamatsu et al. 2009).
Two common aspects regulating pilus gene expression need to be mentioned. First, during investigation of clinical isolates many point mutations in pilus genes were identified leading to either altered pilus protein composition or nonpiliated variants. In S. agalactiae, variability in the expression of pili 1 and 2a among isolates are due to frameshift mutations in the aforementioned regulatory genes. One-base-pair deletions inactivating the rogB gene, or in-frame-stop codon mutations inactivating the sag0644 gene were reported. In a S. suis strain carrying a srtF pilus cluster, a nonsense mutation at the 5′ end of the gene encoding the minor pilin subunit was detected (Fittipaldi et al. 2010).
Second, in S. pyogenes and S. pneumoniae, a rather bistable expression mode was found. Nakata and colleagues reported higher expression levels of the S. pyogenes pilus backbone protein FctA at 30 °C compared to 37 °C (Nakata et al. 2009). Moreover, at 37 °C only 20 % of all S. pyogenes cells expressed pili on their surface. This number increases to 47 % at 30 °C, which is a clear indication of a bistable expression mode. In S. pneumoniae, the bistable expression of type I pili is dependent on the native rlrA promoter, as a nonexpressing population reverts to the previous bimodal distribution, whereas the expressing population retains the same high level expression (Basset et al. 2011). Other studies also reported the positive feedback loop being under control of the transcriptional regulator RlrA expression (De Angelis et al. 2011; Basset et al. 2012).
Together, there are many common themes among transcriptional regulators, their action on pilus gene transcription and their regulatory circuits among different streptococcal species.
3 Streptococcal Two-Component Signal Transduction Systems
3.1 The CovRS/CsrRSS system
The CovRS/CsrRS TCS is the best characterized in S. pyogenes and S. agalactiae. In S. pyogenes, it was first discovered by several groups as a major regulator of capsule gene expression, streptolysin S production, and cysteine protease SpeB expression (Levin and Wessels 1998; Heath et al. 1999; Bernish and van de Rijn 1999). This system is functionally connected to S. pyogenes general stress response, including growth in heat, acid environments, and under salt stress. CovRS is also important for growth under iron starvation, in the presence of antimicrobial peptides, and plays a role in the serotype-dependent S. pyogenes biofilm formation and keratinocyte adherence (Dalton et al. 2006; Froehlich et al. 2009; Sugareva et al. 2010). Moreover, it is apparently the major sensor of S. pyogenes for Mg2+ (Gryllos et al. 2007).
Apart from the above-mentioned S. pyogenes virulence factors and functions, many more are directly or indirectly under control of this important TCS. A total of 15 % of all genes encoded on the S. pyogenes chromosome are repressed by CovR, including its own gene. The activity of CovR on most promoters is direct and involves the consensus binding sequence ATTARA, mutation of which relieved CovR repression. Promoter occlusion is the primary mechanism of repression by CovR (Gusa and Scott 2005). Phosphorylation of CovR is critical to its functional activity and leads to dimerization (Gao et al. 2005; Gusa et al. 2006). However, a subcutaneous mouse infection model revealed that CovR is independent of its cognate sensor kinase CovS to get phosphorylated and exert its control functions, suggesting other sources for phosphotransfer (Dalton et al. 2006). Eukaryotic-type serine/threonine kinases, discussed in the next section, are donors for phosphotransfer. Of note, the HK CovS itself was found to inactivate the RR CovR, thereby allowing S. pyogenes to grow under the above-mentioned stress conditions. Thus, the CovRS system has a Janus-like behavior. CovS acts as a kinase to activate the CovR response regulator, which subsequently acts as gene repressor. However, CovS, upon environmental stimulation, can act as a phosphatase inactivating CovR to permit gene transcription from respective promoters. The importance, functional activity and the Janus-like behavior of this system in S. pyogenes have recently been reviewed (Churchward 2007).
Although CovRS is among the most important TCS in S. pyogenes, it is not a master regulator per se, but is rather integrated into existing regulatory networks and is connected and linked to other S. pyogenes stand-alone regulators and sRNAs (Kreikemeyer et al. 2003; Fiedler et al. 2010a). Roberts and Scott investigated the link between CovRS and the Mga regulon (described elsewhere in this review) and found CovRS to repress RivR, a RALP-family regulator (Ralp4). Adjacent to rivR these authors identified rivX, encoding a small regulatory RNA. RivR enhanced transcription by Mga in vivo and in vitro and the rivRX locus was involved in pathogenesis in a mouse soft tissue infection model (Roberts and Scott 2007). Another S. pyogenes TCS, termed TrxSR, which is homologous to the S. pneumoniae HK07/RR07 system, was found as another CovR repressed system (Leday et al. 2008). TrxR activates transcription of Mga-regulated virulence genes, and thus, TrxR defines a new pathway by which CovR affected virulence via control over Mga. A study conducted by Shelburne and colleagues established partially overlapping transcriptomes of Cov and CcpA mutants, proved that both regulator proteins bound to promoters of co-regulated genes, and identified attenuated phenotypes of the mutants in a myositis model (Shelburne et al. 2010). Moreover, CovRS activity was critical for S. pyogenes growth in human blood, during pharyngitis in cynomolgus macaques and mouse soft tissue infection. Pioneering work of Sumby and colleagues linked CovRS function, observed in vitro and from animal studies, to the real situation in clinical isolates (Sumby et al. 2006). Two fundamentally different transcriptome profiles could be detected during comparison of nine clinical isolates. The pharyngeal transcriptome differed by 10 % from the invasive transcriptome. Complete genome sequencing of an invasive transcriptome isolate uncovered a 7-bp frameshift mutation in the CovS encoding gene. Based on these initial observations, further studies revealed that different in vivo-induced covR mutations led to different transcriptomes and that the CovS-mediated regulation of CovR activity is critical for S. pyogenes cycling between pharyngeal and invasive phenotypes (Trevino et al. 2009). Although also wild-type bacteria undergo extensive transcriptional reprogramming under in vitro and in vivo infection conditions, this rapid host adaptation is not sufficient to survive in the host. Rather the hypervirulent cov mutant strains are able to outcompete the wild type, and thus it can be concluded that mutations and rapid reprogramming are critical steps allowing S. pyogenes to switch infectious phenotypes and to move between niches. This puts sociomicrobiological aspects into the focus of infection research (Aziz et al. 2010).
What are the functions of the CovRS system in S. agalactiae? The genes for the CovRS orthologous system in S. agalactiae are part of a seven-gene operon and mutation led to dramatic phenotypic changes (Lamy et al. 2004). Although the mutant adhered much better to epithelial cells, the survival in human serum was attenuated. Moreover, as also reported for S. pyogenes, the S. agalactiae CovRS system is critical for virulence, as proven by neonate rat sepsis and mouse infection models (Lamy et al. 2004; Jiang et al. 2005). A total of 76 genes are repressed whereas 63 were positively regulated. Transcriptome comparison of several strains uncovered a conserved 39 gene core regulon (Jiang et al. 2008). Moreover, there was a significant overlap of the acid stress transcriptome in S. agalactiae with the CovRS regulon (Santi et al. 2009), and CovRS as well as pH-regulated S. agalactiae adherence to host cells of vaginal, cervical and respiratory origin (Park et al. 2012).
In contrast to CovRS in S. pyogenes, the S. agalactiae system can act bidirectional and is not an exclusive repressor system (Jiang et al. 2008). Direct binding of phosphorylated CovR to many of the target gene promoters has been reported (Lamy et al. 2004; Jiang et al. 2008). However, the binding site differs from the consensus sequence reported for S. pyogenes CovR, although both share a high AT content. It can be concluded that the general classes of genes under control of both systems are identical in S. pyogenes and S. agalactiae. It is currently unknown, whether S. agalactiae CovR is dependent on CovS activity, but there is clear evidence linking CovRS and serine/threonine kinase systems also in S. agalactiae (discussed in Sect. 4).
It is obvious that the CovRS system is central to the virulence of both streptococcal species. Many similar functions have been elucidated, but depending on the pathogens primary niche in the host, both systems also underwent a pathogen-specific evolution and adaptation. It remains to be established if point mutations in the CovRS system in S. agalactiae also occur in a disease specific manner in clinical isolates and if such mutants would be as hypervirulent as their S. pyogenes counterparts.
No CovRS orthologs were found in S. pneumoniae. In S. suis exclusively CovR was identified as an orphan response regulator, lacking the adjacent CovS histidine kinase. Virulence studies characterized the S. suis CovR as a globally acting negative modulator of virulence, as in a mutant most phenotypes changed in the direction of higher virulence potential (Pan et al. 2009).
3.2 The CiaHR System
The CiaHR TCS is widespread among streptococci. Genes encoding this TCS are found on the chromosomes of S. pyogenes, S. agalactiae, S. pneumoniae, and also S. suis. The amino acid sequence identity of CiaH and CiaR from different species ranges between 48–71 and 77–85 %, respectively (Riani et al. 2007). Most data on Cia function have been compiled from studies in S. pneumoniae. An increased cefotaxime resistance and impaired natural competence was noted in a S. pneumoniae CiaH mutant (Guenzi et al. 1994). The influence on competence involves sensing of Ca2+ and oxidative stress (Zahner et al. 2002). A key connection between the S. pneumoniae CiaHR regulon, which has been defined in at least three different strains, and competence is the CiaHR control of HtrA expression. This surface expressed serine protease is down-regulated in CiaHR mutants (Paterson et al. 2006). A restoration of htrA expression in the Cia mutant background alone restored natural competence (Sebert et al. 2005). S. pneumoniae Cia regulons comprise roughly 20 genes and apart from competence genes also stress response genes and operons were identified (Paterson et al. 2006), suggesting natural competence as S. pneumoniae stress factor, which is balanced by Cia activity. Of note, many other virulence genes are found in the Cia regulon, which makes it difficult to clearly define its role in virulence. Further studies have revealed that the CiaH acts as kinase/phosphatase, that CiaR needs to be phosphorylated, that CiaR can obtain phosphates independent from CiaH, and that CiaR acts directly on 15 promoters identified by transcriptional mapping (Halfmann et al. 2007, 2011). Those promoters belong to genes involved in teichoic acid biosynthesis, sugar metabolism, chromosome segregation, and protease maturation. Five of those promoters were identified upstream of small noncoding RNAs (Marx et al. 2010). Matching the observation made in the CovRS TCS in S. pyogenes, clinical S. pneumoniae isolates, particularly those with spontaneous β-lactame resistance, apparently acquired mutations in the CiaH encoding gene. This suggested that ciaH alleles, which overstimulate the CiaR regulon expression, are present in clinical isolates (Muller et al. 2011). Although not studied into such a detail as in S. pneumoniae, it is apparent that the S. pyogenes Cia system has different functions. As S. pyogenes is not naturally competent and lacks large parts of the com operon genes, regulation of competence, and as a consequence, also major regulation of stress response is not attributed to S. pyogenes CiaHR. The CiaH sensor mutants in two S. pyogenes serotype M49 strains allowed identification of up to 120 genes as Cia-controlled, with equal numbers up- and down-regulated (Riani et al. 2007). Among those, genes encoding proteins for divalent cation transport, PTS systems, hemolysins, hyaluronidase, matrix-protein-binding factors, and DNAses were identified. Divergent to observations in S. pneumoniae, metal ions did not affect Cia expression significantly, antibiotic resistance was not affected, and only a small number of six stress response genes were differentially transcribed in the S. pyogenes ciaH mutant (Riani et al. 2007). In S. suis, ciaHR mutants revealed a decreased adherence toward HEp-2 and PIEC cells, a higher susceptibility toward killing by RAW2647 macrophages, and were attenuated in mice and pig animal models of infection (Li et al. 2011). Not unexpected, CiaR was found to promote intracellular survival and resistance to innate immune defences in S. agalactiae. A ciaR mutant had a reduced survival in neutrophils, human macrophages and brain microvascular endothelial cells (BMCE) (Quach et al. 2009). In mouse infection models, the mutant was attenuated and showed decreased survival in blood and brain compared to the wild type (Quach et al. 2009). In summary, particularly in S. pneumoniae CiaHR is crucial for natural competence and general virulence, whereas in the other species virulence control is the major function of Cia.
3.3 The IhK/Irr System
The Ihk/Irr TCS of S. pyogenes is highly important for the pathogenesis of these bacteria and is thus reported to be active during S. pyogenes growth in many body fluids and host niches. As human saliva is one of the first liquids encountered after S. pyogenes droplet-mediated entry into the host oral cavity, growth, and persistence in saliva was tested. The most important TCS in hierarchy in saliva is the SptRS TCS. Among many others, also the genes encoding the Ihk/Irr TCS were up-regulated during S. pyogenes growth in saliva (Shelburne, III et al. 2005). Moreover, a role for Ihk/Irr could be established during S. pyogenes growth in human blood, conditions under which up to 75 % of the genes were differentially transcribed, and during phagocytosis by neutrophils, under which varying sets of genes are time-dependently and differentially transcribed (Voyich et al. 2003; Graham et al. 2005). A more recent study discovered 145 differentially transcribed genes during a 2-h S. pyogenes persistence in macrophages, of which many belong to metabolic and energy dependent processes. Ihk/Irr was again established as a key regulator important for the early processes in S. pyogenes-macrophage interaction (Hertzen et al. 2012). A major shift of response regulator activity from time point 2–6 h during persistence was noted, in which Ihk/Irr was down-regulated and the CovRS system was up-regulated. This hinted toward a TCS driven and fine-tuned temporal expression shift in S. pyogenes intracellular life cycle (Hertzen et al. 2012). In S. suis serotype 2, an Ihk/Irr ortholog was recently characterized (Han et al. 2012). Deletion of this system notably attenuated virulence in mice. Mutants showed reduced adherence to host cells, an increased susceptibility to macrophages killing, and a decreased survival under oxidative stress conditions. Of note, the important S. suis virulence factors suilysin, autolysin, and muraminidase-released protein encoding genes were not under Ihk/Irr control. Rather cell metabolism and superoxide dismutase were repressed in the mutant (Han et al. 2012). It is conceivable, that in the other streptococcal species discussed here, other TCS have similar functions although they cannot be classified as Ihk/Irr orthologs based on sequence homology.
3.4 The VicRK System
The VicRK system is noteworthy as it is present and conserved in many Firmicutes and for a long time was considered essential for growth in all organisms after many failed mutation attempts. In the literature, there are a couple of synonymous names, like YycFG, MicAB, and WalRK. An unconditional vicR insertional mutant generated in S. pyogenes allowed insight into functional aspects of this system (Liu et al. 2006). The mutant grew well in rich lab media, but was severely attenuated in growth in nonimmune human blood and serum, had attenuated virulence, and was unstable in mice. However, as phagocytosis and killing was normal, a general influence on evasion of host defences was excluded. Among Vic-controlled genes, those involved in cell-wall hydrolysis (pcsB), phosphotransferase systems, osmoprotectant transporters, and nutrient uptake were identified by transcriptomics (Liu et al. 2006). A view across the species barriers linked the essentiality in some organisms to VicRK exerted control over functions like murein biosynthesis, cell division, lipid integrity, exopolysaccharide synthesis, biofilm formation, and also virulence factor expression. However, the signals stimulating the Vic-system are largely unknown (Winkler and Hoch 2008). In S. pneumoniae, the essentiality of the Vic-system could only be bypassed by a constitutive PcsB cell-wall hydrolase expression, suggesting that control over cell-wall biosynthesis and osmosis is the critical function (Winkler and Hoch 2008). Recently, also in S. suis in vivo swine infection the VikRK system was found up-regulated together with its target gene pcsB (Li et al. 2010). Since not many virulence factor encoding genes were found to be directly controlled by the VicRK TCS, it cannot be designated a general virulence regulator. However, considering that central cell-wall turnover and metabolic functions are also directly linked to fitness of the pathogens in vivo there is a clear link of VicRK to virulence. Apparently, homologs and orthologs were also found in Actinobacteria like Streptomyces, Mycobacterium, and Corynebacterium species, which places the Vic-system in the focus as excellent target for novel antimicrobial therapy strategy developments (Winkler and Hoch 2008). First structure-based virtual screenings of potential inhibiting compounds from the SPECS library have been done in S. pneumoniae, and interesting target compounds with activity against S. pneumoniae were identified in vitro and also in a mouse sepsis infection model. These compounds decreased mortality in mice and had no general cytotoxic effect (Li et al. 2009).
4 Eukaryotic-Type Serine/Threonine Kinases in Streptococci
Results discussed in the paragraphs above have already indicated that some response regulators can be phosphorylated independently of their cognate kinases, suggesting a potential link to other TCS within the same organism. Just recently, a whole new level of signal recognition and signal processing has been discovered in prokaryotes thanks to advances in genetic strategies and genome sequencing approaches. This includes eukaryotic-like serine/threonine kinases (STKs) which were found to be linked to adjacent phosphatases (STPs). An in-depth review focussing on discovery and functional analysis of these systems in various species has been published (Burnside and Rajagopal 2011). What places these signaling and response systems into the research focus is the fact that their eukaryotic counterparts are excellent targets for therapy. Many STK inhibitors are approved by the United States Food and Drug Administration (USFDA) or at various stages in clinical trials. Thus, the prospect that such inhibitors also work for the bacterial STK/STP systems is promising. The STKs in S. pyogenes, S. pneumoniae, and S. agalactiae have been identified at nearly the same time (Rajagopal et al. 2003; Echenique et al. 2004; Jin and Pancholi 2006). The S. pyogenes STK and STP (SP-STK and SP-STP) were characterized as functional manganese-dependent kinase and phosphatase enzymes (Jin and Pancholi 2006). An altered cell-shape, incomplete cell separation, a loosely associated electron-dense layer, and a tendency to settle during growth in laboratory media were the initial phenotypes after mutation of SP-STK. Increases in doubling time and hemolysis activity were noted. For the virulence of S. pyogenes, the SP-STK was found to be critical for host cell adherence and resistance to phagocytic killing. As a mediator of SP-STK activity on gene regulation, phosphorylation of a 10 kDa histone-like protein was suggested (Jin and Pancholi 2006). The SP-STP was subsequently shown to be involved in phosphorylation of the S. pyogenes VicRK and CovRS TCS (Agarwal et al. 2011). In S. agalactiae, mutants defective in STK and STP were attenuated in a neonatal rat sepsis model (Rajagopal et al. 2003). STK expression is vital for resistance to human blood, neutrophils, and oxidative stress. Transcription of the S. agalactiae ß-hemolysin is mediated via threonine phosphorylation of the CovR response regulator, which improved CovR repression (Rajagopal et al. 2006). This verified a regulatory connection between TCS and STK/STP systems in these pathogenic streptococcal species. STP mutation in S. agalactiae allowed identification of more phenotypes and up to 294 differentially transcribed genes were found. Phosphopeptide enrichment methods allowed identification of 35 STK/STP phosphorylated peptides as part of 27 different proteins (Burnside et al. 2011). In S. pneumoniae, pleiotropic functions are under control of a single STK encoded on the chromosome, including virulence, competence, antibiotic resistance, growth and stress response (Echenique et al. 2004), and functional links to active TCS systems were found (Agarwal et al. 2012). Direct STK phosphorylation of target proteins involved in ion transport, cell division, and RNA-polymerization was proven. Currently, the role of the phosphatase is not known. However, it is tempting to speculate that the S. pneumoniae phosphatase functionally resembles S. pyogenes and S. agalactiae phosphatases. Together, these streptococcal serine/threonine kinase–phosphatase systems play an important role in the physiology and virulence of the species discussed here. They apparently have rather conserved functions in all streptococci. Currently, not much is known about the signals that initiate their activity and where in the regulatory hierarchy in conjunction with stand-alone transcriptional regulators and TCS they play their major role. The success story using inhibitors in their eukaryotic counterparts to fight human diseases raises hope that novel inhibitors counteracting bacterial STK/STP systems will be developed and serve as innovative therapies in combating severe bacterial infections.
5 Noncoding Regulatory RNAs
5.1 Cis-Regulatory RNAs
Diverse cis-regulatory systems controlling virulence factor genes have been described in gram-positive pathogens including RNAIII in Staphylococcus aureus (Novick et al. 1993) and a thermosensor located in the 5’-UTR of the prfA mRNA in Listeria monocytogenes (Johansson et al. 2002). One of the first examples for a regulatory RNA discovered in S. pyogenes was the untranslated 500 bases RNA of the streptococcal pleiotropic effect locus (pel). The region contains the structural gene sagA coding for a precursor of the streptococcal hemolysin SLS. The locus was initially detected following transposon mutagenesis of a S. pyogenes M49 serotype strain. It was found to positively regulate the genes of important streptococcal secreted and surface virulence factors. Loss of the 500 bases transcript decreased transcription of emm and speB genes and reduced secretion of streptokinase. Whether a protein encoded by the locus or an untranslated RNA was responsible for the regulatory effects was not clear at the time (Li et al. 1999). In a later study, it could be shown that pel-dependent virulence factor regulation was mediated by a 459-bases untranslated RNA molecule (Pel). It was demonstrated that regulation by Pel occurs at both transcriptional (e.g. emm and sic) and post-transcriptional (e.g. SpeB) levels (Mangold et al. 2004). Strain specificity of Pel function is indicated by the fact that in a sagA-deficient mutant with an M6 background, emm transcription was not affected (Biswas et al. 2001). Similar results have been obtained in S. pyogenes M1 and M18 Tn916 sagA mutant strains (Betschel et al. 1998). Additionally, pel deletion mutant analysis of four M1T1 S. pyogenes isolates did not confirm any regulatory function of the Pel sRNA in this serotype (Perez et al. 2009).
Expression of ribosomal protein genes (rplJ-rplL) in E. coli is regulated by an autogenous control mechanism involving the 5’-untranslated region of the mRNA. Ribosomal proteins L10 and L12 bind to the leader region of the L10 operon and inhibit translation (Johnsen et al. 1982). The secondary structure of the leader RNA is important for the formation of a stable complex (Christensen et al. 1984). A family of putative ribosomal protein leader autoregulatory structures was also found in B. subtilis and other Firmicutes. For the genus Streptococcus, 393 members of the L10-leader RNA family from 385 species are listed in Rfam (Gardner et al. 2009). Recently, the expression of a L10-leader sRNA, was demonstrated in different growth phases of S. mutans (Xia et al. 2012). Growth phase and pH dependency of expression indicate a regulatory role for the L10-leader in the acid adaptive response in this cariogenic bacterium. Expression of L10-leader RNAs and other ribosomal leader RNAs, including L13-, L20-, and L21-leader RNA, was also observed in S. pyogenes by tiling array analyses (Perez et al. 2009; Patenge et al. 2012) and in S. pneumoniae by tiling array analysis and RNAome sequencing (Kumar et al. 2010; Acebo et al. 2012; Mann et al. 2012).
5.2 FMN-Riboswitch
Riboswitches are regulatory elements found in 5’-untranslated regions of prokaryotic mRNAs. They are acting in cis by controlling expression of their downstream genes through a metabolite-induced alteration of their secondary structure. Doing so, riboswitches play a prominent role in bacterial metabolism control (Nudler and Mironov 2004). The flavin mononucleotide (FMN) riboswitch is a metabolite-dependent riboswitch that directly binds FMN. The element controls expression of genes that encode for FMN biosynthesis and transport proteins (Vitreschak et al. 2002). A link to virulence-related gene expression comes from L. monocytogenes, where the riboflavin analog roseoflavin targets an FMN-riboswitch and blocks L. monocytogenes growth, but also stimulates virulence gene expression and infection (Mansjo and Johansson 2011). Furthermore, two riboswitches acting as noncoding RNAs in trans control expression of the virulence regulator PrfA in L. monocytogenes (Loh et al. 2009). Metabolite-driven trans-regulatory sRNAs form a new class of regulatory noncoding RNAs in bacteria. Expression of FMN-riboswitches has also been detected in streptococci in several recent sRNA expression screens (Perez et al. 2009; Kumar et al. 2010; Patenge et al. 2012; Mann et al. 2012). To date, no involvement of FMN-riboswitches in virulence gene expression has been documented in streptococci. Nevertheless, like other riboswitches, FMN-riboswitches may represent interesting novel drug targets (Blount and Breaker 2006). The natural antibacterial compound roseoflavin binds to FMN-riboswitches and has been shown to inhibit bacterial growth and to regulate gene expression in B. subtilis and L. monocytogenes (Lee et al. 2009; Mansjo and Johansson 2011). The identification of compounds that trigger FMN-riboswitch function in streptococci may be a promising alley for the development of novel antimicrobial drugs.
5.3 Trans-Antisense sRNAs
sRNAs working by trans-antisense binding to their target mRNAs tend to interact with conventional TCS in streptococci. Several examples of sRNA-TCS networks will be described in this chapter.
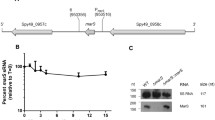
The small regulatory RNA FasX was one of the first characterized sRNAs in streptococci. It was initially identified during the analysis of the two-component type regulator Fas (fibronectin/fibrinogen binding/hemolytic activity/streptokinase regulator) in S. pyogenes (Kreikemeyer et al. 2001). The fasBCA operon in S. pyogenes encodes two potential sensor kinases and one response regulator. It is expressed in a growth phase-dependent manner and controls the production of several secreted virulence factors such as streptokinase and streptolysin S. Downstream of the fasBCA transcriptional unit, an independently transcribed gene, fasX, was found to encode a short RNA molecule. Expression of fasX depends on the RR FasA. Gene replacement of fasX resulted in a phenotype similar to fasBCA or fasA knock-out mutations with prolonged expression of extracellular matrix-protein-binding adhesins and reduced expression of secreted virulence factors. Complementation of the fasX deletion mutant, with fasX expressed in trans from a plasmid, restored the wild-type fasBCA regulation pattern (Kreikemeyer et al. 2001). From these data, it was concluded that FasX, a non-translated RNA, is the main effector molecule of the Fas regulon. Accordingly, S. pyogenes carrying a fasX deletion induced a reduced response of host epithelial cells in terms of cytokine production, apoptosis, and cytotoxicity parameters (Klenk et al. 2005). FasX enhances streptokinase activity in S. pyogenes. Stimulation of streptokinase gene (ska) expression by FasX is achieved by binding to the 5’ end of the ska mRNA and thereby increasing the stability of transcript (Ramirez-Pena et al. 2010). Lack of FasX-ska-mRNA interaction in fasX mutants led to decreased transcript levels and consequently to a decreased streptokinase protein abundance (Ramirez-Pena et al. 2010). Recently, it has been shown that FasX also controls pilus gene expression by pairing to the extreme 5’ end of the pilus biosynthesis operon transcript. In this case, the resulting RNA–RNA interaction reduces the stability of the mRNA, while at the same time inhibiting translation of at least the first gene in the pilus biosynthesis operon. As a consequence of down-regulated pilus expression, adherence to host epithelial cells by S. pyogenes was reduced (Liu et al. 2012). Virulence gene regulation by FasX works by classical antisense binding to target mRNAs, but diverse mechanisms could be identified as depicted in Fig. 1: in one example sRNA-mRNA interaction stabilizes the target (ska expression, Fig. 1a), in the other it destabilizes the target (pilus biosynthesis control, Fig. 1b). This is particularly striking, because up-regulation of Ska as well as pilus down-regulation represent activities related to the transition of S. pyogenes from the colonization stage of infection to the dissemination phase.
Schematic of the differential outcome of FasX binding. The start codons of the respective target mRNAs are indicated with bold letters. a Stabilization of ska transcripts by interaction with FasX, pac man: unknown RNAse molecule. b Destabilization of pilus transcripts and inhibition of cpa-mRNA translation following interaction with FasX, mushroom: ribosome
Another link between a two-component system and sRNAs became evident recently in S. pneumoniae. The ciaRH regulatory network plays a major role in the maintenance of cell-wall integrity. Penicillin-binding protein independent resistance to beta-lactam antibiotics is conferred by the ciaRH regulatory system (Zahner et al. 2002). Genes controlled by the ciaRH system are involved in cell-wall biochemistry. Bacteria carrying mutations in ciaH failed to develop genetic competence due to repression of the comCDE operon region (Mascher et al. 2003). ciaRH mutants were hypersusceptible to a variety of lysis-inducing conditions (Mascher et al. 2006). In two recent studies, a new level of regulation has been introduced to the ciaRH network in S. pneumoniae. Among the genes regulated by CiaR, five sRNA genes have been identified, designated cia-dependent small RNAs (csRNAs). All csRNAs identified so far, show a high sequence similarity and share the same mfold-predicted secondary structure presenting with two stem loops, separated by a stretch of unpaired bases. Deletion mutants lacking csRNA4 and csRNA5, respectively, showed enhanced stationary phase autolysis (Halfmann et al. 2007). The mechanism of csRNA4/5 function is not known yet, but it does not seem to involve interference with LytA and LytC production. Genes for csRNAs have been detected on the basis of CiaR binding site presence over a wide range of streptococcal genomes, including S. mitis, S. oralis, S. sanguinis, and S. pyogenes. Expression of csRNA genes has been demonstrated by Northern blot analyses and suggests that genes for csRNAs belong to the regulon of the response regulator CiaR in all streptococcal species (Marx et al. 2010).
Another excellent example of a regulatory cascade that involves the function of an sRNA is the rivRX (RofA-like protein IV regulator R/X) operon in S. pyogenes. The global transcriptional regulator CovR represses mga and rivRX. RivR activates mga expression and its mechanism has been studied in a covR − background (Roberts and Scott 2007). RivR belongs to the RofA family of transcriptional regulators (described in Sect. 2.4) and contains a DNA-binding site. The protein seems to interact with Mga in order to enhance mga expression. The genes rivR and rivX are co-transcribed but mediate distinct pathways of Mga regulon activation. The rivX sequence contains two putative ORFs. Nonsense mutations in the potential start sites revealed that the rivX transcript rather than peptides encoded by rivX are responsible for the regulatory phenotype. From primer extension analysis, it was concluded that RNA processing could lead to the production of RivX following co-transcription of rivR and rivX explaining the independent function of the two genes. RivX stimulation of mga expression and Mga-activated gene expression depended on the presence of Mga protein. There was no stabilization of mga transcript observed. The mechanism of RivX function seems to involve a direct or indirect interaction with the mga transcript leading to enhancement of Mga translation (Roberts and Scott 2007). The RivR/X system works as integrator of the signals provided by the global CovR and Mga regulatory networks. This cross-talk allows the pathogen to respond to a broad variety of external stimuli with the fine-tuned expression of appropriate virulence factors.
5.4 sRNA Interaction with Proteins
Clustered, regularly interspaced, short palindromic repeat (CRISPR) loci are well-known to provide an adaptive RNA-based immune system in bacteria and archaea. CRISPR protects the cells from horizontal gene transfer originating from phage and plasmid DNA (Marraffini and Sontheimer 2010). Differential RNA sequencing identified a CRISPR/Cas locus in S. pyogenes SF370 (M1 serotype) (Deltcheva et al. 2011) encoded by the system II (Nmeni/CASS4 subtype) (Haft et al. 2005). This locus encodes the trans-activating CRISPR RNA (tracrRNA), which is, in concert with RNAase III and the CRISPR-associated Csn1 protein, responsible for the maturation of CRISPR RNA (crRNA) (Deltcheva et al. 2011). Following crRNA maturation, the cleavage of substrate DNA needs to be initiated. Very recently, it could be shown that the DNA endonuclease Cas9 from the type II CRISPR system in S. pyogenes was guided by a dual RNA molecule to its target DNA. The RNA–RNA complex consisted of the activating tracrRNA and the targeting crRNA, which contained a sequence complementary to the DNA substrate (Jinek et al. 2012). In an independent study, it could be shown that the Cas9-crRNA complex of the S. thermophilus CRISPR/Cas system was able to cleave in vitro an artificial DNA substrate containing a sequence complementary to crRNA (Gasiunas et al. 2012). Bacterial CRISPR/Cas systems seem to be functionally conserved over a wide range of phyla. It could be shown that the S. thermophilus CRISPR/Cas system provided immunity in E. coli (Sapranauskas et al. 2011). An excellent description of the function of the CRISPR system in streptococci is included in a recent review about sRNAs by Le Rhun and Charpentier (Le Rhun and Charpentier 2012).
Formerly regarded as a house keeping RNA, the 4.5S RNA, a component of the bacterial signal recognition particle (SRP), represents another untranslated RNA with influence on streptococcal virulence (Trevino et al. 2010). While the 4.5S RNA gene is not essential for streptococcal growth under laboratory culture conditions, it proved to be essential for S. pyogenes to cause lethal infections in a murine bacteraemia model of infection. Mutation of the 4.5S RNA gene resulted in an altered secretome, including a reduction in secretion of the hemolysin streptolysin O and the SpeB protease. Moreover, remodeling of the S. pyogenes transcriptome was observed following 4.5S RNA mutation. More detailed assessment of gene expression upon loss of the 4.5S RNA gene revealed that differences in abundance of grab, speB, spy0430, and slo were covS-dependent. A further link of the 4.5S RNA gene to virulence was the strong reduction of growth in human saliva of S. pyogenes mutants affected in the gene and decreased virulence of these strains in a murine soft tissue infection model.
5.5 Bioinformatics Prediction Tools for sRNAs
Novel bioinformatics tools and whole-genome expression analyses employing tiling arrays or next generation sequencing helped to study the function of sRNAs in gram-positive pathogens (Mraheil et al. 2010). As knowledge about the role of regulatory RNAs in gram-positive bacteria is rising, new tools are being developed for the analysis of RNA structure and function, e.g., a database focusing on sRNA data from gram-positive bacteria (Pischimarov et al. 2012).
One of the most prominent bioinformatics prediction tools invented for sRNAs was the sRNA identification protocol using high throughput technology (SIPHT) tool, which has been used for many bacterial species (Livny et al. 2005; Livny and Waldor 2007). However, comparison of the prediction results with the actual in vivo expression of sRNAs, often revealed a low overlap between the different screening methods (Perez et al. 2009; Arnvig and Young 2009; Mraheil et al. 2011). This phenomenon is due to a combination of limitations of the prediction programs and the fact that not all sRNAs are expressed under all conditions. Today, the development of sRNA prediction software with improved properties is still ongoing. Several recently published bioinformatics tools have been used for the identification of putative sRNAs in streptococci (Raasch et al. 2010; Sridhar et al. 2010; Pichon et al. 2012).
5.6 Whole-Genome sRNA Expression Screens
In recent years, whole-genome sRNA screens in streptococci employing either tiling array or next generation sequencing approaches, revealed an unexpected number of potential sRNAs (Tsui et al. 2010; Kumar et al. 2010; Mraheil et al. 2011; Beaume et al. 2011; Chen et al. 2011). Based on a previous bioinformatic prediction of putative sRNAs (Livny et al. 2006) expression of 40 putative sRNAs was tested in S. pneumoniae strain D39 by Northern analysis (Tsui et al. 2010). Nine new pneumococcal sRNAs were identified and the previously reported CcnA sRNA (Halfmann et al. 2007) was confirmed. However, functional characterization of deletion mutants and ectopic overexpression constructs of three of the candidate sRNA genes did not reveal strong effects on the phenotypes tested in this study. In a whole-genome approach, a total of 50 sRNAs from the intergenic regions of S. pneumoniae TIGR4 were identified using high-resolution genome tiling arrays (Kumar et al. 2010). In a deep sequencing approach, 88 regulatory sRNAs were identified in the TIGR4 strain of S. pneumoniae (Acebo et al. 2012). Of those, three housekeeping sRNAs were detected, several riboswitches and other cis-regulatory RNAs and 68 novel sRNAs. One of the novel candidates seems to be involved in the modulation of competence regulation in S. pneumoniae (Acebo et al. 2012). In another recent RNAseq study in S. pneumoniae TIGR4, 89 sRNAs were detected, 56 of which were novel. Tn-seq analysis testing relative fitness of bacterial mutants during infection predicted a high number of sRNAs involved in pneumococcal pathogenesis (Mann et al. 2012). Attenuated fitness was predicted during infection of the nasopharynx for 26 sRNAs, in the lung for 28 sRNAs, and in the bloodstream for 18 candidates. Fitness predictions were confirmed by individual targeted deletions in a subset of sRNAs. The high number of sRNA genes involved in pathogenesis underlines the overall importance of sRNAs in streptococcal virulence.
A whole-genome intergenic tiling array screen of S. pyogenes M1T1 identified approximately 40 sRNAs that were expressed during the exponential growth phase in cells cultivated in THY complex medium (Perez et al. 2009). There was a high conservation of sRNA genes among S. pyogenes strains, but the expression of the individual genes was found to be strain dependent. A targeted deletion within the pel region did not confirm the phenotype reported from other S. pyogenes strains. Expression of 16 candidate genes was confirmed by Northern blot analyses. Together with a former bioinformatics prediction (Livny et al. 2006), the number of putative sRNAs in S. pyogenes amounts to 75. In a whole-genome intergenic tiling array screen in S. pyogenes M49, 55 putative sRNAs were identified. A total of 42 sRNAs were novel, whereas 13 RNAs had been described before. The sequences of most of the candidates were conserved over streptococcal genomes. However, comparison of the sRNA expression data to the above-mentioned analysis of the M1T1 strain and to two in silico screening methods revealed a low overlap between the different approaches (Patenge et al. 2012). Thus, the investigation of several conditions and the combination of screening tools will be necessary to gain a comprehensive understanding of the abundance of sRNAs in S. pyogenes. It is to be expected that further analyses of S. pyogenes genomes by RNAseq will lead to the identification of more sRNA genes.
6 Conclusions
The existing and still increasing richness of information on streptococcal transcriptional regulators, two-component signal transduction systems, eukaryotic-like serine/threonine kinase/phosphatase systems, and noncoding regulatory RNAs summarized in this review underscores the importance to decipher the regulatory networks acting in these pathogenic bacteria. A detailed understanding of virulence and pathogenicity mechanisms is critical for the development of novel antibacterial therapies. This review highlighted the presence of many orthologous/homologous regulators and regulatory mechanisms across streptococcal species, of which many have similar functions and are central for the pathogenesis of all the species. In parallel, many have different functions or have undergone a pathogen-specific alteration, including strain- or serotype-specific adaptation. This raises the question whether developing interference strategies targeting regulators and regulatory networks is a promising goal that should be pursued further. In the opinion of the authors, this question can be answered with “yes”. What is required to develop such interference strategies/therapies? (I) The complete operons/regulons of interesting candidates need to be identified. (II) Knowledge needs to be transferred from in vitro to in vivo experiments. (III) Particularly the species-specific regulators/regulatory mechanisms should be kept in focus, as they allow a selective targeting without harmful effects on related species or the residual bacterial flora. In the opinion of the authors the goal of interference therapy should be species-specific virulence/pathogen attenuation rather than killing, as such a strategy allows the immune system of the host to cope with infections and develop immunity. Such novel agents need to be accompanied by available antibiotic approaches. Particular noncoding regulatory RNAs provide a wealth of potential targets, which fulfill above criteria. What are possible means to interfere with their function? In the author’s lab antisense-PNA (peptide nucleic acids) technology is currently explored for specific sRNA-activity interference.
References
Acebo P, Martin-Galiano AJ, Navarro S, Zaballos A, Amblar M (2012) Identification of 88 regulatory small RNAs in the TIGR4 strain of the human pathogen Streptococcus pneumoniae. RNA 18:530–546
Agarwal S, Agarwal S, Pancholi P, Pancholi V (2011) Role of serine/threonine phosphatase (SP-STP) in Streptococcus pyogenes physiology and virulence. J Biol Chem 286:41368–41380
Agarwal S, Agarwal S, Pancholi P, Pancholi V (2012) Strain-specific regulatory role of eukaryote-like serine/threonine phosphatase in pneumococcal adherence. Infect Immun 80:1361–1372
Ahmed NA, Petersen FC, Scheie AA (2008) Biofilm formation and autoinducer-2 signaling in Streptococcus intermedius: role of thermal and pH factors. Oral Microbiol Immunol 23:492–497
Almengor AC, McIver KS (2004) Transcriptional activation of sclA by Mga requires a distal binding site in Streptococcus pyogenes. J Bacteriol 186:7847–7857
Almengor AC, Walters MS, McIver KS (2006) Mga is sufficient to activate transcription in vitro of sof-sfbX and other Mga-regulated virulence genes in the group A Streptococcus. J Bacteriol 188:2038–2047
Almengor AC, Kinkel TL, Day SJ, McIver KS (2007) The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the group A Streptococcus. J Bacteriol 189:8405–8416
Antunes LC, Ferreira RB, Buckner MM, Finlay BB (2010) Quorum sensing in bacterial virulence. Microbiology 156:2271–2282
Arnvig KB, Young DB (2009) Identification of small RNAs in Mycobacterium tuberculosis. Mol Microbiol 73:397–408
Aziz RK, Kansal R, Aronow BJ, Taylor WL, Rowe SL, Kubal M, Chhatwal GS, Walker MJ, Kotb M (2010) Microevolution of group A streptococci in vivo: capturing regulatory networks engaged in sociomicrobiology, niche adaptation, and hypervirulence. PLoS One 5:e9798
Basset A, Turner KH, Boush E, Sayeed S, Dove SL, Malley R (2011) Expression of the type 1 pneumococcal pilus is bistable and negatively regulated by the structural component RrgA. Infect Immun 79:2974–2983
Basset A, Turner KH, Boush E, Sayeed S, Dove SL, Malley R (2012) An epigenetic switch mediates bistable expression of the type 1 pilus genes in Streptococcus pneumoniae. J Bacteriol 194:1088–1091
Bastet L, Dube A, Masse E, Lafontaine DA (2011) New insights into riboswitch regulation mechanisms. Mol Microbiol 80:1148–1154
Beaume M, Hernandez D, Docquier M, Delucinge-Vivier C, Descombes P, Francois P (2011) Orientation and expression of methicillin-resistant Staphylococcus aureus small RNAs by direct multiplexed measurements using the nCounter of NanoString technology. J Microbiol Methods 84:327–334
Beckert S, Kreikemeyer B, Podbielski A (2001) Group A streptococcal rofA gene is involved in the control of several virulence genes and eukaryotic cell attachment and internalization. Infect Immun 69:534–537
Bernish B, van de Rijn I (1999) Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem 274:4786–4793
Bessen DE, Hollingshead SK (1994) Allelic polymorphism of emm loci provides evidence for horizontal gene spread in group A streptococci. Proc Natl Acad Sci U S A 91:3280–3284
Bessen DE, Lizano S (2010) Tissue tropisms in group A streptococcal infections. Future Microbiol 5:623–638
Bessen DE, Manoharan A, Luo F, Wertz JE, Robinson DA (2005) Evolution of transcription regulatory genes is linked to niche specialization in the bacterial pathogen Streptococcus pyogenes. J Bacteriol 187:4163–4172
Betschel SD, Borgia SM, Barg NL, Low DE, De Azavedo JC (1998) Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect Immun 66:1671–1679
Biswas I, Germon P, McDade K, Scott JR (2001) Generation and surface localization of intact M protein in Streptococcus pyogenes are dependent on sagA. Infect Immun 69:7029–7038
Blehert DS, Palmer RJ Jr, Xavier JB, Almeida JS, Kolenbrander PE (2003) Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions. J Bacteriol 185:4851–4860
Blount KF, Breaker RR (2006) Riboswitches as antibacterial drug targets. Nat Biotechnol 24:1558–1564
Brantl S (2009) Bacterial chromosome-encoded small regulatory RNAs. Future Microbiol 4:85–103
Bryan J, Liles R, Cvek U, Trutschl M, Shelver D (2008) Global transcriptional profiling reveals Streptococcus agalactiae genes controlled by the MtaR transcription factor. BMC Genomics 9:607
Burnside K, Rajagopal L (2011) Aspects of eukaryotic-like signaling in gram-positive cocci: a focus on virulence. Future Microbiol 6:747–761
Burnside K, Lembo A, Harrell MI, Gurney M, Xue L, BinhTran NT, Connelly JE, Jewell KA, Schmidt BZ, de los RM, Tao WA, Doran KS, Rajagopal L (2011) Serine/threonine phosphatase Stp1 mediates post-transcriptional regulation of hemolysin, autolysis, and virulence of group B Streptococcus. J Biol Chem 286:44197–44210
Cao M, Feng Y, Wang C, Zheng F, Li M, Liao H, Mao Y, Pan X, Wang J, Hu D, Hu F, Tang J (2011) Functional definition of LuxS, an autoinducer-2 (AI-2) synthase and its role in full virulence of Streptococcus suis serotype 2. J Microbiol 49:1000–1011
Caparon MG, Scott JR (1987) Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci U S A 84:8677–8681
Carapetis JR, Steer AC, Mulholland EK, Weber M (2005) The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694
Carvalho SM, Kloosterman TG, Kuipers OP, Neves AR (2011) CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS One 6:e26707
Caymaris S, Bootsma HJ, Martin B, Hermans PW, Prudhomme M, Claverys JP (2010) The global nutritional regulator CodY is an essential protein in the human pathogen Streptococcus pneumoniae. Mol Microbiol 78:344–360
Chen C, Tang J, Dong W, Wang C, Feng Y, Wang J, Zheng F, Pan X, Liu D, Li M, Song Y, Zhu X, Sun H, Feng T, Guo Z, Ju A, Ge J, Dong Y, Sun W, Jiang Y, Wang J, Yan J, Yang H, Wang X, Gao GF, Yang R, Wang J, Yu J (2007) A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One 2:e315
Chen Y, Indurthi DC, Jones SW, Papoutsakis ET (2011) Small RNAs in the genus Clostridium. MBio 2:00340–0010
Christensen T, Johnsen M, Fiil NP, Friesen JD (1984) RNA secondary structure and translation inhibition: analysis of mutants in the rplJ leader. EMBO J 3:1609–1612
Churchward G (2007) The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol Microbiol 64:34–41
Cunningham MW (2000) Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511
Dramsi S, Dubrac S, Konto-Ghiorghi Y, Da C, V, Couve E, Glaser P, Caliot E, Debarbouille M, Bellais S, Trieu-Cuot P, Mistou MY (2012) Rga, a RofA-like regulator, is the major transcriptional activator of the PI-2a pilus in Streptococcus agalactiae. Microb Drug Resist 18:286–297
Dalton TL, Collins JT, Barnett TC, Scott JR (2006) RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. J Bacteriol 188:77–85
Danne C, Entenza JM, Mallet A, Briandet R, Debarbouille M, Nato F, Glaser P, Jouvion G, Moreillon P, Trieu-Cuot P, Dramsi S (2011) Molecular characterization of a Streptococcus gallolyticus genomic island encoding a pilus involved in endocarditis. J Infect Dis 204:1960–1970
De Angelis G, Moschioni M, Muzzi A, Pezzicoli A, Censini S, Delany I, Lo SM, Sinisi A, Donati C, Masignani V, Barocchi MA (2011) The Streptococcus pneumoniae pilus-1 displays a biphasic expression pattern. PLoS One 6:e21269
Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E (2011) CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471:602–607
Dramsi S, Caliot E, Bonne I, Guadagnini S, Prevost MC, Kojadinovic M, Lalioui L, Poyart C, Trieu-Cuot P (2006) Assembly and role of pili in group B streptococci. Mol Microbiol 60:1401–1413
Echenique J, Kadioglu A, Romao S, Andrew PW, Trombe MC (2004) Protein serine/threonine kinase StkP positively controls virulence and competence in Streptococcus pneumoniae. Infect Immun 72:2434–2437
Federle MJ (2009) Autoinducer-2-based chemical communication in bacteria: complexities of interspecies signaling. Contrib Microbiol 16:18–32
Federle MJ, Bassler BL (2003) Interspecies communication in bacteria. J Clin Invest 112:1291–1299
Fiedler T, Sugareva V, Patenge N, Kreikemeyer B (2010a) Insights into Streptococcus pyogenes pathogenesis from transcriptome studies. Future Microbiol 5:1675–1694
Fiedler T, Kreikemeyer B, Sugareva V, Redanz S, Arlt R, Standar K, Podbielski A (2010b) Impact of the Streptococcus pyogenes Mga regulator on human matrix protein binding and interaction with eukaryotic cells. Int J Med Microbiol 300:248–258
Fittipaldi N, Takamatsu D, de la Cruz Dominguez-Punaro, Lecours MP, Montpetit D, Osaki M, Sekizaki T, Gottschalk M (2010) Mutations in the gene encoding the ancillary pilin subunit of the Streptococcus suis srtF cluster result in pili formed by the major subunit only. PLoS One 5:e8426
Fittipaldi N, Segura M, Grenier D, Gottschalk M (2012) Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol 7:259–279
Froehlich BJ, Bates C, Scott JR (2009) Streptococcus pyogenes CovRS mediates growth in iron starvation and in the presence of the human cationic antimicrobial peptide LL-37. J Bacteriol 191:673–677
Frohlich KS, Vogel J (2009) Activation of gene expression by small RNA. Curr Opin Microbiol 12:674–682
Gamez G, Hammerschmidt S (2012) Combat pneumococcal infections: adhesins as candidates for protein- based vaccine development. Curr Drug Targets 13:323–337
Gao J, Gusa AA, Scott JR, Churchward G (2005) Binding of the global response regulator protein CovR to the sag promoter of Streptococcus pyogenes reveals a new mode of CovR-DNA interaction. J Biol Chem 280:38948–38956
Gardner PP, Daub J, Tate JG, Nawrocki EP, Kolbe DL, Lindgreen S, Wilkinson AC, Finn RD, Griffiths-Jones S, Eddy SR, Bateman A (2009) Rfam: updates to the RNA families database. Nucleic Acids Res 37:D136–D140
Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A 109:E2579–E2586
Giammarinaro P, Paton JC (2002) Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect Immun 70:5454–5461
Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couve E, Lalioui L, Poyart C, Trieu-Cuot P, Kunst F (2002) Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol Microbiol 45:1499–1513
Gogol EB, Rhodius VA, Papenfort K, Vogel J, Gross CA (2011) Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci U S A 108:12875–12880
Gorke B, Vogel J (2008) Noncoding RNA control of the making and breaking of sugars. Genes Dev 22:2914–2925
Gottesman S and Storz G (2011) Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 3:a003798
Gottesman S, McCullen CA, Guillier M, Vanderpool CK, Majdalani N, Benhammou J, Thompson KM, FitzGerald PC, Sowa NA, FitzGerald DJ (2006) Small RNA regulators and the bacterial response to stress. Cold Spring Harb Symp Quant Biol 71:1–11
Graham MR, Virtaneva K, Porcella SF, Barry WT, Gowen BB, Johnson CR, Wright FA, Musser JM (2005) Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am J Pathol 166:455–465
Granok AB, Parsonage D, Ross RP, Caparon MG (2000) The RofA binding site in Streptococcus pyogenes is utilized in multiple transcriptional pathways. J Bacteriol 182:1529–1540
Gryllos I, Grifantini R, Colaprico A, Jiang S, Deforce E, Hakansson A, Telford JL, Grandi G, Wessels MR (2007) Mg(2 +) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol Microbiol 65:671–683
Guedon E, Sperandio B, Pons N, Ehrlich SD, Renault P (2005) Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology 151:3895–3909
Guenzi E, Gasc AM, Sicard MA, Hakenbeck R (1994) A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol Microbiol 12:505–515
Gusa AA, Scott JR (2005) The CovR response regulator of group A streptococcus (GAS) acts directly to repress its own promoter. Mol Microbiol 56:1195–1207
Gusa AA, Gao J, Stringer V, Churchward G, Scott JR (2006) Phosphorylation of the group A Streptococcal CovR response regulator causes dimerization and promoter-specific recruitment by RNA polymerase. J Bacteriol 188:4620–4626
Gutekunst H, Eikmanns BJ, Reinscheid DJ (2003) Analysis of RogB-controlled virulence mechanisms and gene expression in Streptococcus agalactiae. Infect Immun 71:5056–5064
Haanes EJ, Heath DG, Cleary PP (1992) Architecture of the vir regulons of group A streptococci parallels opacity factor phenotype and M protein class. J Bacteriol 174:4967–4976
Haft DH, Selengut J, Mongodin EF, Nelson KE (2005) A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol 1:e60
Halfmann A, Kovacs M, Hakenbeck R, Bruckner R (2007) Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol Microbiol 66:110–126
Halfmann A, Schnorpfeil A, Muller M, Marx P, Gunzler U, Hakenbeck R, Bruckner R (2011) Activity of the two-component regulatory system CiaRH in Streptococcus pneumoniae R6. J Mol Microbiol Biotechnol 20:96–104
Han XG, Lu CP (2009) Detection of autoinducer-2 and analysis of the profile of luxS and pfs transcription in Streptococcus suis serotype 2. Curr Microbiol 58:146–152
Han H, Liu C, Wang Q, Xuan C, Zheng B, Tang J, Yan J, Zhang J, Li M, Cheng H, Lu G, Gao GF (2012) The two-component system Ihk/Irr contributes to the virulence of Streptococcus suis serotype 2 strain 05ZYH33 through alteration of the bacterial cell metabolism. Microbiology 158:1852–1866
Hava DL, Camilli A (2002) Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol 45:1389–1406
Hava DL, Hemsley CJ, Camilli A (2003) Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J Bacteriol 185:413–421
Heath A, DiRita VJ, Barg NL, Engleberg NC (1999) A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun 67:5298–5305
Heidrich N, Chinali A, Gerth U, Brantl S (2006) The small untranslated RNA SR1 from the Bacillus subtilis genome is involved in the regulation of arginine catabolism. Mol Microbiol 62:520–536
Hemsley C, Joyce E, Hava DL, Kawale A, Camilli A (2003) MgrA, an orthologue of Mga, acts as a transcriptional repressor of the genes within the rlrA pathogenicity islet in Streptococcus pneumoniae. J Bacteriol 185:6640–6647
Hendriksen WT, Bootsma HJ, Estev + úo S, Hoogenboezem T, de Jong A, de Groot R, Kuipers OP, Hermans PWM (2008) CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J Bacteriol 190:590–601
Hertzen E, Johansson L, Kansal R, Hecht A, Dahesh S, Janos M, Nizet V, Kotb M, Norrby-Teglund A (2012) Intracellular Streptococcus pyogenes in human macrophages display an altered gene expression profile. PLoS One 7:e35218
Hoch JA (2000) Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3:165–170
Hollingshead SK, Bessen DE (1995) Evolution of the emm gene family: virulence gene clusters in group A streptococci. Dev Biol Stand 85:163–168
Hollingshead SK, Readdy TL, Yung DL, Bessen DE (1993) Structural heterogeneity of the emm gene cluster in group A streptococci. Mol Microbiol 8:707–717
Hondorp ER, McIver KS (2007) The Mga virulence regulon: infection where the grass is greener. Mol Microbiol 66:1056–1065
Hoskins J, Alborn WE Jr, Arnold J, Blaszczak LC, Burgett S, DeHoff BS, Estrem ST, Fritz L, Fu DJ, Fuller W, Geringer C, Gilmour R, Glass JS, Khoja H, Kraft AR, Lagace RE, LeBlanc DJ, Lee LN, Lefkowitz EJ, Lu J, Matsushima P, McAhren SM, McHenney M, McLeaster K, Mundy CW, Nicas TI, Norris FH, O’Gara M, Peery RB, Robertson GT, Rockey P, Sun PM, Winkler ME, Yang Y, Young-Bellido M, Zhao G, Zook CA, Baltz RH, Jaskunas SR, Rosteck PR Jr, Skatrud PL, Glass JI (2001) Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol 183:5709–5717
Iyer R, Baliga NS, Camilli A (2005) Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J Bacteriol 187:8340–8349
Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR (2005) Regulation of virulence by a two-component system in group B streptococcus. J Bacteriol 187:1105–1113
Jiang SM, Ishmael N, Dunning HJ, Puliti M, Tissi L, Kumar N, Cieslewicz MJ, Tettelin H, Wessels MR (2008) Variation in the group B Streptococcus CsrRS regulon and effects on pathogenicity. J Bacteriol 190:1956–1965
Jin H, Pancholi V (2006) Identification and biochemical characterization of a eukaryotic-type serine/threonine kinase and its cognate phosphatase in Streptococcus pyogenes: their biological functions and substrate identification. J Mol Biol 357:1351–1372
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P (2002) An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110:551–561
Johnsen M, Christensen T, Dennis PP, Fiil NP (1982) Autogenous control: ribosomal protein L10–L12 complex binds to the leader sequence of its mRNA. EMBO J 1:999–1004
Joyce EA, Kawale A, Censini S, Kim CC, Covacci A, Falkow S (2004) LuxS is required for persistent pneumococcal carriage and expression of virulence and biosynthesis genes. Infect Immun 72:2964–2975
Kaufman GE, Yother J (2007) CcpA-dependent and -independent control of beta-galactosidase expression in Streptococcus pneumoniae occurs via regulation of an upstream phosphotransferase system-encoding operon. J Bacteriol 189:5183–5192
Kazmierczak KM, Wayne KJ, Rechtsteiner A, Winkler ME (2009) Roles of relSpn in stringent response, global regulation and virulence of serotype 2 Streptococcus pneumoniae D39. Mol Microbiol 72:590–611
Kietzman CC, Caparon MG (2010) CcpA and LacD.1 affect temporal regulation of Streptococcus pyogenes virulence genes. Infect Immun 78:241–252
Kietzman CC, Caparon MG (2011) Distinct time-resolved roles for two catabolite-sensing pathways during Streptococcus pyogenes infection. Infect Immun 79:812–821
Kihlberg BM, Cooney J, Caparon MG, Olsen A, Bjorck L (1995) Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb Pathog 19:299–315
Kinkel TL, McIver KS (2008) CcpA-mediated repression of streptolysin s expression and virulence in the group A Streptococcus. Infect Immun 76:3451–3463
Klenk M, Koczan D, Guthke R, Nakata M, Thiesen HJ, Podbielski A, Kreikemeyer B (2005) Global epithelial cell transcriptional responses reveal Streptococcus pyogenes Fas regulator activity association with bacterial aggressiveness. Cell Microbiol 7:1237–1250
Klinkert B, Narberhaus F (2009) Microbial thermosensors. Cell Mol Life Sci 66:2661–2676
Kratovac Z, Manoharan A, Luo F, Lizano S, Bessen DE (2007) Population genetics and linkage analysis of loci within the FCT region of Streptococcus pyogenes. J Bacteriol 189:1299–1310
Kreikemeyer B, Boyle MD, Buttaro BA, Heinemann M, Podbielski A (2001) Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol Microbiol 39:392–406
Kreikemeyer B, McIver KS, Podbielski A (2003) Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol 11:224–232
Kreikemeyer B, Nakata M, Koller T, Hildisch H, Kourakos V, Standar K, Kawabata S, Glocker MO, Podbielski A (2007) The Streptococcus pyogenes serotype M49 Nra-Ralp3 transcriptional regulatory network and its control of virulence factor expression from the novel eno ralp3 epf sagA pathogenicity region. Infect Immun 75:5698–5710
Kreikemeyer B, Gamez G, Margarit I, Giard JC, Hammerschmidt S, Hartke A, Podbielski A (2011) Genomic organization, structure, regulation and pathogenic role of pilus constituents in major pathogenic Streptococci and Enterococci. Int J Med Microbiol 301:240–251
Kreth J, Chen Z, Ferretti J, Malke H (2011) Counteractive balancing of transcriptome expression involving CodY and CovRS in Streptococcus pyogenes. J Bacteriol 193:4153–4165
Kumar R, Shah P, Swiatlo E, Burgess SC, Lawrence ML, Nanduri B (2010) Identification of novel non-coding small RNAs from Streptococcus pneumoniae TIGR4 using high-resolution genome tiling arrays. BMC Genomics 11:350
Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, Pellegrini E, Glaser P, Kunst F, Msadek T, Trieu-Cuot P, Poyart C (2004) CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol 54:1250–1268
Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME (2007) Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol 189:38–51
Le Rhun A, Charpentier E (2012) Small RNAs in streptococci. RNA Biol 9:414–426
Leday TV, Gold KM, Kinkel TL, Roberts SA, Scott JR, McIver KS (2008) TrxR, a new CovR-repressed response regulator that activates the Mga virulence regulon in group A Streptococcus. Infect Immun 76:4659–4668
Lee ER, Blount KF, Breaker RR (2009) Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA Biol 6:187–194
Levin JC, Wessels MR (1998) Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol 30:209–219
Li Z, Sledjeski DD, Kreikemeyer B, Podbielski A, Boyle MD (1999) Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J Bacteriol 181:6019–6027
Li N, Wang F, Niu S, Cao J, Wu K, Li Y, Yin N, Zhang X, Zhu W, Yin Y (2009) Discovery of novel inhibitors of Streptococcus pneumoniae based on the virtual screening with the homology-modeled structure of histidine kinase (VicK). BMC Microbiol 9:129
Li W, Liu L, Qiu D, Chen H, Zhou R (2010) Identification of Streptococcus suis serotype 2 genes preferentially expressed in the natural host. Int J Med Microbiol 300:482–488
Li J, Tan C, Zhou Y, Fu S, Hu L, Hu J, Chen H, Bei W (2011) The two-component regulatory system CiaRH contributes to the virulence of Streptococcus suis 2. Vet Microbiol 148:99–104
Liu M, Hanks TS, Zhang J, McClure MJ, Siemsen DW, Elser JL, Quinn MT, Lei B (2006) Defects in ex vivo and in vivo growth and sensitivity to osmotic stress of group A Streptococcus caused by interruption of response regulator gene vicR. Microbiology 152:967–978
Liu Z, Trevino J, Ramirez-Pena E, Sumby P (2012) The small regulatory RNA FasX controls pilus expression and adherence in the human bacterial pathogen group A Streptococcus. Mol Microbiol 86:140–154
Livny J, Waldor MK (2007) Identification of small RNAs in diverse bacterial species. Curr Opin Microbiol 10:96–101
Livny J, Fogel MA, Davis BM, Waldor MK (2005) sRNAPredict: an integrative computational approach to identify sRNAs in bacterial genomes. Nucleic Acids Res 33:4096–4105
Livny J, Brencic A, Lory S, Waldor MK (2006) Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2. Nucleic Acids Res 34:3484–3493
Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, Mandin P, Repoila F, Buchrieser C, Cossart P, Johansson J (2009) A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell 139:770–779
Loughman JA, Caparon MG (2006) A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes. EMBO J 25:5414–5422
Loughman JA, Caparon MG (2007) Comparative functional analysis of the lac operons in Streptococcus pyogenes. Mol Microbiol 64:269–280
Luo F, Lizano S, Banik S, Zhang H, Bessen DE (2008) Role of Mga in group A streptococcal infection at the skin epithelium. Microb Pathog 45:217–224
Lyon WR, Madden JC, Levin JC, Stein JL, Caparon MG (2001) Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol Microbiol 42:145–157
Malke H, Ferretti JJ (2007) CodY-affected transcriptional gene expression of Streptococcus pyogenes during growth in human blood. J Med Microbiol 56:707–714
Malke H, Steiner K, McShan WM, Ferretti JJ (2006) Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol 296:259–275
Manetti AG, Koller T, Becherelli M, Buccato S, Kreikemeyer B, Podbielski A, Grandi G, Margarit I (2010) Environmental acidification drives S. pyogenes pilus expression and microcolony formation on epithelial cells in a FCT-dependent manner. PLoS One 5:e13864
Mangold M, Siller M, Roppenser B, Vlaminckx BJ, Penfound TA, Klein R, Novak R, Novick RP, Charpentier E (2004) Synthesis of group A streptococcal virulence factors is controlled by a regulatory RNA molecule. Mol Microbiol 53:1515–1527
Mann B, van OT, Wang J, Obert C, Wang YD, Carter R, McGoldrick DJ, Ridout G, Camilli A, Tuomanen EI, Rosch JW (2012) Control of Virulence by Small RNAs in Streptococcus pneumoniae. PLoS Pathog 8:e1002788
Mansjo M, Johansson J (2011) The riboflavin analog roseoflavin targets an FMN-riboswitch and blocks Listeria monocytogenes growth, but also stimulates virulence gene-expression and infection. RNA Biol 8:674–680
Marouni MJ, Sela S (2003) The luxS Gene of Streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infect Immun 71:5633–5639
Marraffini LA, Sontheimer EJ (2010) Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463:568–571
Marx P, Nuhn M, Kovacs M, Hakenbeck R, Bruckner R (2010) Identification of genes for small non-coding RNAs that belong to the regulon of the two-component regulatory system CiaRH in Streptococcus. BMC Genomics 11:661
Mascher T, Zahner D, Merai M, Balmelle N, de Saizieu AB, Hakenbeck R (2003) The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J Bacteriol 185:60–70
Mascher T, Heintz M, Zahner D, Merai M, Hakenbeck R (2006) The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta-lactam resistance. J Bacteriol 188:1959–1968
McIver KS (2009) Stand-alone response regulators controlling global virulence networks in Streptococcus pyogenes. Contrib Microbiol 16:103–119
McIver KS, Scott JR (1997) Role of mga in growth phase regulation of virulence genes of the group A Streptococcus. J Bacteriol 179:5178–5187
McIver KS, Heath AS, Green BD, Scott JR (1995) Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A Streptococcus. J Bacteriol 177:6619–6624
McIver KS, Thurman AS, Scott JR (1999) Regulation of mga transcription in the group A Streptococcus: specific binding of mga within its own promoter and evidence for a negative regulator. J Bacteriol 181:5373–5383
Mistou MY, Dramsi S, Brega S, Poyart C, Trieu-Cuot P (2009) Molecular dissection of the secA2 locus of group B Streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J Bacteriol 191:4195–4206
Mitchell AM, Mitchell TJ (2010) Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect 16:411–418
Mraheil MA, Billion A, Kuenne C, Pischimarov J, Kreikemeyer B, Engelmann S, Hartke A, Giard JC, Rupnik M, Vorwerk S, Beier M, Retey J, Hartsch T, Jacob A, Cemic F, Hemberger J, Chakraborty T, Hain T (2010) Comparative genome-wide analysis of small RNAs of major Gram-positive pathogens: from identification to application. Microb Biotechnol 3:658–676
Mraheil MA, Billion A, Mohamed W, Mukherjee K, Kuenne C, Pischimarov J, Krawitz C, Retey J, Hartsch T, Chakraborty T, Hain T (2011) The intracellular sRNA transcriptome of Listeria monocytogenes during growth in macrophages. Nucleic Acids Res 39:4235–4248
Muller M, Marx P, Hakenbeck R, Bruckner R (2011) Effect of new alleles of the histidine kinase gene ciaH on the activity of the response regulator CiaR in Streptococcus pneumoniae R6. Microbiology 157:3104–3112
Nakata M, Podbielski A, Kreikemeyer B (2005) MsmR, a specific positive regulator of the Streptococcus pyogenes FCT pathogenicity region and cytolysin-mediated translocation system genes. Mol Microbiol 57:786–803
Nakata M, Koller T, Moritz K, Ribardo D, Jonas L, McIver KS, Sumitomo T, Terao Y, Kawabata S, Podbielski A, Kreikemeyer B (2009) Mode of expression and functional characterization of FCT-3 pilus region-encoded proteins in Streptococcus pyogenes serotype M49. Infect Immun 77:32–44
Narberhaus F, Vogel J (2009) Regulatory RNAs in prokaryotes: here, there and everywhere. Mol Microbiol 74:261–269
Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S (1993) Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975
Nudler E, Mironov AS (2004) The riboswitch control of bacterial metabolism. Trends Biochem Sci 29:11–17
Okada N, Geist RT, Caparon MG (1993) Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol Microbiol 7:893–903
Ou YQ, Ma WL, Liu CH, Shi R, Zheng WL (2005) [Detection of quorum-sensing pathway and construction of LuxS gene deletion mutants of group B Streptococcus]. Di Yi Jun Yi Da Xue Xue Bao 25:1135–1139
Ouyang Q, Ma WL, Liu CH, Shi R, Zheng WL (2006) [Phenotypic analysis of luxS gene deletion mutants and its application in virulence regulation research in group B Streptococcus]. Nan Fang Yi Ke Da Xue Xue Bao 26:117–121
Pan X, Ge J, Li M, Wu B, Wang C, Wang J, Feng Y, Yin Z, Zheng F, Cheng G, Sun W, Ji H, Hu D, Shi P, Feng X, Hao X, Dong R, Hu F, Tang J (2009) The orphan response regulator CovR: a globally negative modulator of virulence in Streptococcus suis serotype 2. J Bacteriol 191:2601–2612
Papenfort K, Vogel J (2010) Regulatory RNA in bacterial pathogens. Cell Host Microbe 8:116–127
Park SE, Jiang S, Wessels MR (2012) CsrRS and environmental pH regulate group B Streptococcus adherence to human epithelial cells and extracellular matrix. Infect Immun 80:3975−3984
Patenge N, Billion A, Raasch P, Normann J, Wisniewska-Kucper A, Retey J, Boisguérin V, Hartsch T, Hain T, Kreikemeyer B (2012) Identification of novel growth phase- and media-dependent small non-coding RNAs in Streptococcus pyogenes M49 using intergenic tiling arrays. BMC Genomics 13:550
Paterson GK, Blue CE, Mitchell TJ (2006) Role of two-component systems in the virulence of Streptococcus pneumoniae. J Med Microbiol 55:355–363
Perez N, Trevino J, Liu Z, Ho SC, Babitzke P, Sumby P (2009) A genome-wide analysis of small regulatory RNAs in the human pathogen group A Streptococcus. PLoS One 4:e7668
Perez-Casal JF, Dillon HF, Husmann LK, Graham B, Scott JR (1993) Virulence of two Streptococcus pyogenes strains (types M1 and M3) associated with toxic-shock-like syndrome depends on an intact mry-like gene. Infect Immun 61:5426–5430
Petersen FC, Ahmed NA, Naemi A, Scheie AA (2006) LuxS-mediated signalling in Streptococcus anginosus and its role in biofilm formation. Antonie Van Leeuwenhoek 90:109–121
Pichon C, du ML, Caliot ME, Trieu-Cuot P, Le BC (2012) An in silico model for identification of small RNAs in whole bacterial genomes: characterization of antisense RNAs in pathogenic Escherichia coli and Streptococcus agalactiae strains. Nucleic Acids Res 40:2846–2861
Pischimarov J, Kuenne C, Billion A, Hemberger J, Cemic F, Chakraborty T, Hain T (2012) sRNAdb: A small non-coding RNA database for gram-positive bacteria. BMC Genomics 13:384
Podbielski A, Flosdorff A, Weber-Heynemann J (1995) The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect Immun 63:9–20
Podbielski A, Woischnik M, Leonard BA, Schmidt KH (1999) Characterization of nra, a global negative regulator gene in group A streptococci. Mol Microbiol 31:1051–1064
Podkaminski D, Vogel J (2010) Small RNAs promote mRNA stability to activate the synthesis of virulence factors. Mol Microbiol 78:1327–1331
Price CE, Zeyniyev A, Kuipers OP, Kok J (2011) From meadows to milk to mucosa—adaptation of Streptococcus and Lactococcus species to their nutritional environments. FEMS Microbiol Rev 36:949–971
Quach D, van Sorge NM, Kristian SA, Bryan JD, Shelver DW, Doran KS (2009) The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses. J Bacteriol 191:2023–2032
Raasch P, Schmitz U, Patenge N, Vera J, Kreikemeyer B, Wolkenhauer O (2010) Non-coding RNA detection methods combined to improve usability, reproducibility and precision. BMC Bioinform 11:491
Rajagopal L (2009) Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol 4:201–221
Rajagopal L, Clancy A, Rubens CE (2003) A eukaryotic type serine/threonine kinase and phosphatase in Streptococcus agalactiae reversibly phosphorylate an inorganic pyrophosphatase and affect growth, cell segregation, and virulence. J Biol Chem 278:14429–14441
Rajagopal L, Vo A, Silvestroni A, Rubens CE (2006) Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol 62:941–957
Ramirez-Pena E, Trevino J, Liu Z, Perez N, Sumby P (2010) The group A Streptococcus small regulatory RNA FasX enhances streptokinase activity by increasing the stability of the ska mRNA transcript. Mol Microbiol 78:1332–1347
Redanz S, Standar K, Podbielski A, Kreikemeyer B (2012) Heterologous expression of sahH reveals that biofilm formation is autoinducer-2 independent in Streptococcus sanguinis, but is associated with an intact AMC. J Biol Chem 287:36111−36122
Riani C, Standar K, Srimuang S, Lembke C, Kreikemeyer B, Podbielski A (2007) Transcriptome analyses extend understanding of Streptococcus pyogenes regulatory mechanisms and behavior toward immunomodulatory substances. Int J Med Microbiol 297:513–523
Ribardo DA, McIver KS (2003) amrA encodes a putative membrane protein necessary for maximal exponential phase expression of the Mga virulence regulon in Streptococcus pyogenes. Mol Microbiol 50:673–685
Ribardo DA, McIver KS (2006) Defining the Mga regulon: comparative transcriptome analysis reveals both direct and indirect regulation by Mga in the group A Streptococcus. Mol Microbiol 62:491–508
Roberts SA, Scott JR (2007) RivR and the small RNA RivX: the missing links between the CovR regulatory cascade and the Mga regulon. Mol Microbiol 66:1506–1522
Romao S, Memmi G, Oggioni MR, Trombe MC (2006) LuxS impacts on LytA-dependent autolysis and on competence in Streptococcus pneumoniae. Microbiology 152:333–341
Rosch JW, Mann B, Thornton J, Sublett J, Tuomanen E (2008) Convergence of regulatory networks on the pilus locus of Streptococcus pneumoniae. Infect Immun 76:3187–3196
Samen U, Heinz B, Boisvert H, Eikmanns BJ, Reinscheid DJ, Borges F (2011) Rga is a regulator of adherence and pilus formation in Streptococcus agalactiae. Microbiology 157:2319–2327
Santi I, Grifantini R, Jiang SM, Brettoni C, Grandi G, Wessels MR, Soriani M (2009) CsrRS regulates group B Streptococcus virulence gene expression in response to environmental pH: a new perspective on vaccine development. J Bacteriol 191:5387–5397
Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V (2011) The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res 39:9275–9282
Schauder S, Shokat K, Surette MG, Bassler BL (2001) The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol 41:463–476
Scott JR, Zahner D (2006) Pili with strong attachments: Gram-positive bacteria do it differently. Mol Microbiol 62:320–330
Sebert ME, Patel KP, Plotnick M, Weiser JN (2005) Pneumococcal HtrA protease mediates inhibition of competence by the CiaRH two-component signaling system. J Bacteriol 187:3969–3979
Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermuller J, Reinhardt R, Stadler PF, Vogel J (2010) The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464:250–255
Sharma CM, Papenfort K, Pernitzsch SR, Mollenkopf HJ, Hinton JC, Vogel J (2011) Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol Microbiol 81:1144–1165
Shelburne SA III, Sumby P, Sitkiewicz I, Granville C, Deleo FR, Musser JM (2005) Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc Natl Acad Sci U S A 102:16037–16042
Shelburne SA, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, Brennan RG, Musser JM (2008) A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci 105:1698–1703
Shelburne SA, Olsen RJ, Suber B, Sahasrabhojane P, Sumby P, Brennan RG, Musser JM (2010) A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog 6:e1000817
Shelver D, Rajagopal L, Harris TO, Rubens CE (2003) MtaR, a regulator of methionine transport, is critical for survival of group B Streptococcus in vivo. J Bacteriol 185:6592–6599
Siller M, Janapatla R, Pirzada Z, Hassler C, Zinkl D, Charpentier E (2008) Functional analysis of the group A streptococcal luxS/AI-2 system in metabolism, adaptation to stress and interaction with host cells. BMC Microbiol 8:188
Solano-Collado V, Espinosa M, Bravo A (2012) Activator role of the pneumococcal mga-like virulence transcriptional regulator. J Bacteriol 194:4197–4207
Sonenshein AL (2005) CodY, a global regulator of stationary phase and virulence in gram-positive bacteria. Curr Opin Microbiol 8:203–207
Sridhar J, Sambaturu N, Sabarinathan R, Ou HY, Deng Z, Sekar K, Rafi ZA, Rajakumar K (2010) sRNAscanner: a computational tool for intergenic small RNA detection in bacterial genomes. PLoS One 5:e11970
Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J (2011) The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol Med Microbiol 62:123–139
Stock AM, Robinson VL, Goudreau PN (2000) Two-component signal transduction. Annu Rev Biochem 69:183–215
Storz G, Vogel J, Wassarman KM (2011) Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891
Stroeher UH, Paton AW, Ogunniyi AD, Paton JC (2003) Mutation of luxS of Streptococcus pneumoniae affects virulence in a mouse model. Infect Immun 71:3206–3212
Sugareva V, Arlt R, Fiedler T, Riani C, Podbielski A, Kreikemeyer B (2010) Serotype- and strain- dependent contribution of the sensor kinase CovS of the CovRS two-component system to Streptococcus pyogenes pathogenesis. BMC Microbiol 10:34
Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM (2006) Genome-wide analysis of group A Streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog 2:e5
Surette MG, Miller MB, Bassler BL (1999) Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A 96:1639–1644
Takamatsu D, Nishino H, Ishiji T, Ishii J, Osaki M, Fittipaldi N, Gottschalk M, Tharavichitkul P, Takai S, Sekizaki T (2009) Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet Microbiol 138:132–139
Telford JL, Barocchi MA, Margarit I, Rappuoli R, Grandi G (2006) Pili in gram-positive pathogens. Nat Rev Microbiol 4:509–519
Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, Deboy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM (2001) Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506
Tettelin H, Masignani V, Cieslewicz MJ, Eisen JA, Peterson S, Wessels MR, Paulsen IT, Nelson KE, Margarit I, Read TD, Madoff LC, Wolf AM, Beanan MJ, Brinkac LM, Daugherty SC, Deboy RT, Durkin AS, Kolonay JF, Madupu R, Lewis MR, Radune D, Fedorova NB, Scanlan D, Khouri H, Mulligan S, Carty HA, Cline RT, Van Aken SE, Gill J, Scarselli M, Mora M, Iacobini ET, Brettoni C, Galli G, Mariani M, Vegni F, Maione D, Rinaudo D, Rappuoli R, Telford JL, Kasper DL, Grandi G, Fraser CM (2002) Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc Natl Acad Sci U S A 99:12391–12396
Thomason MK, Storz G (2010) Bacterial antisense RNAs: how many are there, and what are they doing? Annu Rev Genet 44:167–188
Trappetti C, Potter AJ, Paton AW, Oggioni MR, Paton JC (2011) LuxS mediates iron-dependent biofilm formation, competence, and fratricide in Streptococcus pneumoniae. Infect Immun 79:4550–4558
Trevino J, Perez N, Ramirez-Pena E, Liu Z, Shelburne SA III, Musser JM, Sumby P (2009) CovS simultaneously activates and inhibits the CovR-mediated repression of distinct subsets of group A Streptococcus virulence factor-encoding genes. Infect Immun 77:3141–3149
Trevino J, Perez N, Sumby P (2010) The 4.5S RNA component of the signal recognition particle is required for group A Streptococcus virulence. Microbiology 156:1342–1350
Tsui HC, Mukherjee D, Ray VA, Sham LT, Feig AL, Winkler ME (2010) Identification and characterization of noncoding small RNAs in Streptococcus pneumoniae serotype 2 strain D39. J Bacteriol 192:264–279
Vahling CM, McIver KS (2006) Domains required for transcriptional activation show conservation in the mga family of virulence gene regulators. J Bacteriol 188:863–873
Vanderpool CK, Gottesman S (2005) Noncoding RNAs at the membrane. Nat Struct Mol Biol 12:285–286
Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR (2005) Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol 3:383–396
Vidal JE, Ludewick HP, Kunkel RM, Zähner D, Klugman KP (2011) The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect Immun 79:4050–4060
Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS (2002) Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res 30:3141–3151
Voyich JM, Sturdevant DE, Braughton KR, Kobayashi SD, Lei B, Virtaneva K, Dorward DW, Musser JM, Deleo FR (2003) Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A 100:1996–2001
Wang Y, Zhang W, Wu Z, Zhu X, Lu C (2011) Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence. Vet Microbiol 152:151–160
Waters LS, Storz G (2009) Regulatory RNAs in bacteria. Cell 136:615–628
Willenborg J, Fulde M, de Greeff A, Rohde M, Smith HE, Valentin-Weigand P, Goethe R (2011) Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis. Microbiology 157:1823–1833
Winkler ME, Hoch JA (2008) Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J Bacteriol 190:2645–2648
Xia L, Xia W, Li S, Li W, Liu J, Ding H, Li J, Li H, Chen Y, Su X, Wang W, Sun L, Wang C, Shao N, Chu B (2012) Identification and expression of small non-coding RNA, L10-Leader, in different growth phases of Streptococcus mutans. Nucleic Acid Ther 22:177–186
Yoshida A, Ansai T, Takehara T, Kuramitsu HK (2005) LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol 71:2372–2380
Zahner D, Kaminski K, van der Linden M, Mascher T, Meral M, Hakenbeck R (2002) The ciaR/ciaH regulatory network of Streptococcus pneumoniae. J Mol Microbiol Biotechnol 4:211–216
Zahner D, Gandhi AR, Yi H, Stephens DS (2011) Mitis group streptococci express variable pilus islet 2 pili. PLoS One 6:25124
Zhu J, Patel R, Pei D (2004) Catalytic mechanism of S-ribosylhomocysteinase (LuxS): stereochemical course and kinetic isotope effect of proton transfer reactions. Biochemistry 43:10166–10172
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Patenge, N., Fiedler, T., Kreikemeyer, B. (2012). Common Regulators of Virulence in Streptococci. In: Chhatwal, G. (eds) Host-Pathogen Interactions in Streptococcal Diseases. Current Topics in Microbiology and Immunology, vol 368. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2012_295
Download citation
DOI: https://doi.org/10.1007/82_2012_295
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36339-9
Online ISBN: 978-3-642-36340-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)