Abstract
The heat shock proteins (Hsps) are a family of highly conserved proteins involved in the regulation of numerous cellular processes including those associated with cancer. Inhibiting the function of these Hsps, specifically Hsp70 and Hsp90, is a major strategy used in the development of new cancer therapies. Numerous Hsp90 inhibitors have been evaluated in the clinic, and while some have experienced success, many have produced disappointing results. One reason explaining their failure is that they induce a cytoprotective response that protects cancer cells from the negative effects of Hsp90 inhibition. In order to maximise the therapeutic outcomes, dual inhibition of Hsp70 and Hsp90 can be employed to overcome cell rescue mechanisms induced by monotherapies. In this chapter, we discuss dual inhibition of Hsp70 and Hsp90 using small molecules and evaluate the potential of this strategy for the development of cancer therapeutics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heat shock protein 90 (Hsp90) is a molecular chaperone involved in the maintenance of protein homeostasis in the cell [1–3]. Hsp90 assists in the folding, stabilisation, activation and degradation of numerous cellular proteins. Hsp90 interacts with over 400 client proteins [4], many of which are associated with cancer [1]. Upon activation, these client proteins assist in disease progression, which makes Hsp90 a regulator of many disease-causing pathways. Consequently, Hsp90 inhibition has emerged as a promising strategy for the treatment of diseases involving aberrant protein structure and function, including cancer.
Hsp90 exists as a homodimer, where each monomer contains an amino (N-) terminus, a middle domain and a carboxy (C-) terminus (Fig. 1). The N-terminus contains an ATP-binding site, the middle domain contains binding sites for client proteins and co-chaperones, and the C-terminus serves as the dimerisation domain and also contains binding sites for co-chaperones. It is well understood that clinical Hsp90 inhibitors or “classical inhibitors” target the N-terminal ATP-binding site of Hsp90, impacting Hsp90’s protein folding cycle (Fig. 1).
Hsp90’s protein folding cycle and the mechanism of classical Hsp90 inhibition. (a) Unfolded client proteins are delivered to Hsp90 by Hsp40 and Hsp70 via interactions with the co-chaperone Hsp70/Hsp90 organising protein (HOP). (b) Hsp90 utilises ATP hydrolysis to change conformation. (c) Fully folded client protein is released
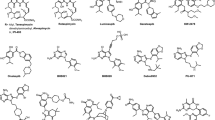
The first Hsp90 inhibitor, geldanamycin, was identified in 1994, and its derivative tanespimycin (17-AAG) entered the clinic as a cancer therapeutic in 1999 [5] (Fig. 2). Both of these analogs are from the ansamycin class of compounds. Since 1995, Hsp90 inhibitor drug candidates have been steadily entering the clinic, with a total of 15 different drugs being tested as monotherapies in clinical trials since 1999 [6, 7]. The ansamycin molecules are reported to inhibit ATP from binding to Hsp90 (Fig. 1b). However, recent evidence shows that they are highly promiscuous and, as such, are likely targeting many proteins, not just Hsp90, which would explain their failure in clinical trials.
More recent Hsp90 inhibitor drug candidates mimic the ATP molecule (Fig. 2). Several of these molecules are still in clinical trials and are being used to treat many cancer types including breast cancer, non-small cell lung cancer (NSCLC), melanoma, renal cell carcinoma (RCC), multiple myeloma (MM), gastrointestinal stromal tumour (GIST), castrate-resistant prostate cancer (CRPC) and several types of leukaemia [6, 7]. Clinical inhibitors block ATP from binding, leading to the inhibition of protein folding. However, clinical trial results showed that when used as single agents, these Hsp90 inhibitors were not highly efficacious and have generated disappointing patient outcomes.
As a single agent, 17-AAG had significant side effects [8] and was subsequently dropped from clinical trials. Three other recent monotherapy regiments in clinical trials involved (1) 17-DMAG to treat CRPC, melanoma or acute myeloid leukaemia; (2) ganetespib to treat breast cancer and NSCLC; and (3) IPI-504 to treat NSCLC and GIST [9, 10] (Fig. 2). However, patient’s responses were modest, where 17-DMAG caused a response in 2 of 28 patients (7%) HER2+ breast cancer patients, ganetespib caused a partial response (PR) in 2 of 22 (9%) breast cancer patients, and IPI-504 caused a PR in 5 of 76 (7%) NSCLC patients and 1 in 36 (3%) GIST patients [7, 10, 11]. 17-DMAG was also reported to cause a complete response (CR) in a single patient that had CRPC, three CR in acute myeloid leukaemia and one PR in melanoma [8, 11]. Thus, Hsp90 inhibitors have shown positive clinical benefit for patients, although the low response rate is a significant concern. Classical Hsp90 inhibitors continue to enter into clinical trials as both single and combination therapies. Currently, there are 32 active studies evaluating the effects of these drugs on numerous cancer types (Table 1).
The low response rate coupled with hepatotoxicity, ocular toxicity and in one case mortality has caused the suspension of most clinical trials using Hsp90 inhibitors as single-agent chemotherapeutics [12, 13]. The limited effectiveness of these Hsp90 inhibitors appears to be due to several key factors. The first is that the ATP-binding site, where these clinical molecules interact with Hsp90, has a binding pocket that is similar to several classes of proteins, specifically DNA polymerases and tyrosine kinases [14]. Thus, classical inhibitors appear to bind to other proteins in addition to Hsp90, thereby producing off-target effects [15–25] and potentially contributing to the observed toxicity associated with these drugs.
Second, resistance and anti-apoptotic pathways are activated immediately upon patient treatment with these clinical Hsp90 inhibitors. This resistance is a result of the specific types of Hsp90 inhibitors activating the cellular heat shock response (HSR) [26]. The HSR is an evolutionary response that is triggered when the cell is under stress and was first discovered by subjecting cells to high temperatures. Triggering a HSR induces high levels of heat shock proteins (Hsps), which are responsible for refolding the aggregated and misfolded proteins that accumulate in the stressed or rapidly growing cell, and they aid in protein degradation [27]. The HSR facilitates cell survival by activating resistance mechanisms and anti-apoptotic pathways [28, 29].
Specifically, cellular stress leads to releasing heat shock factor 1 (HSF-1) from Hsp90 (Fig. 3b) [30–34]. Transport of HSF-1 into the cytoplasm is inhibited leading to a build-up of HSF-1 in the nucleus [35]. HSF-1 then forms a trimer in the nucleus, which is extensively phosphorylated [36]. The HSF-1 trimer binds to specific sequences known as heat shock elements (HSE) in DNA promoters and induces transcription of genes encoding for itself and multiple cellular chaperones, including heat shock protein 27 (Hsp27), heat shock protein 40 (Hsp40) and heat shock protein 70 (Hsp70), in order to rescue the cell from the accumulating unfolded proteins [37, 38] (Fig. 3c). In the absence of stress, promoters for these genes are occupied and unavailable [39, 40]. The mRNAs encoding for inducible and constitutive Hsp70 (HSPA1A and HSPA8, respectively) are produced during the HSR, as well as mRNA that encodes for HSF-1, Hsp40 and Hsp27 (Fig. 3d). These Hsps attempt to rescue the cell from the unfolded protein that is accumulating. The high levels of Hsps refold the aggregated and misfolded proteins that accumulate in the stressed cell, and selected Hsps can also aid in protein degradation [27].
Depiction of the widely accepted model of heat shock and the induction of the heat shock response (HSR). (a) Stress from heat shock or clinical Hsp90 inhibitors triggers an accumulation of unfolded proteins in the cell. (b) The Hsp90 protein complex collects these unfolded proteins, which causes the release of HSF-1 from the protein complex. (c) HSF-1 then forms an active trimer, which translocates to the nucleus and binds to DNA. (d) The mRNA of the heat shock proteins is transcribed from the DNA. (e) The mRNA is then translated into the heat shock proteins, which can then facilitate folding of the previously accumulated unfolded proteins
Similar to the stress caused by high temperatures, the excessive growth of cancer creates stress in cells, and thus cancer cells produce high levels of Hsps. These Hsps maintain protein folding and protein degradation and repair the large quantity of proteins required for rapid cell division, as well as stabilising mutated oncoproteins [27, 41]. This high level of Hsps, including Hsp90, is why Hsp90 inhibitors are a promising treatment for cancer. However, inhibiting Hsp90 function using the clinical inhibitors is well established to produce high levels of Hsp70; indeed, Hsp70 is often used as a pharmacodynamic marker to determine if Hsp90 is being inhibited by classical inhibitors [42–44].
Hsp70 assists in the delivery of specific clients to Hsp90 [45], as well as functioning as an independent chaperone that facilitates protein translocation; stabilises anti-apoptotic proteins; plays a key role in cellular resistance; and prevents apoptosis [46–49]. Thus, inducing high levels of Hsp70 (>6-fold over background) such as those observed when treating cells and patients with the classical Hsp90 inhibitors is problematic for killing cancer cells. Indeed, the high production of Hsp70 likely plays a key role in the disappointing clinical results [20–24]. In response to these poor results for classical inhibitors, two strategies have been employed.
The first approach, which is the development of Hsp90 inhibitors that target sites other than the ATP-binding site of Hsp90, offers alternative mechanisms for blocking Hsp90’s activity. Specifically, inhibiting activity at the C-terminus of Hsp90 does not induce a HSR, nor lead to the upregulation of the Hsps [20–24, 50–55]. Thus, this approach may succeed as a single treatment as it does not produce the anti-apoptotic or resistance observed with the classical inhibitors. The second approach, which is already being used in the clinic, is a combination of classical Hsp90 inhibitors with other forms of therapy. This second approach is discussed in this chapter.
Dual inhibition is a rapidly developing area, and there are a large number of clinical trials and patents being reported in this field. Of the 32 active clinical trials, 17 are studying the effects of Hsp90 inhibitors with one or more other drugs (www.clinicaltrials.gov database). Recent patents include the use of combination treatments utilising Hsp90 inhibitors with Hsp27 or Hsp70 inhibitors (patent number WO-2007041294). Yukimasa patented results using a classic Hsp90 inhibitor (KW-2478) in combination with an Hsp70, Hsp27 or BCL2 cancer treatment drug (patent number WO-2007028387). Kyowa Hakko Kogyo patented the treatment of a classical inhibitor 17-AAG being used in combination with a kinase inhibitor such as gefitinib or a proteasome inhibitor such as bortezomib (patent number WO-2008108386). Astex Therapeutics has patented the drug combination of the classical inhibitor AT9283 with cyclin-dependent kinase inhibitors or aurora kinase inhibitors (WO2008044045). Patent activity on dual inhibitors shows that this line of investigation is being vigorously pursued.
2 Hsp90 Inhibition-Based Combination Treatment
Hsp90 is vital for most cancer cells because of its pivotal role in modulating protein conformation and maturation [56–60]. To date, more than 400 proteins are regulated by or associated with Hsp90, and as such they are called Hsp90 client proteins [4]. About half of these clients are critical for cancer cell growth [61], including transmembrane tyrosine kinases (HER2 and EGFR), metastable signalling proteins (Akt, K-ras and Raf-1), mutated signalling proteins (p53 and v-Src), chimeric signalling proteins (Bcr-Abl), cell cycle regulators (Cdk4 and Cdk6) and steroid receptors (androgen, oestrogen and progesterone receptors) [62–67]. When mutated or deregulated, these clients promote cancer growth. Cancer cell proliferation and survival [68, 69] are facilitated by Hsp90 by maintaining tumours and homeostasis and helping cells to adapt to unfavourable or stressful microenvironments that include heat, hypoxia, free radical production, radiation and chemotherapy [68–71].
Because of its key roles in tumour development, Hsp90 has emerged as a promising target for cancer therapy [63, 72–74]. Inhibiting Hsp90 has involved targeting all three domains: the N-, middle and C-domains as a paradigm of network-oriented drug discovery [63, 71, 75, 76]. Indeed, success at suppressing cancer cell growth has been reported in both preclinical and clinical studies [5, 50, 51, 54, 61, 72–74, 77–80]. Although there are currently 32 clinical trials involving Hsp90 inhibitors, only three unique structures are involved in these studies and are being tested on patients. All three target the N-terminal ATP-binding site of Hsp90 [54] and more than half of these clinical trials are using the compounds in conjunction with other therapies [81–84].
Given Hsp90’s central regulating role in cancer development and its close relationship with numerous key oncogenic proteins, studies are now exploring if Hsp90 inhibitors can sensitise tumours to other chemotherapeutic agents. Developing combination therapies using Hsp90 inhibitors and other types of anticancer agents with a distinct mechanism of action is one avenue that is currently being investigated. Encouragingly, Hsp90 inhibition-based combination treatments of cancer have proven to be more effective and more successful than monotherapies in clinical trials, indicating a promising future for anticancer treatment. In this section, we focus on the investigation and achievement of combination treatments based on direct Hsp90 and Hsp70 dual inhibition.
2.1 Dual Hsp90 and Hsp70 Inhibition
The disappointing clinical results of Hsp90 inhibitors are likely connected to induction of the HSR, which upregulates Hsp70 and Hsp27 as well as HSF-1 [74, 85, 86]. Induction of Hsp70 produces the undesirable effect of counteracting the efficiency of Hsp90-based treatment, and it has been identified as a hallmark of N-terminal Hsp90 inhibitors [87–97] (Fig. 4a). The C-terminal modulators, which do not target the ATP site on Hsp90, do not induce HSF-1 nor the HSR [50, 51, 54, 77–80, 98] (Fig. 4b and c). Herein we discuss two approaches to dual inhibition of Hsp70 and Hsp90 including combining small-molecule inhibitors of both Hsp70 and Hsp90 and combining Hsp70 silencing with Hsp90 inhibitors.
Hsp90 inhibitors in clinical trials. (a) Structures of current Hsp90 inhibitors in clinical trials, all targeting the ATP-binding site at the N-terminus. (b) Structures of Hsp90 inhibitors targeting alternative sites on Hsp90. (c) Diagram of Hsp90 showing the binding locations of each inhibitor. (d) The interactions shown (ATP binding and TPR-containing co-chaperones) are modulated by the inhibitors
There are several rescue mechanisms that are induced with the HSR. First, Hsp90 is induced and can still perform its protein folding and regulatory role. Second, Hsp70 is also induced and may compensate for some of Hsp90’s inhibited functions by assisting in protein folding, preventing protein aggregation and regulating protein complex assembly or disassembly [99, 100]. Third, Hsp70 actively participates in the protection of cancer cells from both extrinsic and intrinsic apoptosis [99]. Ectopic overexpression or induced endogenous levels of Hsp70 promote cancer cell survival by effectively inhibiting lysosomal membrane permeabilization [49], death receptor pathway [48], mitochondria-initiated signalling for caspase-dependent apoptosis [46, 47, 101–104] as well as AIF-associated caspase-independent apoptosis [105, 106].
Evidence of Hsp70’s critical role in apoptosis was seen when silencing Hsp70 expression using antisense oligonucleotides or ectopic transfection produced extensive apoptotic cancer cell death [48, 107, 108]. Furthermore, Hsp70 inhibition triggers an antitumour immune response by blocking the Hsp70-induced activation of myeloid suppressive cells (MDSC), which have the capacity to suppress both the cytotoxic activities of natural killer (NK) and NKT cells and the adaptive immune response mediated by CD4+ and CD8+ T cells [109–113]. All of these factors make dual inhibition of Hsp90 and Hsp70 an optimal cancer therapy.
Using a combination treatment of Hsp90 and Hsp70 inhibitors may not only neutralise the issues associated with N-terminal Hsp90 inhibition, but it may also amplify their anticancer efficiency based on their multiple and independent mechanisms of action. Encouragingly, Hsp70 silencing using siRNA (small interfering RNA), shRNA (small hairpin RNA) or cDNA (complementary DNA in the reversed orientation) of Hsp70 has proven to successfully and synergistically potentiate Hsp90-based anticancer treatment in both solid tumours and leukaemia [48, 89, 90, 95]. However, only a few scientific studies on dual inhibition using small-molecule inhibitors have been published, mainly because only a limited number of compounds effectively modulate Hsp70’s function [54, 90, 114]. MAL3-101, Pifithrin-μ and VER-155008 (Fig. 5c) are the only three drugs that specifically target Hsp70 and show synergism or an additive effect in combination treatment with Hsp90 inhibitors both in vitro and in vivo [89, 93, 115–117].
VER-155008 binds to the ATP-binding site in Hsp70, blocking Hsp70’s access to ATP and halting Hsp70’s function by denying it energy to perform (Fig. 5a). VER-155008 was developed through a structure-based X-ray crystallographic design [118]. It is also the first molecule to target the ATP-binding domain of Hsp70 protein [116]. Treatment of cancer cells with VER-155008 showed antiproliferative activity in many types of human cancer cells, including colon cancer [116], breast cancer [118], multiple myeloma [89] and acute myeloid leukaemia [117]. As expected, VER-155008 shows synergistic or additive combination effects with Hsp90 inhibitors in preclinical cancer treatments [89, 116, 117].
Pifithrin-μ binds to the substrate-binding domain of Hsp70 and blocks other substrates from effectively interacting with that site (Fig. 5). Pifithrin-μ specifically targets the inducible isoform of Hsp70, without binding to the constitutive Hsp70 or to Hsp90 [119]. It interferes with the C-terminal substrate-binding domain of Hsp70 and disrupts its association with client proteins, causing cell cycle arrest and significant apoptosis at low micromolar concentrations. This leads the loss of Hsp70 function, as it can no longer interact with substrates.
Like Pifithrin-μ, MAL3-101 (Fig. 5) binds to Hsp70 at the substrate-binding site. It blocks Hsp70’s essential cellular function by inhibiting the ability of Hsp40 co-chaperones to stimulate Hsp70 ATPase activity [120, 121] (Fig. 5b). Hsp40 docks to Hsp70 during substrate transfer of unfolded client proteins (Fig. 1); thus, inhibiting this binding event halts the transfer of unfolded proteins and impacts protein homeostasis, thereby inducing cell death.
2.1.1 Combination of VER-155008 and NVP-AUY922 in Multiple Myeloma Treatment
VER-155008-based Hsp70 inhibition has been relatively successful in the treatment of multiple myeloma (MM) [89]. VER-155008 significantly decreased the cellular viability in MM cell lines, including INA-6, MM.1S, L363, KMS11 and JJN-3. The sensitivity of MM cells to VER-155008 differed between cell lines with IC50 values from 2.5 to 17 μM, and the drug concentrations that induce near complete cell demise in all studied cell lines are between 10 and 30 μM. VER-155008-induced Hsp70 inhibition led to apoptosis in MM with substantial accumulation of apoptosis-inducing factor (AIF) in the nucleus and with increased cleavage of pro-caspases 9/3 and the caspase substrate poly(ADP-ribose) polymerase 1 (PARP 1). Additionally, VER-155008 treatment simultaneously degraded many Hsp90 client proteins involved in a number of oncogenic signalling pathways including Ras/Raf/MAPK, JAK/STAT3, PI3K/Akt and the IKK/NFkB pathways.
When a dual inhibition approach of VER-155008 and NVP-AUY-922 was implemented into INA-6, MM.1S and primary MM cells, a synergistic mode of action was observed [89]. Specifically, the combination treatment with two inhibitors significantly enhanced apoptosis induction, where the combination effect on INA-6 cells and MM.1S cells was synergistic and additive, respectively, with combination indices (CI) less than 1 for all effect levels calculated [89, 90] (Table 2).
2.1.2 Combination of VER-155008 and 17-DMAG in Leukaemia Treatment
Acute myeloid leukaemia (AML) is a biologically heterogeneous malignancy characterised by bone marrow infiltration of immature leukaemic blasts [122]. Hsp90 has emerged as a potent therapeutic target in AML, and the Hsp90 inhibitor 17-DMAG is effective in killing AML cells in vitro preclinical tests [117]. However, Hsp90 inhibition showed limited antileukaemic effects in phase I clinical trials [11, 123]. One major reason for this is the compensatory Hsp70 upregulation, which is induced by HSF-1 [74, 95, 124]. This is supported by the observation that, in a manner observed with other Hsp90 inhibitors that target the ATP pocket, 17-DMAG-based AML treatment increased Hsp70 and Hsp90 production [11].
However, combination treatment with Hsp90 and Hsp70 inhibitors to neutralise the induced Hsp70 proteins shows promise for human AML [117]. In primary human AML cells from 19 unselected patients, the Hsp70 inhibitor VER-155008 itself showed significant antileukaemic activity at 10 μΜ, causing a dose-dependent inhibition of cancer cell proliferation, where growth was inhibited by 72%. Additionally, Hsp90 inhibitor 17-DMAG was used to treat cells at 50 nM, which also resulted in growth inhibition of 58%. The combination treatment with both inhibitors in primary AML cells decreased cell growth to 82%, indicating an additive growth inhibition effect on AML cells. Moreover, although both VER-155008 (10 μM) and 17-DMAG (50 nM) were able to cause an inhibition in AML colony formation for most patients, the strongest and most significant decrease in colony number was observed when the two drugs were combined.
VER-155008-mediated Hsp70 inhibition in AML cells did not induce any compensatory increase in other Hsps; in fact it caused a significant reduction of both Hsp70 and Hsp90 expressions when used alone. In contrast, 17-DMAG-mediated Hsp90 inhibition resulted in a significant increase in Hsp70 and Hsp90 levels. When VER-155008 was used in combination with 17-DMAG, Hsp90 and Hsp70, expression levels increased to the same level as when cells were treated with 17-DMAG alone [117]. Thus, in contrast to silencing Hsp70 using siRNA, chemical inhibition of Hsp70 by VER-155008 fails to regulate the Hsp70 and Hsp90 protein increase that is induced by Hsp90 inhibition. These data explain why the combined effect of VER-155008 and 17-DMAG is only additive and not synergistic. Furthermore, it is possible that given that VER-155008 targets an ATP-binding pocket, it may have off-target effects.
2.1.3 Combination of VER-155008 and SM122 in Colon Cancer Treatment
SM122 (Fig. 4b) is a unique Hsp90 inhibitor that modulates the C-terminus and does not induce a HSR or produce an accumulation of Hsp70 in the human colon cancer cell line (HCT116). Recent work by Wang and McAlpine investigated the effects of combining SM122 with Hsp70 inhibitor VER-155008 on chaperone-mediated protein folding and the induction of apoptosis, compared to a combination of 17-AAG and VER-155008 [20, 21]. Synergistic effects for both SM122/VER-155008 and 17-AAG/VER-155008 treatments were observed in multiple cell lines including HCT116, human lung adenocarcinoma epithelial cells (A549), human cervical cancer cells (HeLa) and human pancreatic cancer cells (MiaPaca-2). In addition to showing synergism, both combination treatments displayed tumour-specific effects with an acceptable therapeutic window. Analysis of chaperone-mediated protein folding was achieved using a rabbit reticulocyte lysate (RRL)-based luciferase-refolding assay. Individually, SM122 and 17-AAG have a similar impact on protein folding, where they both have an IC50 value of ~2 μM. However, the most effective inhibition of protein folding was observed when Hsp90 and Hsp70 were concomitantly inhibited. Combinations of 20 μM VER-155008 with increasing concentrations of either SM122 or 17-AAG showed very strong synergism. Interestingly, while 17-AAG and SM122 have very different GI50 values of 50 nM and 8 μM in HCT116 cells, respectively, they inhibit protein folding at a similar concentration [20, 21].
Combination treatments of SM122 or 17-AAG with VER-155008 both showed synergism in their ability to kill multiple cancer cell types and had a similar impact on protein folding. However, each combination induced apoptosis via a unique mechanism [20, 21]. HCT116 cells were treated with 50 μM VER-155008 and either SM122 or 17-AAG at two- to threefold over their GI50. Apoptosis was induced in 75% of the cells treated with SM122/VER-155008, while only 50% apoptosis was induced in cells treated with 17-AAG/VER-155008. Cell death occurred via a caspase 3/7-dependent pathway with PARP-1 cleavage in both dual treatments. Interestingly, while the 17-AAG/VER-155008 treatment showed a better capacity to activate caspase 3/7, SM122/VER-155008 induced higher levels of early and late apoptosis [20, 21]. This data suggests that the primary mechanism through which 17-AAG/VER-155008 induces cell death is via caspase pathways; however, SM122/VER-155008 triggers cell death through additional pathways simultaneously, which may be beneficial in reducing the chance of tumour cells developing resistance.
The individual mechanisms by which SM122 and 17-AAG trigger cell death explains the differences in the apoptosis observed as they induce apoptosis via different cellular pathways when used in combination with VER-155008 [20–24]. Each drug combination has distinct impacts on HSR pathways. Evaluating the impact of SM122 and 17-AAG with VER-155008 on mRNA transcription, translation and protein expression levels of Hsps provided evidence of their individually unique mechanism of action [20, 21]. Activation of the HSR is characterised by an accumulation of Hsps including Hsp70, Hsp40 and Hsp27. When HCT116 cells were treated with 17-AAG and VER-155008 individually, Hsp70 mRNA levels increased by 45- and 250-fold, respectively. In contrast, SM122 produced a twofold decrease in Hsp70 mRNA [20]. These data show that Hsp70 inhibition and N-terminal Hsp90 inhibition triggers the HSR at a transcriptional level.
When HCT116 cells were treated with 17-AAG/VER-155008 and SM122/VER-155008, Hsp70 mRNA levels increased by 3,500- and 1,500-fold, respectively [20, 21]. The SM122/VER-155008 treatment did not trigger the HSR as rapidly as 17-AAG/VER-155008, which is likely because SM122 suppresses and/or delays the transcription of Hsp70 mRNA when used in combination with VER-155008.
These phenotypic differences between SM122 and 17-AAG are also observed at the translational level, where SM122/VER-155008 synergistically inhibits protein translation, while 17-AAG/VER-155008 has no impact on translation [20]. Evaluation of heat shock protein expression levels (Hsp27, Hsp70 and Hsp40) showed that treating HCT116 cells with 17-AAG, VER-155008 or 17-AAG/VER-155008 in combination produced a large increase in Hsp70, Hsp40 and Hsp27. As discussed earlier, cells treated with SM122 decreased these protein levels [20, 21]. Dual treatment with SM122 and VER-155008 produced higher protein levels than when cells were treated with SM122 alone; however, the levels were no higher than cells treated with VER-155008 alone, showing that SM122 did not contribute to the rescue mechanism.
These results show that C-terminal modulators and N-terminal Hsp90 inhibitors have distinct mechanisms when used in combination with an Hsp70 inhibitor. Dual treatments are synergistic and induce rapid cell death in numerous cancer cell lines far more effectively than monotherapies. Thus, dual therapies have great potential as cancer treatment regimens, particularly those involving C-terminal Hsp90 modulators like SM122, which has the added benefit of reducing the HSR and limiting the ability of the cancer cell to rescue itself following treatment.
2.1.4 Combination Treatment of MAL3-101 with 17-AAG
MAL3-101 binds to an interface between Hsp40 and Hsp70, thereby impacting Hsp40-mediated stimulation of Hsp70’s ATPase activity [120, 125]. Using MAL3-101 to inhibit Hsp70 alone has successfully treated preclinical MM primary tumour cells and endothelial progenitor cells (EPCs) obtained from MM patients [126]. Specifically, MAL3-101 treatment led to the inhibition of proliferation and survival in NCI-H929 cells with an IC50 value of 8.3 μM at 40-h exposure using an MTS assay. Cell cycle analysis showed that after 48-h treatment, MAL3-101 caused a 2.5-fold decrease at G2/M phase, with a nearly threefold increase at sub-G0/G1 phase in NCI-H929 cells, which indicated an activation of an apoptotic pathway. Confirmation by FACS analysis showed that cells treated with MAL3-101 increased apoptosis, cleavage of caspase-3 and PARP in a time-dependent manner.
In contrast to MM cells being treated with VER 155008 and 17-DMAG inhibitors, treatment of MM cells with MAL3-101 and 17-AAG led to apoptosis [126]. Specifically, in NCI-H929 MM cell line, 10 μM of MAL3-101 significantly decreased the IC50 of 17-AAG from 400 to 30 nM. The isobologram analysis of 10 μM MAL3-101 and 17-AAG with five different concentrations (25, 50, 100, 500 and 1,000 nM) showed tremendous synergistic effect, with combination index (CI) values from 0.008 to 0.12, where CI < 0.1 is “very strong synergism” and 0.1 ≤ CI ≥ 0.3 is “strong synergism” (Table 2). These data support the hypothesis that VER-155008 may have off-target effects and is not only targeting Hsp70, whereas MAL3-101s may have a more selective impact on Hsp70’s activity.
2.1.5 Combination Treatment of PFT-μ with 17-AAG
Pifithrin-μ (PFT-μ) has been identified as a potent Hsp70 inhibitor specifically targeting the inducible isoform of Hsp70, without binding to Hsp90 [119]. It interferes with the C-terminal substrate-binding domain of Hsp70 and disrupts its association with client proteins, causing cell cycle arrest and significant apoptosis at low micromolar concentrations in acute myeloid leukaemia (AML), acute lymphoblastic leukaemia (ALL) and primary AML blasts [119]. Importantly, normal haematopoietic cells and stromal cells exhibited a remarkably high resistance to PFT-μ compared to leukaemic blasts [115]. In bone marrow stromal cells (BMSC), the median IC50 value was ~4-fold higher than that in leukaemic blast cancer cells [115], which indicates that a therapeutic index can be achieved using PFT-μ.
Combination treatment with PFT-μ and the Hsp90 inhibitor 17-AAG showed synergism in reducing cell viability of all studied acute leukaemia cells including NALM-6, TOM-1 and KG-1a. Among the three cell lines, KG-1a was the least sensitive to PFT-μ and 17-AAG individual treatment, with 81% and 72% cell viability after exposure to 10 μM of PFT-μ and 5 μM of 17-AAG, respectively. However, this cell line had the most significant response to treatment by both inhibitors, showing only 29% cell viability upon treatment with these two concentrations. For the NALM-6 leukaemia cell line, their viability when treated with PFT-μ and 17-AAG monotherapies was 70% (2 μM of PFT-μ) and 70% (2 μM of 17-AAG), respectively, versus 42% when used in combination. For the TOM-1 cell line, viability was 85% (3 μM of PFT-μ) and 57% (1 μM of 17-AAG) when using monotherapies versus 36% when treating cells with both drugs.
2.1.6 Combination Treatment of AIF-Derived Peptide with 17-AAG
ADD70 is a designed peptide constructed from the amino acid residues in the AIF protein that bind to Hsp70 (amino acids 150–228) (Fig. 6). ADD70 sensitises cancer cells to apoptosis induction by capturing and neutralising the endogenous Hsp70 protein in the cytosol. ADD70 does not exert any apoptotic effects by itself [105, 127, 128]. ADD70 displayed significant anti-tumorigenic and anti-metastatic properties, as well as the ability to enhance cancer cell immunogenicity by facilitating the induction of a tumour-specific immune response, which increased the number and cytotoxic activity of CD8+ tumour-infiltrating T cells [127].
The expression of ADD70 showed an additive effect when 17-AGG was used in the rat colon cancer ProB cells and mouse melanoma cancer B16F10 cells. These two distinct models of tumours were developed in syngeneic rodents. The additive effect observed when using ADD70 and 17-AAG appears to be related to the reduction of inducible Hsp70 protein by ADD70, where low levels of Hsp70 protein allowed AIF-mediated caspase-independent apoptotic pathways (Fig. 6) to induce pro-apoptotic functions [105, 106, 129]. Inducing AIF-mediated apoptosis is unique to ADD70 and is not seen with any of the small molecules described above. It is thought that, since ADD70 contains the AIF sequence that binds to Hsp70, ADD70 disrupts the AIF-Hsp70-binding event inducing apoptosis via the AIF pathway. Release of AIF facilitates apoptosis and an indirect induction of the Apaf-1-mediated caspase-dependent apoptosis (Fig. 6).
Interestingly, ADD70 significantly enhanced the chemosensitizing effect of 17-AAG on cisplatin-mediated chemotherapy [127]. For example, the combination of 17-AAG and cisplatin only showed additive anticancer effects on several cancer cells. However, in the presence of ADD70, the impact of cisplatin on cell death was strongly enhanced in both cell lines, indicating that the expression of ADD70 can efficaciously potentiate the chemosensitizing effect of 17-AAG. Thus, the study of ADD70 and 17-AAG provided evidence that simultaneous targeting Hsp70 and Hsp90 can effectively provide anticancer therapy.
2.1.7 Combining Hsp70 Silencing with Hsp90 Inhibition in Human Solid Tumours
Constitutive heat shock cognate 70 (Hsc70) and inducible heat shock protein 72 (Hsp72) are two major cytoplasmic isoforms of the Hsp70 multigene family, and they have different expression patterns in mammalian cells. In non-tumour tissues, Hsc70 is abundantly and ubiquitously expressed, whereas Hsp72 is present at relatively low levels. However, under stressed conditions, Hsp72 is overexpressed, while Hsc70 is minimally impacted [89, 95, 130–132]. Selectively knocking down either Hsp72 or Hsc70 isoform using siRNA had no impact on cell proliferation in multiple cancer cells [95]. However, silencing Hsp72 significantly enhanced the antiproliferative effect of 17-AAG-mediated Hsp90 inhibition on colon cancer HCT116 cells, inducing a fivefold increase in apoptosis [95]. In contrast, when Hsc70 was silenced, there was no improved apoptosis or response to 17-AAG in any cancer cell line [95].
The differential effects of selective Hsp70 isoform silencing on the combination treatment with 17-AAG indicate that although both Hsc70 and Hsp72 can bind to Hsp90, both are induced after 17-AAG-mediated Hsp90 inhibition [92, 133–136]. Hsp72 appears to play the most important role in maintaining cell viability. These data are supported by recent evidence that 17-AAG induces 80–100-fold increases in Hsp72 mRNA levels, but only ~6-fold increase in Hsc70 [21, 23]. Thus, it appears the cell protection effects are primarily produced by an increase in Hsp72, and this isoform is heavily induced by 17-AAG. The protective effects can be silenced by knocking down Hsp72, and indeed this is the most effective route for enhancing Hsp90 inhibitors [48, 137]. Furthermore, coupling treatment of Hsp72/Hsc70 inhibition with 17-AAG also shows no effect on non-tumour cells. This observation suggests that inhibiting Hsp72 in combination with an Hsp90 inhibitor may offer a reasonable treatment with a potential therapeutic window [138].
3 Conclusions
Highlighted in this chapter are examples that indicate Hsp90 inhibition is a more effective treatment when used in combination with other chemotherapies. Successfully combining Hsp90 inhibitors with other chemotherapy drugs including molecules that target Hsps produces rapid apoptosis and cell death, which can avoid resistance and cancer metastasis. Specifically, combining Hsp90 and Hsp70 inhibitors produces large increases in apoptosis and potency of up to 92% compared to using single inhibitors. A major reason for this combination being so effective is that inhibiting Hsp90 using classical inhibitors increases the production of Hsp70 protein, which is pro-survival. We also describe how using C-terminal Hsp90 inhibitors is more effective than a classical N-terminal inhibitor when used as a dual therapy. Thus, targeting multiple points in the cell protection mechanism known as the HSR is likely to produce a highly effective new therapeutic approach (Fig. 7).
(a) Using N-terminal inhibitors (i.e. classical inhibitors) promotes production of Hsp70 and the heat shock response, whereas using a C-terminal modulator inhibits co-chaperones from binding to Hsp90 and induces cell death. (b) Inhibiting both Hsp90 and Hsp70 stops the function of both proteins simultaneously, blocking the rescue response and inducing massive cancer cell death
References
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332
Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C (2007) Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol 81(1):15–27
Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5(10):781–791
Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S (2012) Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150(5):987–1001
Whitesell L, Mimnaugh EG, De Costa B, Meyers CE, Neckers LM (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 91(18):8324–8328
Jhaveri K, Modi S (2012) HSP90 inhibitors for cancer therapy and overcoming drug resistance. Adv Pharmacol 65:471–517
Jhaveri K, Taldone T, Modi S, Chiosis G (2012) Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta 1823(3):742–755
Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U, Roels B, Peachey H, Aherne W, de Bono JS, Raynaud F, Workman P, Judson I (2011) A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res 17(6):1561–1570
Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D’Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C (2011) HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 17(15):5132–5139
Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R, Hafeez N, Sweeney J, Walker JR, Fritz C, Ross RW, Grayzel D, Engelman JA, Borger DR, Paez G, Natale R (2010) Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol 28(33):4953–4960
Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL, Baer MR (2010) Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia 24:699–705
Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L, Mannargudi B, Figg WD, Houk BE, Shnaidman M, Brega N, Giaccone G (2011) A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res 17(21):6831–6839
Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J, Patterson J, Penders C, Pink M, Read MA, Sang J, Woodward C, Zhang Y, Grayzel DS, Wright J, Barrett JA, Palombella VJ, Adams J, Tong JK (2006) Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci U S A 103(46):17408–17413
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90(1):65–75
Chavany C, Mimnaugh E, Miller P, Bitton R, Nguyen P, Trepel J, Whitesell L, Schnur R, Moyer J, Neckers L (1996) p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem 271:4974–4977
Johnson RD, Haber A, Rinehart KLJ (1974) Geldanamycin biosynthesis and carbon magnetic resonance. J Am Chem Soc 96:3316–3317
Li YH, Lu QN, Wang HQ, Tao PZ, Jiang JD (2012) Geldanamycin, a ligand of heat shock protein 90, inhibits herpes simplex virus type 2 replication both in vitro and in vivo. J Antibiot (Tokyo) 65:509–512
Rinehart KL, Sasaki K, Slomp G, Grostic MF, Olson EC (1970) Geldanamycin. I. Structure assignment. J Am Chem Soc 92:7591–7593
Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, DiOrio CI, Barbacci EG, Miller PE, Pollack VA, Savage DM, Sloan DE, Pustilnik LR, Moyer JD, Moyer MP (1995) erbB-2 oncogene inhibition by geldanamycin derivatives: synthesis, mechanism of action, and structure-activity relationships. J Med Chem 38:3813–3820
Wang Y, McAlpine SR (2015) C-terminal heat shock protein 90 modulators produce desirable oncogenic properties. Org Biomol Chem 13:4627–4631
Wang Y, McAlpine SR (2015) Combining an Hsp70 inhibitor with either an N-terminal and C-terminal hsp90 inhibitor produces mechanistically distinct phenotypes. Org Biomol Chem 13:3691–3698
Wang Y, McAlpine SR (2015) Heat shock protein 90 inhibitors: will they ever succeed as chemotherapeutics? Future Med Chem 7(2):87–90
Wang Y, Mcalpine SR (2015) N-terminal and C-terminal modulation of Hsp90 produce dissimilar phenotypes. Chem Commun 51:1410–1413
Wang Y, McAlpine SR (2015) Regulating the cytoprotective response in cancer cells using simultaneous inhibition of Hsp90 and Hsp70. Org Biomol Chem 13:2108–2116
Yamaki H, Suzuki H, Choi EC, Tanaka N (1982) Inhibition of DNA synthesis in murine tumor cells by geldanamycin, an antibiotic of the benzoquinoid ansamycin group. J Antibiot (Tokyo) 35:886–892
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Anckar J, Sistonen L (2011) Regulation of HSF1 in the heat stress response: implications in aging and disease. Annu Rev Biochem 80:1089–1115
Chiosis G, JHuezo H, Rosen N, Mimgaugh E, Whitesell L, Neckers L (2003) Binding affinity and potent cell activity-finding an explanation. Mol Cancer Ther 2:123–129
Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ (2009) Targeting HSP90 for cancer therapy. Br J Cancer 100:1523–1529
Ali A, Bharadwaj S, O’Carroll R, Ovsenek N (1998) Hsp90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol 18:4949–4960
Bharadwaj S, Ali A, Ovsenek N (1999) Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 in vivo. Mol Cell Biol 19:8033–8041
Guo Y, Guettouche T, Fenna M, Boellmann F, Pratt WB (2001) Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J Biol Chem 276:45791–45799
Morimoto RI (2002) Dynamic remodelling of transcription complexes by molecular chaperones. Cell 110:281–284
Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP complex) that forms a stress-sensitive complex with HSF1. Cell 94:471–480
Vujanac M, Fenaroli A, Zimarino V (2005) Constitutive nuclear import and stress-regulated nucleocytoplasmic shuttling of mammalian heat-shock factor 1. Traffic 6:214–229
Kline MP, Morimoto RI (1997) Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol 17:2107–2115
Sandqvist A, Bjork JK, Akerfelt M, Chitikova Z, Grichine A (2009) Heterotrimerization of heat shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol Biol Cell 20:1340–1347
Xiao H, Perisic O, Lis JT (1991) Cooperative binding of Drosophila heat shock factor to arrays of conserved 5 bp unit. Cell 64:585–593
Core LJ, Lis JT (2008) Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science 319:1791–1792
Rougvie AE, Lis JT (1988) The RNA polymerase II molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell 54:795–804
Morimoto RI, Tissieres A, Georgopoulos C (1990) The stress response, function of the proteins and perspectives. In: Morimoto RI, Tissieres A, Georgopoulos C (eds) Stress protein in biology and medicinal. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 1–36
Day JEH, Sharp SY, Rowlands MG, Aherne W, Hayes A, Raynaud FI, Lewis W, Roe SM, Prodromou C, Pearl LH, Workman P, Moody CJ (2011) Targeting the Hsp90 molecular chaperone with novel macrolactams. Synthesis, structural, binding, and cellular studies. ACS Chem Biol 6(12):1339–1347
Powers MV, Valenti M, Miranda S, Maloney A, Eccles SA, Thomas G, Clarke PA, Workman P (2013) Mode of cell death induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent on the expression of pro-apoptotic bax. Oncotarget 4(11):1963–1975
Workman P, Al-Lazikani B (2013) Drugging cancer genomes. Nat Rev Drug Discov 12(12):889–890
Powers MV, Jones K, Barillari C, Westwood I, van Montfort RL, Workman P (2010) Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle 9:1542–1550
Beere HM (2004) “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475
Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, Annavarapu S, Mouttaki A, Sondarva G, Wei S, Wu J, Djeu J, Bhalla K (2005) Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood 105:1246–1255
Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Høyer-Hansen M, Weber E, Multhoff G, Rohde M, Jäättelä M (2004) Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 200:425–435
Ardi VC, Alexander LD, Johnson VA, McAlpine SR (2011) Macrocycles that inhibit the binding between heat shock protein 90 and TPR-containing proteins. ACS Chem Biol 6:1357–1367
Eskew JD, Sadikot T, Morales P, Duren A, Dunwiddie I, Swink M, Zhang X, Hembruff S, Donnelly A, Rajewski RA, Blagg B, Manjarrez JR, Matts RL, Holzbeierlein JM, Vielhauer GA (2011) Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer 11:468
Koay YC, McConnell JR, Wang Y, Kim SJ, McAlpine SR (2014) Chemically accessible Hsp90 inhibitor that does not induce a heat shock response. ACS Med Chem Lett 5:771–776
Kunicki JB, Petersen MN, Alexander LD, Ardi VC, McConnell JR, McAlpine SR (2011) Synthesis and evaluation of biotinylated sansalvamide A analogs and their modulation of Hsp90. Bioorg Med Chem Lett 21:4716–4719
McConnell JM, Alexander LD, McAlpine SR (2014) A heat shock protein inhibitor that modulates immunophilins and regulates hormone receptors. Bioorg Med Chem Lett 24:661–666
Shelton SNS, Matthews ME, Lu SB, Donnelly Y, Szabla AC, Tanol K, Vielhauer M, Rajewski GA, Matts RA, Blagg RL, Robertson BS (2009) KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells. Mol Pharmacol 76:1314–1322
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858
Horibe T, Kohno M, Haramoto M, Ohara K, Kawakami K (2011) Designed hybrid TPR peptide targeting Hsp90 as a novel anticancer agent. J Transl Med 9:8
Neckers L, Mimnaugh E, Schulte TW (1999) Hsp90 as an anti-cancer target. Drug Resist Updat 2:165–172
Scott MD, Frydman J (2003) Aberrant protein folding as the molecular basis of cancer. Methods Mol Biol 232:67–76
Workman P, Burrows F, Neckers L, Rosend N (2007) Drugging the cancer chaperone Hsp90: combinational therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci 1113:202–216
Yi F, Regan L (2008) A novel class of small molecule inhibitors of Hsp90. ACS Chem Biol 3:645–654
Falsone SF, Gesslbauer B, Tirk F, Piccinini AM, Kungl AJ (2005) A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Lett 579:6350–6354
Horibe T, Torisawa A, Kohno M, Kawakami K (2012) Molecular mechanism of cytotoxicity induced by Hsp90-targeted Antp-TPR hybrid peptide in glioblastoma cells. Mol Cancer 11:59
Kamal A, Boehm MF, Burrows FJ (2004) Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med 10:283–290
McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J (2007) Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approach. Cell 131:121–135
Welch WJ (1991) The role of heat-shock proteins as molecular chaperones. Curr Opin Cell Biol 3:1033–1038
Westerheide SD, Morimoto RI (2005) Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem 280:33097–33100
Pearl LH, Prodromou C (2000) Structure and in vivo function of Hsp90. Curr Opin Struct Biol 10:46–51
Young JC, Moarefi I, Hartl FU (2001) Hsp90: a specialized but essential protein-folding tool. J Cell Biol 154:267–273
Horibe T, Kawamoto M, Kohno M, Kawakami K (2012) Cytotoxic activity to acute myeloid leukemia cells by Antp-TPR hybrid peptide targeting Hsp90. J Biosci Bioeng 114:96–103
Isaacs JS, Xu W, Neckers L (2003) Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 3:213–217
Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC (2010) Heat shock protein 90: inhibitors in clinical trials. J Med Chem 53:3–17
Janin YL (2010) ATPase inhibitors of heat-shock protein 90, second season. Drug Discov Today 15:342–353
Powers MV, Clarke PA, Workman P (2009) Death by chaperone: HSP90, HSP70 or both? Cell Cycle 8:518–526
Butcher EC (2005) Can cell systems biology rescue drug discovery? Nat Rev Drug Discov 4:461–467
Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J (2006) Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel 9:483–495
Alexander LD, Partridge JR, Agard DA, McAlpine SR (2011) A small molecule that preferentially binds the closed Hsp90 conformation. Bioorg Med Chem Lett 21:7068–7071
Kusuma BR, Peterson LB, Zhao H, Vielhauer G, Holzberlein J, Blagg BS (2011) Targeting the heat shock protein 90 dimer with dimeric inhibitors. J Med Chem 54:6234–6253
Vasko RC, Rodriguez RA, Cunningham CN, Ardi VC, Agard DA, McAlpine SR (2010) Mechanistic studies of Sansalvamide A-amide: an allosteric modulator of Hsp90. ACS Med Chem Lett 1:4–8
Yu XM, Shen G, Cronk B, Marcu M, Holzberlein J, Neckers LM, Blagg BSJ (2005) Hsp90 inhibitors identified from a library of novobiocin analogues. J Am Chem Soc 127:12778–12779
Gandhi N, Wild AT, Chettiar ST, Aziz K, Kato Y, Gajula RP, Williams RD, Cades JA, Annadanam A, Song D, Zhang Y, Hales RK, Herman JM, Armour E, DeWeese TL, Schaeffer EM, Tran PT (2013) Novel Hsp90 inhibitor NVP-AUY922 radiosensitizes prostate cancer cells. Cancer Biol Ther 14:347–356
Goldman JW, Raju RN, Gordon GA, El-Hariry I, Teofilivici F, Vukovic VM, Bradley R, Karol MD, Chen Y, Guo W, Inoue T, Rosen LS (2013) A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer 13:152–161
Graham B, Curry J, Smyth T, Fazal L, Feltell R, Harada I, Coyle J, Williams B, Reule M, Angove H, Cross DM, Lyons J, Wallis NG, Thompson NT (2012) The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci 103:522–527
Modi S, Saura C, Henderson C, Lin NU, Mahtani R, Goddard J, Rodenas E, Hudis C, O’Shaughnessy J, Baselga J (2013) A multicenter trial evaluating retaspimycin HCL (IPI-504) plus trastuzumab in patients with advanced or metastatic HER2-positive breast cancer. Breast Cancer Res Treat 139:107–113
Song D, Chaerkady R, Tan AC, García-García E, Nalli A, Suárez-Gauthier A, López-Ríos F, Zhang XF, Solomon A, Tong J, Read M, Fritz C, Jimeno A, Pandey A, Hidalgo M (2008) Antitumor activity and molecular effects of the novel heat shock protein 90 inhibitor, IPI-504, in pancreatic cancer. Mol Cancer Ther 7:3275–3284
Zhang H, Chung D, Yang YC, Neely L, Tsurumoto S, Fan J, Zhang L, Biamonte M, Brekken J, Lundgren K, Burrows F (2006) Identification of new biomarkers for clinical trials of Hsp90 inhibitors. Mol Cancer Ther 5:1256–1264
Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A, Guo Y, Wu N, de Stanchina E, White J, Gross SS, Ma Y, Varticovski L, Melnick A, Chiosis G (2009) Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A 106:8368–8373
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172
Chatterjee M, Andrulis M, Stühmer T, Müller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H, Kressmann S, Einsele H, Bargou RC (2013) The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 98:1132–1141
Davenport EL, Zeisig A, Aronson LI, Moore HE, Hockley S, Gonzalez D, Smith EM, Powers MV, Sharp SY, Workman P, Morgan GJ, Davies FE (2010) Targeting heat shock protein 72 enhances Hsp90 inhibitor-induced apoptosis in myeloma. Leukemia 24(10):1804–1807
Gaspar N, Sharp SY, Eccles SA, Gowan S, Popov S, Jones C, Pearson A, Vassal G, Workman P (2010) Mechanistic evaluation of the novel HSP90 inhibitor NVP-AUY922 in adult and pediatric glioblastoma. Mol Cancer Ther 9:1219–1233
Maloney A, Clarke PA, Naaby-Hansen S, Stein R, Koopman J-O, Akpan A, Yang A, Zvelebil M, Cramer R, Stimson L, Aherne W, Banerji U, Judson I, Sharp S, Powers M, deBilly E, Salmons J, Walton M, Burlingame A, Waterfield M, Workman P (2007) Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res 67:3239–3253
McCollum AK, TenEyck CJ, Sauer BM, Toft DO, Erlichman C (2006) Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res 66:10967–10975
Mosser DD, Morimoto RI (2004) Molecular chaperones and the stress of oncogenesis. Oncogene 23:2907–2918
Powers MV, Clarke PA, Workman P (2008) Dual targeting of Hsc70 and Hsp72 inhibits Hsp90 function and induces tumor-specific apoptosis. Cancer Cell 14:250–262
Stühmer T, Chatterjee M, Grella E, Seggewiss R, Langer C, Müller S, Schoepfer J, Garcia-Echeverria C, Quadt C, Jensen MR, Einsele H, Bargou RC (2009) Anti-myeloma activity of the novel 2-aminothienopyrimidine Hsp90 inhibitor NVP-BEP800. Br J Haematol 47:319–327
Stühmer T, Zöllinger A, Siegmund D, Chatterjee M, Grella E, Knop S, Kortüm M, Unzicker C, Jensen MR, Quadt C, Chène P, Schoepfer J, García-Echeverría C, Einsele H, Wajant H, Bargou RC (2008) Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia 22:1604–1612
Wahyudi H, Wang Y, McAlpine SR (2014) Utilizing a Dimerization strategy to inhibit the dimer protein Hsp90: synthesis and biological activity of a sansalvamide A dimer. Org Biomol Chem 12:765–773
Goloudina AR, Demidov ON, Garrido C (2012) Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett 325:117–124
Whitesell L, Santagata S, Lin NU (2012) Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med 12:1108–1124
Creagh EM, Sheehan D, Cotter TG (2000) Heat shock proteins--modulators of apoptosis in tumour cells. Leukemia 14:1161–1173
Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 17:6124–6134
Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES (2000) Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2:476–483
Takayama S, Reed JC, Homma S (2003) Heat-shock proteins as regulators of apoptosis. Oncogene 22:9041–9047
Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, Kouranti I, Spahr C, Pance A, Kroemer G, Garrido C (2003) Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 22:6669–6678
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3:839–843
Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jäättelä M (2000) Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A 97:7871–7876
Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jäättelä M (2002) Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res 62:7139–7142
Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, Hizuta A, Tanaka N, Srivastava PK, Nakayama E (1999) Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol 162:1303–1309
Multhoff G (2002) Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia 18:576–585
Rérole AL, Gobbo J, De Thonel A, Schmitt E, Pais de Barros JP, Hammann A, Lanneau D, Fourmaux E, Deminov O, Micheau O, Lagrost L, Colas P, Kroemer G, Garrido C (2011) Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res 71(2):484–495
Srivastava PK (2008) New jobs for ancient chaperones. Sci Am 299:50–55
Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G (2011) Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A 108(2):733–738
Evans CG, Chang L, Gestwicki JE (2010) Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem 53:4585–4602
Kaiser M, Kühnl A, Reins J, Fischer S, Ortiz-Tanchez J, Schlee C, Mochmann LH, Heesch S, Benlasfer O, Hofmann WK, Thiel E, Baldus CD (2011) Antileukemic activity of the HSP70 inhibitor pifithrin-μ in acute leukemia. Blood Cancer J 1(7):e28. doi:10.1038/bcj.2011.28
Massey AJ, Williamson DS, Browne H, Murray JB, Dokurno P, Shaw T, Macias AT, Daniels Z, Geoffroy S, Dopson M, Lavan P, Matassova N, Francis GL, Graham CJ, Parsons R, Wang Y, Padfield A, Comer M, Drysdale MJ, Wood M (2010) A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol 66(3):535–545
Reikvam H, Nepstad I, Sulen A, Gjertsen BT, Hatfield KJ, Bruserud Ø (2013) Increased antileukemic effects in human acute myeloid leukemia by combining HSP70 and HSP90 inhibitors. Expert Opin Investig Drugs 22:551–563
Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, Macias AT, Murray JB, Parsons R, Shaw T, Surgenor AE, Terry L, Wang Y, Wood M, Massey AJ (2009) Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem 52:1510–1513
Leu JI, Pimkina J, Frank A, Murphy ME, George DL (2009) A small molecule inhibitor of inducible heat shock protein 70. Mol Cell 36(1):15–27
Fewell SW, Smith CM, Lyon MA, Dumitrescu TP, Wipf P, Day BW, Brodsky JL (2004) Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. J Biol Chem 279(49):51131–51140
Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, Litz J, Clement CC, Kang Y, She Y, Wu N, Felts S, Wipf P, Massague J, Jiang X, Brodsky JL, Krystal GW, Chiosis G (2007) Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat Chem Biol 3:498–507
Estey EH (2012) Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 87:89–99
Kaufmann SH, Karp JE, Litzow MR, Mesa RA, Hogan W, Steensma DP, Flatten KS, Loegering DA, Schneider PA, Peterson KL, Maurer MJ, Smith BD, Greer J, Chen Y, Reid JM, Ivy SP, Ames MM, Adjei AA, Erlichman C, Karnitz LM (2011) Phase I and pharmacological study of cytarabine and tanespimycin in relapsed and refractory acute leukemia. Haematologica 96:1619–1626
Reikvam H, Ersvaer E, Bruserud O (2009) Heat shock protein 90 - a potential target in the treatment of human acute myelogenous leukemia. Curr Cancer Drug Targets 9:761–776
Wisen S, Bertelsen EB, Thompson AD, Patury S, Ung P, Chang L, Evans CG, Walter GM, Wipf P, Carlson HA, Brodsky JL, Zuiderweg ER, Gestwicki JE (2010) Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol 5(6):611–622
Braunstein MJ, Scott SS, Scott CM, Behrman S, Walter P, Wipf P, Coplan JD, Chrico W, Joseph D, Brodsky JL, Batuman O (2011) Antimyeloma effects of the heat shock protein 70 molecular chaperone inhibitor MAL3-101. J Oncol 2011:232037
Schmitt E, Maingret L, Puig PE, Rerole AL, Ghiringhelli F, Hammann A, Solary E, Kroemer G, Garrido C (2006) Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res 66:4191–4197
Schmitt E, Parcellier A, Gurbuxani S, Cande C, Hammann A, Morales MC, Hunt CR, Dix DJ, Kroemer RT, Giordanetto F, Jäättelä M, Penninger JM, Pance A, Kroemer G, Garrido C (2003) Chemosensitization by a non-apoptogenic heat shock protein 70-binding apoptosis-inducing factor mutant. Cancer Res 63(23):8233–8240
Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J (2005) Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab 25:899–910
Daugaard M, Rohde M, Jäättelä M (2007) The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett 581:3702–3710
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (2006) Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 5:2592–2601
Sherman M, Multhoff G (2007) Heat shock proteins in cancer. Ann N Y Acad Sci 1113:192–201
Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I (2005) Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 23:4152–4161
Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P (2005) Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res 11:7023–7032
Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C (2005) Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol 23:1078–1087
Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA (2001) Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res 61:4003–4009
Gabai VL, Budagova KR, Sherman MY (2005) Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene 24:3328–3338
Yoon YJ, Kim JA, Shin KD, Shin DS, Han YM, Lee YJ, Lee JS, Kwon BM, Han DC (2011) KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J Biol Chem 286(3):1737–1747
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Buckton, L.K., Wang, Y., McConnell, J.R., McAlpine, S.R. (2015). Evaluating Dual Hsp90 and Hsp70 Inhibition as a Cancer Therapy. In: McAlpine, S., Edkins, A. (eds) Heat Shock Protein Inhibitors. Topics in Medicinal Chemistry, vol 19. Springer, Cham. https://doi.org/10.1007/7355_2015_96
Download citation
DOI: https://doi.org/10.1007/7355_2015_96
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32605-4
Online ISBN: 978-3-319-32607-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)