Abstract
Dermal papilla cells (DPCs) are associated with development of hair follicles (HFs) and regulation of the hair cycle. However, primary DPCs are known to lose their ability to induce HFs after culture in standard media for fibroblasts. We examined a new culture condition for DPCs including addition of Wnt-10b, which promoted proliferation and maintained their HF induction ability for more than ten passages. These results suggest that Wnt-10b plays a pivotal role in proliferation and maintenance of DPCs in vitro.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords
1 Introduction
Dermal papilla cells (DPCs), specialized fibroblasts located in hair follicles (HFs), are deeply associated with HF development and regulation of the hair cycle, and known to be an important target for elucidating the mechanism of HF induction and hair growth [1–3]. Technical advances in isolation of DPCs from HFs and subsequent in vitro cultures have made it possible to investigate the ability of these cells to induce HFs and promote hair growth [4–6].
DPCs lose their ability for HF induction and do not promote hair growth after being cultured in standard medium for fibroblasts [4, 6, 7], thus establishment of an effective culture method by which the functions of DPCs are maintained is considered to be important. Previously, DPCs cocultured with Wnt-3a-producing cells were shown to sustain their ability to induce HFs [5], suggesting that canonical Wnts may be important for DPC growth. Another study provided supportive evidence concerning the role of Wnts, as it found that a GSK inhibitor (BIO) acted favorably for maintaining DP function [8]. However, there are no reports documenting the direct effects of Wnt proteins on DPCs. In our previous study, we investigated the effects of Wnt-10b on primary DPCs prepared from DP cultures and DPCs after serial passages, and found that Wnt-10b promoted their proliferation and maintained HF induction ability [9]. In the present protocol, we describe more detail of the culture method, by which DPCs can be maintained over several passages with their sustained function.
2 Materials
2.1 Isolation of DPCs
-
1.
Inbred 4-week-old C3H/HeN mice.
-
2.
Dissection instrument (micro-scissors, fine forceps).
-
3.
Stereo microscope.
-
4.
Saline (Otsuka, Tokyo, cat. no. D05352).
-
5.
Plastic petri dishes.
-
6.
Dulbecco’s modified Eagle’s medium (DMEM; Wako, Osaka, cat. no. 043-30085) containing 10 % fetal bovine serum (FBS; PAA Laboratory, Morningside QLD, cat. no. A15002).
2.2 Preparation of Wnt-10b
-
1.
Wnt-10b-secreting COS cell line (Wnt-COS cells) (see Note 1 ).
-
2.
Plastic cell culture dishes (10 cm) (BD, Bedford, MA, cat. no. 353003).
-
3.
Phosphate-buffered saline (PBS), pH 7.4 (Wako, cat. no. 048-29805).
-
4.
DMEM medium containing 10 % FBS.
-
5.
Penicillin and streptomycin (Wako, cat. no. 168-23191).
-
6.
Plastic tubes (50 mL) (BD, cat. no. 352070).
-
7.
Filter membrane (0.22 μm) (TPP, Switzerland, cat. no. 99522).
2.3 DPC Culture
-
1.
Plastic cell culture dishes (6 cm) (BD, cat. no. 353002).
-
2.
PBS.
-
3.
DMEM medium containing 10 % FBS.
-
4.
Penicillin and streptomycin.
-
5.
Trypsin–EDTA (0.25 %) (Invitrogen, Burlington, ON, Canada, cat. no. 25200-056).
-
6.
Wnt-COS supernatant prepared from Wnt-COS cells (see Subheading 3.2).
2.4 Characterization of DPCs
2.4.1 ALP staining
-
1.
PBS.
-
2.
Alkaline phosphatase kit (Sigma, St Louis, MO, cat. no. 85L3R).
-
3.
Distilled water.
-
4.
Light microscope (CarlZeiss; model no. Axivert 40CFL).
2.4.2 Cell Proliferation Assay
-
1.
Flat-bottom 96-well plastic plates (BD, cat. no. 353072).
-
2.
PBS.
-
3.
Trypsin–EDTA (0.25 %).
-
4.
Trypan blue (Invitrogen, cat. no. 15250061).
-
5.
Hemocytometer (Waken Btech, Kyoto, Japan, cat. no. WC2-100).
-
6.
Light microscope.
2.4.3 RT-PCR
-
1.
PBS.
-
2.
TRIzol (Invitrogen, cat. no. 15596-026).
-
3.
Random primer (Takara, Otsu, Japan, cat. no. 3801).
-
4.
DNase (Takara, cat. no. 2215A).
-
5.
M-MLV reverse transcriptase (Promega, Madison, WI, cat.no. M1861).
-
6.
PCR primers (see Table 1).
Table 1 Gene-specific primers used in the present study -
7.
PCR rTaq enzyme (Takara, cat. no. R001A).
-
8.
Thermal cycler (MJ Research; model no. PTC-200).
-
9.
Agarose (Nacalai Tesque, Osaka, cat. no. 011-53).
-
10.
Tris–acetate–EDTA (TAE) electrophoresis buffer (Nippon Gene, Toyama, Japan, cat. no. 313-90035).
-
11.
DNA molecular weight markers (Takara, cat. no. 3407A).
-
12.
Ethidium bromide (Bio-Rad, cat. no. 161-0433).
-
13.
Horizontal gel electrophoresis apparatus (COSMO BIO, model no. MyRun system).
3 Methods
3.1 Isolation of DPCs
-
1.
Isolate dermal papilla (DP) from vibrissa of 4-week-old mice using microdissection method. Cut open the mystacial pad, invert the skin, and remove follicles using micro-scissors (Fig. 1a).
Fig. 1 Isolation and cultivation of DP cells. (a) Vibrissa follicles isolated from 4-week-old C3H/HeN mice. Scale bar = 100 μm (b) The collagen capsule surrounding each follicle was removed to expose the follicle base, then the DP (arrow) was dissected. Scale bar = 250 μm (c) On day 4, DPCs appeared only around the DP specimens (arrow). Scale bar = 100 μm (d) During the following 2 days, DP cells showed rapid outgrowth. Scale bar = 100 μm
-
2.
Remove collagen capsules surrounding vibrissae follicles to expose the follicle base and dissect DP using fine forceps (Fig. 1b).
-
3.
Place DP specimens into 6-cm dishes and culture quietly for 4 days in DMEM containing 10 % FBS (Fig. 1c).
-
4.
DP cells (DPCs) appear only around the DPs and begin to show rapid outgrowth over the following 2 days (Fig. 1d). By day 10, DPCs show spreading throughout all areas of the dishes.
3.2 Preparation of Wnt-10b
-
1.
Collect Wnt-COS cells cultured in DMEM containing 10 % FBS using trypsin–EDTA.
-
2.
Seed Wnt-COS cells into 10-cm dishes at a density of 2 × 105 cells/cm2.
-
3.
The next day, confirm confluent cell growth in the dishes. To prepare conditioned medium, irradiate the cells using X-ray (40 Gy) and rinse with PBS, then add 20 mL of fresh DMEM medium containing 10 % FBS.
-
4.
After 48 h, collect the supernatants.
-
5.
Centrifuge samples at 250 × g for 5 min and collect the supernatants.
-
6.
Filtrate supernatants with 0.22-μm filter membrane and aliquot 10-mL samples into 15-mL tubes (see Note 2 ).
3.3 DPC Cultures
Figure 2 shows outlines of the DP and DPC culture protocols.
Experimental design and procedures. DP culture: DPs were isolated from vibrissa of C3H/HeN mice, then placed in 6-cm dishes and quietly cultured in DMEM containing 10 % FBS. DPC cultures: Primary DPCs harvested from DP cultures were seeded into 6-cm culture dishes and cultured in Wnt-COS supernatant (Wnt-COS sup), then harvested on day 10. DPCs cultured in Wnt-COS sup underwent passaging every 10 days until the end of the tenth culture
-
1.
Culture primary DPCs from DPs in DMEM medium containing 10 % FBS for 10 days to allow for proliferation (see Subheading 3.1).
-
2.
Wash DPCs with PBS and harvest with 0.25 % trypsin–EDTA.
-
3.
Subject samples to centrifuging at 250 × g for 5 min and collect DPCs.
-
4.
Seed 1 × 103 DPCs into 6-cm culture dishes with Wnt-COS supernatant (see Subheading 3.2).
-
5.
Change medium every 4 days.
-
6.
DPCs cultured in Wnt-COS supernatant undergo passaging every 10 days (see Note 3 )
-
7.
Harvest DPCs on day 10 using 0.25 % trypsin–EDTA and use for various experiments (e.g., transplantation).
3.4 Characterization of DPCs
3.4.1 ALP Staining
-
1.
Culture DPCs with Wnt-COS supernatant for 10 days to allow for proliferation (see Subheading 3.3).
-
2.
Wash cells twice in PBS and fix in fixation solution at room temperature for 30 s.
-
3.
Remove fixation solution and wash with water.
-
4.
Remove water and add dye solution, then incubate for 30 min at room temperature.
-
5.
Rinse cells with water 3–4 times and observe under a light microscope (see Note 4 and Fig. 3a).
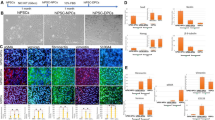
Fig. 3 Properties maintained by Wnt-10b in serially passaged DP cells. (a) DPC-10p formed multilayered clumps and retained ALP activity in a manner similar to DPCs after two passages in Wnt-COS supernatant (DPC-2p). Scale bar = 100 μm (b) DPC-10p proliferated in a manner similar to DPC-2p in regard to cell number. (c) RT-PCR findings showed clear expressions of Lef-1 and versican, and slight expression of Ptc-1
3.4.2 Cell Proliferation Assay
-
1.
Culture DPCs with Wnt-COS supernatant to allow for proliferation (see Subheading 3.3.).
-
2.
Wash DPCs with PBS and harvest with 0.25 % trypsin–EDTA.
-
3.
Centrifuge samples at 250 × g for 5 min and collect DPCs.
-
4.
Seed DPCs into flat-bottom 96-well plastic plates at a density of 50 cells per well with Wnt-COS supernatant.
-
5.
Change medium every 4 days.
-
6.
Harvest DPCs on day 10 using 0.25 % trypsin–EDTA and resuspend pellets with DMEM.
-
7.
Transfer 100 μL of cells into new 1.5-mL tubes and add 400 μL of 0.4 % trypan blue (final concentration 0.08 %). Mix gently.
-
8.
Using a pipette, take 100 μL of the trypan blue-treated cell suspensions and examine with hemocytometer.
-
9.
Using a hand tally counter, count live (unstained) cells (live cells do not take up trypan blue) in a single set of 16 squares (see Note 5 and Fig. 3b).
3.4.3 RT-PCR
-
1.
Culture DPCs with Wnt-COS supernatant to allow for proliferation (see Subheading 3.3).
-
2.
Wash DPCs with PBS twice, add 1.0 mL of TRIzol to attached DPCs to extract total RNA, and purify using the protocol recommended by the manufacturer.
-
3.
Prepare cDNA by reaction of random primer and M-MLV reverse transcriptase with 1 μg of DNase-treated total RNA.
-
4.
For PCR analysis, use 0.5 μg of cDNA as a template and perform amplification using primer sequences shown in Table 1. General PCR conditions: 25–30 cycles at 94 °C for 2 min, 94 °C for 30 s, 52–62 °C for 30 s, and 72 °C for 1 min.
-
5.
Run PCR products on 1.5 % agarose gels and visualize by ethidium bromide staining. (see Note 6 and Fig. 3c )
4 Notes
-
1.
In order to obtain Wnt-10b protein, we constructed a plasmid carrying the Wnt-10b cDNA gene and transfected it into COS-7 cells [10]. The clone that produced recombinant Wnt-10b protein most abundantly was selected and termed Wnt-COS. Supernatant from cultures of Wnt-COS cells contained secreted recombinant Wnt-10b, which inhibited the differentiation of a pre-adipocyte cell line (3 T3-L1) into lipid-carrying adipocytes.
-
2.
The culture supernatant of Wnt-COS cells (Wnt-COS supernatant) contains bioactive Wnt-10b protein. The aliquots may be stored at 4 °C for up to 1 week or at −80 °C for a much longer period.
-
3.
We examined DPCs after ten passages in cultures with Wnt-COS supernatant (DPC-10p). DPC-10p cells proliferated in a manner similar to DPCs after two passages in Wnt-COS supernatant (DPC-2p), and retained their ALP activity and gene expressions (see Subheading 3.4 and Fig. 3). It was shown possible that growth can continue for more than ten passages [9].
-
4.
DPC-10p cells retained ALP activity in a manner similar to DPCs after two passages in cultures with Wnt-COS supernatant (DPC-2p) (see Fig. 3a).
-
5.
DPC-10p cells proliferated in a manner similar to DPCs after two passages in cultures with Wnt-COS supernatant (DPC-2p) (see Fig. 3b).
-
6.
Isolated DPs expressed all of the target genes (Versican, Lef-1, Ptc-1, Gli-1). Both DPC-10p and DPC-2p cells cultured with Wnt-COS supernatant distinctly expressed Versican and Lef-1, while they showed faint Ptc-1 expression and none of Gli-1 (see Fig. 3c).
References
du Cros DL, LeBaron RG, Couchman JR (1995) Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol 105(3):426–431
Millar SE (2002) Molecular mechanisms regulating hair follicle development. J Invest Dermatol 118(2):216–225. doi:10.1046/j.0022-202x.2001.01670.x, 1670 [pii]
Botchkareva NV, Ahluwalia G, Shander D (2006) Apoptosis in the hair follicle. J Invest Dermatol 126(2):258–264. doi:10.1038/sj.jid.5700007, 5700007 [pii]
Jahoda C, Oliver RF (1981) The growth of vibrissa dermal papilla cells in vitro. Br J Dermatol 105(6):623–627
Kishimoto J, Burgeson RE, Morgan BA (2000) Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 14(10):1181–1185
Osada A, Iwabuchi T, Kishimoto J, Hamazaki TS, Okochi H (2007) Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng 13(5):975–982. doi:10.1089/ten.2006.0304
Inamatsu M, Matsuzaki T, Iwanari H, Yoshizato K (1998) Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J Invest Dermatol 111(5):767–775. doi:10.1046/j.1523-1747.1998.00382.x
Yamauchi K, Kurosaka A (2009) Inhibition of glycogen synthase kinase-3 enhances the expression of alkaline phosphatase and insulin-like growth factor-1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Arch Dermatol Res 301(5):357–365. doi:10.1007/s00403-009-0929-7
Ouji Y, Ishizaka S, Yoshikawa M (2012) Dermal papilla cells serially cultured with Wnt-10b sustain their hair follicle induction activity after transplantation into nude mice. Cell Transplant. doi:10.3727/096368912X636867
Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S (2006) Wnt-10b promotes differentiation of skin epithelial cells in vitro. Biochem Biophys Res Commun 342(1):28–35. doi:10.1016/j.bbrc.2006.01.104, S0006-291X(06)00177-X [pii]
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Ouji, Y., Yoshikawa, M. (2016). Maintenance of Dermal Papilla Cells by Wnt-10b In Vitro. In: Turksen, K. (eds) Stem Cell Heterogeneity. Methods in Molecular Biology, vol 1516. Humana Press, New York, NY. https://doi.org/10.1007/7651_2016_319
Download citation
DOI: https://doi.org/10.1007/7651_2016_319
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-6549-6
Online ISBN: 978-1-4939-6550-2
eBook Packages: Springer Protocols