Abstract
The human orexin/hypocretin receptors (hOX1R and hOX2R) are G protein-coupled receptors (GPCRs) that mediate the diverse functions of the orexin/hypocretin neuropeptides. Orexins/hypocretins produced by neurons in the lateral hypothalamus stimulate their cognate GPCRs in multiple regions of the central nervous system to control sleep and arousal, circadian rhythms, metabolism, reward pathways, and other behaviors. Dysfunction of orexin/hypocretin signaling is associated with human disease, and the receptors are active targets in a number of therapeutic areas. To better understand the molecular mechanism of the orexin/hypocretin neuropeptides, high-resolution three-dimensional structures of hOX1R and hOX2R are critical. We have solved high-resolution crystal structures of both human orexin/hypocretin receptors bound to high-affinity antagonists. These atomic structures have elucidated how different small molecule antagonists bind with high potency and selectivity, and have also provided clues as to how the native ligands may associate with their receptors. The orexin/hypocretin receptor coordinates, now available to the broader academic and drug discovery community, will facilitate rational design of new therapeutics that modulate orexin/hypocretin signaling in humans.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Goals Behind Solving Structures of hOX1R and hOX2R
The motivation for structural studies of the orexin/hypocretin receptors is twofold. First, we would like to understand at the atomic level how the native ligands (orexin-A and orexin-B) and synthetic antagonists bind to the receptors, and how ligand binding stabilizes distinct receptor conformations. This goal is fundamentally a problem of understanding GPCR allostery for the specific case of the orexin/hypocretin receptors. Second, by studying receptor–ligand interactions, we hope to create knowledge and tools that translate into the design of more potent and selective modulators of orexin/hypocretin signaling.
hOX1R and hOX2R exhibit different physiological functions and pharmacology [1]. The two receptor subtypes are expressed differentially in various CNS regions [2], and pharmacological and genetic studies have uncovered differences in their behavioral functions. The hOX2R is the more evolutionarily ancient of the two subtypes [3], and plays a more significant role in controlling circadian rhythms, sleep, and arousal [4, 5]. Thus modulation of hOX2R has become an attractive therapeutic strategy for sleep and wake disorders such as insomnia (e.g., the FDA-approved drug suvorexant) and narcolepsy (for potential small molecule orexin mimetics). The hOX1R functions in modulating reward [6, 7], nociception [8], and stress [9], and inhibition of this subtype has developed into an active therapeutic area for disorders such as addiction [10].
2 Challenges for Solving High-Resolution Crystal Structures of GPCRs
The key to determining high-resolution structures of the hOX1R and hOX2R was the ability to obtain diffraction-quality crystals. A decade ago, ligand-activated GPCRs were thought to be largely intractable targets for structure determination. However crystallization of GPCRs has recently become possible due to a number of breakthrough technologies that were developed for GPCRs and other integral membrane proteins. First, methods of expression and purification from recombinant systems such as Spodoptera frugiperda (Sf9 insect cells) [11], Pichia pastoris (yeast) [12, 13], and mammalian cells (such as HEK293) [14] have allowed labs to purify milligram quantities of many GPCRs. Second, protein engineering methods including crystallizable domain chimeras [15], thermostabilizing mutations [16], and antibodies [12, 17] and nanobodies [18, 19] have made purified GPCRs more stable and amenable to forming three-dimensional crystals. Third, detergents such as the neopentyl glycols [20] were developed which stabilize GPCRs during solubilization, purification, and reconstitution. Finally, lipid-mediated crystallization techniques such as lipidic cubic phase (LCP) [21] and bicelles [22] have facilitated GPCR crystal formation by promoting lateral crystal contacts between the receptors’ transmembrane (TM) regions.
Despite these advances, GPCRs are still highly challenging targets for X-ray crystallography. To highlight this point, the only previous successful crystallography efforts for GPCRs in the β branch of the rhodopsin family were for thermostabilized mutants of the rat neurotensin receptor (rNTSR1) bound to neurotensin agonist peptides [23–25]. In order to crystallize hOX1R and hOX2R, we solubilized the protein out of Sf9 cell membranes in lauryl maltose neopentyl glycol (LMNG), and purified milligram quantities of the receptors to homogeneity. Further, we created chimeras with domains such as T4L that had previously facilitated GPCR crystallization [15]. After using these methods, we were still not able to obtain diffraction-quality crystals. To finally overcome this barrier, we had to identify and develop a new fusion domain – PGS (Pyrococcus abysii glycogen synthase) – that yielded high-resolution diffracting crystals for both hOX1R [26] and hOX2R [27] when fused at the third intracellular loop (ICL3). The ICL3 fusion strategy is successful for GPCR crystallization because it removes an inherently flexible region of the receptor (ICL3), which may hinder crystal contact formation, and adds a stable folded structure in its place that can successfully mediate lattice contacts [15]. While other crystallizable domains have been developed as fusion protein partners [28], the new PGS domain proved indispensible for our efforts to crystallize both orexin receptor subtypes.
3 Comparison of hOX1R and hOX2R to Other GPCRs
The crystal structures that we obtained of the orexin/hypocretin receptors each have high-affinity antagonists bound (see Sect. 5). The extracellular surfaces of hOX1R and hOX2R broadly resemble those of other peptide-binding GPCRs. Figure 1 shows identical views of four different GPCRs activated by peptide hormones that have been structurally characterized: hOX2R [27], rat neurotensin receptor rNTSR1 [25], opioid receptor μOR [29], and the chemokine receptor CXCR4 [30]. Along with other related examples [31, 32], these structures support the idea that all peptide-activated GPCRs in the rhodopsin family (Class A) contain a short β-hairpin (two antiparallel β-strands) in the second extracellular loop (ECL2) situated above the orthosteric pocket. In the “partially active” conformation shown for rNTSR1, the agonist peptide Neurotensin8-13 packs against the β-hairpin motif. This motif may be a general platform for GPCR-peptide interaction due to the large flat surface area it presents, although other peptide-bound structures are needed to confirm this prediction. The sites of several of the most deleterious reported mutations for orexin/hypocretin affinity and potency are on this β-hairpin [33]. In contrast, in the secretin family of GPCRs (Class B), high-affinity peptide recognition requires a large folded N-terminal extracellular domain [34].
As illustrated in Fig. 1, small molecule antagonists for hOX2R, μOR, and CXCR4 are each positioned in the orthosteric pocket that is created by the TM α-helices embedded within the membrane. In fact, the position of the drug suvorexant (used for co-crystallization of both hOX1R and hOX2R) is very similar to the binding site for β-blocker inverse agonists such as carazolol in the β2 adrenergic receptor (β2AR), the most extensively studied model system for ligand-activated GPCRs (Fig. 2a). Structures of β2AR with antagonists [15], agonists [18, 35], and G proteins [19] led to a model in which agonist-induced inward movements of the TM α-helices at the orthosteric binding pocket initiate conformational changes through the transmembrane 7TM bundle. These changes ultimately lead to outward movement of TMs 5 and 6 at the intracellular surface, which is necessary for the engagement of G proteins. Based on this model, also recapitulated in other members of the GPCR superfamily [36], binding of antagonists such as suvorexant (Fig. 2b) are hypothesized to stabilize the inactive conformation by preventing inward movement of the TM α-helices at the orthosteric site, as well as competing for binding surfaces with the native orexin/hypocretin ligands. Comparison of the published structures of inactive [23] and partially active [25] conformations of rNTSR1 showed that a peptide agonist can promote an outward shift of TM6 at the intracellular surface, through binding of a surface that overlaps with suvorexant’s binding site in the orexin receptors.
4 Overall Structures of hOX1R and hOX2R
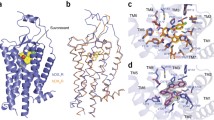
The global structures of hOX1R and hOX2R are shown in Fig. 3. To date, we have reported the structure of hOX1R bound to the dual orexin receptor antagonist (DORA) suvorexant at 2.75 Å resolution (Fig. 3a); the structure of hOX1R bound to the type 1-selective antagonist SB-674042 at 2.8 Å resolution (Fig. 3b); and the structure of hOX2R bound to suvorexant at 2.5 Å resolution (Fig. 3c) [27, 37]. The root mean squared deviation (rmsd) between superimposed hOX1R and hOX2R is 0.4 Å over 282 Cα’s, indicating that these two receptors (with 64% sequence identity) are very similar in three-dimensional structure (Fig. 3d). Beyond this similarity, the rmsd between the hOX2R structure (Fig. 3c) and the inactive-state structure of the β2AR (with 23% sequence identity) is only 2.2 Å, highlighting the strong structural conservation within the GPCR superfamily. The major difference between the crystal structures lies in the extracellular region containing the ECL2 and N-terminus. The hOX1R has a short α-helix preceding TM1, which packs against the ECL2 (Fig. 2a, b). We did not observe such a motif in our hOX2R structure (Fig. 3c); however, this α-helix may exist but have too much flexibility to visualize in the crystal’s electron density. We believe that this N-terminal α-helix is directly involved in orexin/hypocretin recruitment and receptor activation (see Sect. 6).
Structures of orexin receptors bound to small molecules. (a) hOX1R (wheat cartoon) bound to suvorexant (balls and sticks with gray carbons). (b) hOX1R (green cartoon) bound to SB-674042 (balls and sticks with magenta carbons). (c) hOX2R (cyan cartoon) bound to suvorexant (balls and sticks with gray carbons). (d) Superposition of hOX1R and hOX2R
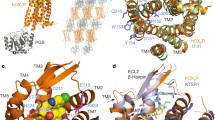
Like all GPCRs, hOX1R and hOX2R translate agonist binding into functional responses through receptor-mediated activation of intracellular heterotrimeric G proteins, principally Gq/11 and Gi/o for the orexin/hypocretin receptors [38]. Based on previous structural and biophysical studies of other GPCRs [18, 35, 36], the binding of agonists should stimulate outward movement of the TM α-helices at the cytoplasmic surface to facilitate binding of G proteins [19]. In the inactive state of the β2AR, TM5 and TM6 pack against TM3 bearing the conserved DRY motif at the intracellular surface, blocking epitopes involved in G protein binding. The DRY motif is conserved throughout Class A GPCRs, and these residues are important for maintaining a stable inactive-state conformation with low basal activity [39, 40]. For hOX1R and hOX2R, the DRY motif is changed to DRWY, and these residues are tightly packed against residues from TM5 and TM6 (Fig. 4), analogous to the antagonist-bound M3 acetylcholine receptor (another Gq-coupled GPCR). Therefore we can conclude that the antagonist-bound crystal structures of hOX1R and hOX2R represent inactive conformations [26, 27].
Structures of inactive-state GPCRs at the intracellular surface of the membrane. Superposition of suvorexant-bound hOX1R (wheat cartoon), suvorexant-bound hOX2R (cyan cartoon), and tiotropium-bound M3 muscarinic acetylcholine receptor (orange cartoon, PDB 4DAJ), viewed from intracellular side. Intracellular loops are removed for clarity. The DRWY residues on TM3 and interacting residues on TMs 5 and 6 are shown as magenta sticks. Arrow indicates outward movement of TM6 during activation
5 Binding of Small Molecule Antagonists
Orexin receptor antagonists are prospective therapeutics for a number of different human diseases, as detailed elsewhere in this volume. So far, the only such molecule to be approved by the FDA is suvorexant (Belsomra) [41] for treatment of insomnia. An important goal of characterizing the structures of the receptors is to understand the precise mechanisms by which antagonists bind and prevent activation. The solvent-exposed orthosteric binding sites where small molecule antagonists bind to hOX1R and hOX2R are well ordered in the crystal structures, along with the bound small molecules. Figure 5 shows the ligand binding poses and detailed interactions with the receptors for all three crystal structures we have reported: hOX1R with suvorexant (Fig. 5a), hOX1R with SB-674042 (Fig. 5b), and hOX2R with suvorexant (Fig. 5c) [26, 27]. The binding sites include contributions from the extracellular ends of all TMs except TM1, as well as from the ECL2. Several of the amino acids that make the greatest contact with the ligands (in terms of buried surface area) have been previously characterized in mutagenesis studies as contributing greatly to antagonist affinity, adding further functional support to our structural data [42–45].
Binding pockets and antagonist interactions with the orexin/hypocretin receptors. (a) Contact residues within 4 Å between suvorexant (balls and sticks with gray carbons) and hOX1R (gray cartoon with wheat sidechains). (b) Contact residues within 4 Å between SB-674042 (balls and sticks with magenta carbons) and hOX1R (gray cartoon with green sidechains). (c) Contact residues within 4 Å between suvorexant (balls and sticks with gray carbons) and hOX1R (gray cartoon with cyan sidechains). (d) Superposition of binding pocket residues of suvorexant-bound hOX1R (wheat sticks) and suvorexant-bound hOX2R (cyan sticks). Labels use Ballesteros–Weinstein numbering in superscript. The two divergent binding site residues are displayed as magenta sticks for hOX1R and yellow sticks for hOX2R
The two ligands that we have so far co-crystallized with the orexin receptors both adopt a compact horseshoe-like bound conformation, in which two aromatic groups, separated by a spacer group, engage in intramolecular aromatic stacking interactions (Fig. 5). For suvorexant analogs, a related 3D conformation of the isolated ligand in solution was previously reported, and suggested to be relevant to the receptor-binding conformation [46]. Our structures support the idea that these molecules and related antagonists prepay some of the entropic cost of ligand binding by constraining their 3D conformations through intramolecular packing. Indeed, a large number of the small molecule orexin/hypocretin receptor antagonists discovered by different laboratories have the same basic form, in which two aromatic moieties are separated and presented by a small ring scaffold [47]. We predict that many of these molecules will bind in a similar mode as we have elucidated in our crystal structures.
In conjunction with solving the hOX1R structures, we carried out several different computational analyses to better understand how subtype-selective ligands such as SB-674042 discriminate between hOX1R and hOX2R. These studies demonstrated how the very highly conserved orthosteric binding pockets, which have only two subtle differences in amino acid composition (Fig. 4d), create small differences in pocket volume and shape that can be exploited to achieve selectivity toward either subtype [26]. The hOX1R-selective SB-674042 occupies slightly more space in the orthosteric pocket and clashes with the two larger divergent residues (Thr111 and Thr135) in the resulting slightly smaller hOX2R pocket. In contrast, docking and simulation of the hOX2R-selective antagonist 2-SORA-DMP indicated that better shape complementarity and van der Waals contacts with hOX2R lead to greater affinity compared to hOX1R with its larger orthosteric pocket. While our structural observations and calculations are consistent with the subtype selectivity of these antagonists, one caveat is that the crystallographic coordinates represent saturated complexes with high ligand occupancy and do not inform kinetic mechanisms that influence binding selectivity. Intriguingly, the antagonist-bound orexin receptor structures revealed a “lid” over the binding pocket formed by multiple salt bridges, leaving only a constricted solvent channel to the orthosteric site. This feature implies that the receptor’s extracellular surface must breath in order to allow access for antagonists, which may influence both association and dissociation. The precise contributions of binding pocket residues and the extracellular structure to ligand binding kinetics can now be probed with pharmacological studies of mutant receptors guided by the high-resolution structures. Another important factor that is not captured by our structures is ligand and binding pocket desolvation that occurs during complex formation [48]. In future studies, interactions of water molecules with the receptor, ligand, and bound complex can be simulated using our crystallographic coordinates as a framework, to achieve a more complete understanding of the large differences in subtype affinity displayed by selective orexin receptor antagonists.
6 Clues for Orexin/Hypocretin Interaction with hOX1R and hOX2R
We currently lack a clear understanding of how the orexin/hypocretin neuropeptides bind and activate their cognate GPCRs. One intriguing clue from the hOX1R structure was the ordered N-terminal region prior to TM1, containing a short amphipathic α-helix that is positioned over the orthosteric binding pocket (Fig. 6a). This region is conserved in all known vertebrate orexin/hypocretin receptor sequences, from fish and amphibians to humans [49] (Fig. 6b). Given that the NMR structures of orexin-A and orexin-B also revealed amphipathic α-helices [50, 51], we hypothesized that the structured N-terminal region is involved in binding and recruitment of the neuropeptides through interactions of α-helices. Using a combination of binding and receptor activation assays, we showed that this element is essential for potent orexin-A activation for both hOX1R and hOX2R. We also found that mutation of a polar residue in the orthosteric binding pocket (hOX1R N318 or hOX2R N324) severely diminished orexin-A potency [26]. Previous site-directed mutagenesis experiments demonstrated that residues in the ECL2 β-hairpin (e.g., hOX1R D203A or hOX2R D211A) are critical for orexin-A potency [33]. Putting these findings together, we propose that orexin-A binds to hOX1R or hOX2R through a polytopic interface involving all three of these receptor sites (Fig. 6a). In this context, the published result that 17 or more amino acids in the orexin/hypocretin neuropeptides are required to reach low-nanomolar potency [52] can be easily rationalized. A detailed picture of how this interface forms and influences receptor conformation will await structures of the neuropeptide-bound GPCRs. It is worth noting that juxtamembrane N-terminal regions are involved in binding peptide agonists for a number of other rhodopsin family GPCRs, including formyl peptide receptor [53], cholecystokinin (CCK) receptor [54], and the tachykinin receptors [55]. The N-terminal region also plays a key role in chemokine receptors, providing an extended epitope that interacts with the folded globular domain of the chemokine hormone [56, 57].
Structure of the N-terminal extracellular region of hOX1R. (a) The amphipathic α-helix at the N-terminus preceding TM1 in hOX1R structure (cyan cartoon, sidechains with gray carbons). (b) Sequence alignment of orexin receptor N-termini, including Mus musculus (mouse), Homo sapiens (human), Bos Taurus (bovine), Danio rerio (fish), Xenopus laevis (frog), and Canis lupus (dog) sequences (adapted from Yin et al. [26])
7 Conclusions and Future Prospects
The crystal structures of the human orexin/hypocretin receptors have provided an atomic-level framework for understanding binding and subtype selectivity of small molecule antagonists, including the clinically used suvorexant and the hOX1R-selective SB-674042 [26, 27]. In addition, the structures have revealed a previously unknown role for the receptor N-terminal region in recruitment of the orexin/hypocretin neuropeptides [26]. We anticipate that our publicly deposited coordinates (PDB accession codes 4S0V, 4ZJ8, 4ZJC) and future co-crystal structures using our reported constructs and protocols will aid the design and optimization of orexin/hypocretin receptor antagonists with improved affinity and subtype selectivity.
To fully elucidate the mechanism for orexin hormone activation of the orexin receptors, we must obtain structures bound to agonists, as well as complexes with G proteins or G protein-mimetic antibodies. Crystallographic and biophysical studies of β2AR other ligand-activated GPCRs have shown that extracellular agonist binding and intracellular conformational changes leading to signaling are weakly coupled [35, 58]. Therefore structures of the receptors bound to the orexin neuropeptides alone (akin to Egloff et al. [23]) may not reveal the propagated conformational changes through the membrane that ultimately result in signal propagation. In studies of active β2AR [18], M3 muscarinic acetylcholine receptor [36], and μ opioid receptor [59], this problem was overcome by selecting nanobodies (small single-chain antibody domains derived from llamas) that stabilize the active conformation by binding at the G protein coupling site [60]. Discovery of active state-stabilizing nanobodies for the orexin receptors may similarly enable structure determination of an active neuropeptide-bound GPCR, illuminating how the peptide agonist allosterically promotes the active conformation. Further, the structures of the orexin receptors in different conformations will allow design of biophysical experiments to measure the dynamic changes between states, for example, by fluorescence [61] and NMR spectroscopy [62]. Finally, the biochemical precedents for homogeneous purification and crystallization of the orexin receptors may facilitate attempts to co-crystallize these GPCRs with G proteins, such as the Gq heterotrimer. While this goal is highly challenging (there is only one GPCR-G protein complex structure solved to date, for β2AR) [19], only a complex with a G protein will ultimately explain how orexin neuropeptides stimulate G protein signaling. Importantly, these developments would pave the way for structure-guided design of small molecule activators of orexin signaling, which have so far been extremely challenging to isolate. The coming years promise to be an exciting time for biophysical characterization and manipulation of orexin/hypocretin signaling.
References
Li J, Hu Z, de Lecea L (2014) The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol 171:332–350. doi:10.1111/bph.12415
Marcus JN, Aschkenasi CJ, Lee CE et al (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25
Wong KKY, Ng SYL, Lee LTO et al (2011) Orexins and their receptors from fish to mammals: a comparative approach. Gen Comp Endocrinol 171:124–130. doi:10.1016/j.ygcen.2011.01.001
Lin L, Faraco J, Li R et al (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98:365–376
Willie JT, Chemelli RM, Sinton CM et al (2003) Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron 38:715–730
Boutrel B, Kenny PJ, Specio SE et al (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102:19168–19173. doi:10.1073/pnas.0507480102
Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. doi:10.1038/nature04071
Bingham S, Davey PT, Babbs AJ et al (2001) Orexin-A, an hypothalamic peptide with analgesic properties. Pain 92:81–90
Johnson PL, Truitt W, Fitz SD et al (2010) A key role for orexin in panic anxiety. Nat Med 16:111–115. doi:10.1038/nm.2075
Aston-Jones G, Smith RJ, Moorman DE, Richardson KA (2009) Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology 56(Suppl 1):112–121. doi:10.1016/j.neuropharm.2008.06.060
Kobilka BK (1995) Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal Biochem 231:269–271
Hino T, Arakawa T, Iwanari H et al (2012) G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 482:237–240. doi:10.1038/nature10750
Shimamura T, Shiroishi M, Weyand S et al (2011) Structure of the human histamine H1 receptor complex with doxepin. Nature 475:65–70. doi:10.1038/nature10236
Kang Y, Zhou XE, Gao X et al (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523:561–567. doi:10.1038/nature14656
Rosenbaum DM, Cherezov V, Hanson MA et al (2007) GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318:1266–1273. doi:10.1126/science.1150609
Vaidehi N, Grisshammer R, Tate CG (2016) How can mutations thermostabilize G-protein-coupled receptors? Trends Pharmacol Sci 37:37–46. doi:10.1016/j.tips.2015.09.005
Rasmussen SGF, Choi H-J, Rosenbaum DM et al (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450:383–387. doi:10.1038/nature06325
Rasmussen SGF, Choi H-J, Fung JJ et al (2011) Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469:175–180. doi:10.1038/nature09648
Rasmussen SGF, DeVree BT, Zou Y et al (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555. doi:10.1038/nature10361
Chae PS, Rasmussen SGF, Rana RR et al (2010) Maltose–neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods 7:1003–1008. doi:10.1038/nmeth.1526
Caffrey M, Cherezov V (2009) Crystallizing membrane proteins using lipidic mesophases. Nat Protoc 4:706–731. doi:10.1038/nprot.2009.31
Faham S, Boulting GL, Massey EA et al (2005) Crystallization of bacteriorhodopsin from bicelle formulations at room temperature. Protein Sci 14:836–840. doi:10.1110/ps.041167605
Egloff P, Hillenbrand M, Klenk C et al (2014) Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli. Proc Natl Acad Sci U S A 111:E655–E662. doi:10.1073/pnas.1317903111
Krumm BE, White JF, Shah P, Grisshammer R (2015) Structural prerequisites for G-protein activation by the neurotensin receptor. Nat Commun 6:7895. doi:10.1038/ncomms8895
White JF, Noinaj N, Shibata Y et al (2012) Structure of the agonist-bound neurotensin receptor. Nature 490:508–513. doi:10.1038/nature11558
Yin J, Babaoglu K, Brautigam CA et al (2016) Structure and ligand-binding mechanism of the human OX1 and OX2 orexin receptors. Nat Struct Mol Biol 23:293–299. doi:10.1038/nsmb.3183
Yin J, Mobarec JC, Kolb P, Rosenbaum DM (2015) Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature 519:247–250. doi:10.1038/nature14035
Chun E, Thompson AA, Liu W et al (2012) Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20:967–976. doi:10.1016/j.str.2012.04.010
Manglik A, Kruse AC, Kobilka TS et al (2012) Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 485:321–326. doi:10.1038/nature10954
Wu B, Chien EYT, Mol CD et al (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330:1066–1071. doi:10.1126/science.1194396
Granier S, Manglik A, Kruse AC et al (2012) Structure of the δ-opioid receptor bound to naltrindole. Nature 485:400–404. doi:10.1038/nature11111
Tan Q, Zhu Y, Li J et al (2013) Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 341:1387–1390. doi:10.1126/science.1241475
Malherbe P, Roche O, Marcuz A et al (2010) Mapping the binding pocket of dual antagonist almorexant to human orexin 1 and orexin 2 receptors: comparison with the selective OX1 antagonist SB-674042 and the selective OX2 antagonist N-ethyl-2-[(6-methoxy-pyridin-3-yl)-(toluene-2-sulfonyl)-amino]-N-pyridin-3-ylmethyl-acetamide (EMPA). Mol Pharmacol 78:81–93. doi:10.1124/mol.110.064584
Pioszak AA, Xu HE (2008) Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc Natl Acad Sci U S A 105:5034–5039. doi:10.1073/pnas.0801027105
Rosenbaum DM, Zhang C, Lyons JA et al (2011) Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 469:236–240. doi:10.1038/nature09665
Kruse AC, Ring AM, Manglik A et al (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504:101–106. doi:10.1038/nature12735
Yin J, Li L, Shaw N et al (2009) Structural basis and catalytic mechanism for the dual functional endo-beta-N-acetylglucosaminidase A. PLoS One 4:e4658. doi:10.1371/journal.pone.0004658
Zhu Y, Miwa Y, Yamanaka A et al (2003) Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci 92:259–266
Ballesteros JA (2001) Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem 276:29171–29177. doi:10.1074/jbc.M103747200
Rasmussen SG, Jensen AD, Liapakis G et al (1999) Mutation of a highly conserved aspartic acid in the beta2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol Pharmacol 56:175–184
Cox CD, Breslin MJ, Whitman DB et al (2010) Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem 53:5320–5332. doi:10.1021/jm100541c
Heifetz A, Morris GB, Biggin PC et al (2012) Study of human orexin-1 and -2 G-protein-coupled receptors with novel and published antagonists by modeling, molecular dynamics simulations, and site-directed mutagenesis. Biochemistry 51:3178–3197. doi:10.1021/bi300136h
Langmead CJ, Jerman JC, Brough SJ et al (2004) Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol 141:340–346. doi:10.1038/sj.bjp.0705610
Putula J, Kukkonen JP (2012) Mapping of the binding sites for the OX1 orexin receptor antagonist, SB-334867, using orexin/hypocretin receptor chimaeras. Neurosci Lett 506:111–115. doi:10.1016/j.neulet.2011.10.061
Tran D-T, Bonaventure P, Hack M et al (2011) Chimeric, mutant orexin receptors show key interactions between orexin receptors, peptides and antagonists. Eur J Pharmacol 667:120–128. doi:10.1016/j.ejphar.2011.05.074
Cox CD, McGaughey GB, Bogusky MJ et al (2009) Conformational analysis of N,N-disubstituted-1,4-diazepane orexin receptor antagonists and implications for receptor binding. Bioorg Med Chem Lett 19:2997–3001. doi:10.1016/j.bmcl.2009.04.026
Lebold TP, Bonaventure P, Shireman BT (2013) Selective orexin receptor antagonists. Bioorg Med Chem Lett 23:4761–4769. doi:10.1016/j.bmcl.2013.06.057
Biela A, Nasief NN, Betz M et al (2013) Dissecting the hydrophobic effect on the molecular level: the role of water, enthalpy, and entropy in ligand binding to thermolysin. Angew Chem Int Ed Engl 52:1822–1828. doi:10.1002/anie.201208561
Isberg V, Vroling B, van der Kant R et al (2014) GPCRDB: an information system for G protein-coupled receptors. Nucleic Acids Res 42:D422–D425. doi:10.1093/nar/gkt1255
Kim H-Y, Hong E, Kim J-I, Lee W (2004) Solution structure of human orexin-A: regulator of appetite and wakefulness. J Biochem Mol Biol 37:565–573
Lee JH, Bang E, Chae KJ et al (1999) Solution structure of a new hypothalamic neuropeptide, human hypocretin-2/orexin-B. Eur J Biochem 266:831–839
German NA, Decker AM, Gilmour BP et al (2013) Truncated orexin peptides: structure-activity relationship studies. ACS Med Chem Lett 4:1224–1227. doi:10.1021/ml400333a
Perez HD, Vilander L, Andrews WH, Holmes R (1994) Human formyl peptide receptor ligand binding domain(s). Studies using an improved mutagenesis/expression vector reveal a novel mechanism for the regulation of receptor occupancy. J Biol Chem 269:22485–22487
Kennedy K, Gigoux V, Escrieut C et al (1997) Identification of two amino acids of the human cholecystokinin-A receptor that interact with the N-terminal moiety of cholecystokinin. J Biol Chem 272:2920–2926
Valentin-Hansen L, Park M, Huber T et al (2014) Mapping substance P binding sites on the neurokinin-1 receptor using genetic incorporation of a photoreactive amino acid. J Biol Chem 289:18045–18054. doi:10.1074/jbc.M113.527085
Burg JS, Ingram JR, Venkatakrishnan AJ et al (2015) Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science 347:1113–1117. doi:10.1126/science.aaa5026
Qin L, Kufareva I, Holden LG et al (2015) Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science 347:1117–1122. doi:10.1126/science.1261064
Manglik A, Kim TH, Masureel M et al (2015) Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161:1101–1111. doi:10.1016/j.cell.2015.04.043
Huang W, Manglik A, Venkatakrishnan AJ et al (2015) Structural insights into μ-opioid receptor activation. Nature 524:315–321. doi:10.1038/nature14886
Steyaert J, Kobilka BK (2011) Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol 21:567–572. doi:10.1016/j.sbi.2011.06.011
Yao X, Parnot C, Deupi X et al (2006) Coupling ligand structure to specific conformational switches in the beta2-adrenoceptor. Nat Chem Biol 2:417–422. doi:10.1038/nchembio801
Nygaard R, Zou Y, Dror RO et al (2013) The dynamic process of β(2)-adrenergic receptor activation. Cell 152:532–542. doi:10.1016/j.cell.2013.01.008
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Yin, J., Rosenbaum, D.M. (2016). The Human Orexin/Hypocretin Receptor Crystal Structures. In: Lawrence, A.J., de Lecea, L. (eds) Behavioral Neuroscience of Orexin/Hypocretin. Current Topics in Behavioral Neurosciences, vol 33. Springer, Cham. https://doi.org/10.1007/7854_2016_52
Download citation
DOI: https://doi.org/10.1007/7854_2016_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57534-6
Online ISBN: 978-3-319-57535-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)