Abstract
The structure, synthesis, reactivity and applications of 1,2,3-triazoles fused to aromatic rings are described. These compounds have been classified in two groups by a structural approach: (a) fused 1,2,3-triazoles without a bridgehead nitrogen atom and (b) fused 1,2,3-triazoles with a bridgehead nitrogen atom. Although both systems present a similar structure, the synthetic procedures and their reactivity are different.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Fused 1,2,3-triazoles represent a large family of compounds that are applied in different scientific fields, covering from organic synthesis until copper conservation or highly energetic materials. The scope of the fused triazoles treated in this chapter involves systems with two aromatic rings of which one is a 1,2,3-triazole ring and the other is a six-membered aromatic ring. Such compounds have extensively been reviewed in the Comprehensive Heterocyclic Chemistry collection [1, 2]. In order to classify these compounds a structural approach has been reported: (a) Compounds without any bridged nitrogen atom, and the simplest structure is benzotriazole (Bt, 1) reported by Chattaway and Orton in 1901 [3] (Fig. 1). This compound was initially named as azimido benzene. (b) Compounds with one of the three nitrogen acting as a bridge atom, with [1,2,3]triazolo[1,5-a]pyridine (Tp, 2) as the simplest compound of the group. The first report concerning this structure was the corresponding protonated compound reported in 1953 by Kuhn and Munzing [4]. Although both systems present a similar structure, the synthetic approaches towards them and their reactivity show themselves to be completely different.
2 1,2,3-Triazoles Fused to Aromatic Rings, Structure and Classification
Fused 1,2,3-triazoles having no nitrogen bridge atoms are a large family of compound with benzotriazole 1 as the most studied. The fusion of 5- and 6-membered aromatic rings, with the former 1,2,3-triazole, allows the possibility of another heterocyclic ring (i.e. pyridine) replacing benzene. [1,2,3]Triazolo[4,5-b] pyridine (3) or [1,2,3]triazolo[4,5-c]pyridine (4) are the closest systems to 1 reported in the literature (Fig. 2). [1,2,3]Triazolo[4,5-d]pyrimidine (5) and [1,2,3]-triazolo[4,5-d]pyridazine (6) systems are also included in this group (Fig. 2). Although these compounds represent the majority of the reported structures, some examples of fused 5+5 aromatic ring 7 can also be found in the literature; however, they are rare structures not deeply investigated compared to the 5+6 family.
The second family of fused 1,2,3-triazoles involves the sharing of one of the 1,2,3-triazole nitrogen atoms by both aromatic rings (either 6+5 or 5+5). As mentioned before, the simplest compound of this group is [1,2,3]triazolo[1,5-a] pyridine (2, Tp). In this family, pyrimidine [1,5-a] (8a) and [1,5-c] (8b), pyrazine (9) and pyridazine (10) derivatives have also been reported and even some examples of 5+5 systems 11. This family has been significantly less applied in comparison to the benzotriazole analogues, although they have interesting properties due to the presence of the nitrogen atom in both aromatic rings.
3 Group A: Fused 1,2,3-Triazoles Without a Bridgehead Nitrogen Atom
3.1 Structure: Tautomerism and Ring-Chain Isomerization
The particular arrangement of the three nitrogen atoms of benzotriazole gives rise to a special feature for these compounds. Firstly, benzotriazole shows proton tautomerism (Scheme 1). 1H and 2H Bt structures are in equilibrium. Wofford et al. reported in 1982 that in solution the 1H tautomer is the major compound [5]. However, several studies indicate that the 2H tautomer is observed in the gas phase at 0°K, with tautomer 1H increases its population at higher temperature. In terms of lone pair repulsion, it is clear that the 2H tautomer is more stable; however, theoretical calculations indicate that the more stable is the 1H tautomer [6].
This particular characteristic has consequences for the benzotriazole nomenclature, and as long as two 1H structures are possible (Scheme 2), the mixture of them must be specified, for example, 5(6)-substituted-1H-benzotriazole (12).
This kind of phenomena is also present in N-alkylated benzotriazoles (Scheme 3). Known as cationtropic tautomerism and initially reported by Katritzky, this peculiarity allows the equilibrium between 1N and 2N alkylated Bt. N-Dialkylmethyl-aminobenzotriazole 13 exists as the N1 isomer in the solid state; however, in solution (in nonpolar solvents) or the gas phase, both isomers are present in a 2:1 ratio [7]. Analogues with oxygen 14a [8] or sulphur 14b have also the same feature [9]; however, with these heteroatoms, the interconversion is less fast and both 1N and 2N systems can be isolated.
Benzotriazoles also present a particular property in their structures that has been less studied. Indeed they present an opened form in equilibrium with a closed form. Normally this equilibrium is completely on the closed form because of its larger stability. The open form may correspond to a molecule with resonance structures like an ortho-quinoid diazoimine and a benzene ring with a diazonium and an amide as substituents (Scheme 4). Although the detection of the opened forms remains difficult, Katritzky has reported one example with a compound that requires this form as the intermediate to explain the observed equilibrium between the two structures. The only possibility to go from structure 15A to structure 15B is through such opened form [10]. This isomerization, being anecdotic in the benzotriazole family, is very common in triazolopyridines (Scheme 4).
For example, compound 16 is in equilibrium between A and B forms; hence it must be necessary to go through the open form [11]. Compound 17A is also a good example of this ring-chain isomerization. In the presence of ammonia at 150°C, it converts into 17B by means of an opened diazo system [12, 13].
3.2 Synthesis of Benzotriazoles and Triazolopyridines
The preparation of benzotriazole can be realized by different strategies, either by [2+4] cycloaddition from an aryne or by the azotation of ortho-disubstituted diaminobenzene (Scheme 5). Interestingly, the most employed methodology relies on the use of ortho-diaminobenzenes as building blocks. Peterson reported in 1940 the protocol that has been employed for the preparation of benzotriazole and substituted benzotriazoles [14].
Some examples report the use of ortho-nitro anilines that are in situ reduced to obtain the diamine. Oxygenated derivative 18 was obtained during the formation of the triazole ring with hydrazine from 2-chloronitrobenzene [15].
Applying a similar approach with the corresponding diamino derivatives, pyridine (19, 20) and pyrimidine (21) derivatives were also obtained [16–19] (Scheme 6). This strategy can be applied to a large family of compounds and has allowed the preparation of more complex molecules derived from diamino pyridines [19, 20].
In the literature there are a few examples involving the aryne approach; however, they remain less employed. This 2+4 cycloaddition to obtain benzotriazoles was first reported by Kulagowski [21] (Scheme 7). Azide derivative 22 reacts with the corresponding aryne to form benzotriazole 23.
Despite these two strategies being the most common, some alternatives are possible with more complex heterocyclic compounds, based on the initial presence of the triazole ring in the reagent (Scheme 8). For example, triazole 24 undergoes cyclization to form compound 25 [22]. Treatment in acetone of compound 26 allows the formation of triazolopyridine 27 [23]. When 2 heteroatoms are present on the 6-membered ring, other strategies can be employed. In Scheme 8 we show that 1,2,3-triazoles 28 and 29 react with hydrazine to afford compounds 30 [24] and 31 [25].
3.3 Reactivity of Benzotriazoles and Triazolopyridines
The chemical reactivity of benzotriazoles and triazolopyridines can be presented in two parts: (1) functionalization of the triazole ring and the (2) functionalization of the benzene or heterocyclic (commonly pyridine) ring.
3.3.1 Functionalization of the Triazole Ring
3.3.1.1 Alkylation
When Bt is reacted with alkyl halides, up to three compounds can be observed (Scheme 9): compounds derived from monosubstitution at position 1/3N (major) or 2N and, in some cases, 1,3-disubstituted compounds. Direct methylation of Bt affords with high yield (95%), a mixture between 1N-32 and 2N-33 methylated compounds in a 72/28 ratio [26]. However, the complexity of these reactions increases when benzotriazole has different substituents on the benzene ring (i.e. nitro substituent, compound 34) (Scheme 9). In this compound N1 and N3 are no longer the same and the simplest reaction (i.e. methylation) affords at least 3 different compounds 35, 36 and 37 [27].
3.3.1.2 Arylation
Bt acts as a nucleophile in aromatic nucleophilic substitution; exclusive substitution at 1N is observed. Reactions with chloronitrobenzenes are described, affording compounds 38 and 39 in high yields (Scheme 10) [28, 29].
With triazolopyridine derivatives, this behaviour has also been observed. Although no direct reaction with methylene iodide has been reported, reactions with chloronitrobenzene or chloronitropyridine are present in the literature (Scheme 11). Triazolopyridine 3 reacts with either chloronitrobenzene or chloronitropyridine to form compounds 40, 41 and 42 or 43 and 44 [30, 31]. In both cases the nitrogen atom from the triazole ring is more reactive than the one on the pyridine nitrogen. Triazolopyridine 4 reacts under similar conditions affording exclusively compound 45. Triazolopyrimidine 21 affords 46 as a single compound in 60% [24].
3.3.1.3 N1 Functionalization by Different Substituents
Reaction of Bt with acyl chlorides allows the preparation of ketones/amides 47 [32]. Even the introduction of a cyano substituent has been achieved. When the corresponding sodium salt of Bt is treated with ClCN [33] or BrCN [34], compound 48 is obtained (65 and 90%) (Scheme 12).
The introduction of other atoms has also been reported at position 1 (Scheme 13). Chlorination affords compound 49 in good yield [35]; fluorination [36] takes place with moderate yields affording 50 in 25%. Silylation and borylation have also been reported in the literature to form compounds 51 and 52 with moderate to good yields [37]. Amino and phosphorus derivatives 53 and 54 have also been reported in moderate to good yield [38, 39].
Some of these types of reactions have also been reported with triazolopyridines; shown in Fig. 3 are some of these less common compounds [38–42]. Compounds 55–57 are in agreement with the regioselectivity indicated before (Scheme 10).
At this point of the chapter, it is important to remark that almost all applications of Bt in organic synthesis deal with N-substituted Bt. Katritzky has reported many of these original contributions (more than 700) and has written several reviews covering the preparation and application of these compounds [32].
3.3.2 Functionalization of the Benzene or Pyridine Ring
The introduction of functional groups on the benzotriazole trends to be performed on the benzene ring prior to the formation of the triazole ring. Nevertheless there are some reactions that are carried out on the benzotriazole ring that allow the functionalization of the benzene ring.
3.3.2.1 Nitration
Direct nitration of benzotriazole does indeed proceed at positions 4 and 5 with preference at the 4th position [10, 43]; thus compound 58 is the major isomer compared to 59 (Scheme 14). With chlorine-substituted systems 60, mononitration [44] and dinitration [45] can be realized by increasing the temperature from 60 to 120°C, leading to compounds 61 and 62. None of these reactions have been reported in the literature for triazolopyridines.
3.3.2.2 Amination
It remains essential to remark that no direct amination has been reported with benzotriazole. The reduction of nitro groups is the most employed strategy towards the synthesis of amino benzotriazoles (Scheme 15). Hydrazine or Pd/H2 reductions are the most common methodologies to prepare 4-aminobenzotriazole 63 [10]. The derivative at position 5 compound 64 is less common and has been reported by reduction either with hydrazine or with SnCl2 under acid medium [44, 46]. Those reduction conditions did not affect the benzene ring.
3.3.2.3 Halogenation
Direct halogenation has been mainly achieved with bromine leading either to dibromation at positions 5 and 6 (Scheme 16, compound 65) or tetrabromation (compound 66) with the harshest conditions [47]. The only example reported of chlorination is the reaction of 4,7-dimethyl benzotriazole 67 with NaOCl in acid medium [48]. Compound 68 is obtained under these conditions. No direct fluorination or iodination is reported.
An alternative approach towards the preparation of iodo derivatives relies on diazonium salts. Treatment of 64 with NaNO2 affords the corresponding diazonium salt 69 that undergoes reaction with potassium iodide yielding the monoiodine derivative 70 in low yield (32%) (Scheme 17) [49].
3.3.2.4 Oxidation and Reduction
Oxidation and reduction are performed on the substituents attached to the benzotriazole ring. It has been reported that strong oxidation of 5-methyl benzotriazole 71 leads to the corresponding acid 72 in good yield (Scheme 18) [50]; however, extreme oxidant conditions can result in the complete destruction of the benzene ring as it will be shown later.
The structure of benzotriazole resists classical Fischer esterification conditions (H2SO4). Thus ester 73 has been reported [51]. Furthermore typical reduction reagents like LiAlH4 allow reduction of functional groups without modification of the aromatic core, affording compound 74 [51] (Scheme 18).
3.3.2.5 Methylation
Methylation of benzotriazole has also been achieved in 80% yield by reaction with (H3CO)2P(O)CH3 yielding to compound 75 (Scheme 19) [52]. These kinds of reactions are rarely reported in the literature with triazolopyridines; however, the regioselective methylation of compound 76 towards 77 [53, 54] has been described.
3.3.2.6 Aromatic Nucleophilic Substitution
Aromatic nucleophilic substitution has also been achieved with benzotriazoles but almost all examples required nitro groups to activate the system [45]. Compounds 78 and 79 are obtained by means of this reaction (Scheme 20). Some examples are also reported with chlorinated triazolopyridines. Compound 80 undergoes aromatic nucleophilic substitution with primary amines leading to 81 [54].
3.3.2.7 Lithiation
Benzotriazole can also be functionalized by reaction with BuLi; however, it requires Boc protection of 1N (Scheme 21). The only reported example is from compound 53. After Boc protection (compound 82), regioselective lithiation and subsequent trapping have been performed, affording compound 83 [38].
3.3.2.8 Hydrogenation
As apparent from the examples above, the benzene ring from Bt resists many different conditions. No references concerning the hydrogenation of this compound or derivatives have been reported. However, triazolopyridine derivatives undergo hydrogenation under particular conditions (Scheme 22). Compounds 84 and 19 under reducing conditions result in triazolopiperidines 85 and 86 [16, 56].
3.3.3 Triazole Ring-Opening Reaction
The triazole ring in Bt is very stable as it can be seen by means of the reaction conditions reported in the previous examples. As an example, it is interesting to show that extreme oxidative conditions lead to the destruction of the benzene ring instead of the triazole one. Compound 87 affords triazole 88 under strongly oxidative conditions (Scheme 23) [57].
However, some reactions are reported that involve the destruction of the triazole ring leading to a benzene system. For example, tetrachlorobenzotriazole 89 reacts under strongly reducing conditions, affording the corresponding diamine 90 [58] (Scheme 24).
A second example of a ring-opening reaction starts from nitro compound 91 [59]. The presence of the nitro substituent destabilizes the triazole ring, and some ring-opening reactions have been reported [60]. Adduct 92 can be obtained in excellent yield via azo coupling reaction of intermediate diazonium salt 93 with the basic form of napht-2-ol. The formation of this compound can be explained through an open form of 91 with the structure 93. This ionic form can undergo direct ArNs, leading to compound 94 that was subsequently transformed into a triazole by means of click chemistry (compound 95). Finally, compound 91 has also been reported as a precursor of exotic heterocyclic compounds like 96 (Scheme 25).
1-Aminobenzotriazole 53 has a particular and interesting behaviour [61, 62] (Scheme 26) giving diiodobenzene 97 and dibromobenzene 98 in moderate yields by radical reactions.
This amino derivative allows the generation of an aryne as the intermediate in the presence of lead acetate (Scheme 27). Despite not being the most employed aryne source, some examples have been reported [63–65]. Adduct 99 that combines two arynes can be obtained in moderate yield. In a similar way, reaction with furan or oxazole leads to the corresponding cycloaddition adducts 100 and 101 in good yields.
Even more surprisingly, diaminobistriazole 102 also shows this behaviour [66] (Scheme 28). This compound performs double-aryne generation, affording more complex structures in moderate to good yields. Adducts 103 and 104 are obtained with good yields.
Another approach towards the cleavage of the triazole ring relies on the preparation of salts. Grignard addition to compound 105 generates intermediate 106 that in the presence of water decomposes towards the diamine derivative 107 (Scheme 29) [67]. However, the presence of the ether moiety is required for this reactivity.
An alternative towards the activation of the triazole ring is the photochemical approach; nevertheless, benzotriazole shows itself to be extremely stable, and low yields are obtained of the corresponding photodegradation products from nitrogen elimination [68] (Scheme 30).
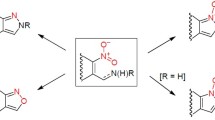
4 Group B: Fused 1,2,3-Triazoles with a Bridgehead Nitrogen Atom
Fused 1,2,3-triazoles with a bridgehead nitrogen atom are those systems when one of the three nitrogen atoms from the triazole ring belongs also to the second aromatic ring. As it has been outlined before, these compounds are represented by the parent [1,2,3]triazolo[1,5-a]pyridine (Fig. 4). This compound Tp is the simplest member of this family, and although having similar features like benzotriazole, it has also particular characteristics that are not present in the Bt family.
4.1 Structure: Tautomerism and Ring-Chain Isomerization
Compared to the benzotriazole family, these compounds do not present H-tautomerism. However, they indeed show also a ring-chain isomerization. This phenomenon is even more common than in the Bt family (Scheme 31) [69]. The open chain form of these systems is a classic diazo compound. Triazolopyrimidine 8 also presents this equilibrium [70]. As it will be described later, these compounds can react like a diazo compound.
This phenomenon became even more interesting when the substituent R is a 2-pyridyl [71] 108 or 2-quinolyl [72] 109, because then there are 2 structures in equilibrium. Through an open intermediate with a diazo structure, the cyclization can take place, involving one of the two different nitrogen atoms. Similarly to what has been reported with Bt, the most electron-rich nitrogen (or the less hindered) is preferred for the triazole ring formation (Scheme 32).
For 3-(2-pyridyl)-triazolopyridine 108, both structures are exactly the same; however, in the case of potential equilibrium 109A and 109B, there is an interesting difference in the structure of both isomers. There are some studies about the ring-chain isomerism of 7-substituted-3-(2-pyridyl)-triazolopyridines 110 and 9-substituted-3-(2-pyridyl)-triazoloquinolines 111 [71, 72].
Traditionally these structures had been noted as A compounds for those bearing the substitution on the triazolopyridine (or triazoloquinoline) ring and B for those obtained after the isomerization. In compound 110 with a methyl group as substituent, a mixture of A/B products is observed, and although they can easily be identified by NMR (Scheme 33), these systems cannot be separated because they isomerize at room temperature. Theoretical calculations support these findings. Electronic properties of the substituent were found determinant. Electron-withdrawing substituents favour a B structure. Electron donors tend to result in the A structure.
Triazoloquinolinepyridines 111 behave similarly. However, initially both A and B structures are nonequivalent and only A is observed (Scheme 34). The introduction of a substituent modifies the A/B ratio. Also in this case, electron-withdrawing and bulky substituents afford the B structure, and small and donor substituents afford the A structure.
4.2 Synthesis
All examples concerning the synthesis of these compounds involve the preparation of alpha-substituted pyridines. Indeed pyridines are the major starting reagents for the preparation of Tp. There are several methodologies reported. The most common approach involves 2-pyridyl aldehydes or ketones that react with hydrazine leading to hydrazone 112 that then is submitted to oxidation (analogous to the Staudinger approach to diazo compounds) affording the desired compounds Tp 2 (Scheme 35). This strategy is also employed for the other members of the families like triazolo[1,5-a] or [1,5-c]pyrimidines 8 [70, 73].
Boyer et al. [74] published the first synthesis of [1,2,3]triazolo[1,5-a]pyridines of type 2. Hydrazones 112 were oxidized using Ag2O to give the diazo intermediates which undergo an intramolecular cyclization, affording [1,2,3]triazolo[1,5-a]pyridines 2. Although Ag2O provided triazolopyridines in good yields, Boyer and Ramage [75] replaced it by potassium ferrocyanide. However, along with Tp several side reaction products were obtained. Many other oxidants, like nickel peroxide, lead tetraacetate and copper (I) salts, have been tested [76, 77]. Comparing all published synthetic ways to obtain [1,2,3]triazolo[1,5-a]pyridines using this methodology, the oxidation with manganese (IV) oxide (MnO2) due to its low cost and the good and reproducible yields made it the reagent of choice. Manganese oxide was successfully employed by Abarca [78] to prepare triazolopyridines on gram scale (Scheme 36).
In order to avoid the oxidation step that can show incompatibilities with other functional groups, Boyer and Goebel [79] developed another variant of Bamford-Stevens approach to obtain triazolopyridines. They were synthesized after condensation of tosylhydrazine with the corresponding 2-pyridyl aldehydes or ketones. This reaction led to tosylhydrazones 113. Following a basic treatment with NaOH or KOH, derivatives 114 were obtained. In this way, they succeeded in the synthesis of 3-phenyl, 3-picolinoyl and [1,2,3]triazolo[1,5-a]pyridines in high yields without use of oxidizing agent (Scheme 37). Other bases like morpholine were also employed to prepare, for example, 7-methyltriazolopyridine [80], 5-methoxytriazolopyridine [81] or their bromine analogues [82].
A third original approach relies on the reaction with azides. From 2-acylmethylpyridines 115 with tosylazide (TsN3) in the presence of sodium ethoxide, Regitz obtained triazolopyridine derivatives with moderate to high yields (50–80%) [83, 84], compounds 116 were obtained by this procedure. From compound 117 and other different azides, like 2-azido-1-ethylpyridinium tetrafluoroborate 118 [85] or 2-azido-3-ethylbenzothiazolium tetrafluoroborate 119, the cyano derivative 120 was obtained (Scheme 38) [86].
The previous strategies are the most commonly employed; however, some alternatives have also appeared that allow the formation of the 1,2,3-triazole ring with substitution at N1 [87]. This approach employs a diazo compound 121 that reacts with the anion 122 leading to a substituted hydrazine 123. Oxidation with copper (II) perchlorate leads to the 1-subtituted triazolopyridinium perchlorate salt 124 in moderate yield (Scheme 39).
These strategies have been applied with different starting reagents. Although the number of examples synthesized is significantly smaller that in Bt family, some interesting structures 8–11 and 125 –127 have been reported [70, 73, 74, 88–90] (Fig. 5).
4.3 Reactivity of the Triazolopyridines Family
4.3.1 Functionalization of the Triazole Ring
4.3.1.1 Halogenation
This family has significant differences in terms of reactivity regarding Bt. Triazolopyridine with no substituent at position 3 can be bromated or iodinated in basic medium, giving compounds 128 and 129 with moderate yields [91, 92]. It is also important to remark that the proton in position 3 is acidic and exchanges with deuterium just by heating in D2O, leading to compound 130 (Scheme 40).
4.3.1.2 Alkylation
Alkylation to give triazolopyridinium salts has also been reported on these systems. Normally they are alkylated at nitrogen 2, obtaining structures like compound 131. However, with large substituents at position 3 [93, 94], like tert-butyl derivative 132, mixtures of 133 (N2) and 134 (N1) alkylated products are observed (Scheme 41).
4.3.1.3 Nitration
Nitration of these systems with no substitution at position 3 can also be performed, affording nitrotriazolopyridines 135 [95] and nitrotriazoloquinolines 136 [96]. However, these reactions gave low yields and side products derived from the opening of the triazole ring (Scheme 42) (see 4.3.3).
4.3.2 Functionalization of the Pyridine Ring
4.3.2.1 Lithiation
This family of compounds presents a general reactivity that is completely different from benzotriazoles. Indeed all triazolopyridines undergo regioselective metallation at position 7 with butyllithium [80]. Trapping with electrophiles allows the preparation of 7-substituted derivatives 137. This regioselectivity can be explained by the directed effect by N1 towards peri-metallation (Scheme 43).
The regioselective metallation at position 7 has been employed for the preparation of a large variety of compounds. This includes also all compounds that were studied in the ring-chain isomerization (see Sect. 4.1 Schemes 33 and 34). This position is extremely activated, undergoing lithiation even when a methyl group is at this place, for example, in compound 138 leading to 139 [80, 97]. This result indicates how different can be the reactivity of Bt and Tp families. Some studies have been performed [81] by introducing ortho-directing groups to metallation on the triazolopyridine ring, like compound 140, trying to get lithiation in different positions. Nevertheless the metallation with LDA provided exclusively 7-substitued triazolopyridines 141 (Scheme 44).
So far, only few examples of metallation at other positions were described in the literature. Nevertheless, in 1995, Jones reported the reaction of 3-cyano-[1,2,3]triazolo[1,5-a]pyridine 120 with LDA [98]. Although this reaction provides a complex mixture of products after trapping with trimethylsilyl chloride, 4-substituted triazolopyridine 142 was identified. However, the low yield remained an important drawback from a synthetic point of view (compound from dimetallation 143 was also isolated and some other side products like 144 and 145) (Scheme 45).
As reported also by Jones, 4-substitution could be achieved with 7-trimethylsilyl-3-carboxamide-[1,2,3]triazolo[1,5-a]pyridine (146) but lead to compound 147 in very low yield (5%) [98] (Scheme 46).
One important reaction reported with lithium derivatives is the dimerization. Under specific conditions, heterocyclic π-deficient compounds can undergo dimerization. This kind of aryl–aryl coupling is known, but it was not intensively investigated. The reaction of dimerization of [1,2,3]triazolo[1,5-a]pyridines was observed by Abarca and Ballesteros for the first time in 1997 (Scheme 47). When 3-methyl-[1,2,3]triazolo[1,5-a]pyridine (148) was treated with LDA at −40°C in THF followed by trapping with 2-pyridylcarboxaldehyde, expected compound 149 was obtained. However, the corresponding dimer 150 was also obtained as a side product [99].
Due to the interesting structure of this dimer, the authors modified the reaction conditions in order to favour dimerization [82]. They found out that the optimal conditions were LDA (1 eq)/THF/−70°C. After 9 h, the dimer 150 was obtained in a 50% yield, but surprisingly, another side product (1-(3-methyl-[1,2,3]triazolo[1,5-a]pyridin-7-yl)-4-(5-methyl-1H-1,2,3-triazol-4-yl)-1,3-buta-dienyl) (151) was formed in a non-negligible amount (25%) (Scheme 48).
Other compounds of this group of fused 1,2,3-triazoles with a N-bridgehead tend to have particular reactivity. Indeed triazoloquinolines 126 can be metallated at position 3 with LiTMP giving compounds 152, after trapping with electrophiles, in good yield. However, when 3 equiv. of BuLi are employed, double lithiation can be achieved, affording, after trapping, 3,9-disubstituted triazoloquinolines 153 [100] (Scheme 49).
4.3.2.2 Reactions with Nucleophiles
The [1,2,3]triazolo[1,5-a]pyridines do not react directly with nucleophiles. However, some ArNs reactions with the halogenated derivatives 7-bromo-3-substituted-[1,2,3]triazolo[1,5-a]pyridines (154) and 5-bromo-3-substituted-[1,2,3]triazolo[1,5-a]pyridines (155) are described [82, 101]. Compounds 154 react with nucleophiles like sodium methoxide, sodium 4-methoxyphenolate or sodium benzenethiolate in DMF at 90°C to give substituted compounds 156 in high yields. Also, in ethanol at 80°C, sodium hydrazine and sodium piperidine afforded substitution products 156 in respectively 60 and 65% yield. No reaction occurs with sodium azide and potassium cyanate [101] (Scheme 50).
5-Bromotriazolopyridine 157 reacts with nucleophiles allowing the functionalization at the C5 position leading to compounds like 158 [82] (Scheme 51). No reaction occurs when the reaction is carried out with the chlorinated derivative or with 6-bromo-[1,2,3]triazolo[1,5-a]pyridine.
Position 7 of Tp 2 has also been reported as suitable for direct CH activation in the presence of Ni(COD)2 and disubstituted alkynes leading to compounds 159 in good yields (85–90%) [102] (Scheme 52).
4.3.2.3 Hydrogenation Reactions of Triazolopyridines
In 1999, Abarca et al. published a study on the hydrogenation of several triazolopyridines 2 by means of heterogeneous catalysis under mild conditions (Pd/C, methanol, 25°C, atmospheric pressure) and obtained 4,5,6,7-tetrahydrotriazolopyridines 160 as indicated in Scheme 53 [103]. This particular feature is completely different from benzotriazoles where the hydrogenation remains difficult and only was reported with some pyridine derivatives.
When the triazolopyridine is substituted by a methyl group in position 3, the reactions lead to the formation of the tetrahydro derivative in good yield. However, if the methyl group is on the pyridine ring, no hydrogenation product was observed and the starting material was recovered. When the pyridine is substituted with a thiophene at position 3, the 4,5,6,7-hydrogenated product was obtained in low yield (32%) even with increased catalyst charge. This can be explained by the poisoning effect of sulphur towards palladium. On the other hand, the authors highlighted that the presence of electron-withdrawing substituents at the C3 position decreases the reactivity towards hydrogenation.
Recently Glorius has reported a homogeneous hydrogenation of substituted triazolopyridines 138 in excellent yields and high enantiomeric ratio (e.r) by means of Ru NHC complexes to obtain triazolopiperidines 161 (Scheme 54) [97].
4.3.3 Opening Reactions of the Triazole Ring in Triazolopyridines
Triazolopyridine 2 and its derivatives undergo triazole ring-opening reaction with loss of dinitrogen in many different conditions. These compounds tend to afford pyridines in the presence of acids. The first paper about this was published by Boyer and Wolford in 1958. In their study [104], with carboxylic acids at high temperature, the triazole ring degrades with loss of dinitrogen to provide pyridine esters 162 in moderate yields (Scheme 55).
Jones performed an exhaustive and methodological study [105] about the ring-opening reaction with loss of nitrogen molecule with electrophiles like sulphuric acid, acetic acid, halogens (Cl2 and Br2) and selenium dioxide (Table 1).
Reaction with bromine and iodine has also described with compound 163, giving the formation of the corresponding derivatives 164 and 165 [106] (Scheme 56).
Abarca and Ballesteros also studied the ring-opening reaction of triazolopyridine dienic derivatives 166 and 167 in sulphuric acid, acetic acid and selenium (IV) oxide [99] (Scheme 57). These reactions afford the corresponding alcohols 168 and 169 with sulphuric acid; esters 170 and 171 were obtained with acetic acid. With selenium oxide, however, compound 166 does not react, but 167 gives the corresponding ketone 172.
As it has been noted before, the triazolopyridines can be in equilibrium with an opened form. This form is a diazo compound; thus reactivity similar to diazo compounds should be observed. This behaviour was initially reported by Wentrup in the 1960s–1970s [91]. Flash vacuum thermolysis of compound 173 affords complex compound 174 that is explained by means of the chemistry of diazo form 175 (Wolff rearrangement) [107] (Scheme 58).
Other reaction reported in the literature by Wentrup is the thermal treatment of 2 in presence of fumaronitrile, leading to cyclopropane 176. This result can be explained by the formation of a carbene intermediate from the diazo derivative [108], (Scheme 59).
Abarca and Ballesteros also reported of the generation of carbenes in the course of their study on the thermal decomposition of 7-bromotriazolopyridine 177 [109]. The carbene intermediate can be generated by loss of dinitrogen in the corresponding diazo compound before electrophile attack, as indicated in Scheme 59. In this work traces of compound 178 were isolated, and cyclopropanes 179 and 180 were also formed probably by “cyclopropanation” between the carbene and 178 (Scheme 60).
More recently Gevorgyan reported the reaction of 7-chlorotriazolopyridine 181 with rhodium acetate and alkynes or nitriles to afford indolizines 182 and imidazopyridines 183. Its formation is explained through a diazo intermediate [110] (Scheme 61).
There are also some examples of triazole opening reaction with triazolopyridinium ylides. Initially, these experiments were performed with [1,2,3]triazolo[1,5-a]pyridinium ylide 184 and acetylenic esters [111–114]. The authors found out that these reactions were extremely solvent polarity dependent and the results could vary according to the acetylenic ester (Scheme 62).
When the synthesis was performed in toluene with methyl propiolate, indolizines 185 were obtained, providing a new way to synthesize this heterocycle [113]. When dimethyl acetylenedicarboxylate (DMAD) was used as dipolarophile in toluene, pyrazolo[1,5-a]pyridines 186 were obtained after the addition of two molecules of DMAD. In both cases cleavage of the N2–N3 bond occurred, leading to the triazole ring opening, and a 1,3-dipolar cycloaddition was observed. The structure of these compounds was confirmed by single X-ray [112]. However, when acetonitrile was used as solvent, the reaction of the ylides 184 with methyl propiolate gives in each case two products characterized as 1:1 187 and 1:2 188 adducts, with ylide structure and without triazole ring opening.
2-Dicyanomethyl-3-methyl-[1,2,3]triazolo[1,5-a]pyridinium ylide 189 and 2-dicyanomethyl-7-methyl-[1,2,3]triazolo[1,5-a]pyridinium ylide 190 were also studied. The reactivity of these compounds towards acetylenic esters is different depending on the dipolarophile [115], but always produces the triazole ring-opening reaction with loss of nitrogen. 3-Methylated (R1=CH3, R2=H) ylide 189 reacts with methyl propiolate in acetonitrile as solvent to provide indolizine 191 and cyclizine 192. The reaction performed with the 7-methylated (R1=H, R2=CH3) ylide 190 provided exclusively the indolizine. 7-Methylated ylide reacted with DMAD to afford 4H-4,4-dicyan-2,3-dimethoxycarbonyl-6-methylquinolizine 193 (Scheme 63).
4.4 Reactivity of Triazolodiazines
Triazolodiazines represent a less explored family. These compounds are depicted in Scheme 64. In all cases those compounds are obtained by hydrazine/oxidation methodology of the corresponding aldehyde [70, 73]. Despite of not being extensively studied from a chemical point of view, many of these structures are evaluated in pharmacological studies.
The presence of the two nitrogen atoms in the six-membered ring induces instability of these systems which are, contrary to almost all of the previous examples, water sensitive. For compound 10 only ring-opening reaction with acetic acid has been reported yielding to acetate 194 [73]. Triazolo[1,5-c]pyrimidine 8a has been extensively studied by Abarca and Jones and behaves similar to triazolopyridine 2 [116]. The compound 8a of acid leads to a ring-opening reaction affording alcohol 195 with H2SO4 and ketone 196 with selenium dioxide. Monohalogenation can be achieved with HBr, leading to compound 197. Jones reported also on the dehalogenation of this molecule with molecular bromine, affording compound 198 [80]. However, in these reactions the presence of a large amount of side products is reported. This has been associated to the instability of these compounds. Indeed, the presence of nucleophiles induces the ring opening of the pyrimidine moiety, leading to triazoles 199 [116] (Scheme 65).
The reactivity of compound 8b has not been studied; it is only known that dimethyl derivative 200 undergoes ring-opening reaction with bromine, leading to compound 201. In a similar way, when 200 is treated with ICl, halogenated compound 202 is obtained in moderate yield [117] (Scheme 66).
The reaction of pyrazine 9 with acetic acid has also been reported yielding compound 203 in good yield [73]. Despite not being deeply studied, Wentrup reported on the deuteration of this compound with D2O [91], being able to introduce 3 deuterium atoms (204). Of this particular structure, more conjugated analogues, like compound 205, have been reported [118]. Compound 205 reacts with nucleophiles in moderate yields affording 206 [119] (Scheme 67).
5 Applications of Fused 1,2,3-Triazoles
5.1 Benzotriazole Applications
Benzotriazoles resist hot sulphuric acid or melted KOH treatment; even strong oxidants or reductants (KMnO4, LiAlH4) do not affect this system. Taking into consideration that benzotriazole is relatively cheap and stable up to 400°C, several applications have been reported in different fields.
5.1.1 Organic Synthesis
Katritzky is the main researcher on the application of these compounds in organic synthesis [32]. Several reviews, patents and research articles are reported in the literature just concerning its application in organic synthesis [120–122]. The key point of benzotriazole is that it can be easily introduced into different molecules by means of different reactions. Substitution [32], addition or even three-component reactions had been reported for this purpose. Once Bt is attached to a molecule, it can be used under different approaches: as leaving group [123, 124], ortho-directing group [125], as cation stabilizer [67], radical precursor [126], etc. Today more than 1,000 publications employ benzotriazoles as a synthetic tool. Schematic examples about the use of Bt in organic synthesis are shown in Fig. 6.
In this field it is important to stress the utility of some benzotriazoles in peptide synthesis [127] (Fig. 7). The compounds used in this synthesis are derivatives of hydroxy triazoles 18 [15, 128]. Compounds 207, 208 and 209 are commercially available and largely employed for amide bond formation with a high degree of racemization suppression [129–131].
5.1.2 Medicinal Applications
The benzotriazole structure (5+6 aromatic rings) displays similarities with the natural bases adenine and guanine (Fig. 8). For this reason it is not surprising that it is considered as a preferential scaffold in pharmaceutical chemistry as long as it allows subsequent derivatization. In particular pyrimidine derivatives tend to be employed [132–134].
5.1.3 Coordination Chemistry and Metal Organic Frameworks
Benzotriazole and its derivatives had also been applied in coordination chemistry and metal organic frameworks [135–138], in particular, carboxylic derivatives 73 [139] or even more complex molecules 214 [137] (Fig. 9). The particular arrangement of the nitrogen atoms allows coordination with different angles; thus coordination polymers and metal organic frameworks have been obtained. Although this is not the most common nitrogenated ligand employed, its reports reveal particular features that are difficult to obtain with other compounds. Furthermore, these structures are stable.
5.1.4 Photostabilizers, Photographic Application and Sensors
Hydroxyphenylbenzotriazole 215 [140, 141] has been used as photostabilizer of polymers. With the addition of this compound, their stability towards light increases. In a similar way, compound 216 is employed as a fog inhibitor in the processing of silver photographic material [142] (Fig. 10). The particular facility towards the formation of benzotriazole 218 from the ortho-diamine 217 has been employed as a switch on sensor for NO. The initial molecule presents almost zero emission, but when NO is in the atmosphere, the formation of the benzotriazole ring in 218 leads to a strong emission [143].
5.1.5 Copper Conservation
Benzotriazole has been reported as a copper corrosion inhibitor. Indeed copper and copper alloys are treated with a benzotriazole solution [144, 145]. This method has also been applied for brass, steel, cast iron or aluminium to prevent corrosion [146].
5.2 Applications of Triazolopyridines with Nitrogen as Bridgehead Atom
Their reactivity has been shown to be a powerful tool to get access to extremely important compounds in many different yields.
5.2.1 Organic Synthesis
5.2.1.1 Synthesis of 2,6-Asymmetrically Disubstituted Pyridines and Quinolines
The triazole ring has been employed as an activating and protecting group of 2 aldehyde/ketone pyridines or quinolone. The combination of the triazole ring formation, lithiation, trapping with electrophiles and ring-opening reactions is a powerful strategy to prepare 2,6-asymmetrically disubstituted pyridines or 2,8- asymmetrically disubstituted quinolines. These kinds of molecules are difficult to obtain by other procedures. However, by means of the triazolopyridine chemistry, several compounds have been obtained [80, 82, 100, 147].
5.2.1.2 A New Route to 2,2′-Bipyridines
As has been described, the usual reaction between triazolopyridines and lithium reagents at −40°C gives a 7-lithio derivative that can be trapped by electrophiles [80, 147]. This reaction is temperature dependent, and at −70°C in THF as solvent, a new reaction occurs, giving two products, the 7,7′-bitriazolopyridine 150 and the butadiene 151 [82] (see Scheme 48). Like all simple triazolopyridines, bitriazolopyridines 150 react with electrophiles to produce 2,2′-bipyridines 219 (Scheme 68). With these reactions, a general route to 2,2′-bipyridines has been discovered with a variety of substituents in the 6 and 6′ positions [82]. These compounds have use in supramolecular chemistry because of their great complexing power for metal ions, and, in particular, 2,2′-disubstituted-6,6′-bipyridines are useful building blocks for oligo-bipyridines, which spontaneously form helical metal complexes [148].
5.2.1.3 Synthesis of Pyridylcarbonylpyridines
Pyridyl carbonyl pyridyl triazolopyridine 220 is obtained from 3-(2-pyridyl)-triazolopyridine by the typical reaction of lithiation and trapping the lithio derivative by 2-PyCHO/air or 2-PyCO2Et or 2-PyCN. Its triazole ring-opening reaction with SeO2 formed a bis-pyridylcarbonyl-pyridine 221 [149] (Scheme 69). This compound undergoes hydration or reaction with methanol, leading to compounds 221A, 221B and 221C.
The discovery of this synthesis of compound 221, using triazolopyridines as building blocks, has been the beginning of a new study looking for new polynitrogenated potential helicating ligands or coordination supramolecular compounds from triazolopyridines with potential magnetic or photochemical properties [150]. The aim of this study was the synthesis of oligopyridylcarbonylpyridines 222 and related compounds. In Fig. 11 there are some examples of the synthesized compounds with this methodology in different conditions [149–152].
Pyridylcarbonylpyridine (PyCOPyCOPy) 221 is a ligand very often used in coordination chemistry to form clusters or helicates with different structures and very interesting magnetic properties. Figure 12 shows the molecular formula of some examples synthesized from 221 and with application in these fields.
Complexes with silver 237 and 238 and copper 239 and 240 [153] and with iron 241–244 [154], the first icosanuclear Co cluster exhibiting superparamagnetic relaxation 245 [155], an S-shaped pentanuclear CuII cluster 246 [156], clusters of CuII 4 247 and CoII 4 248 with ferromagnetic interactions [157], a NiII 5 cluster 249 with a S = 5 ground state exhibiting slow magnetic relaxation and a high spin-reversal barrier have been described [158]; complexes 250–252 (CuII 4, CoII 4 and NiII 6) are also synthesized in the presence of sodium azide with very interesting ferromagnetic intramolecular interactions [159]. Structural, magnetic and spectroscopic studies have been done with 253 (FeIII) [160]. Isomorphous replacement of MII ions in MII-GdII dimers 254 (MII=CuII (a), MnII (b), NiII (c), CoII (d), ZnII (e) [161], FeII (f) [162]) has been studied; magnetic susceptibility measures indicate a ferromagnetic interaction for (a), antiferromagnetic for (b–e) and weakly ferromagnetic for (f).
There is a second-generation family of ligands derived from metal ion-assisted reactivity of di-2,6-(2-pyridylcarbonyl)pyridine 221. A MnII/III 4 rhombus was synthesized by nucleophilic attack of the carbanion –CH2COCH3 at the carbonyl carbon atoms of (py)CO(py)CO(py), in the presence of Mnn+ ions under basic conditions; the cationic cluster [Mn4(OH)2(L)2(H2O)2](ClO4)4 255, where L2− is the (py)C(CH2COCH3)(O–)(py)C(CH2COCH3)(O–)(py) dianion, was synthesized and characterized [163]. Complex 255 is antiferromagnetically coupled with an unusual S = 2 ground state resulting from spin frustration effects within the triangular Mn3 subunits of the cluster.
5.2.2 Triazolopyridines as Building Blocks in Supramolecular Chemistry
All triazolopyridines have interesting ligand properties to form polynuclear complexes with different metal ions. These molecules may also have the ability to complex other cationic, neutral or anionic species of biomedical or environmental relevance to form supramolecular compounds, which may have interesting magnetic or fluorescent properties, and could act as luminescent molecular chemosensors.
The following are the preliminary experiments accomplished in supramolecular chemistry, with some of the compounds described in this chapter.
X-ray single-crystal studies and magnetic, photomagnetic and colorimetric measurements of a series of iron(II)-3-(2-pyridyl)-triazolopyridine (TP) complexes [Fe(TP)3](BF4)2 256, [Fe(TP)2](NCS)2·2CHCl3 257, [Fe(TP)2](NCS)2·H2O 258 and [Fe(TP)2](NCSe)2 259 have been studied and have been characterized as new mononuclear spin crossover compounds [164].
A molecular chemosensor for metal ions, anions and amino acids has been described, the Zn(II) complex of compound 231b [165]. This system permits the direct detection of anions without using competitive reactions or dyes. One of the most interesting aspects is the discrimination between nitrite and nitrate anions. The ability of the Zn(II) complex to interact and quantify amino acids has been explored for l-glutamate and l-aspartate.
Triazolopyridine 223 (TPT) (Fig. 11) possessing fluorescent properties has been studied as molecular chemosensor for Zn(II), nitrite and cyanide anions. The fluorescence behaviour of TPT was checked in the presence of the divalent transition metal ions Co2+, Ni2+ and Cu2+ and of the post-transition metal ions Zn2+, Cd2+ and Pb2+. Zn(TPT)2+ 1:1 complex in solution was checked with different monovalent anions (F−, Cl−, Br−, I−, CN−, SCN−, NO2 −, NO3 −). In all cases, quenching of the emission was produced. Complex Zn(TPT)2+ is a sensor for anions specially cyanide and nitrite [166].
A tetranuclear complex of Cu(II) with compound 110B (R = 2-PyCO-) with magnetic properties has been described; the structure shows a cubane tetrameric complex of copper(II) with the hemiacetalate of the 2-pyridyl-[1,2,3]triazolo[1,5-a]pyrid-7-ylmethanone and a S 4 symmetry. The Cu4O4 core corresponds to a distorted cubane [167]. The magnetic behaviour of the complex is typical for compounds displaying significant intramolecular antiferromagnetic coupling.
5.2.3 Pharmacological Studies
There are no [1,2,3]triazolo[1,5-a]pyridines used as pharmaceutical compounds. This section reports preliminary studies of the pharmacological interest of some triazolopyridines.
5.2.3.1 Synthesis and Evaluation of 7-Arylhydroxymethyltriazolopyridines as Potential Cardiovascular Agents
7-Arylhydroxymethyltriazolopyridines might be considered as structural analogues of benzyltetrahydroisoquinoline and bisbenzyltetrahydroisoquinoline alkaloids that have the ability to block calcium channels and/or antagonize α1-adrenoreceptors, and may have applications in the treatment of cardiovascular disorders. A series of these triazolopyridine derivatives 260 have been synthesized (Fig. 13), and the activity as relaxants of vascular smooth muscle has been tested in isolated aortic rings precontracted by noradrenaline looking for activity as antagonists of the α1-adrenoreceptors present in this tissue and stimulated by noradrenaline. The lack of a relaxant action excludes the possibility that these compounds act as α1-adrenoreceptors antagonists.
Addition of depolarizing solution to the aortic ring induces a sustained contractile response in the absence of endothelium. In these conditions, opening of voltage-sensitive calcium channels and calcium entry promotes this contractile response. Subsequent addition of these compounds in cumulative concentrations, once the contractile plateau induce by depolarizing solution had been reached, did not modify the tone, thus suggesting that none of the compounds tested can block calcium entry through voltage-dependent calcium channels [168].
5.2.3.2 Biological Evaluation of [1,2,3]Triazolo[1,5-a]pyridines as New Neural Nitric Oxide Synthase Inhibitors
The importance of nitric oxide (NO) as a biological messenger in numerous physiological processes has been demonstrated to a growing extent over the last decades. This molecule is indeed involved in various fundamental functions such as neurotransmission [169], blood pressure and blood flow regulation [170] and platelet aggregation and inflammation [171]. Overproduction of nitric oxide plays a role in a variety of disorders. Nitric oxide is synthesized in several cell types from L-arginine by different isoforms of nitric oxide synthase (NOS).
A series of inhibitors of this enzyme is constituted by heterocycles such as substituted indazoles or imidazoles. The 3- or 7-substituted indazoles are potent nNOS inhibitors [172, 173]. [1,2,3]Triazolo[1,5-a] pyridines can be considered as aza-analogues of indazoles, and some studies have been done to test the possibility that the triazolopyridines can be (NO) synthase inhibitors. A number of 3- and 7- substituted triazolopyridines 261 and 262 (Fig. 13) have been synthesized and have been tested [174]. The triazolopyridines evaluated have small activity, and the results indicate that a NH group is necessary for the interaction with the NOS.
Abbreviations
- 2-PyCHO:
-
2-Pyridylcarboxyaldehyde
- AIBN:
-
Azobisisobutyronitrile
- ArNs:
-
Aromatic nucleophilic substitution
- Boc:
-
Tertbutoxycarbonyl
- Bt:
-
1H-1,2,3-Benzotriazol
- BuLi:
-
Butyllithium
- Cod:
-
Cyclooctadiene
- DMAD:
-
Dimethyl acetylenedicarboxylate
- DMF:
-
Dimethylformamide
- El:
-
Electrophile
- Et:
-
Ethyl
- FVT:
-
Flash vacuum thermolysis
- LDA:
-
Lithium diisopropylamide
- LiTMP:
-
Lithium tetramethylpiperidine
- NBS:
-
N-Bromosuccinimide
- NHC:
-
N-heterocyclic carbene
- NMR:
-
Nuclear magnetic resonance
- NOS:
-
Nitric oxide synthase
- Nu:
-
Nucleophile
- OAc:
-
Acetoxy
- Ph:
-
Phenyl
- THF:
-
Tetrahydrofuran
- TMSCN:
-
Trimethylsilyl cyanide
- TMSN3 :
-
Trimethylsilyl azide
- Tp:
-
[1,2,3]triazolo[1,5-a]pyridine
- TPT:
-
Triazolopyridine-pyridine-triazolopyridine
- TsN3 :
-
Tosyl azide
References
Zhdankin VV ed (2008) Five-membered rings: triazoles, oxadiazoles, thiadiazoles and their fused carbocyclic derivatives In: Katritzky AR, Ramsde CA, Scriven EFV, Taylor RJK (eds) Comprehensive heterocyclic chemistry III and previous collections I and II, vol 5. Elsevier, Oxford
Cossy J ed (2008) Bicyclic 5–5 and 5–6 fused ring systems with at least one bridgehead (ring junction) N. In: Katritzky AR, Ramsde CA, Scriven EFV, Taylor RJK (eds) Comprehensive heterocyclic chemistry III and previous collections I and II, vol 11. Elsevier, Oxford
Chattaway FD, Orton KJP (1901) The action of acetylchloro- and acetylbromo-aminobenzenes on amines and phenylhydrazine. J Chem Soc Trans 79:461
Kuhn R, Munzing W (1953) N-Halogen-acrylamide zur Darst, ellung von Tetrszolium-, Triazolium- und 8-Aza-indazolium-Salzen N-Haloacylamides for the preparation of tetrazolium-, triazolium-, and 7a-azaindazolium salts. Chem Berichte 86:858
Wofford DS, Korkey DM, Russell JG (1982) 15N NMR spectroscopy: prototropic tautomerism of azoles. J Org Chem 47:5133
Tomás F, Catalán J, Perez P, Elguero J (1994) Influence of lone pair repulsion vs resonance energy on the relative stabilities of molecular structures: a theoretical approach to the equilibrium between 1H and 2H-benzotriazole tautomers. J Org Chem 59:2799
Katritzky AR, Yannakopoulou K, Kuzmierkiewicz W, Aurrecoechea JM, Palenik GJ, Koziol AE, Szczesniak M, Skarjune R (1987) The chemistry of N-substituted benzotriazoles. Part 7. The isomeric composition and mechanism of interconversion of some N-(aminomethyl)benzotriazole derivatives. J Chem Soc Perkin Trans 1 12:2673
Katritzky AR, Bayyuk SI, Rachwal S (1991) An efficient synthesis of ketone enol ethers mediated by N-(1-alkoxyalkyl)benzotriazoles. Synthesis 4:279
Katritzky AR, Kuzmierkiewicz W, Perumal S (1991) Isomerization of N-[α-(alkylthio)alkyl]- and N-[α-(arylthio)alkyl]benzotriazoles. Hel Chim Acta 74:1936
Katritzky AR, Ji F-B, Fan W-Q, Gallos JK, Greenhill JV, King RW (1992) Novel Dimroth rearrangements of the benzotriazole system:4-amino-1-(arylsulfonyl) benzotriazoles to 4-[(Arylsulfonyl)amino]benzotriazoles. J Org Chem 57:190
De Roos KB, Salemink CA (1971) Deazapurine derivatives. VII. Synthesis of substituted imidazo- and triazolopyridines. Recueil des Travaux Chimiques des Pays-Bas. 90:1166
Temple C, Smith BH, Montgomery JA (1972) Preparation and properties of some isomeric v-triazolopyridines-1- and 3-deaza-8-azapurines. J Org Chem 37:3601
Temple C, Smith BH, Montgomery JA (1973) Preparation and properties of isomeric diamino-v-triazolopyridines. 1- and 3-Deaza-2,6-diamino-8-azapurines. J Org Chem 38:1095
Damschroder RE, Peterson WD (1940) 1,2,3-Benzotriazole. Org Synt 20:6
Leonard NJ, Golankiewicz K (1969) Thermolysis of substituted 1-acetoxy benzotriazoles. Carbon-to-oxygen migration of an alkyl group. J Org Chem 34:359
Vaughan JR Jr, Krapcho J, English JP (1949) Triazolo- and imidazopyridines. J Am Chem Soc 71:1885
Patel PD, Pater MR, Kocsis B, Kocksis E, Graham SM, Warren AR, Nicholsin SM, Billack B, Fronczek FR, Talele TT (2010) Design, synthesis and determination of antifungal activity of 5(6)-substituted benzotriazoles. Eur J Med Chem 45:2214
Torrini I, Zecchini GP, Agrosì F, Paradisi MP (1986) Applications of 1-alkoxycarbonyl- and 1-acyl-v-triazolo[4,5-b]pyridines as acylating reagents. J Heterocycl Chem 23:1459
Holt J, Fiksdahl A (2006) N-acyl and N-alkoxycarbonyl derivatives of 1H-1,2,3-triazolo[4,5-c]pyridine; preparation and application. J Heterocycl Chem 43:417
Roblin Jr RO, Lampen, JO, English JP, Cole QP, Vaughan Jr JR (1945) Chemotherapy. VIII. Methionine and purine antagonists and their relation to the sulfonamides. J Am Chem Soc 67:290
Mitchell G, Rees CW (1987) Cyclo-octa[def]carbazole, a new paratropic ring system. J Chem Soc Perkin Trans 1 2:403
Varma KS, Havaldar F, Nanavati S, Patel B, Fernandes PS (1985) Synthesis of 2H-1,2,3-triazolo[4,5-c][1,2,4]triazolo[4,3-a]pyridines and related systems. Liebigs Annalen Chem 9:1922
L'abbé G, Vandendriessche A, Weyns N (1988) A new general synthetic method for [1,2,3]triazolo[4,5-b]pyridines. Bull Soc Chim Belg 97:85
Ramanaiah KCV, Stevens ED, Trudell ML, Pagoria PF (2000) Synthesis of 1-substituted [1,2,3]triazolo[4,5-d]pyridazines as precursors for novel tetraazapentalene derivatives. J Heterocycl Chem 37:1597
Ried W, Laoutidis J (1988) Synthesis of new triazolo-annulated 8-azapurines. Liebigs Annalen Chem 11:1107
Katritzky AR, Kuzmierkiewicz W, Greenhill JV (1991) An improved method for the N-alkylation of benzotriazole and 1,2,4-triazole. Recueil Travaux Chimiques Pays-Bas 110:369
Carta A, Palomba M, Paglietti G, Molicotti P, Paglietti B, Cannas S, Zanetti S (2007) [1,2,3]Triazolo[4,5-h]quinolones. A new class of potent antitubercular agents against multidrug resistant Mycobacterium tuberculosis strains. Bioorg Med Chem Lett 17:4791
Le Z-G, Chen Z-C, Hu Y, Zheng Q-G (2004) Organic reactions in ionic liquids: a simple highly regioselective or regiospecific substitutions of benzotriazole. Heterocycles 63:1077
Huynh MHV, Hiskey MA, Chavez DE, Gilardi RD (2005) Tetraazapentalene chemistry: unexpected intramolecular electron rearrangement induced by highly reactive ψ-dinitroso substituents. Angew Chem Int Ed 44:7089
Maquestiau A, Biemans R, Flammang-Barbieux M, Vilain E (1986) Synthesis of pyridinobenzotetraazapentalenes. Bull Soc Chim Belg 95:1107
Balachari D, Trudell ML (1997) Synthesis of new dipyridotetraazapentalenes. Tetrahedron Lett 38:8607
Katritzky AR, Lan X, Yang JZ, Denisko OV (1998) Properties and Synthetic Utility of N-Substituted Benzotriazoles. Chem Rev 98:409
Hermes ME, Marsh FD (1967) 1-Cyano-1,2,3-triazole-α-diazo-N-cyanoimine tautomers from cyanogen azide and acetylenes. J Am Chem Soc 89:4760
Katritzky AR, Akue-Gedu R, Vakulenko, AV (2007) C-Cyanation with 1-cyanobenzotriazole. ARKIVOC iii:5
Hughes TV, Hammond SD, Cava MP (1998) A convenient new synthesis of 1-cyanobenzotriazole and its use as a C-cyanating reagent. J Org Chem 63:401
Gakh AA, Romaniko SV, Ugrak BI, Fainzilberg AA (1991) N-Fluorination with cesium fluoroxysulfate. Tetrahedron 47:7447
Niedenzu K, Woodrum KR (1989) Boron-nitrogen compounds. 121. Triazaboles and related triazole derivatives of boron. Inorg Chem 28:4022
Knight DW, Little PB (2000) 1-Aminobenzotriazole functionalisation using directed metallation: new routes to chromanes and chromenes using intramolecular benzyne trapping by alcohols. J Chem Soc Perkin Trans 1 15:2343
Efremov DA, Tebby JC, Zavlin PM (1994) The phosphorylation of organic compounds by phosphoric anhydride. Part 2. Phosphorylated azoles. Phosphorus Sulfur Silicon Relat Elem 92:167
Ruefenacht K (1975) Phosphates and thiophosphates with a heterocyclic substituent. 9. Aza analogs. I. Aza analogs of phthalimide, benzotriazole, and 1,2,3,-benzotriazin-4(3H)-one derivatives. Helvetica Chim Acta 58:1521
Zecchini GP, Torrini I, Paradisi MP (1985) A new route to N2- and N3-substituted-2,3-diaminopyridines. Synthesis of 1- and 3-alkoxycarbonyl-v-triazolo[4,5-b]pyridines. J Heterocycl Chem 22:313
Benko P, Berenyi E, Messmer A, Hajos G, Pallos L (1976) Condensed as-triazines. V. Pyrido[4,3-e]-as-triazines. Acta Chim Acad Scientiarum Hungaricae 90:405
Reid AK, McHugh CJ, Richie G, Graham D (2006) Electron-deficient benzotriazoles for the selective N-acetylation of nucleosides. Tetrahedron Lett 47:4201
Carta A, Piras S, Boatto G, Paglietti G (2005) 1H,6H-Triazolo[4,5-e]benzotriazole 3-oxides and 5,5′′-(Z)-diazene-1,2-diylbis(2-methyl-2H-1,2,3-benzotriazole) derived from chloronitrobenzotriazoles and hydrazine. Heterocycles 65:2471
Srinivas D, Ghule VD, Tewari SP, Muralidharan K (2012) Synthesis of amino, azido, nitro, and nitrogen-rich azole substituted derivatives of 1H-benzotriazole for high-energy materials application. Chem Eur J 18:15031
Graham D, McAnally G (1999) Synthesis of aminobenzotriazoles. Heterocycl Commun 5:377
Kopanska K, Najda A, Zebrowska J, Chomicz L, Piekarczyk J, Myjak P, Bretner M (2004) Synthesis and activity of 1H-benzimidazole and 1H-benzotriazole derivatives as inhibitors of Acanthamoeba castellanii. Bioorg Med Chem 12:2617
Canada J, Claramunt RM, De Mendoza J, Elguero J (1985) On the possibility of chlorotropy in aromatic azoles: the case of 1,2,3-triazoles and benzotriazoles. Heterocycles 23:2225
Wright JL, Gregory TF, Kesten SR, Boxer PA, Serpa KA, Meltzer LT, Wise LD, Espitia SA, Konkoy CS, Whittemore ER, Woodward RM (2000) Subtype-selective N-methyl-D-aspartate receptor antagonists: synthesis and biological evaluation of 1-(Heteroarylalkynyl)-4-benzylpiperidines. J Med Chem 18:3408
Chen H, Wang H (2007) Method for synthesizing benzotriazolecarboxylic acid as copper corrosion inhibitor. Faming Zhuanli Shenqing Gongkai Shuomingshu 101029031
Katritzky AR, Ji F-B, Fan W-Q, Delprato I (1993) Synthesis of 5,5-Di-(Benzotriazol-5-ylmethyl)-2,2-dimethyl-1,3-dioxane-4,6-dione and 5-(Benzotriazol-5-ylmethyl)-2,2,5-trimethyl-1,3-dioxane-4,6-dione. Synth Commun 23:2019
Hayashi M, Yamauchi K, Kinoshita M (1976) Esters of phosphorus oxy acids as alkylating agents. IV. N-Alkylation of imidazole and its analogs with alkyl esters of phosphonic and phosphinic acids. Bull Chem Soc Jpn 49:283
Yutilov YM, Smolyar NN (1996) Radical C-alkylation of 1-substituted 1,2,3-triazolo[4,5-c]pyridines. Zhurnal Organicheskoi Khimii 32:1085
Yutilov YM, Smolyar NN (1996) Radical C-alkylation of 1-substituted 1,2,3-triazolo[4,5-c]pyridines by tert-butyl alcohol. Zhurnal Organicheskoi Khimii 32:1412
Svertilova IA, Smolyar NN, Yutilov Y (1996) Synthesis of 4-(arylamino)- and 4-(alkylamino)-1H-imidazo[4,5-c]pyridines and −1,2,3-triazolo[4,5-c]pyridines. Ukrainskii Khimicheskii Zhurnal (Russien Edition) 62:64
Yutilov YM, Smolyar NN, Astashkina NV (2002) Reduction of imidazo[4,5-c]pyridine and [1,2,3]Triazolo[4,5-c]pyridine derivatives to spinaceamines and 2-azaspinaceamines. Russian J Org Chem (Translation of Zhurnal Organicheskoi Khimii) 38:419
Plaut GWE (1954) The preparation of 1,5,6-trimethylbenzotriazole and 1-methyl-v-triazole-4,5-dicarboxylic acid. J Am Chem Soc 76:5801
Burton DE, Lambie AJ, Lane DWJ, Newbold GT, Percival A (1968) Halo-o-phenylenediamines and derived heterocycles. I. Reductive fission of benzotriazoles to o-phenylenediamines. J Chem Soc [Sect] C Org 1268
Ketari R, Foucaud A (1982) Synthesis of 4-bromo-1-nitropyrazoles and 1-nitrobenzotriazoles (N-nitroazoles). Synthesis 10:844
Uhde M, Ziegler T (2010) Reaction of N-nitro-benzotriazole with nucleophiles. Synth Commun 40:3046
Birkett MA, Knight DW, Little PB, Mitchell MB (2000) A new approach to dihydrobenzofurans and dihydrobenzopyrans (chromans) based on the intramolecular trapping by alcohols of benzynes generated from 7-substituted-1-aminobenzotriazoles. Tetrahedron 56:1013
Campbell CD, Rees CW (1969) Reactive intermediates. III. Oxidation of 1-aminobenzotriazole with oxidants other than lead tetraacetate. J Chem Soc [Sect] C Org 752
Cresp TM, Wege D (1986) The addition of benzyne to azulene. Tetrahedron 42:6713
Whitney SE, Rickborn B (1988) Isolation of a 1:1 oxazole-benzyne cycloadduct. An improved method for generating benzyne and a new approach to isobenzofuran. J Org Chem 53:5595
Whitney SE, Winters M, Rickborn B (1990) Benzyne-Oxazole cycloadducts: isolation and Retro–Diels–Alder reactions. J Org Chem 55:929
Hart H, Ok D (1986) Synthesis of 1,5-diamino-1,5-dihydrobenzo[1,2-d:4,5-d′] bistriazole (DABT) and its use as a 1,4-benzadiyne equivalent. J Org Chem 51:979
Katritzky AR, Rachwal S, Rachwal B (1989) Reactions of 1-(α-alkoxyalkyl)- and 1-(α-(aryloxy)alkyl)benzotriazoles with the Grignard reagents. A new and versatile method for the preparation of ethers. J Org Chem 54:6022
Wang H, Burda C, Persy G, Wirz J (2000) Photochemistry of 1H-Benzotriazole in aqueous solution: a phototalent base. J Am Chem Soc 122:5849
Blanco F, Alkorta I, Elguero J, Cruz V, Abarca B, Ballesteros R (2008) [1,2,3] Triazolo[1,5-a]pyridines. A theoretical (DFT) study of the ring-chain isomerization. Tetrahedron 64:11150
Tennant G, Vevers RJS (1974) Diazoalkylideneamine-1,2,3-triazole tautomerism in 1,2,3-triazolo[1,5-a]pyrimidines at elevated temperatures. J Chem Soc Chem Commun 16:671
Abarca B, Alkorta I, Ballesteros R, Blanco F, Chadlaoui M, Elguero J, Mojarrad F (2005) 3-(2-pyridyl)-[1,2,3]triazolo[1,5-a]pyridines. An experimental and theoretical (DFT) study of the ring-chain isomerization. Org Biomol Chem 3:3905
Ballesteros-Garrido R, Blanco F, Ballesteros R, Leroux FR, Abarca B, Colobert F, Alkorta I, Elguero J (2009) 3-(Pyridin-2-yl)[1,2,3]triazolo[1,5-a]quinoline: a theoretical and experimental analysis of ring-chain isomerisation. Eur J Org Chem 33:5765
Maury G, Meziane D, Srairi D, Paugan JP, Paugam R (1982) 1,2,3-Triazolo[1,5]azines et autres hétérocylcs azotés dérivés dàzine-carboxaldéhydes. Bull Soc Chim Belgique 91:153
Boyer JH, Borgers R, Wolford LT (1957) The azomethine linkage of pyridine in ring-closure isomerizations. J Am Chem Soc 79:678
Bower JD, Ramage GR (1957). Heterocyclic systems related to pyrrocoline. II. Preparation of polyazaindenes by dehydrogenative cyclizations. J Chem Soc 4506
Jones G, Sliskovic DR (1983) The chemistry of the triazolopyridines. Adv Heterocycl Chem 34:79
Jones G (2002) The chemistry of the triazolopyridines: an update. Adv Heterocycl Chem 83:1
Abarca B, Ballesteros R, Houari N, Samadi A (1998) The reaction between triazolobenzopyridinium and triazolothiazolium ylides with dimethyl acetylenedicarboxylate. Tetrahedron 54:3913
Boyer JH, Goebel N (1960) The identification of C12H8N4O, an Oxidation Product from α-Pyridyl Monohydrazone. J Org Chem 25:304
Jones G, Sliskovic DR (1982) Triazolopyridines. Part 2. Preparation of 7-substituted triazolo[1,5-a]pyridines by directed lithiation. J Chem Soc Perkin Trans 1 4:967
Abarca B, Hayles DJ, Jones G, Sliskovic DR (1983). Triazolopyridines. Part 3. Attempts to introduce substituents into the six-membered ring of 1,2,3-triazolo[1,5-a] pyridine. J Chem Res (Synopses) 144:1341
Jones G, Pitman MA, Lunt E, Lythgoe DJ, Abarca B, Ballesteros R, Elmasnaouy M (1997) Triazolopyridines. 18. Nucleophilic substitution reactions on triazolopyridines; a new route to 2,2′-bipyridines. Tetrahedron 53:8257
Regitz M (1965) Reactions between active methylene compounds and azides. IX. Synthesis of v-triazolo [3,4-a]pyridines and 1,2,3-triazoles by diazo group transfer with tosyl azide. Angew Chem 77:428
Regitz M, Liedhegener A (1966). Reaktionen aktiver Methylenverbindungen mit Aziden, XII. Synthese von Diacyl-diazomethanen durch Diazogruppen-Übertragung. Chem Beritche 66:2918
Balli H, Loew R, Mueller V, Rempfler H, Sezen-Gezgin A (1978) Azidiniumsalze. 19. Mitteilung [1]. Einführung der Diazogruppe in reaktive Methylenverbindungen mit Azidiniumsalzen. Helvetica Chim Acta 61:97
Monteiro HJ (1987) Preparation of α-Diazo-β-Ketosulfones by Diazo transfer reaction with an in situ generated azidinium salt. A safe and efficient procedure for the Diazo-transfer reaction in neutral medium. Synthc Commun 17:983
Robbins TF, Qian H, Su X, Hughes RP, Aprahamian I (2013) Cyanide detection using a triazolopyridinium salt. Org Lett 15:2386
Jones G, Ollivierre H, Fuller LS, Young JH (1991) 1,2,3-triazolo[5,1-b]thiazoles; synthesis and properties. Tetrahedron 47:2851
Adam R, Ballesteros-Garrido R, Vallcorba O, Abarca B, Ballesteros R, Leroux FR, Colobert F, Amigó JM, Rius J (2013) Synthesis and structural properties of hexaaza[5]helicene containing two [1,2,3]triazolo[1,5-a]pyridine moieties. Tetrahedron Lett 54:4316
Reimlinger H, Lingier WRF, Merényi R (1975) Kondensierte Isochinoline, XIV. Synthese von v-Triazolo[5,1-a]isochinolinen. Chem Berichte 108:3794
Wentrup C (1978) [1,2,3]Triazoloazine/(diazomethyl)azine valence tautomers from 5-azinyltetrazoles. Helvetica Chim Acta 61:1755
Abarca B, Aucejo R, Ballesteros R, Blanco F, García-España E (2006) Synthesis of novel fluorescent 3-aryl- and 3-methyl-7-aryl-[1,2,3]triazolo[1,5-a]pyridines by Suzuki cross-coupling reactions. Tetrahedron Lett 47:8101
Asensio A, Abarca B, Jones G, Hursthouse MB, Abdul Malik KM (1993) Triazolopyridines. 14. Substitution reactions of 7-amino[1,2,3]triazolo[1,5-a]pyridines. Tetrahedron 49:703
Jones G, Richardson CM, Yates PC, Hajos G, Timari G (1993) Theoretical interpretations of some experimental observations in reactions of triazolopyridines and their quaternary salts. Tetrahedron 49:4307
Davies LS, Jones G (1970) Quinolizines. XIII. Rearrangement of quinolizinium-1-diazonium salts into v-triazolo[1,5-a]pyridines. J Chem Res [Sect] C Org 5:688
Abarca B, Gomez-Aldaravi E, Jones G (1984) Triazolopyridines. Part 4. Directed lithiation using 1,2,3-triazolo[1,5-a]quinoline. J Chem Res Synopses 5:140
Ortega N, Tang D-TD, Urban S, Zhao D, Glorius F (2013) Ruthenium–NHC-catalyzed asymmetric hydrogenation of indolizines: access to indolizidine alkaloids. Angew Chem Int Ed 52:9500
Jones G, Mouat DJ, Pitman MA, Lunt E, Lythgoe DJ (1995) Triazolopyridines. 16 1. lithiation of 3-cyano[1,2,3]triazolo[1,5-a]-pyridine. Tetrahedron 51:10969
Abarca B, Ballesteros R, Elmasnaouy M (2002) Triazolopyridines. 21. The stereochemistry of 1-[1,2,3]triazolo[1,5-a]pyridin-7-yl-4-(2H-[1,2,3]triazol-4-yl)-1,3-buta dienes and triazolo ring opening derivatives. ARKIVOC vi:146
Ballesteros-Garrido R, Leroux FR, Ballesteros R, Abarca B, Colobert F (2009) The deprotonative metalation of [1,2,3]triazolo[1,5-a]quinoline. Synthesis of 8-haloquinolin-2-carboxaldehydes. Tetrahedron 65:4410
Abarca B, Ballesteros R, Jones G, Mojarrad F (1986) Nucleophilic substitutions on bromotriazolopyridines an improved route to 2,6-disubstituted pyridines and to 1,3-disubstituted isoquinolines. Tetrahedron Lett 27:3543
Liu S, Sawicki J, Driver TG (2012) Ni-catalyzed alkenylation of triazolopyridines: synthesis of 2,6-disubstituted pyridines. Org Lett 14:3744
Abarca B, Ballesteros R, Elmasnouy M (1999) Triazolopyridines 20. Hydrogenation reactions. Tetrahedron 55:12881
Boyer JH, Wolford LT (1958) Alkylation of organic acids with pyridotriazole. J Am Chem Soc 80:2741
Jones G, Mouat DJ, Tonkinson DJ (1985) Triazolopyridines. Part 6. Ring opening reactions of triazolopyridines. J Chem Soc Perkin Trans 1 12:2719
Eisbert B, Schade W (1958) Über das Diazoketon, Azi–pyridil. Chem Ber 91:1411
Plüg C, Kuhn A, Wntrup C (2002) Quinilizine-2,4-diones by reversible dimerisation 2-pyridylketenes. J Chem Soc Perkin Trans 1 1366
Wentrup C (1974) Thermochemistry of carbene and nitrene rearrangements. Tetrahedron 30:1301
Abarca B, Ballesteros R, Blanco F (2007) Pyridylcarbene formation by thermal decomposition of 7-bromo-3-methyl-[1,2,3]triazolo[1,5-a]pyridine under pressure. ARKIVOC iv:297
Chuprakov S, Hwang FW, Gevorgyan V (2007) Rh-catalyzed transannulation of pyridotriazoles with alkynes and nitrile. Angew Chem Int Ed 46:4757
Abarca B, Ballesteros R, Mojarrad F, Metni MR, Garcia-Granda S, Perez-Carreno E, Jones G (1991) Triazolopyridines. Part 11. Ylides derived from 2-acylmethyl triazolo pyridinium salts. Tetrahedron 47:5277
Abarca B, Ballesteros R, Metni MR, Jones G, Ando DJ, Hursthouse MB (1991) A remarkable rearrangement during reaction between triazolopyridinium ylides and dimethyl acetylenedicarboxylate. Tetrahedron Lett 32:4977
Abarca B, Ballesteros R, Metni MR, Jones G (1992) Triazolopyridines. Part 12. A new synthesis of indolizines from triazolopyridinium ylides. Heterocycles 33:203
Abarca B, Ballesteros R, Jones G (1993) Triazolopyridines. 15. Reactions between triazolopyridinium ylides and alkenes. Heterocycles 35:851
Abarca B, Ballesteros R, Muñoz A, Jones G (1996) Triazolopyridines. 17. N2-dicyanomethylides: Synthesis, structure and reactivity with acetylenic dipolarophiles. Tetrahedron 52:10519
Abarca B, Ballesteros R, Chadlaoui M, Miralles J, Murillo JV, Colonna D (2001) The chemistry of [1,2,3]triazolo[1,5-c]pyrimidine. Tetrahedron 57:10111
Novinson T, Dea P, Okabe T (1976) Ring opening of 5,7-dimethyl-v-triazolo[1,5-a]pyrimidine by halogenating agents. J Org Chem 41:385
Vogel M, Lippmann E (1987) Quinoxalines. XXIV. Synthesis of 4-substituted 1,2,3-triazolo[1,5-a]quinoxalines. J fuer Praktische Chem (Leipzig) 329:101
Vogel M, Lippmann E (1987) Quinoxalines XXV. Reactions of 4-chloro-1,2,3-triazolo[1,5-a]quinoxalines with bases. Zeitschrift fuer Chem 27:38
Katritzky AR, Rachwal S (2009) Synthesis of heterocycles mediated by benzotriazole. 1. Monocyclic systems. Chem Rev 110:1564
Katritzky AR, Kuanar M, Slavov S, Hall CD, Karelson M, Kahn I, Dobchev DA (2010) Quantitative correlation of physical and chemical properties with chemical structure: utility for prediction. Chem Rev 110:5714
Katritzky AR, Huang L, Chahar M, Sakhuja R, Hall CD (2012) The chemistry of N-hydroxyamidoximes, N-aminoamidoximes, and hydrazidines. Chem Rev 112:1633
Katritzky AR, Avan I, Tala SR (2009) Efficient preparation of aminoxyacyl amides, aminoxy hybrid peptides, and α-aminoxy peptides. J Org Chem 74:8690
Katritzky AR, El-Gendy BE-DM, Todadze E, Abdel-Fattah AAA (2008) (α-aminoacyl)amino-substituted heterocycles and related compounds. J Org Chem 73:5442
Katritzky AR, Abdel-Fattah AAA, Gromova AV, Witek R, Steel PJ (2005) α-Nitro ketone synthesis using N-acylbenzotriazoles. J Org Chem 70:9211
Kaim LE, Meyer C (1996) An unprecedented radical reaction of benzotriazole derivatives. A new efficient method for the generation of iminyl radicals. J Org Chem 61:1556
Panda SS, Hall CD, Scriven E, Katritzky AR (2013) Aminoacyl benzotriazolides: versatile reagents for the preparation of peptides and their mimetics and conjugates. Aldrichimica Acta 46:43
Carpino LA (1993) 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J Am Chem Soc 115:4397
Coste J, Le-Nguyen D, Castro B (1990) PyBOP®: a new peptide coupling reagent devoid of toxic by-product. Tetrahedron Lett 31:205
Carpino LA, Imazumi H, El-Faham A, Ferrer FJ, Zhang C, Lee Y, Foxman BM, Henklein P, Hanay C, Mügge C, Wenschuh H, Klose J, Beyermann M, Bienert M (2002) The Uronium/Guanidinium peptide coupling reagents: finally the true uronium salts. Angew Chem Int Ed 41:441
Carpino LA, Imazumi H, Foxman BM, Vela MJ, Henklein P, El-Faham A, Klose J, Bienert M (2000) Comparison of the effects of 5- and 6-HOAt on model peptide coupling reactions relative to the cases for the 4- and 7-isomers. Org Lett 2:2253
Suma BV, Natesh NN, Madhavan V (2011) Benzotriazole in medicinal chemistry: an overview. J Chem Pharm Res 3:375
Palumbo P, Guarcello A (2010) Bioactive compounds containing benzoxadiazole, benzothiadiazole, benzotriazole. Curr Bioactive Comp 6:266
Holý A, Dvořáková H, Jindřich J, Masojídková M, Buděšínský M, Balzarini J, Andrei G, De Clercq E (1996) Acyclic nucleotide analogs derived from 8-azapurines: synthesis and antiviral activity. J Med Chem 39:4073
Aromí G, Barrios LA, Roubeau O, Gamez P (2011) Triazoles and tetrazoles: prime ligands to generate remarkable coordination materials. Coord Chem Rev 255:485
Shen Y-C, Li Z-J, Cheng J-K, Qin Y-Y, Yao Y-G (2007) Benzotriazole controlled unusual building blocks in two zinc complexes and their fluorescence properties. Inorg Chem Commun 10:888
Biswas S, Grzywa M, Nayek HP, Dehnen S, Senkovska I, Kaskel S, Volkmer D (2009) A cubic coordination framework constructed from benzobistriazolate ligands and zinc ions having selective gas sorption properties. Dalton Trans 33:6487
Han Z-B, Lu R-Y, Liang Y-F, Zhou Y-L, Chen Q, Zeng M-H (2011) Mn(II)-based porous metal–organic framework showing metamagnetic properties and high hydrogen adsorption at low pressure. Inorg Chem 51:674
Xiao J, Wu Y, Li M, Liu B-Y, Huang X-C, Li D (2013) Crystalline structural intermediates of a breathing metal-organic framework that functions as a luminescent sensor and gas reservoir. Chem Eur J 19:1891
Vogl O, Li S (1983) Di- and tri(benzotriazolyl)trihydroxybenzenes. USA Patent, US 539493
Stoeber L, Sustic A, Simonsick WJ, Vogl O (2000) Functional polymers 64. Potassium ionisation of desorbed species (K+IDS) of 2(2-hidroxyphenyl)2H-benzoriazoles. J Macromol Sci A 37:1269
Ohashi M, Horii S (1990) Method for processing photographic material containing antifoggant. Japan Patent US 4,920,043
Gabe Y, Urano Y, Kikuchi K, Kojima H, Nagano T (2004) Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore rational design of potentially useful bioimaging fluorescence probe. J Am Chem Soc 126:3357
MacLeod ID (1987) Conservation of corroded copper alloys: a comparison of new and traditional methods for removing chloride ions. Stud Conserv 32:25
Sease C (1978) Benzotriazole: a review for conservators. Stud Conserv 23:76
Madsen HB (1967) A preliminary note on the use of benzotriazole for stabilizing bronze objects, 12/4 1967. Stud Conserv 12:163
Jones G, Sliskovik DR (1980) [1,2,3]Triazolo[1,5-a]pyridine-A synthon for 6-disubstituted pyridine-2-carboxaldehydes. Tetrahedron Lett 21:4529
Lehn JM (1995) Supramolecular chemistry. VCH, Weinheim (Bundersrepublik Deutschland)
Abarca B, Ballesteros R, Elmasnaouy M (1998) A facile route to potential helicating ligands. Tetrahedron 54:15287
Abarca B, Ballesteros R, Chadlaoui M (2004) Triazolopyridines. Part 24: new polynitrogenated potential helicating ligands. Tetrahedron 60:5785
Abarca B, Aucejo R, Ballesteros R, Chadlaoui M, García-España E, Ramírez de Arellano C (2005) X-ray characterization of 3-methyl-6,8-di(2-pyridyl)-[1,2,3]triazolo [5′,1′:6,1]pyrido[2,3-d]pyrimidine. ARKIVOC xiv:71
Abarca B, Ballesteros R, Chadlaoui M (2008) Synthesis of novel polypyridyl carbonylpyridines from triazolopyridines. Building blocks in supramolecular chemistry. ARKIVOC vii:73
Chen XD, Mak TCW (2005) Molecular and supramolecular architectures of silver(I) and copper(I) complexes of 2,6-pyridinediylbis(2-pyridinyl)methanone. J Mol Struct 748:183
Chen XD, Du M, He F, Chen XM, Mak TCW (2005) Molecular and supramolecular architectures of silver(I) and copper(I) complexes of 2,6-pyridinediylbis(2-pyridinyl) methanone. Polyhedron 1047
Boudalis AK, Raptopoulou CP, Abarca B, Ballesteros R, Chadlaoui M, Tuchages JP, Terzis A (2006) CoII Chemistry of 2,6-Bis(2-pyridylcarbonyl)-pyridine: an icosanuclear co cluster exhibiting superparamagnetic relaxation. Ange Chem Int Ed 45:432
Boudalis AK, Raptopoulou CP, Psycharis V, Sanakis Y, Abarca B, Ballesteros R, Chadlaoui M (2007) An “S”-shaped pentanuclear CuII cluster derived from the metal-assisted hydrolysis of pyCOpyCOpy: structural, magnetic and spectroscopic studies. Dalton Trans 36:3582
Boudalis AK, Raptopoulou CP, Psycharis V, Abarca B, Ballesteros R (2008) Ferromagnetism in CuII 4 and CoII 4 complexes derived from metal-assisted solvolysis of Di-2,6-(2-pyridylcarbonyl)pyridine: synthesis, structures, and magnetic properties. Eur J Inorg Chem 3796
Boudalis AK, Pissas M, Raptopoulou CP, Psycharis V, Abarca B, Ballesteros R (2008) Slow magnetic relaxation of a ferromagnetic Ni cluster with an S = 5 ground state. Inorg Chem 47:10674
Georgopoulou AN, Raptopoulou CP, Psycharis V, Ballesteros R, Abarca B, Boudalis AK (2009) Ferromagnetic Cu, Co, and Ni azido complexes derived from metal-assisted methanolysis of Di-2,6-(2-pyridylcarbonyl)pyridine. Inorg Chem 48:3167
Georgopoulou AN, Adam R, Sanakis Y, Raptopoulou CP, Psycharis V, Ballesteros R, Abarca B, Boudalis AK (2009) A diferric complex from metal-assisted methanolysis of di-2,6-(2-pyridylcarbonyl)pyridine: structural, magnetic and spectroscopic (Mössbauer, EPR) study. Polyhedron 28:3251, Corrigendum (2010) Polyhedron 29:1189
Georgopoulou AN, Adam R, Raptopoulou CP, Psycharis V, Ballesteros R, Abarca B, Boudalis AK (2010) Isomorphous replacement of MII ions in MII- GdIII dimers (MII = CuII, MnII, NiII, CoII, ZnII): magnetic studies of the products. Dalton Trans 39:5020
Georgopoulou AN, Adam R, Raptopoulou CP, Psycharis V, Ballesteros R, Abarca B, Boudalis AK (2011) Expanding the 3d-4f heterometallic chemistry of the (py)2CO and pyCOpyCOpy ligands: structural, magnetic and Mössbauer spectroscopic studies of two FeII-GdII complexes. Dalton Trans 40:8199
Stamatatos TC, Adam R, Raptopoulou CP, Psycharis V, Ballesteros R, Abarca B, Perlepes SP, Boudalis AK (2012) The first member of a second generation family of ligand derived from metal.ion assisted reactivity of di-2,6-(2-pyridylcarbonyl)pyridine: synthesis and characterization of a MnII/III 4 rhombus. Inorg Chem Comm 15:73
Niel V, Gaspar AB, Muñoz MC, Abarca B, Ballesteros R, Real JA (2003) Spin crossover behaviour in the Iron(II)-2-pyridyl[1,2,3]triazolo[1,5-a]pyridine system: X-ray structure, calorimetric, magnetic, and photomagnetic studies. Inorg Chem 42:4782
Chadlaoui M, Abarca B, Ballesteros R, Ramírez de Arellano C, Aguilar J, Aucejo R, García-España E (2006) Properties of a Triazolopyridine System as a Molecular Chemosensor for Metal Ions, Anions, and Amino Acids. J Org Chem 71:9030
Ballesteros-Garrido R, Abarca B, Ballesteros R, Ramírez de Arellano C, Leroux FR, Colobert F, García-España E (2009) [1,2,3]Triazolo[1,5-a]pyridine derivatives as molecular chemosensors for zinc(II), nitrite and cyanide anions. New J Chem 33:2102
Abarca B, Ballesteros R, Chadlaoui M, Ramírez de Arellano C, Real JA (2007) [(Pyridylcarbonyl)-pyridyl]triazolopyridines, useful ligands for the construction of polynuclear coordination compounds- synthesis, crystal structure and magnetic properties of a novel tetranuclear copper(II) cubane. Eur J Inorg Chem 4574
Abarca B, Ballesteros R, Elmasnaouy M, D’Ocon P, Ivorra MD, Valiente M (2002) Evaluation and synthesis of 7-arylhydroxymethyltriazolopyridines as potential cardiovascular agents. ARKIVOC x:9
Kiss JP, Vizi ES (2001) Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 24:211
Kadekaro M, Summy-Long JY (2000) Centrally produced nitric oxide and the regulation of body fluid and blood pressure homeostases. Clin Exp Pharmacol Physiol 27:450
Bredt DS (1999) Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic Res 31:577
Schumann P, Collot V, Hommet Y, Gsell W, Dauphin F, Sopkova J, MacKenzie E, Duval D, Boulouard M, Rault S (2001) Inhibition of neuronal nitric oxide synthase by 7-methoxyindazole and related substituted indazoles. Bioorg Med Chem Lett 11:1153
Tuynman A, Pérollier C, Frapart Y, Schumann P, Collot V. Rault S, Boucher JL (2003) Inhibitory effects and spectral interactions of isomeric methoxyindazoles on recombinant nitric oxide synthases. Nitric Oxide 9:86
Abarca B, Ballesteros R, Blanco F, Collot V, Rault S, Schumann-Bard P (2003) Pharmacological evaluation of [1,2,3]Triazolo[1,5-a]pyridines as new compounds inhibitors of neuronal nitric oxide synthase. XIII Congreso Nacional de la Real Sociedad Española de Química. Terapéutica, Santiago de Compostela (Spain)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Abarca, B., Ballesteros-Garrido, R. (2014). 1,2,3-Triazoles Fused to Aromatic Rings. In: Dehaen, W., Bakulev, V. (eds) Chemistry of 1,2,3-triazoles. Topics in Heterocyclic Chemistry, vol 40. Springer, Cham. https://doi.org/10.1007/7081_2014_121
Download citation
DOI: https://doi.org/10.1007/7081_2014_121
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07961-5
Online ISBN: 978-3-319-07962-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)