Abstract
Selenium (Se) is an essential element to humans and animals. Since its diverse functions were discovered, its importance has been more and more revealed. After the discovery of selenium as a key component of many proteins, significant research efforts have been focused on its therapeutic and nutritional potentials and possible impacts on human and animal health. To date, 25 selenoprotein genes have been identified in humans and animals, where selenium exists in the form of an amino acid, selenocysteine (Sec). Sec incorporation into selenoproteins occurs through a unique process by recognizing UGA stop codon via the Sec-charged tRNA for delivering Sec into proteins. Most selenoproteins are antioxidant proteins and play critical roles in maintaining redox balance in living systems by removing reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). These reactive species are major threats to bio-macromolecules, such as DNAs, RNAs, proteins, lipids, and carbohydrates. They may cause molecular oxidation and DNA damage/mutation, alter gene expression patterns, weaken the immune system, and even lead to neurological disorders and other pathological conditions. As potential drugs and/or nutritional supplements, selenium-functionalized molecules (SeFMs) have shown benefits in preventing and/or treating many diseases, such as cancer, cardiovascular diseases, thyroid diseases, diabetes, AIDS, rheumatoid arthritis (RA), and neurodegenerative diseases. These promising results and therapeutic potentials of SeFMs have attracted tremendous attention from academia and pharmaceutical and biotech industries. However, further fundamental research and investigation are required to discover the ideal chemical, biochemical, and biological forms of selenium, appropriate dosages, and mechanisms of the selenium biological actions, since the tolerance window of selenium in humans may be limited.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- DNA and RNA
- Drug and supplement
- Essential element
- Nucleic acids
- Nucleotides
- Selenium functionalization and modification
- Selenium-derivatized nucleosides
1 Introduction
Selenium is an essential trace element for humans and animals. It was discovered in 1817 by Jöns Jakob Berzelius (a Swedish physician and chemist), when he was trying to uncover the etiology of a mysterious disease among workers at a H2SO4 plant in Gripsholm (Sweden). The reason that selenium research has become an intense field of research is because this trace element with nutritional importance has unique sets of biological activities. During early years of the twentieth century, selenium was reported as a toxic element that was manifested by feeding livestock forage grown in seleniferous soil. The first evidence of selenium essentiality in living systems appeared in the middle of the twentieth century. Selenium was reported as a nutritional factor in the formation of formic dehydrogenase in E. coli and Aerobacter aerogenes bacteria [1] and in prevention of exudative diathesis in chicks [2]. Around the same time, Klaus Schwarz and Calvin M. Foltz reported that selenium could prevent liver necrosis in rats fed with torula yeast grown in a selenium-rich medium [3]. Soon thereafter, selenium deficiency was implicated as a factor for the development of several diseases, such as white muscle disease of calves, lambs and foals, and exudative diathesis of poultry. In the late 1960s, epidemiological surveys in different parts of the United States suggested an inverse correlation between the incidences of certain cancers (particularly breast cancer) and the selenium content of the plants growing in different regions. It took about 15 years after the establishment of selenium essentiality in human health to determine one biological role of selenium. The real excitement appeared in 1973 when selenium was found as a natural part of a redox enzyme, glutathione peroxidase (GPx) [4]. Subsequently, two other selenium-containing redox enzymes, formate dehydrogenase [5] and glycine reductase of Clostridium thermoaceticum (belonging to the glutathione peroxidase family), were identified [6]. Analysis of the glycine-reductase complex labeled with 75Se found an oxygen-labile chromophore, which implied the ionized -SeH group of the selenocysteine (Sec) residue [7]. Later, many other studies confirmed the existence of selenium in selenoproteins in the chemical form of selenocysteine [8, 9]. Sec is a cysteine (Cys) analog that differs from Cys by only one atom (selenium versus sulfur), and yet this switch dramatically influences the important aspects of the enzyme reactivity [10]. As Sec is invariably recognized by a codon and has its unique Sec-tRNA and other translation factors, Sec is referred as the 21st amino acid. Besides the glutathione peroxidase family, type 1 iodothyronine deiodinase (a Sec-containing enzyme), which catalyzes the hepatic conversion of thyroxine (T4) to triiodothyronine, was reported in the late 1980s [11, 12].
The puzzle of how selenium incorporates into the amino acid Sec and how Sec is stably co-translated into selenoprotein became clear in 1986. The mRNA sequences of glutathione peroxidase (gpx) of mouse [13] and formate dehydrogenase (fdhF) of E. coli [14] revealed an in-frame UGA codon (termination codon), which encodes for Sec. Simultaneously, another cis-acting element called Sec-insertion sequence (SECIS) element was identified at the 3′-untranslated region of mRNA, which forms the secondary structure and helps Sec-tRNASec to get position at the UGA codon [15, 16]. To date, 25 selenoproteins (Table 1) have been identified and characterized with various functions, such as hormone metabolism, selenium transport, antioxidant/redox properties, anti-inflammatory properties, etc. In every selenoprotein, the Sec residue invariably occupies the core position of the active site and plays very diverse but crucial role in enzymatic catalysis. Besides Sec, another selenium-containing amino acid (selenomethionine, Se-Met) also presents in proteins of all genera. But the incorporation of Se-Met into proteins is not regulated as Sec. Se-Met is randomly incorporated into protein in place of Met, and its incorporation rate depends on the availability of Se-Met during protein synthesis. Se-Met replaces methionine (Met) by charging with the same methionine tRNA (tRNAMet), which is efficiently recognized by all translational factors [54, 55]. The stably incorporated Se-Met does not significantly alter the protein structure, but may influence the activity of enzymes, if Se-Met replaces Met in the vicinity of the active site.
The natural occurrence of selenium is also reported in certain tRNAs from bacteria, archaea, and eukaryotes. The modified 5-methylaminomethyl-2-selenouridine (mnm5Se2U) has been reported to commonly occupy at the wobble position of the anticodons of tRNAGlu, tRNAGln, and tRNALys [56–59]. It is thought to play a critical role in fine-tuning of codon–anticodon base pairing by positioning the Se-modified nucleotide at the first position of the anticodon. The proposed selenium function at the wobble position is supported by the recent research from Zhen Huang and co-workers [60–62]. In addition, the selenium introduction as a heavy atom via Se-Met into protein provides a magnificent tool to solve the phase problem in X-ray crystal structure determination of proteins. In the structure determination, selenium acts as a suitable resonance center for anomalous dispersion in MAD and SAD experiments, which require synchrotron radiation with precise beam wavelength control [63–68]. For nucleic acid structure studies, Zhen Huang and co-workers have developed selenium nucleic acids [61, 62, 68–72] to derivatize nucleic acids and protein–nucleic acid complexes for nucleic acid–protein X-ray crystallography [68].
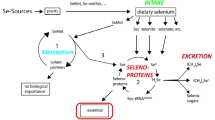
The selenoproteins have a wide range of pleiotropic effects, ranging from antioxidant and anti-inflammatory properties to the production of active thyroid hormone. In the past 10 years, a large number of research projects have been dedicated to discover the disease-associated polymorphism in selenoprotein genes. Selenium is an essential microelement because of its participation in many important enzymes, and its deficiency hampers or reduces the activities of many enzymes essential for proper physiological functions. The two most notable classes of selenoproteins are antioxidants and deiodinases. The roles of antioxidant proteins are to scavenge or neutralize reactive oxygen species (ROS) and reactive nitrogen species (RNS) from the body, where they are continuously generated during normal metabolic and respiratory processes. These ROS and RNS are the major oxidation threats to bio-macromolecules, and they or their by-products may also cause alkylation of DNA, RNA, proteins, lipids, and/or many other biomolecules, which are potentially linked with many diseases, such as cardiovascular diseases, cancers, AIDS, neurodegenerative diseases, thyroid diseases, rheumatoid arthritis, etc. Selenoproteins have been reported to prevent these diseases by removing ROS and RNS. Selenium supplementation may help to maintain a proper level of selenoproteins, thereby reducing the threat of ROS and/or RNS in plasma and preventing many deadly diseases. Selenoproteins have a number of biological functions in oxidoreductions, redox signaling, antioxidant defense, thyroid hormone metabolism, and immune responses. This review focuses on the recent advancement and a few pathological conditions and/or diseased states that may be prevented by or even cured with selenium-functionalized molecules (SeFM; Fig. 1).
Potential metabolic mechanisms of Se-functionalized molecules (SeFMs) in the human body. SeFM enters the body in inorganic (I), organic (II), and/or bio-organic (III) forms. Selenium inorganic forms (selenate and selenite taken as supplements) are converted into hydrogen selenide (less reactive) through several reductions (a–c). The hydrogen selenide is phosphorylated and converted into selenophosphate by selenophosphate synthetase enzyme in an ATP-driven process (n). The highly active selenium donor (selenophosphate) donates selenium functionality to the serine-charged tRNASec, catalyzed by selenocysteine synthase, and produces Sec-tRNASec. The Sec-tRNASec is positioned on an in-frame UGA codon of an mRNA containing SECIS secondary hairpin structure during translation process (q), generating selenoproteins. The most common forms of the organic seleno-compounds are Sec and Se-Met, which are derived from the hydrolysis of proteins ingested as diet. Se-Met can be readily incorporated into proteins nonspecifically (i). Sec, which can be formed from Se-Met by the trans-selenization process (h) and derived from hydrolysis of selenoproteins, undergoes several chemical transformations and forms hydrogen selenide via elemental selenium (l and m). Methylation of the hydrogen selenide could readily occur and produces methyl selenol (CH3SeH) (k), which can also be formed from Se-Met (e). Further methylation of methyl selenol (CH3SeH) produces dimethyl selenide [(CH3)2Se] and trimethyl selenonium ion [(CH3)3Se+], which are excreted from the body through the breath and urine, respectively. The non-amino acid selenium compounds (including organic and bio-organic SeFMs) may also act as potential drugs and/or nutritional supplements [73, 74] in order to supply selenium for maintaining the proper level of selenoproteins and producing beneficial physiological effects [75, 76]
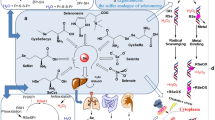
2 Naturally Occurring Selenium Proteins
Twenty-five selenoproteins have been identified in humans with different functions in different subcellular locations (Table 1). Selenoproteins are mostly enzymes that constitute two major metabolic pathways (Fig. 2), namely, redox metabolism and thyroid hormone metabolism (discussed later). The selenoproteins with redox activities are ubiquitous and help to maintain redox balance along with other pathways in the body. The selenoprotein GPx operates with a small ubiquitous peptide called glutathione (GSH) and forms one of the major pathways to neutralize or reduce reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are generated continuously during metabolic and respiratory processes. In the GSH–GPx redox system, the sulfur-containing peptide glutathione (GSH) is a cofactor and acts as a reducing substrate in the peroxidase reaction. During the GPx catalytic cycle, the selenenic acid form (GPx–Se-OH) reacts with GSH and generates GPx–selenenyl-sulfide adduct (GPx–Se-SG). Subsequently, the adduct reacts with an additional GSH to regenerate the active selenol (GPx–SeH), which reduces a peroxide molecule (Fig. 2) [77, 78]. Another selenoprotein family, involved in redox defense system, consists of thioredoxin, thioredoxin reductase, and peroxiredoxin. This is also a major redox pathway and plays a diverse role in metabolism with broader substrate specificity. This powerful system is involved in many central intracellular and extracellular processes, including cell proliferation, the redox regulation of gene expression and signal transduction, protection against oxidative stress, anti-apoptotic functions, growth factor and co-cytokine effects, and regulation of the redox state of the extracellular environment [79, 80] (Fig. 2).
General catalytic mechanisms of human selenoproteins. Selenoproteins constitute two major classes of enzymes: deiodinases (a) and antioxidants (b and c). Deiodinases are responsible for converting the less-active thyroid hormones T4 into active form T3. Among the several redox systems in humans, glutathione–glutathione peroxidase (b; n = 1, 2, 3, 4, or 6) and thioredoxin–thioredoxin reductase (c) redox systems are very important for detoxification of ROS in the body. In the glutathione–glutathione peroxidase redox system, glutathione is a cofactor and acts as a reducing substrate. During the catalytic cycle, oxidized di-glutathione is reduced by the enzyme, glutathione reductase. Thioredoxin–thioredoxin reductase (c) system can also detoxify ROS, but this system has broader substrate specificity
Selenium in proteins exists as amino acid selenocysteine (Sec) and selenomethionine (Se-Met). The presence of selenium atom in proteins confers many advantages in protein functionality. Selenium belongs to the same elemental family (VI A) as oxygen and sulfur in the periodic table. Although the selenium atom (atomic radius, 1.16 Å) is bigger than oxygen (atomic radius, 0.73 Å) and sulfur (atomic radius, 1.02 Å) atoms, their chemical and electronic properties are closely related. As a result of this functional similarity with oxygen and sulfur, but bigger atomic size on the other hand, incorporation of selenium into biomolecules offers many unique and important advantages. Due to the larger electron density, one of the major advantages is that selenium can exist in multiple oxidation states, such as elemental selenium (0), selenide (−2), selenite (+4), and selenate (+6), which largely depend on the chemical properties of the environment. The reactivity of a selenoprotein arises from the selenol (-SeH) side chain of the Sec residue. This SeH functionality provides unique properties to selenoprotein over other residues in the protein. The pK a value of the SeH group in Sec is lower (pK a 5.2), compared to the SH group in Cys (pK a 8.5). As a result of lower pK a, the SeH side chain is more acidic and remains deprotonated at physiological pH, which makes the SeH a more powerful reductant and stronger nucleophile. Most of the selenoproteins are enzymes, and their Sec residues are conveniently located in or near an active site in the close proximity with Cys residue. The catalytic function of a selenoprotein enzyme is dependent on the interplay or shuttling of electrons among SeH, SH groups, and cofactors (if any).
On the other hand, selenium in Se-Met exists as –Se–CH3 side chain, and proteins containing Se-Met do not completely depend on the Se function for their activity. Se-Met is an essential amino acid, and it cannot be synthesized de novo in humans. It must be ingested in the form of Se-Met or protein containing Se-Met. Plants and microorganisms can introduce selenium in place of sulfur during Met biosynthesis. In eukaryotes, if Se-Met is available, it can be charged onto tRNAMet, which is recognized by all other translational components and stably incorporated into proteins in place of Met. Se-Met incorporation into protein is random, and it depends on the availability of Se-Met [81–83]. Se-Met does not significantly alter the protein structure, but may influence the activity of enzymes, if Se-Met replaces Met in the vicinity of the active site. In contrast, Sec may remain in the free form and cannot be introduced into protein readily. It is incorporated into protein via a specific translational control process, which is unique in relation to the standard protein translation [84]. The Sec incorporation into selenoproteins is discovered in all three domains of life: bacteria, archaea, and eukarya [85]. The pathway for selenocysteine incorporation into protein in archaea and eukarya is very similar to E. coli. Selenoprotein synthesis in E. coli begins with the acylation of the 3′-end of a special tRNASec (encoded by selC gene) with l-serine by seryl-tRNA ligase, thus producing seryl-tRNASec (Ser-tRNASec) [86]. The tRNASec has many unique and unusual features, such as formation of O-phosphoseryl-tRNA [87], its anticodon (UCA) suppressing UGA termination codon [88], longer in size (90 nucleotides in eukaryotes), relatively fewer base modifications [89, 90], and the unique cloverleaf model with a long extra arm [91]. The serine residue on the Ser-tRNASec is converted into Sec and forms Sec-tRNASec in two-step reactions by the pyridoxal 5′-phosphate-dependent enzyme, selenocysteine synthase (product of selA gene, EC 2.9.1.1). In the first step, a Schiff base is formed between α-amino group of serine and carbonyl of pyridoxal 5′-phosphate, followed by 2,3-elimination of a water molecule to yield enzyme-bound dehydroalanyl-tRNASec intermediate. In the second step, nucleophilic addition of monoselenophosphate to the double bond of the dehydroalanyl residue produces selenocystyl-tRNASec [92–95]. The highly active Se donor (monoselenophosphate) is formed by transferring γ-phosphate of ATP to hydrogen selenide, which is catalyzed by selenophosphate synthetase (product of selD) [96]. Once Sec-tRNASec is formed, the Sec-tRNASec is recognized by a cis-acting element, Sec-insertion sequence (SECIS), a secondary structure on the selenoproteins mRNA that helps in docking Sec-tRNASec at UGA codon and directs selenocysteine incorporation into protein [97–99]. A Sec-specific elongation factor (product of selB gene) binds to Sec-tRNASec and SECIS and guides the incorporation of Sec onto nascent selenoprotein [100–102]. In archaea and eukarya, O-phosphoseryl-tRNASec kinase phosphorylates the hydroxyl group of serine on seryl-tRNASec [103, 104], and the resulting O-phosphoseryl-tRNASec is subsequently converted to selenocysteinyl-tRNASec by Sec synthetase.
3 Naturally Occurring Selenium Nucleic Acids
The natural occurrence of selenium in certain tRNAs was also reported in bacteria [59, 105], archaea [58, 106], mammals [107], and plants [108]. The discovery of the presence of naturally occurring selenium in tRNAs further provides the evidence at the molecular level for the nutritional role of selenium in life. It is not clear whether selenium can be directly incorporated into nucleosides naturally. Nonetheless, the presence of seleno-modified uridine residue (5-methylaminomethyl-2-selenouridine, mnm5Se2U) in tRNAs was reported, and mnm5Se2U occupies the wobble (first) position of the anticodon of the tRNAs for glutamate (tRNAGlu), glycine (tRNAGln), and lysine (tRNALys) in bacteria [56, 59, 109]. This modified nucleoside is thought to play a critical role in the fine-tuning of codon–anticodon base pairing by positioning the Se modification at the first position of the anticodon. In the anaerobic microorganism, Clostridium sticklandii, the most prominent selenium-containing tRNA was identified as the major glutamate-accepting species in the bulk tRNA preparation. It has been proposed that the presence of selenium in this tRNAGlu is essential for its aminoacylation activity [58, 106, 110].
4 Artificially Seleno-Modified Nucleosides and Nucleotides and Selenium Nucleic Acids (SeNA)
The presence of the Se modification in natural tRNA has encouraged Huang and co-workers to pioneer and incorporate the selenium functionalities at various selected positions of RNA and DNA, namely, selenium-derivatized nucleic acids (SeNA) [68–71, 111–115]. Their experimental data have demonstrated that nucleic acid oxygen in various positions can be stably replaced with selenium for structure and function studies, especially the facilitation of phase solution, crystallization, and high-resolution structure determination in X-ray crystallography. Their studies imply that selenium functionalities on nucleobases might improve the accuracy and efficiency of translation, RNA transcription, and even DNA replication by enhancing base-pair interaction, selectivity, and fidelity. For instance, the biochemical study of RNase H with the Se-modified DNA/RNA duplexes showed that the Se-modified nucleobase functionality can enhance the enzyme turnover rate by manyfold [68]. The supporting evidence from X-ray crystallographic structures has revealed that as a larger atom, the selenium functionality causes local unwinding of DNA/RNA substrate duplex. The Se-functionalized DNA acts as a guiding sequence shifting the scissile phosphate of the RNA substrate closer to the active site, therefore further activating the attacking nucleophilic water molecule [68]. Previously, it has been reported that the selenium-modified nucleic acids can resist nuclease digestion [116–118]. Thus, this Se-atom-specific mutagenesis (SAM) has opened a new research area to further explore and design nucleic acid-based novel therapeutics as well as nutritional supplements.
Therapeutic nucleosides, nucleotides, and nucleic acids can be used to block or interfere with specific pathological processes or pathways. The most common targets of these therapeutics are cancers and viruses. The potential oligonucleotide therapeutics, such as small interference RNAs (siRNA), antisense DNAs, or CRISPR-RNA (crRNA) [119, 120], are based on sequence complementarity to the target RNAs (such as mRNAs) or DNAs (such as genomic DNA). This is an intensely advanced area, because the short oligonucleotides can target a specific RNA very precisely and hydrolyze the RNA by Dicer–Argonaute system [121–125] or target a specific DNA by crRNA and Cas system [119, 120, 126]. Potential application of the Se functionalities in short oligonucleotides could offer additional benefits to the oligonucleotides by improving the base-pair stability, selectivity, and fidelity, by enhancing the catalytic rate, and by conferring resistance to nuclease digestion. However, extensive in vivo research is needed in order to take full advantage of all Se modifications, especially the Se-nucleobase functionalities [61, 68, 72, 127].
As we know, nucleoside analogs are a major class of chemotherapeutic agents that disrupt DNA and/or RNA synthesis (or repair) in targeting malignant cancer cells or viruses. Because the proliferative status of a malignant cell is higher than that of a normal cell, the malignant cell always maintains an elevated concentration of nucleosides. Thus, the malignant cells are generally more sensitive to modified nucleoside agents disrupting nucleic acid synthesis. However, research on the Se-modified therapeutic nucleosides is limited; few Se-modified nucleosides have been investigated to target cancers and viruses. Recently, after pioneering and developing selenium nucleic acids (SeNA) for the function and X-ray crystal structure studies [68–71, 111–115], Huang and co-workers have also investigated several Se-modified nucleosides for anticancer activity against various tumor cell lines. They reported that the MeSe nucleosides have relatively fine solubility in aqueous solution, have anticancer effects, and can generally inhibit cancer cell growth (Fig. 3). Among them, 5′-Se-thymidine (3 in Fig. 3a) is more active than the other Se nucleosides against these prostate cancer cell lines [128]. Recently, Kim and co-workers [129] reported the synthesis of two Se-modified nucleosides: 5-phenylselenyl-methyl-2′-deoxyuridine (5-PhSe-T) and 5-methylselenyl-methyl-2′-deoxyuridine (5-MeSe-T). They have been evaluated for the cytotoxic effect on human cancer cells (Fig. 3b and c). Their caspase activity study suggested that these nucleoside derivatives induced apoptosis by interfering with caspase-2 and caspase-3 pathways and, to a lesser extent, caspase-8 pathway. Another study on HL-60 cells by the same group suggested that the apoptosis induced by 5-PhSe-T and 5-MeSe-T is attributed to p38 pathway. This p38 pathway served as a link between ROS generation and DNA damage/caspase activation [129]. Moreover, several other Se-modified nucleosides have been investigated against HIV as potent inhibitors of reverse transcriptase (Fig. 3d–f) [130].
Chemical structures of selenium-modified nucleosides used as anticancer and antivirus therapy. (a) Among these three MeSe nucleosides, compound 3 was more effective as cytotoxic agent. (b) and (c) PhSe-T and MeSe-T mediate apoptosis induced by p38 pathway. (d) 4′-SelenoddN, (e) 4′-seleno-AZT, and (f) 4′-selenothymidine; they are inhibitors of reverse transcriptase
5 Selenium Deficiency and Diseases
5.1 Thyroid Health
The role of selenium in thyroid function has been established for a long time. In thyroid gland, the amount of selenium per gram of the tissue is the highest among all organs in human body [131, 132]. As an essential micronutrient, selenium is as important as iodine for the production of thyroid hormone thyroxine (or triiodothyronine, T3) and the maintenance of thyroid hormone homeostasis [133]. Thyroid peroxidase (TPO) is the major enzyme produced by the thyroid gland [134, 135], and it is a heme-containing oxidoreductase that catalyzes stepwise iodination of the tyrosine residue on thyroglobulin to produce thyroxine or 3,3′,5,5′-tetraiodothyronine (T4). The selenoenzyme iodothyronine deiodinase converts inactive thyroxine (T4) to its more active 3,5,3′-triiodothyronine form (T3) by removing one iodine atom [136, 137]. Three isozymes of the selenoproteins, iodothyronine deiodinase, types 1, 2, and 3 (DIO1, DIO2, and DIO3), have been characterized and cloned [20, 138, 139]. The expression and function of these isozymes are tightly regulated in a tissue-specific manner and represent a new family of eukaryotic selenoproteins. Two other seleno-enzymes, the antioxidant enzyme (such as glutathione peroxidase) and the redox enzyme (such as thioredoxin reductase), are present in thyroid glands in order to maintain proper environment for thyroid activity [140]. The role of glutathione peroxidase in the gland is to protect the thyroid cells from oxidative damage by catalyzing the reduction of H2O2 with the help of thiol co-substrates, such as glutathione (GSH). The human TRxR also plays important roles in detoxifying ROS in the thyroid gland [132, 141, 142]. Therefore, adequate supply of both iodine and selenium is necessary for thyroid homeostasis and thyroid health. One of the most common thyroid diseases is hyperthyroidism, also called Graves’ disease, often referred to as an overactive thyroid. Hyperthyroidism is a condition where the thyroid gland produces and secretes excessive amounts of thyroid hormones that circulate in the blood [143]. A number of studies have demonstrated an increase in oxidative stress and generation of ROS in Graves’ disease and also reported an increased production of malondialdehyde found in urine [144].
In Graves’ disease, the balance between intracellular and extracellular oxidants and antioxidants appears to be disturbed, and overall GPx activity has been reported to be diminished [145]. In treating hyperthyroidism, methimazole (MMI), propylthiouracil (PTU), and methyl thiouracil (MTU) are the commonly prescribed drugs for adult and pediatric patients. Recently, the FDA has issued a warning that use of PTU is associated with a higher risk for clinically serious or fatal liver injury compared to MMI in both adult and pediatric patients. There are also clinical study reports on the resistance of MMI in patients with hyperthyroidism in spite of good compliance [146]. In recent years there have been renewed interest is grown in developing selenium analogs of antithyroid drugs, such as, MMI (MSeI), PTU (PSeU), and MTU (MSeU) (Fig. 6). Besides toxicity and drug resistance, there are several other initial assumption was made for the selection of the selenium analog over thiol containing compounds. Selenium is a better nucleophile than sulfur; therefore, the seleno-compound may exhibit higher inhibitory effect toward ID-1, compared to the sulfur drug. As these compounds are anticipated to react with the selenenyl iodide intermediate of ID-1, the formation of Se–Se bond may occur more readily than the formation of Se–S bond [147]. In addition, the seleno-compounds may have a significant effect in neutralizing H2O2. There are a few other low-molecular-weight seleno-compounds listed in Fig. 4 that were designed and synthesized by several different groups to mimic Gpx activity as ebselen. These compounds are generated either by modifying the basic structure of ebselen or by incorporating some structural features of the native enzyme. The synthetic GPX mimics reported in the literature include benzoselenazolinones, selenenamide, diaryl selenide, various diselenides, hydroxyalkyl selenides, a selenocysteine derivative, and a selenenate ester (Fig. 4).
Selenium deficiency is implicated in several other thyroid diseases. Chronic lymphocytic thyroiditis is the most common thyroid autoimmune disease where iodine supply is insufficient. Administration of selenium in the form of selenomethionine or sodium selenite along with levothyroxine substitution therapy improves the pathogenic conditions [148, 149]. The thyroid disease myxoedematous cretinism is also found to associate with selenium deficiency. Myxoedematous cretinism is characterized by the persistence of hypothyroidism despite of supplement with iodine. It has been reported that supplementation with iodine alone has no effect, but when together with selenium, it improves the pathogenic condition of myxoedematous cretinism [150].
5.2 Cardiovascular Disease
Cardiovascular disease (also called heart disease) is the number one disease causing death and disability worldwide [151, 152]. There are diverse causes for cardiovascular disease, but the main causes are arteriosclerosis and hypertension [153]. The manifestation and progression of arteriosclerosis involve the triad: hyperlipidemia, oxidative stress, and inflammation [154]. Hyperlipidemia is the condition of elevated level of low-density lipoprotein (LDL) in the blood, which is eventually deposited in adipose tissues. Lipids are the primary targets for oxidative modification (oxidative stress consequence) enzymatically or by free radicals, because they are the main repository of oxidizable olefinic or double bond [155]. Subsequently, these oxidized lipids activate an NFκB-like transcription factor and induce the expression of genes containing NFκB binding sites, which initiate the production of various cytokines [156, 157] involved in inflammation. The inflammatory response leads to the development of the fatty streak and atherosclerotic plaque. Many studies have clearly demonstrated that selenium can greatly reduce cardiovascular risks (such as arteriosclerosis, myocardial hypertrophy, etc.) and help to maintain cardiac health by enhancing antioxidant role of selenoproteins, especially glutathione peroxidases. These cardioprotective effects are thought to confer through the antioxidant actions of selenoprotein enzymes, which directly limit the levels of ROS (such as hydrogen peroxide, H2O2) and reverse the oxidative damages to lipids and proteins [158–160]. It is generally accepted that the GPx level is decreased by the instability of GPx mRNA at low selenium intakes [161, 162].
In the glutathione–GPx redox system, the sulfur-containing peptide glutathione (GSH) is a cofactor and acts as a reducing substrate in the reduction of peroxidases. During the GPx catalytic cycle, the selenenic acid of GPx reacts with GSH to generate GPx–selenenyl-sulfide adduct (GPx–Se-SG). Subsequently, the adduct reacts with an additional GSH to generate the active selenol (GPx–SeH), which in turn reduces peroxide [77, 78]. Another selenoprotein family, involved in redox defense system, consists of thioredoxin, thioredoxin reductase (TRxR), and peroxiredoxin, which play a pivotal role in both hematopoiesis and heart function. Among three types of TRxR in humans, the cytosolic TRxR1 and mitochondrial TRxR2 are required for protecting heart from ROS or free radical-mediated damages [163, 164]. The primary function of the TRxR system in normal cell is to keep thioredoxin (Trx) in reduced state, serve as a redox regulator for cell signaling, and contribute to the antioxidant defense to cells [165, 166].
SeFMs as potential therapeutics and nutritional supplements have been reported to prevent cardiovascular risks and/or to treat cardiovascular diseases. Some SeFMs (Fig. 5) have been studied for preventing or treating cardiovascular diseases by many research laboratories. Though the exact mechanisms of actions for these compounds are not clear, it has been proposed that the cardioprotective effects may be attributed to the antioxidant defense [167]. One particular organo-seleno-compound, diphenyl diselenide (DPDS), has been reported to inhibit human LDL oxidation, and this phenomenon was related to the thiol-peroxidase activity [168]. Another SeFM, ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one), has been investigated for a long time and is a nontoxic organo-seleno-compound that has anti-inflammatory, anti-atherosclerotic, and cytoprotective properties. Ebselen acts by mimicking the active site of GPx, and it is also an excellent scavenger of peroxynitrite. Moreover, it inhibits activities of cyclooxygenase and lipoxygenases at the micromolar concentrations [169, 170].
5.3 SeFMs for Diabetes Treatment
Diabetes mellitus (DM) affects over 170 million people worldwide, with more than 90% of the patients suffering from type 2 diabetes (T2D) [171]. T2D is a heterogeneous metabolic disorder characterized by increased blood sugar level resulting from the resistance of liver, skeletal muscle, and fat tissue to insulin. These conditions cause dyslipidemia, hyperglycemia, and an increase in insulin secretion by pancreatic beta cells for compensation of the poor insulin response by major target tissues [172–175]. T2D is a major risk factor for the incidence of cardiovascular disease characterized by arteriosclerosis and hypertension [153, 176, 177]. In the United States, 12.9% of the adults older than 20 have T2D, of which 39.8% remain undiagnosed [178, 179]. The relationship between glucose metabolism and selenium status in the body is conflicting [180]. In spite of the antioxidant effect of many selenoproteins in maintaining redox balance in the cellular system, some studies indicate a deleterious effect of selenium in T2D. A cross-sectional study on almost 9,000 American adults, along with other analysis, showed a positive link between high selenium levels and T2D [181, 182]. A similar study performed on 7,182 women from Northern Italy has indicated that the increased dietary selenium intake is associated with an increased risk of T2DM [183]. It has also been suggested that selenium impairs hepatic insulin sensitivity through opposite regulation of ROS [184].
In the insulin signaling pathway, binding of insulin to its receptors initiates a signaling cascade with a mild oxidative burst, where H2O2 acts as secondary messenger [180]. In turn, H2O2 oxidizes redox-regulated Cys residues, therefore leading to the deactivation of tyrosine phosphatase 1B (PTP-1-B) and tensin homolog protein (PTEN). PTP-1B deactivates insulin receptor substrate (IRS), whereas PTEN inhibits phosphatidylinositol 3-kinase (PI3K). This results in overall stimulation of the signaling pathway for glucose uptake [185–187]. The selenoproteins (such as glutathione peroxidase 1) reduce H2O2; therefore, the presence of antioxidant proteins produces an inhibitory action on the signaling cascade [188, 189]. However, it has been observed that insulin combined with selenium significantly decreased blood glucose levels and maintained the expression of insulin receptor substrate (IRS-1), phosphatidylinositol-3 kinase (PI3K), and glucose transporter 4 (GLUT4) in skeletal muscles of diabetic rats [190]. This experimental result indicates that the relation between insulin and selenium still remains a challenging mystery, which requires more investigation.
5.4 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune condition with unknown etiology. It is characterized by chronic inflammatory polyarthritis with progressive erosion of tissues within and surrounding the joints. It affects 1% of the population and is associated with significant morbidity and increased mortality [191]. Recent studies indicate a possible role of selenium on the etiology and disease progression of RA. Clinical data shows that patients with RA have a lower level of selenium in serum, compared to healthy controls [192, 193]. The exact mechanism is unknown, but it is assumed that selenium deficiency affects the signaling pathway of nuclear factor kappa-B (NF-κB), which is correlated with interleukin-6 and TNF-α production [194, 195]. Recently, diaryl isoselenazole compounds (4,5-diaryl isoselenazoles) have been reported (Fig. 6) as multiple target nonsteroidal anti-inflammatory drugs (MTNSAIDs) for the inhibition of inflammatory processes [196]. This SeFM could be a safe alternative as a nonsteroidal anti-inflammatory drug (NSAID), which is associated with serious side effects, such as hypertension, gastrointestinal hemorrhage, ulceration, and kidney failure [197]. The mechanism of NSAIDs is to inhibit cyclooxygenase enzymes (COX-1 and COX-2), which catalyze the biotransformation of arachidonic acid to the prostaglandins [198, 199]. But the new class MTNSAIDs (4,5-diaryl isoselenazoles) have been described as dual COX/LO inhibitors with potential radical scavenging by the selenium moiety. This compound is related to the well-known SeFM ebselen with a variety of beneficial properties, such as antioxidant function, radical inactivation, and lipid peroxidase inhibition.
5.5 SeFMs for Cancer Prevention and Treatment
Cancer is becoming an increasingly deadly disease, and there are seven million deaths occurring around the globe each year. According to World Cancer Research Fund and American Institute for Cancer Research, 40% of all cancer can be prevented by the combination of appropriate diets and physical activities. Therefore, cancer management becomes an ideal approach for reduction of cancer mortality by preventing, delaying, or even reversing cancer incidence. Since the initial discovery of selenium as an essential element in humans, the nutritional aspect of selenium has become an intense field of research for connecting the possible role of selenium in cancer prevention. Extensive epidemiological and specific studies have started after the identification of selenoproteins decades ago. Since then, many selenol compounds have been examined, and some have demonstrated potential clinical and molecular role in cancer prevention. A few selenol compounds have been found very effective against many types of cancers, such as lung cancer [200], bladder cancer [201], colorectal cancer [202], liver cancer [203], esophageal cancer [204], gastric–cardiac cancer [205], thyroid cancer [206], and prostate cancers [202, 207–210]. The pivotal role of selenium in cancer prevention is the antioxidant defense, which protects the cells against ROS. Both inorganic forms (such as sodium selenite: Na2SeO3) and organic forms of selenium (such as selenocysteine or selenomethionine) have shown effectiveness in antioxidant defense [76]. Several mechanisms have been proposed for the selenium-mediated anticancer effects. The major mechanisms are (1) the reduction of DNA damage [211, 212], oxidative stress [213–218], and inflammation [219], (2) induction of phase II conjugating enzymes that detoxify carcinogens [220, 221], (3) Se incorporation into selenoproteins, (4) alteration in DNA methylation status of tumor suppressor genes [222–224], and (5) induction of apoptosis and kinase modulation [225–227].
In addition to chemopreventive and anticarcinogenic properties, several seleno-compounds have been reported to halt progression and metastasis of various types of cancers [228–230]. Among them, prostate cancer has been studied most extensively with SeFMs. Administration of oral capsules of sodium selenate to patients with prostate cancer significantly boosts the activity of the protein PP2A phosphatase and impedes tumor neovascularization and angiogenesis [231]. Selenium effect has also been examined in ovine pulmonary adenocarcinoma (OPA), as an animal model for studying lung cancer, by treating animals with sodium selenate [232]. Selenium at higher concentration suppresses expression of TrxR in tissues with lung cancer and causes rapid cell death [233]. The possible mechanism is due to an impaired function of SBP2 (SECIS binding protein 2), therefore reducing TrxR production and activity [234]. Methylselenol, a selenium metabolite, has been reported to induce cell cycle arrest in G1 phase and mediate apoptosis via inhibiting extracellular-regulated kinase 1/2 (ERK1/2) pathway and c-Myc expression [235].
More SeFMs are listed in Fig. 7, and they have been examined as cytotoxic agents with anticancer properties. A clinical trial report revealed that the administration of 1,4-phenylenebis(methylene)selenocyanate (p-XSC) to 40 patients with end-stage cancers has increased predicted survival time in 76% of these cases [236]. The cytotoxic effect of this compound is thought to function by modulating TrxR activity through mitochondrial dysfunction [237]. Another selenol derivative cytotoxic compound acting by Akt3 signaling modulation is isoselenocyanate-4 (ISC-4, similar to ISC-3 in Fig. 7) with a four-carbon alkyl chain. Topical application of ISC-4 on xenografted melanoma mice resulted in reducing tumor cell expansion and development by 80% and increasing apoptosis rates by threefold [238]. Another heterocyclic selenium compound, 1,2-[bis(1,2-benzisoselenazolone-3-(2H)-ketone)]ethane (BBSKE), has been reported to induce apoptosis of the tongue cancer Tca8113 cells by activating caspase-3 pathway. BBSKE is structurally related to ebselen, a thioredoxin reductase inhibitor (Fig. 7) [239].
Examples of seleno-compounds with cytotoxic effect on cancer cells. The Se nucleoside analogs for cancer chemotherapy are in Fig. 3
5.6 SeFMs for HIV Treatment
HIV belongs to the retrovirus family. It carries a single copy of genomic RNA and RNA genes required for reproduction and pathogenesis. The HIV infection process begins with attachment of viral protein gp120 to the specific type of CD4 receptor and a co-receptor on the surface of the CD4 cell. Upon its entry into cells, the viral RNA genome is reversely transcribed into DNA (cDNA) by reverse transcriptase (viral protein), followed by integration of the double-stranded DNA into the host genome. The picture of the HIV-infected population is dire. According to the recent estimates from UNAIDS, there were 35.3 million people living with HIV in 2012, which was up from 29.4 million in 2001. HIV is a leading cause of death worldwide, and the number one cause of death by disease in Africa. Sub-Saharan Africa is most heavily affected by HIV and AIDS than any other regions in the world. An estimated 22.9 million people live with HIV in this region, which is approximately two-thirds of the total number. In 2012, 1.6 million deaths were caused by AIDS worldwide. Though enormous research has been performed on many aspects of HIV/AIDS, critical research on nutritional aspect (especially micronutrient) on HIV epidemic has not been done thoroughly. There are growing concerns on why HIV infection, disease progression, and mortality rates are much higher in the underdeveloped areas in the world. Recently, a randomized clinical trial on the effect of micronutrient supplementation on HIV-infected ART (antiviral treatment) has been reported. It has been found that the combined supplementation with selenium and vitamins B, C and E largely improved the CD4 cell counts of the HIV-infected patients. A supplement containing multivitamins and selenium was found to be safe. The supplement significantly reduced the risk of immune decline and morbidity, if the supplement treatment was started in the early stages of HIV infection [240].
The HIV-infected individuals are immunocompromised. The mechanisms on why vitamins and selenium exert immune stimulatory effects are not fully elucidated, but the role of each micronutrient has been documented. The role of selenium in HIV infection, activation, and disease progression has been investigated in human and animal models extensively [241]. Like many other pathological conditions, oxidative stress is also a potent inducer of HIV activation and DNA damage to the infected cells [242]. The antioxidant properties of selenoproteins are assumed to play an important regulatory role in immune response [243, 244]. Since HIV infection causes the inflammatory response, many inflammatory cytokines are elevated during HIV infection [245], which include tumor necrosis factor-alpha (TNFα), interferon-α, and various interleukins (IL-1, IL-4, IL-6, IL-10) [246]. Elevation of TNFα was related with viral activation, a high level of free radicals, and DNA damage [242].
Oxidative stress is also reported to upregulate the activation of viral replication through the actions of nuclear factor kappa-B (NFκB) [247, 248]. All of these free radical productions lead to body-wide inflammation and depletion of primary antioxidants that cells use to protect themselves against free radicals. In addition, various free radicals generated from inflammatory response cause hypermutation of the viral genome, which lead to the generation of HIV with new pathogenesis. Several identified selenoproteins can decrease the viral replication by mitigating the oxidative stress. It has been found that the levels of selenoproteins that are expressed in T cells, including thioredoxin reductase, glutathione peroxidase, and phospholipid hydroperoxide glutathione peroxidases, are increased in the presence of selenium supplements [249–253]. Unlike some Se-containing compounds used as potential antioxidant supplements, several Se-modified nucleosides have been reported as potential antiviral agents. The 2′,3′-dideoxy-4′-selenonucleosides [254] and 4′-seleno-AZT [255], which are bioisosteric analogs of 2′,3′-dideoxynucleosides (ddNs) and AZT, have been reported as potential inhibitors of HIV reverse transcriptase (Fig. 3).
5.7 SeFMs for Tropical Disease Treatment
Tropical diseases refer to diseases that occur solely or principally in the tropics, such as tuberculosis, malaria, leishmaniasis, schistosomiasis, onchocerciasis, lymphatic filariasis, Chagas disease, African trypanosomiasis, and dengue [256, 257]. As a result of selenium functionality being a natural component of antioxidants and redox proteins in mammals, many experiments have been designed to observe the effect of selenium on tropical diseases as a preventive agent and cure. Among the different diseases that constitute tropical disease, only tuberculosis (TB) and leishmaniasis are discussed here in relation to potential selenium drugs.
Leishmaniasis is a tropical disease, prevalent in the Southeast Asia and East Africa. It occurs all over the world, but the form of leishmaniasis is different from one region to another [258, 259]. Leishmaniasis is caused by several species of flagellated protozoa belonging to the genus Leishmania in the Trypanosomatidae family, whose members are characterized by the presence of the kinetoplast, a unique form of mitochondrial DNA [260]. Leishmaniasis can be caused by over 20 Leishmania species, and it is transmitted to humans through the bite of infected female phlebotomine sand flies [261, 262]. The World Health Organization (WHO) estimates that leishmaniasis results in two million new cases a year and threatens 350 million people in 88 countries. There are 12 million people currently infected worldwide [261, 263]. Recently, the trace element selenium has been identified as a protective agent against oxidative damage caused by Leishmania infection [264, 265]. The biochemical pathways that constitute the cellular targets for selenium are still under investigation.
A class of compounds, which were originally developed as anticancer drugs, has been found very effective as antiprotozoal agents (Fig. 8) [265–268]. These seleno-compounds were synthesized and reported as potential antileishmanial drugs by Carmen Sanmartín’s laboratory. It has also been reported that between the selenocyanide (Fig. 8a) and diselenide (Fig. 8b) derivatives, the diselenide compound with aryl ring substitution (especially with 4-aminophenyl) showed better cytotoxic effects, compared to selenocyanide [265]. Another class of imidothiocarbamate and imidoselenocarbamate compounds (Fig. 8c) has been synthesized and tested against leishmaniasis. Among various substitutions, the imidoselenocarbamate compound (especially with X = Se, Y = N, R = methyl, and R′ = 2Cl groups) was very effective as an antileishmaniasis agent [264]. In 2013, the same laboratory has synthesized and tested a new class of compounds for the treatment of leishmaniasis. The new compound was designed based on the 4,4′-diselanediyldianiline (Fig. 8d) with sulfonamide moieties bound to different rings (Fig. 8e) [269]. Their data suggest that compounds with R group, such as 4-methylphenyl, 4-flurophynyl, or 8-quinolinyl, exhibit high leishmanicidal activity and low cytotoxicity, which are required for antileishmanial drugs.
Selenium containing antiprotozoal agents. (a) Selenocyanide, (b) Diselenide derivatives (best cytotoxic effect with aryl ring substitution with 4-aminophenyl), (c) Imidothiocarbamate and imidoselenocarbamate compounds (among various substitutions, X = Se, Y = N, R = methyl, R’ = 2Cl was shown very effective), (d) 4,4′-diselanediyldianiline (e) 4,4′-diselanediyldianiline with sulfonamide moieties bound to different rings
Tuberculosis (TB) is a highly infectious disease. The etiological agent of TB is Mycobacterium tuberculosis. The notoriety of this highly infectious disease is rested on the fact that the Mycobacterium tuberculosis could develop multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains within the long treatment regimen [270]. The bacteria have evolved drug resistance mechanisms through a persistent phenotype and mutations in drug target genes [271]. Recently, a new target, the antigen 85 (Ag85) protein family consisting of Ag85-A, Ag85-B, and Ag85-C, has been identified. Ag85 has mycoloyl transferase activity, which produces an envelope lipid called trehalose dimycolate (TDM) and cell wall with arabinogalactan-linked mycolic acids, and it is required for cell wall synthesis [272–274]. The organoselenium compound ebselen has been reported to inactivate the Ag85 activity by covalently linking with a cysteine residue located near the mycoloyl transferase active site. Mutational and crystal structure studies indicate that linking ebselen to the cysteine residue disrupts the hydrogen bonding network within the active site that is essential for enzymatic activity [275]. Tuberculosis often coincides with nutritional deficiencies. The effect of selenium on tuberculosis has also been explored in the nutritional aspect. It has been reported that a 2-month intervention with vitamin E and selenium supplementation reduced oxidative stress and improved the outcome in patients undergoing TB chemotherapy [276, 277].
5.8 SeFMs for Neurodegenerative Disease Treatment
Free radicals or, more generally, reactive oxygen species are generated continuously during the process of normal oxidative metabolism. There is growing evidence that free radicals play an important role in onset and progression of several neurodegenerative diseases. Those diseases include cerebral ischemia, Parkinson’s disease, Huntington’s disease, epilepsy, amyotrophic lateral sclerosis, Down’s syndrome, and Alzheimer’s disease (AD) [278–281]. Among these neurodegenerative diseases, Alzheimer’s disease (AD) is among the most pressing health concerns. The pathology of AD is characterized by the formation of large insoluble plaques made of misfolded amyloid beta (Aβ) protein. Amyloid beta (Aβ) protein is a short peptide that is a proteolytic by-product of the transmembrane protein amyloid precursor protein (APP), whose function is unclear, but thought to be involved in neuronal development [282, 283]. The experimental data suggests that beta-amyloid aggregation and toxicity are probably caused by the cellular oxidative stress, which plays a key role in the conversion of soluble to insoluble beta-amyloid, suggesting that the oxidative stress is primary to the beta-amyloid cascade [284, 285].
The effect of selenium on neurodegenerative diseases has been investigated. It has been found that the brain is enriched with selenium and its level declines with age, which may be linked to cognitive impairment and AD development. Administration of selenium in a rat model of acute dementia is found to reduce tau phosphorylation and improve memory loss, which implies the neuroprotective role of selenium [37, 286]. It has also been reported that selenium could attenuate Aβ-induced neuronal death by reducing amyloidogenic β- and γ-secretase activities [286]. Gene knockdown experiments have revealed a potent effect of glutathione peroxidase 4, which protects cells against lipid hydroperoxide damage, the characteristic feature of neurodegenerative disease and AD [287]. A number of studies suggest that GPx4 is an antioxidant enzyme possessing broader substrate specificity than other glutathione peroxidases. It accepts many reductant substrates in addition to glutathione and reacts with a wide array of organic and inorganic peroxides [25, 288, 289]. The neuroprotective role of SelP protein has also been investigated. SelP protects neuronal cells from Aβ-induced toxicity, suggesting a neuroprotective role for SelP in preventing neurodegenerative disorders in AD [290, 291]. Consistently, several studies have demonstrated the significantly lower Se levels (micronutrient status) in plasma, erythrocytes, and nails in AD patients [292]. The therapeutic effect of p,p′-methoxyl-diphenyl diselenide [(MeOPhSe)2], a SeFM, against streptozotocin (STZ)-induced sporadic dementia of Alzheimer’s type (SDAT) in rats has been evaluated [293]. Several interesting observations from (MeOPhSe)2 supplementation on SDAT rats have been reported. The results indicate that dietary supplementation with (MeOPhSe)2 reverts STZ-induced memory impairment in rats and also reverts oxidative stress in the STZ group by decreasing reactive species and tyrosine nitration levels and enhancing nonprotein thiol levels [294]. The dietary supplementation with (MeOPhSe)2 has also shown to normalize acetylcholinesterase (AChE) activity, which was reported to enhance by STZ injection, but did not revert the deficit in cerebral energy metabolism caused by STZ [293, 295].
Huntington’s disease (HD) is also a fatal neurodegenerative disorder caused by CAG-repeat expansion encoding a polyglutamine tract in the huntingtin (Htt) protein characterized by motor and psychiatric disturbances and cognitive decline leading to dementia [296, 297]. In HD neurodegeneration, an increase in intracellular reactive oxygen species (ROS) and caspase-3 activity has been reported. Oxidative stress from ROS is primarily implicated to the impaired activity of the major endogenous glutathione redox system. This impaired system causes oxidative damage and formation of 8-hydroxydeoxyguanosine (8-OH-dG) [298, 299] as a DNA oxidative damage marker, malondialdehyde (MDA) [300] as a lipid peroxidation marker, and protein oxidation [301]. The role of antioxidant defense by glutathione peroxidases in HD has been demonstrated via genome-wide screening, two suppressors of Htt encode glutathione peroxidases, and their overexpression could ameliorate the toxicity of a mutant Htt fragment in yeast [302]. Moreover, studies indicated that mGPx1 overexpression and ebselen treatment mimicing GPx activity can lower the ROS production and significantly reduce caspase-3/caspase-7 activation.
Selenium supplementation and therapeutics have also been examined in treating bipolar disease. Recently, ebselen has been examined as a potential drug for bipolar disease. They reported ebselen as an effective drug candidate inhibiting inositol monophosphatase (IMPase) activity and acting as a lithium mimetic in mouse models of bipolar disorder [303]. Lithium has been used as the most effective mood stabilizer for the treatment of bipolar disorder, but it is toxic at only twice the therapeutic dosage and has many undesirable side effects [304, 305]. The selenium moiety of ebselen irreversibly inhibits IMPase by forming a selenylsulfide (–Se–S–) bond with the cysteine residue, which is conserved in both human and mouse isoforms. It has also been demonstrated that ebselen permeates blood–brain barrier and inhibits endogenous inositol monophosphatase in mouse brain with no selenium toxicity at pharmacological dosage.
Epilepsy, which affects about 2–3% of the general population, is a common chronic neurological disorder with various etiological factors [306]. Several causes have been suggested to be responsible for idiopathic epilepsy, such as imbalances in trace elements [307] and genetic reasons, including mutations in ion and non-ion channel genes [308, 309] and mutations in genes involved in the antioxidant system [310]. The impaired antioxidant system, which generates oxidative stress by producing free radicals, has been implicated as a leading cause to seizures or the risk of their recurrence in idiopathic epilepsy [311, 312]. A control case study suggests that the Se and Zn levels are significantly decreased in patients with epilepsy and there are no significant differences in gender or age, which implies the possible cause for impaired antioxidant system in epilepsy patients [313].
6 SeFMs in Clinical Trials
After discovery of several selenocysteine-containing key enzymes in humans and vertebrates, tremendous attention has been paid to evaluate synthetic Se compounds for pharmaceutical applications. Among many Se compounds that have been studied, ebselen is studied and tested most extensively for its therapeutic value in many pathological conditions. Ebselen [2-phenyl-1,2-benzoisoselenazol-3(2H)-one] was first synthesized in 1924. In 1984, it was first reported that ebselen has the properties of thiol peroxidase and antioxidant and acts by mimicking glutathione peroxidase (GPx; Fig. 9) [315, 316]. Since then, a large number of research articles have been published: approximately 1000 ebselen-related publications may be found in the NCBI database. Due to its properties as antioxidant [317, 318] and anti-inflammatory [319, 320] and its low toxicity in vivo [321], ebselen was first clinically tested for using in the stroke treatment by Daiichi Pharmaceuticals, Japan, in 1997 [322]. Unfortunately, it was not authorized (as harmokisane) for registration by MHLW, the Japanese regulatory authority in therapeutics [323]. Ebselen has a broad range of biological activities due to the GPx-like activity, which catalyzes the reduction of hydrogen peroxide and other hydroperoxides by utilizing glutathione and a variety of other thiols [324]. Ebselen can also reduce the peroxynitrite (ONOO−) to nitrite, thereby diminishing harmful effect of peroxynitrite to cellular constituents in host, when the concentration of peroxynitrite is too high [325]. Ebselen is in the National Institutes of Health Clinical Collection, a chemical library of bioavailable drugs considered clinically safe but without proven use.
Mechanism of glutathione peroxidase-like activity of ebselen. R = polyunsaturated phospholipids, cholesterol esters, cholesterol, or any other organic hydroperoxide or hydrogen. R′ = glutathione, dihydrolipoate, N-acetylcysteine, or thiol protein residue [314]
Ebselen was studied clinically for treating the late stages of development of stroke. The pathological condition of stroke involves a variety of destructive mechanisms, which include oxygen radical generation [326], lipid peroxidation [326], calcium overload [327, 328], and the generation of inflammatory mediators [329–331]. In vitro and animal studies showed that Ebselen can mimic glutathione peroxidase, react with peroxynitrite, and inhibit acute ischemic stroke-related enzymes, such as cyclooxygenase [332], lipoxygenases [169], NO synthases [333, 334], NADPH oxidase [335–337], and H(+)/K(+)-ATPase [317].
Ebselen is the most characterized for its high bioavailability. It can readily cross the blood–brain barrier and also produce promising results in clinical trials for noise-induced hearing loss (NIHL) along with stroke. NIHL is manifested by the acute exposure to loud noise, which affects several structural elements in auditory hair cells, including cell membrane and intracellular biochemical pathways [338]. Loud noise can reduce GSH and increase the level of oxidized glutathione in the inner ear, therefore leaving it prone to mediated cell damages, which also overwhelm resident detoxification and other antioxidant mechanisms [339, 340]. Intriguingly, GPx activity, which regenerates reduced GSH, has been reported to decrease following noise exposure. Therefore, administration of ebselen improves NIHL conditions by mimicking GPx activity [341–343].
7 Conclusion and Perspective
Because it is a natural part of proteins and tRNAs, selenium is an essential trace element for living systems. Many selenoproteins (such as GPx, TRxR, and Sel proteins) modulate the redox status and systems. Therefore, selenium deficiency will lead to insufficient and/or nonfunctional selenoprotein production, which disrupts the balance of cellular redox systems, thereby resulting in pathological conditions, such as cardiovascular disease, thyroid disease, cancer, neurological disease, depression, diabetes, pancreatitis, and tropical diseases. Furthermore, the deiodinase selenoprotein family is responsible for maintaining a circulating level of thyroid hormone. As an atypical element, selenium has been successfully incorporated into numerous potential therapeutic agents. Approximately 30,000 research articles on selenium have been published and deposited into the NCBI database, indicating the importance of selenium in human health. However, the tolerance range of elemental selenium in humans is narrow: from 55 μg/day (the adequate intake level) to 400 μg/day (the tolerable upper intake level). Epidemiological and pharmacological studies and randomized clinical trials should be conducted to further investigate the link between Se supplement intake and health benefits.
Clearly, selenium function in living systems and its effects on human health are essential. As selenium naturally occurs in protein and tRNA via replacement of oxygen or sulfur atom(s), we predict that the selenium functionality may also present in genomic DNA to assist genome functions, including DNA condensation and accuracy and efficiency of DNA replication and RNA transcription. Thorough research is required to discover and fully understand the selenium functionalities in living organisms. Moreover, selenium-atom-specific modification (SAM) will transform the design of potential oligonucleotide therapeutics via antisense DNA, siRNA, miRNA, and crRNA strategies, by improving the molecular specificity and substrate cleavage efficiency [68].
References
Pinsent J (1954) Biochem J 57:10
Schwarz K, Bieri JG, Briggs GM, Scott ML (1957) Proc Soc Exp Biol Med 95:621
Schwarz K, Foltz CM (1957) J Am Chem Soc 79:3292
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Science 179:588
Andreesen JR, Ljungdahl LG (1973) J Bacteriol 116:867
Turner DC, Stadtman TC (1973) Arch Biochem Biophys 154:366
Cone JE, Del Rio RM, Davis JN, Stadtman TC (1976) Proc Natl Acad Sci U S A 73:2659
Jones JB, Stadtman TC (1981) J Biol Chem 256:656
Motsenbocker MA, Tappel AL (1982) Biochim Biophys Acta 704:253
Hondal RJ, Marino SM, Gladyshev VN (2013) Antioxid Redox Signal 18:1675
Beckett GJ, Beddows SE, Morrice PC, Nicol F, Arthur JR (1987) Biochem J 248:443
Behne D, Kyriakopoulos A, Meinhold H, Kohrle J (1990) Biochem Biophys Res Commun 173:1143
Chambers I, Frampton J, Goldfarb P, Affara N, McBain W, Harrison PR (1986) EMBO J 5:1221
Zinoni F, Birkmann A, Stadtman TC, Bock A (1986) Proc Natl Acad Sci U S A 83:4650
Zinoni F, Heider J, Bock A (1990) Proc Natl Acad Sci U S A 87:4660
Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD et al (1991) Nature 353:273
Leonard JL, Rosenberg IN (1978) Endocrinology 103:274
Maciel RM, Ozawa Y, Chopra IJ (1979) Endocrinology 104:365
Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC (2000) Endocrinology 141:4309
Baqui M, Botero D, Gereben B, Curcio C, Harney JW et al (2003) J Biol Chem 278:1206
Bera S, Weinberg F, Ekoue DN, Ansenberger-Fricano K, Mao M et al (2014) Cancer Res 74:5118
Florian S, Wingler K, Schmehl K, Jacobasch G, Kreuzer OJ et al (2001) Free Radic Res 35:655
Kipp A, Banning A, Brigelius-Flohe R (2007) Biol Chem 388:1027
Baez-Duarte BG, Mendoza-Carrera F, Garcia-Zapien A, Flores-Martinez SE, Sanchez-Corona J et al (2014) Arch Med Res 45:375
Imai H, Nakagawa Y (2003) Free Radic Biol Med 34:145
Januel C, El Hentati FZ, Carreras M, Arthur JR, Calzada C et al (2006) Biochim Biophys Acta 1761:1228
Kryukov GV, Kryukov VM, Gladyshev VN (1999) J Biol Chem 274:33888
Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W et al (2000) J Biol Chem 275:3693
Karimpour S, Lou J, Lin LL, Rene LM, Lagunas L et al (2002) Oncogene 21:6317
Miranda-Vizuete A, Damdimopoulos AE, Pedrajas JR, Gustafsson JA, Spyrou G (1999) Eur J Biochem 261:405
Prasad R, Chan LF, Hughes CR, Kaski JP, Kowalczyk JC et al (2014) J Clin Endocrinol Metab 99:E1556
Lee SR, Kim JR, Kwon KS, Yoon HW, Levine RL et al (1999) J Biol Chem 274:4722
Novoselov SV, Kryukov GV, Xu XM, Carlson BA, Hatfield DL, Gladyshev VN (2007) J Biol Chem 282:11960
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O et al (2003) Science 300:1439
Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K et al (2011) J Immunol 186:2127
Shchedrina VA, Everley RA, Zhang Y, Gygi SP, Hatfield DL, Gladyshev VN (2011) J Biol Chem 286:42937
Reeves MA, Bellinger FP, Berry MJ (2010) Antioxid Redox Signal 12:809
Dikiy A, Novoselov SV, Fomenko DE, Sengupta A, Carlson BA et al (2007) Biochemistry 46:6871
Castets P, Bertrand AT, Beuvin M, Ferry A, Le Grand F et al (2011) Hum Mol Genet 20:694
Han SJ, Lee BC, Yim SH, Gladyshev VN, Lee SR (2014) PLoS One 9:e95518
Schomburg L, Schweizer U, Holtmann B, Flohe L, Sendtner M, Kohrle J (2003) Biochem J 370:397
Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF et al (2003) J Biol Chem 278:13640
Hill KE, Zhou J, McMahan WJ, Motley AK, Burk RF (2004) J Nutr 134:157
Liu J, Rozovsky S (2013) Biochemistry 52:5514
Olsson M, Olsson B, Jacobson P, Thelle DS, Bjorkegren J et al (2011) Metabolism 60:114
Steinbrenner H, Hotze AL, Speckmann B, Pinto A, Sies H et al (2013) J Mol Endocrinol 50:31
Whanger PD (2009) Biochim Biophys Acta 1790:1448
Vendeland SC, Beilstein MA, Yeh JY, Ream W, Whanger PD (1995) Proc Natl Acad Sci U S A 92:8749
Xu XM, Carlson BA, Irons R, Mix H, Zhong N et al (2007) Biochem J 404:115
Tamura T, Yamamoto S, Takahata M, Sakaguchi H, Tanaka H et al (2004) Proc Natl Acad Sci U S A 101:16162
Kasaikina MV, Fomenko DE, Labunskyy VM, Lachke SA, Qiu W et al (2011) J Biol Chem 286:33203
Veres Z, Kim IY, Scholz TD, Stadtman TC (1994) J Biol Chem 269:10597
Prast-Nielsen S, Huang HH, Williams DL (2011) Biochim Biophys Acta 1810:1262
Engler R, Lombart C, Jayle MF (1972) Biochem Biophys Res Commun 46:1483
Dosseto M, Rohner C, Pierres M, Goridis C (1981) J Immunol Methods 41:145
Ching WM, Alzner-DeWeerd B, Stadtman TC (1985) Proc Natl Acad Sci U S A 82:347
Ching WM, Tsai L, Wittwer AJ (1985) Curr Top Cell Regul 27:497
Ching WM, Wittwer AJ, Tsai L, Stadtman TC (1984) Proc Natl Acad Sci U S A 81:57
Wittwer AJ, Tsai L, Ching WM, Stadtman TC (1984) Biochemistry 23:4650
Sun H, Sheng J, Hassan AE, Jiang S, Gan J, Huang Z (2012) Nucleic Acids Res 40:5171
Sheng J, Gan J, Soares AS, Salon J, Huang Z (2013) Nucleic Acids Res 41:10476
Sun H, Jiang S, Caton-Williams J, Liu H, Huang Z (2013) RNA 19:1309
Hendrickson WA, Pahler A, Smith JL, Satow Y, Merritt EA, Phizackerley RP (1989) Proc Natl Acad Sci U S A 86:2190
Yang W, Hendrickson WA, Crouch RJ, Satow Y (1990) Science 249:1398
Gonzalez A (2007) J Synchrotron Radiat 14:43
Ouerdane L, Mester Z (2008) J Agric Food Chem 56:11792
Ferre-D’Amare AR, Zhou K, Doudna JA (1998) Nature 395:567
Abdur R, Gerlits OO, Gan J, Jiang J, Salon J et al (2014) Acta Crystallogr D Biol Crystallogr 70:354
Carrasco N, Ginsburg D, Du Q, Huang Z (2001) Nucleosides Nucleotides Nucleic Acids 20:1723
Lin L, Sheng J, Huang Z (2011) Chem Soc Rev 40:4591
Wen Z, Abdalla HE, Zhen H (2013) Sci China Chem 56:273
Sheng J, Zhang W, Hassan AE, Gan J, Soares AS et al (2012) Nucleic Acids Res 40:8111
Rahmanto AS, Davies MJ (2012) IUBMB Life 64:863
Fleet JC (1997) Nutr Rev 55:277
Fleming J, Ghose A, Harrison PR (2001) Nutr Cancer 40:42
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK et al (1996) JAMA 276:1957
Hayes JD, McLellan LI (1999) Free Radic Res 31:273
Yao JK, Leonard S, Reddy R (2006) Dis Markers 22:83
Shahpiri A, Svensson B, Finnie C (2008) Plant Physiol 146:789
Biaglow JE, Miller RA (2005) Cancer Biol Ther 4:6
Yang X, Tian Y, Ha P, Gu L (1997) Wei Sheng Yan Jiu 26:113
Schrauzer GN (2000) J Nutr 130:1653
Guo X, Wu L (1998) Ecotoxicol Environ Saf 39:207
Leinfelder W, Forchhammer K, Zinoni F, Sawers G, Mandrand-Berthelot MA, Bock A (1988) J Bacteriol 170:540
Bock A, Stadtman TC (1988) Biofactors 1:245
Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Bock A (1988) Nature 331:723
Hatfield D, Portugal FH (1970) Proc Natl Acad Sci U S A 67:1200
Diamond A, Dudock B, Hatfield D (1981) Cell 25:497
Hatfield DL, Carlson BA, Xu XM, Mix H, Gladyshev VN (2006) Prog Nucleic Acid Res Mol Biol 81:97
Ishii TM, Kotlova N, Tapsoba F, Steinberg SV (2013) J Biol Chem 288:13337
Ohama T, Yang DC, Hatfield DL (1994) Arch Biochem Biophys 315:293
Tormay P, Wilting R, Lottspeich F, Mehta PK, Christen P, Bock A (1998) Eur J Biochem 254:655
Forchhammer K, Leinfelder W, Boesmiller K, Veprek B, Bock A (1991) J Biol Chem 266:6318
Commans S, Bock A (1999) FEMS Microbiol Rev 23:335
Atkinson GC, Hauryliuk V, Tenson T (2011) BMC Evol Biol 11:22
Leinfelder W, Forchhammer K, Veprek B, Zehelein E, Bock A (1990) Proc Natl Acad Sci U S A 87:543
Engelberg-Kulka H, Liu Z, Li C, Reches M (2001) Biofactors 14:61
Copeland PR (2005) Genome Biol 6:221
Fourmy D, Guittet E, Yoshizawa S (2002) J Mol Biol 324:137
Tormay P, Sawers A, Bock A (1996) Mol Microbiol 21:1253
Rother M, Wilting R, Commans S, Bock A (2000) J Mol Biol 299:351
Keeling PJ, Fast NM, McFadden GI (1998) J Mol Evol 47:649
Kaiser JT, Gromadski K, Rother M, Engelhardt H, Rodnina MV, Wahl MC (2005) Biochemistry 44:13315
Carlson BA, Xu XM, Kryukov GV, Rao M, Berry MJ et al (2004) Proc Natl Acad Sci U S A 101:12848
Wittwer AJ, Stadtman TC (1986) Arch Biochem Biophys 248:540
Politino M, Tsai L, Veres Z, Stadtman TC (1990) Proc Natl Acad Sci U S A 87:6345
Mizutani T, Watanabe T, Kanaya K, Nakagawa Y, Fujiwara T (1999) Mol Biol Rep 26:167
Huang KX, An YX, Chen ZX, Xu HB (2001) Biol Trace Elem Res 82:247
Chen CS, Stadtman TC (1980) Proc Natl Acad Sci U S A 77:1403
Ching WM, Stadtman TC (1982) Proc Natl Acad Sci U S A 79:374
Hassan AE, Sheng J, Zhang W, Huang Z (2010) J Am Chem Soc 132:2120
Zhang W, Huang Z (2011) Org Lett 13:2000
Sheng J, Huang Z (2010) Chem Biodivers 7:753
Salon J, Gan J, Abdur R, Liu H, Huang Z (2013) Org Lett 15:3934
Thompson A, Spring A, Sheng J, Huang Z, Germann MW (2015) J Biomol Struct Dyn 33:289
Brandt G, Carrasco N, Huang Z (2006) Biochemistry 45:8972
Carrasco N, Caton-Williams J, Brandt G, Wang S, Huang Z (2006) Angew Chem Int Ed Engl 45:94
Caton-Williams J, Huang Z (2008) Chem Biodivers 5:396
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA (2010) Science 329:1355
Li H (2015) Structure 23:13
Schmitter D, Filkowski J, Sewer A, Pillai RS, Oakeley EJ et al (2006) Nucleic Acids Res 34:4801
Castellano L, Stebbing J (2013) Nucleic Acids Res 41:3339
Song JJ, Smith SK, Hannon GJ, Joshua-Tor L (2004) Science 305:1434
Hutvagner G, Zamore PD (2002) Science 297:2056–2060
Macrae IJ, Zhou K, Li F, Repic A, Brooks AN et al (2006) Science 311:195
Liu T, Li Y, Wang X, Ye Q, Li H et al (2015) Nucleic Acids Res 43:1044
Salon J, Sheng J, Jiang J, Chen G, Caton-Williams J, Huang Z (2007) J Am Chem Soc 129:4862
Lin L, Sheng J, Momin RK, Du Q, Huang Z (2009) Nucleosides Nucleotides Nucleic Acids 28:56
Kim BM, Rode AB, Han EJ, Hong IS, Hong SH (2012) Apoptosis 17:200
Goudgaon NM, Schinazi RF (1991) J Med Chem 34:3305
Drutel A, Archambeaud F, Caron P (2013) Clin Endocrinol (Oxf) 78:155
Kohrle J (1999) Biochimie 81:527
Schomburg L, Kohrle J (2008) Mol Nutr Food Res 52:1235
Contempre B, Duale NL, Dumont JE, Ngo B, Diplock AT, Vanderpas J (1992) Clin Endocrinol (Oxf) 36:579
Duntas LH (2002) Thyroid 12:287
Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Endocr Rev 23:38
Sellitti DF, Suzuki K (2014) Intrinsic regulation of thyroid function by thyroglobulin. Thyroid 24:625–638. doi:10.1089/thy.2013.0344
Chiu-Ugalde J, Wirth EK, Klein MO, Sapin R, Fradejas-Villar N et al (2012) Antioxid Redox Signal 17:902
Berry MJ, Banu L, Larsen PR (1991) Nature 349:438
Poncin S, Van Eeckoudt S, Humblet K, Colin IM, Gerard AC (2010) Am J Pathol 176:1355
Zagrodzki P, Nicol F, Arthur JR, Slowiaczek M (2001) Biofactors 14:223
Mitchell JH, Nicol F, Beckett GJ, Arthur JR (1996) J Mol Endocrinol 16:259
Pizzulli A, Ranjbar A (2000) Biol Trace Elem Res 77:199
Guerra LN, Rios de Molina Mdel C, Miler EA, Moiguer S, Karner M, Burdman JA (2005) Clin Chim Acta 352:115
Lassoued S, Mseddi M, Mnif F, Abid M, Guermazi F et al (2010) Biol Trace Elem Res 138:107
Li H, Okuda J, Akamizu T, Mori T (1995) Endocr J 42:697
Taurog A, Dorris ML, Guziec LJ, Guziec FS Jr (1994) Biochem Pharmacol 48:1447
Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D (2010) Thyroid 20:1163
Wiersinga WM (2014) Nat Rev Endocrinol 10:164
Contempre B, Dumont JE, Denef JF, Many MC (1995) Eur J Endocrinol 133:99
Guilbert JJ (2003) Educ Health (Abingdon) 16:230
Mathers CD, Lopez AD, Murray CJL (2006) The burden of disease and mortality by condition: data, methods and results for 2001. In: Lopez AD, Mathers CD, Ezzati M, Murray CJL, Jamison DT (eds) Global burden of disease and risk factors. Oxford University Press, New York, pp 45–240
Clauser M, Altenberger J (2013) Herz 38:610
Alexander RW (1995) Hypertension 25:155
Lei C, Niu X, Wei J, Zhu J, Zhu Y (2009) Clin Chim Acta 399:102
Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL et al (1995) Circulation 91:2488
Liao F, Andalibi A, deBeer FC, Fogelman AM, Lusis AJ (1993) J Clin Invest 91:2572
Hoffmann FW, Hashimoto AS, Lee BC, Rose AH, Shohet RV, Hoffmann PR (2011) Arch Biochem Biophys 512:38
Nogales F, Ojeda ML, Fenutria M, Murillo ML, Carreras O (2013) Reproduction 146:659
Reeves MA, Hoffmann PR (2009) Cell Mol Life Sci 66:2457
Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ et al (1995) Biochem J 311(Pt 2):425
Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ et al (1996) Biol Trace Elem Res 51:211
Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A et al (2004) Mol Cell Biol 24:9414
Stanley BA, Sivakumaran V, Shi S, McDonald I, Lloyd D et al (2011) J Biol Chem 286:33669
Rundlof AK, Arner ES (2004) Antioxid Redox Signal 6:41
Fujino G, Noguchi T, Takeda K, Ichijo H (2006) Semin Cancer Biol 16:427
Battin EE, Brumaghim JL (2009) Cell Biochem Biophys 55:1
de Bem AF, Farina M, Portella Rde L, Nogueira CW, Dinis TC et al (2008) Atherosclerosis 201:92
Schewe C, Schewe T, Wendel A (1994) Biochem Pharmacol 48:65
Baran H, Vass K, Lassmann H, Hornykiewicz O (1994) Brain Res 646:201
Rathmann W, Giani G (2004) Diabetes Care 27:2568, author reply 9
Pillay TS, Makgoba MW (1991) S Afr Med J 79:607
Olefsky JM (1993) Adv Exp Med Biol 334:129
Muoio DM, Newgard CB (2008) Nat Rev Mol Cell Biol 9:193
Olefsky JM, Nolan JJ (1995) Am J Clin Nutr 61:980S
Wilson PW (1998) Am J Kidney Dis 32:S89
Shara NM, Wang H, Valaitis E, Pehlivanova M, Carter EA et al (2011) Am J Cardiol 107:399
Song M (2010) J Cardiovasc Nurs 25:93
Kadulina AA, Kurash TP, Kuskova LM, Grinberg EM, Vorob’ev VI (1982) Probl Endokrinol (Mosk) 28:11
Steinbrenner H, Speckmann B, Pinto A, Sies H (2011) J Clin Biochem Nutr 48:40
Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E (2009) Circ Cardiovasc Qual Outcomes 2:369
Wiernsperger N, Rapin J (2010) Diabetol Metab Syndr 2:70
Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E et al (2010) BMC Public Health 10:564
Wang X, Zhang W, Chen H, Liao N, Wang Z et al (2014) Toxicol Lett 224:16
Miki H, Funato Y (2012) J Biochem 151:255
Tsou RC, Bence KK (2012) Front Neurosci 6:192
Tiganis T (2013) FEBS J 280:445
Loh K, Deng H, Fukushima A, Cai X, Boivin B et al (2009) Cell Metab 10:260
Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG (2008) Diabetologia 51:1515
Xu T, Liu Y, Meng J, Zeng J, Xu X, Mi M (2013) Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 29:1245
Kaplan MJ (2010) Rheum Dis Clin North Am 36:405
Peretz AM, Neve JD, Famaey JP (1991) Semin Arthritis Rheum 20:305
Peretz A, Neve J, Famaey JP, Molle L (1987) J Pharm Belg 42:395
Sehnert B, Burkhardt H, Wessels JT, Schroder A, May MJ et al (2013) Proc Natl Acad Sci U S A 110:16556
Prabhu KS, Zamamiri-Davis F, Stewart JB, Thompson JT, Sordillo LM, Reddy CC (2002) Biochem J 366:203
Scholz M, Ulbrich HK, Dannhardt G (2008) Eur J Med Chem 43:1152
McCabe CJ, Akehurst RL, Kirsch J, Whitfield M, Backhouse M et al (1998) Pharmacoeconomics 14:191
Dannhardt G, Kiefer W (2001) Eur J Med Chem 36:109
Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL (1994) J Pharmacol Exp Ther 271:927
Zhuo H, Smith AH, Steinmaus C (2004) Cancer Epidemiol Biomarkers Prev 13:771
Amaral AF, Cantor KP, Silverman DT, Malats N (2010) Cancer Epidemiol Biomarkers Prev 19:2407
Peters U, Takata Y (2008) Mol Nutr Food Res 52:1261
Kim IW, Bae SM, Kim YW, Liu HB, Bae SH et al (2012) Biol Trace Elem Res 148:25
Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW et al (2004) Am J Clin Nutr 79:80
Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H et al (2000) J Natl Cancer Inst 92:1753
Glattre E, Thomassen Y, Thoresen SO, Haldorsen T, Lund-Larsen PG et al (1989) Int J Epidemiol 18:45
Rayman MP (2009) Biochim Biophys Acta 1790:1533
Etminan M, FitzGerald JM, Gleave M, Chambers K (2005) Cancer Causes Control 16:1125
Penney KL, Schumacher FR, Li H, Kraft P, Morris JS et al (2010) Cancer Prev Res (Phila) 3:604
Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC et al (2004) J Natl Cancer Inst 96:696
Battin EE, Zimmerman MT, Ramoutar RR, Quarles CE, Brumaghim JL (2011) Metallomics 3:503
Prabhu P, Bag PP, Singh BG, Hodage A, Jain VK et al (2011) Free Radic Res 45:461
Mohamed J, Wei WL, Husin NN, Alwahaibi NY, Budin SB (2011) Pak J Biol Sci 14:1055
Reszka E (2012) Clin Chim Acta 413:847
Brozmanova J (2011) Klin Onkol 24:171
Selenius M, Rundlof AK, Olm E, Fernandes AP, Bjornstedt M (2010) Antioxid Redox Signal 12:867
Reid ME, Duffield-Lillico AJ, Slate E, Natarajan N, Turnbull B et al (2008) Nutr Cancer 60:155
Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH et al (2003) BJU Int 91:608
Krehl S, Loewinger M, Florian S, Kipp AP, Banning A et al (2012) Carcinogenesis 33:620
Ip C, Lisk DJ (1997) Nutr Cancer 28:184
Duntas LH (2006) Thyroid 16:455
Davis CD, Uthus EO (2004) Exp Biol Med (Maywood) 229:988
Davis CD, Uthus EO (2003) J Nutr 133:2907
Zeng H, Combs GF Jr (2008) J Nutr Biochem 19:1
Sanmartin C, Plano D, Sharma AK, Palop JA (2012) Int J Mol Sci 13:9649
Park SH, Kim JH, Chi GY, Kim GY, Chang YC et al (2012) Toxicol Lett 212:252
Sanmartin C, Plano D, Font M, Palop JA (2011) Curr Cancer Drug Targets 11:496
Alwahaibi N, Mohamed J, Alhamadani A (2010) J Trace Elem Med Biol 24:119
Rayman MP (2005) Proc Nutr Soc 64:527
Peters U, Chatterjee N, Church TR, Mayo C, Sturup S et al (2006) Cancer Epidemiol Biomarkers Prev 15:315
Corcoran NM, Hovens CM, Michael M, Rosenthal MA, Costello AJ (2010) Br J Cancer 103:462
Humann-Ziehank E, Wolf P, Renko K, Schomburg L, Ludwig Bruegmann M et al (2011) J Trace Elem Med Biol 25(Suppl 1):S30
Selenius M, Fernandes AP, Brodin O, Bjornstedt M, Rundlof AK (2008) Biochem Pharmacol 75:2092
Papp LV, Lu J, Striebel F, Kennedy D, Holmgren A, Khanna KK (2006) Mol Cell Biol 26:4895–4910
Zeng H, Wu M, Botnen JH (2009) J Nutr 139:1613
Hertz N, Lister RE (2009) J Int Med Res 37:1961
Poerschke RL, Moos PJ (2011) Biochem Pharmacol 81:211
Nguyen N, Sharma A, Sharma AK, Desai D, Huh SJ et al (2011) Cancer Prev Res (Phila) 4:248
Xing F, Li S, Ge X, Wang C, Zeng H et al (2008) Oral Oncol 44:963
Baum MK, Campa A, Lai S, Sales Martinez S, Tsalaile L et al (2013) JAMA 310:2154
Cirelli A, Ciardi M, de Simone C, Sorice F, Giordano R et al (1991) Clin Biochem 24:211
Baruchel S, Wainberg MA (1992) J Leukoc Biol 52:111
Stone CA, Kawai K, Kupka R, Fawzi WW (2010) Nutr Rev 68:671
Hoffmann PR, Berry MJ (2008) Mol Nutr Food Res 52:1273
Makropoulos V, Bruning T, Schulze-Osthoff K (1996) Arch Toxicol 70:277
Roux-Lombard P, Modoux C, Cruchaud A, Dayer JM (1989) Clin Immunol Immunopathol 50:374
Westendorp MO, Shatrov VA, Schulze-Osthoff K, Frank R, Kraft M et al (1995) EMBO J 14:546
Peterhans E (1997) J Nutr 127:962S
Taylor EW, Cox AG, Zhao L, Ruzicka JA, Bhat AA et al (2000) J Acquir Immune Defic Syndr 25(Suppl 1):S53
Ferencik M, Ebringer L (2003) Folia Microbiol (Praha) 48:417
Stambullian M, Feliu S, Slobodianik NH (2007) Br J Nutr 98(Suppl 1):S140
Lopez-Herce JA (1998) An Med Interna 15:623
Taylor EW, Nadimpalli RG, Ramanathan CS (1997) Biol Trace Elem Res 56:63
Jeong LS, Choi YN, Tosh DK, Choi WJ, Kim HO, Choi J (2008) Bioorg Med Chem 16:9891
Alexander V, Choi WJ, Chun J, Kim HO, Jeon JH et al (2010) Org Lett 12:2242
Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R et al (2014) PLoS Negl Trop Dis 8:e2865
Cattand P, Desjeux P, Guzmán MG, Jannin J, Kroeger A, Medici A, Musgrove P, Nathan MB, Shaw A, Schofield CJ (2006) Tropical diseases lacking adequate control measures: dengue: leishmaniasis, and African trypanosomiasis. In: Disease control priorities in developing countries. 2nd edn. World Bank, Washington DC
Postigo JA (2010) Int J Antimicrob Agents 36(Suppl 1):S62
Carvalho LP, Passos S, Schriefer A, Carvalho EM (2012) Front Immunol 3:301
da Silva MS, Monteiro JP, Nunes VS, Vasconcelos EJ, Perez AM et al (2013) PLoS One 8:e81397
Tiwananthagorn S, Bhutto AM, Baloch JH, Soomro FR, Kawamura Y et al (2012) Parasitol Res 111:125
Bates PA (2007) Int J Parasitol 37:1097
Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K (2009) Curr Med Chem 16:599
Moreno D, Plano D, Baquedano Y, Jimenez-Ruiz A, Palop JA, Sanmartin C (2011) Parasitol Res 108:233
Plano D, Baquedano Y, Moreno-Mateos D, Font M, Jimenez-Ruiz A et al (2011) Eur J Med Chem 46:3315
Fuertes MA, Nguewa PA, Castilla J, Alonso C, Perez JM (2008) Curr Med Chem 15:433
Sharma U, Singh S (2008) J Vector Borne Dis 45:255
Haouas N, Pesson B, Boudabous R, Dedet JP, Babba H, Ravel C (2007) Am J Trop Med Hyg 77:1054
Baquedano Y, Moreno E, Espuelas S, Nguewa P, Font M et al (2014) Eur J Med Chem 74:116
van den Boogaard J, Kibiki GS, Kisanga ER, Boeree MJ, Aarnoutse RE (2009) Antimicrob Agents Chemother 53:849
Connolly LE, Edelstein PH, Ramakrishnan L (2007) PLoS Med 4:e120
Kremer L, Maughan WN, Wilson RA, Dover LG, Besra GS (2002) Lett Appl Microbiol 34:233
Warrier T, Tropis M, Werngren J, Diehl A, Gengenbacher M et al (2012) Antimicrob Agents Chemother 56:1735
Elamin AA, Stehr M, Oehlmann W, Singh M (2009) J Microbiol Methods 79:358
Favrot L, Grzegorzewicz AE, Lajiness DH, Marvin RK, Boucau J et al (2013) Nat Commun 4:2748
Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E et al (2008) J Infect Dis 197:1499
Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, Pourmogaddam M (2008) Respirology 13:294
Gutteridge JM (1994) Ann N Y Acad Sci 738:201
Tabet N, Mantle D, Orrell M (2000) Adverse Drug React Toxicol Rev 19:127
Atwood CS, Huang X, Moir RD, Tanzi RE, Bush A (1999) Met Ions Biol Syst 36:309
Bus AI, Huang X, Fairlie DP (1999) Neurobiol Aging 20:33, discussion 9–42
Forlenza OV, Diniz BS, Teixeira AL, Stella F, Gattaz W (2013) Rev Bras Psiquiatr 35:284
Maccioni RB, Lavados M, Maccioni CB, Mendoza-Naranjo A (2004) Curr Alzheimer Res 1:307
Riederer BM, Leuba G, Elhajj Z (2013) Exp Biol Med (Maywood) 238:519
Butterfield DA (2002) Free Radic Res 36:1307
Gwon AR, Park JS, Park JH, Baik SH, Jeong HY et al (2010) Neurosci Lett 469:391
Yoo MH, Gu X, Xu XM, Kim JY, Carlson BA et al (2010) Antioxid Redox Signal 12:819
Thomas JP, Geiger PG, Maiorino M, Ursini F, Girotti AW (1990) Biochim Biophys Acta 1045:252
Thomas JP, Maiorino M, Ursini F, Girotti AW (1990) J Biol Chem 265:454
Chen P, Wang C, Ma X, Zhang Y, Liu Q et al (2013) PLoS One 8:e66384
Takemoto AS, Berry MJ, Bellinger FP (2010) Ethn Dis 20:S1–S92
Cardoso BR, Ong TP, Jacob-Filho W, Jaluul O, Freitas MI, Cozzolino SM (2010) Br J Nutr 103:803
Pinton S, Bruning CA, Sartori Oliveira CE, Prigol M, Nogueira CW (2013) J Nutr Biochem 24:311
Pinton S, da Rocha JT, Gai BM, Prigol M, da Rosa LV, Nogueira CW (2011) Cell Biochem Funct 29:235
Pinton S, da Rocha JT, Zeni G, Nogueira CW (2010) Neurosci Lett 472:56
Barron LH, Rae A, Holloway S, Brock DJ, Warner JP (1994) Hum Mol Genet 3:173
Warner JP, Barron LH, Brock DJ (1993) Mol Cell Probes 7:235
Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM et al (2007) Biochem Biophys Res Commun 359:335
Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A et al (2006) Neurology 66:250
Stoy N, Mackay GM, Forrest CM, Christofides J, Egerton M et al (2005) J Neurochem 93:611
Sorolla MA, Rodriguez-Colman MJ, Vall-llaura N, Tamarit J, Ros J, Cabiscol E (2012) Biofactors 38:173
Mason RP, Casu M, Butler N, Breda C, Campesan S et al (2013) Nat Genet 45:1249
Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R et al (2013) Nat Commun 4:1332
Okusa MD, Crystal LJ (1994) Am J Med 97:383
Groleau G, Barish R, Tso E, Whye D, Browne B (1987) Am J Emerg Med 5:527
Hauser WA, Annegers JF, Rocca WA (1996) Mayo Clin Proc 71:576
Hamed SA, Abdellah MM, El-Melegy N (2004) J Pharmacol Sci 96:465
Child ND, Benarroch EE (2014) Neurology 82:989
Armijo JA, Shushtarian M, Valdizan EM, Cuadrado A, de las Cuevas I, Adin J (2005) Curr Pharm Des 11:1975
Naziroglu M (2009) Neurochem Res 34:2181
Willmore LJ, Rubin JJ (1981) Neurology 31:63
Savaskan NE, Brauer AU, Kuhbacher M, Eyupoglu IY, Kyriakopoulos A et al (2003) FASEB J 17:112
Seven M, Basaran SY, Cengiz M, Unal S, Yuksel A (2013) Epilepsy Res 104:35
Haenen GR, De Rooij BM, Vermeulen NP, Bast A (1990) Mol Pharmacol 37:412
Muller A, Cadenas E, Graf P, Sies H (1984) Biochem Pharmacol 33:3235
Wendel A, Fausel M, Safayhi H, Tiegs G, Otter R (1984) Biochem Pharmacol 33:3241
Schewe T (1995) Gen Pharmacol 26:1153
Yoshikawa T, Naito Y, Kondo M (1993) J Nutr Sci Vitaminol (Tokyo) 39(Suppl):S35
Renson M, Dereu N (1990) J Pharm Belg 45:322
Brune B, Diewald B, Ullrich V (1991) Biochem Pharmacol 41:1805
Sies H (1993) Free Radic Biol Med 14:313–323
Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y et al (1998) Stroke 29:12
Parnham MJ, Sies H (2013) Biochem Pharmacol 86:1248
Cotgreave IA, Morgenstern R, Engman L, Ahokas J (1992) Chem Biol Interact 84:69
Masumoto H, Kissner R, Koppenol WH, Sies H (1996) FEBS Lett 398:179
Braughler JM, Hall ED (1989) Free Radic Biol Med 6:289
De Ley G, Weyne J, Demeester G, Stryckmans K, Goethals P, Leusen I (1989) Stroke 20:357
Nakase T, Sohl G, Theis M, Willecke K, Naus CC (2004) Am J Pathol 164:2067
Barone FC, Feuerstein GZ (1999) J Cereb Blood Flow Metab 19:819–834
Worthmann H, Tryc AB, Goldbecker A, Ma YT, Tountopoulou A et al (2010) Cerebrovasc Dis 30:85
Rosenzweig S, Carmichael ST (2013) Stroke 44:2579
Pratta MA, Ackerman NR, Arner EC (1998) Inflamm Res 47:115
Zembowicz A, Hatchett RJ, Radziszewski W, Gryglewski RJ (1993) J Pharmacol Exp Ther 267:1112
Kim HR, Kim JW, Park JY, Je HD, Lee SY et al (2001) J Auton Pharmacol 21:23
Smith SM, Min J, Ganesh T, Diebold B, Kawahara T et al (2012) Chem Biol 19:752
Yoshizumi M, Fujita Y, Izawa Y, Suzaki Y, Kyaw M et al (2004) Exp Cell Res 292:1
Tepperman BL, Chang Q, Soper BD (1999) Br J Pharmacol 128:1268–1274
Plontke S, Zenner HP (2004) GMS Curr Top Otorhinolaryngol Head Neck Surg 3:Doc06
Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS (2000) J Assoc Res Otolaryngol 1:243
Henderson D, Bielefeld EC, Harris KC, Hu BH (2006) Ear Hear 27:1
Kil J, Pierce C, Tran H, Gu R, Lynch ED (2007) Hear Res 226:44
Lynch ED, Gu R, Pierce C, Kil J (2004) Laryngoscope 114:333
Pourbakht A, Yamasoba T (2003) Hear Res 181:100
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Abdur, R., Huang, Z. (2015). Selenium-Functionalized Molecules (SeFMs) as Potential Drugs and Nutritional Supplements. In: Schwarz, J. (eds) Atypical Elements in Drug Design. EGC 2015. Topics in Medicinal Chemistry, vol 17. Springer, Cham. https://doi.org/10.1007/7355_2015_87
Download citation
DOI: https://doi.org/10.1007/7355_2015_87
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27740-0
Online ISBN: 978-3-319-27742-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)