Abstract
When it comes to bioplastics, it is important to differentiate between the biopolymer in its form as a macromolecule and the resulting bioplastic material as a ready-to-use material. Furthermore, a distinction must be made between bio-based and biodegradable plastics. Bio-based refers to the raw material origin of the polymer feedstock, while biodegradability describes an end of life option. However, both features are independent of each other. Although biodegradability describes a material property that depends on the microstructure and the chemical structure of the material, in practice biodegradability is a system feature, since there are a variety of environmental conditions, from industrial composting facilities to sewage treatment plants, soils in a variety of climatic regions, the beach and the seabed, or even the human body. It is, therefore, necessary to provide clear information about the environmental conditions and the point in time at which a material or product is biodegradable. In the area of compostability, some test standards for bioplastics and other organic substances cover various environmental conditions well, while test standards and also the understanding of degradation mechanisms in other areas, such as degradability in soil or in marine systems, are only available in small numbers and do not reflect the complex environmental conditions well.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaerobic digestion
- Aquatic degradability
- Biodegradability
- Biodegradable plastics
- Bioplastics
- Compostability
- Environmental conditions
- Marine degradability
- Material microstructure
- Oxo-degradability
- Standards for biodegradability
1 Introduction
The number of newly developed bioplastics has increased continuously in recent years but the market volume is still less than 1% of the total plastics market. Bioplastics are not, however, a completely new kind of material, but rather a rediscovered class of materials within the familiar group of materials known as plastics.
The first polymer materials synthesized by man were all based on renewable materials (e.g., caseins, gelatin, shellac, celluloid, cellophane, linoleum, rubber, etc.) because at that time there were simply no petrochemical feedstocks available. Apart from a few exceptions (cellulose- and rubber-based materials), these first bio-based plastics were almost completely replaced by today’s petrochemical plastics.
Bioplastics are now experiencing a renaissance: this is particularly because of ecological aspects as well as limited petrochemical resources and also, in part, innovative property profiles like their biodegradability. This is combined with an increasing awareness amongst the public, politicians, industry and, in particular, research and development. These biopolymers or bioplastics are, however, still very much at the start of their development.
2 Wording
There is still a lot of confusion about the terms “biopolymer”, “bioplastic”, “biodegradable plastic”, “plastics from renewable resources”, etc., because biodegradable plastics can be based on petrochemical as well as on renewable resources and biobased feedstock can lead to degradable as well as durable plastics. The best general definition for biopolymers so far describes a polymer material that fulfills at least one of the two following properties [1]:
-
Fully or partly made from bio-based (renewable) raw materials
-
In some way biodegradable
Given that, the following three fundamental groups of biopolymers exist:
-
1.
Degradable petro-based biopolymers
-
2.
Degradable (mainly) bio-based biopolymers
-
3.
Non-degradable bio-based biopolymers
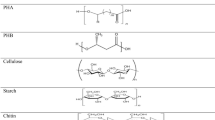
Biologically degradable plastics can be based on petrochemical raw materials as well as on renewable raw materials. Degradability in polymeric materials is ultimately influenced only by the chemical and physical microstructure of the polymer, and neither by the origin of the raw materials used nor by the process used for manufacturing these polymers or different products made out of them. This means that biopolymers need not necessarily be made exclusively from renewable materials. Biologically degradable plastics can also be produced from petrochemical ingredients such as polyvinyl alcohols, polycaprolactone, various polyesters, polyesteramides, etc. (Fig. 1, bottom right). Conversely, not all biopolymers based on renewable ingredients are necessarily biologically degradable; for example, highly substituted cellulose acetates, vulcanized rubber, casein plastics, linoleum or bio-based PE, PET, PA, etc. (Fig. 1, top left). Typical examples of the group of bio-based and biologically degradable bioplastics are starch-based plastic blends, polyhydroxyalkanoates (PHA), and PLA (polylactic acid).
Bioplastics and the three fundamentally different biopolymer groups ([1], modified)
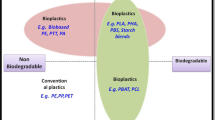
To avoid misunderstandings when speaking of biopolymers or bioplastics, it is imperative that the most precise nomenclature possible is used. Therefore, it is advisable to speak specifically of biodegradable or bio-based bioplastics. Degradability here means a functional property or disposal option at the end of the materials’ life cycle, irrespective of the origin of the raw materials, whilst, conversely, bio-based describes exclusively the origin of the raw ingredients of the polymers at the beginning and provides no statement whatsoever regarding its degradability. These two different approaches are still being pursued and form the technical basis for a variety of bioplastics (Fig. 2).
Raw material basis and degradability of bioplastics ([2], modified)
2.1 Degradable Petroleum-Based Biopolymers
Biopolymers based on petrochemical feedstock, as well as their petro-based secondary products (e.g., polyols, carboxylic acids), are based on hydrocarbon monomers and oligomers gained from crude oil, natural gas, or coal by various methods of fractionated distilling and targeted cracking, as are conventional plastics. The property profile of conventional polymers can be varied by any number of basic resins, polymerization mechanisms, processing parameters, or additives and adapted to any number of applications. Similarly, the property profile of polymer materials can be expanded for degradability by incorporating various heteroatoms (especially oxygen and nitrogen) into their molecules. For conventional plastics, a desirable property was a high level of resistance to chemicals and microbiological or ecologically determined influences. The goal for degradable petrochemical based biopolymers is to design molecules and materials that are not very resistant to environmental influences but rather biodegrade and depolymerize easily under natural influences.
2.2 Degradable Bio-Based Biopolymers
Within the last 25 years the term biopolymers were defined by polymer materials entered the marketplace that are based on renewable resources and which are compostable. Especially cellulose, starch, sugar, vegetable oils and their secondary products like acids or alcohols, as well as some lignins and proteins are renewable resources that can be used as basic components for production of bio-based and biodegradable biopolymers.
2.3 Non-degradable Bio-Based Biopolymers
These biopolymeric materials have been known for a long time. The first engineering polymer materials were based on renewable materials, such as cellulose and natural latex. Raw materials availability was the main feature of these materials. During the materials manufacturing process, these readily available natural resources were modified in such a way that the resulting polymeric materials exhibited property profiles that were utterly new in those times. The main achievement was that for the first time it was possible to turn perishable organic raw materials into durable polymer materials. In the late 1900s, while industrialization continued its advance, petrochemical raw materials were not yet available. Thus, more than 100 years ago, initial, non-degradable biopolymers based on renewable resources were manufactured, although they were not explicitly termed biopolymers. The long-term availability of CO2-neutral raw materials has recently become the main reason for the development of modern bio-based biopolymers. This is not “only” about the climate-neutral and acute or immediate availability of raw materials, but also about a strategic independence from exhaustible, globally unfavorably distributed petrochemical raw materials for the production of plastics.
2.4 Polymerblends and Copolymers
There are many co- and terpolymers, as well as mixtures, i.e. blends or the so-called polymer alloys, combining various materials from the previously mentioned biopolymer groups.
Besides their main raw materials, biopolymers almost always contain additives suitable for tailoring a particular property profile. These additives are classified by the same method used previously to define biopolymers but often these additives used to upgrade the basic polymers are still petro-based and non degradable.
The increasing use of bio-based, but non-degradable polymers as additives in biopolymer blends tends to impair the biodegradability of these blends. For co- and terpolymers, the increasing use of non-bio-based blend components or petrochemical monomer raw material necessarily leads to a reduction in the amount of bio-based carbon in the final polymer material. Currently, no minimum content levels have been established for bio-based material components in biopolymer blends and co- or terpolymers. Therefore, polypropylene–starch blends, various copolyetsers or even so-called wood plastic composites (WPC) are considered biopolymers, even though they are non-biogradable and their bio-based content is significantly smaller than their petrochemical content.
2.5 Old and New Economy Bioplastics
The first technical, industrially-used polymer materials, the development of which began over 100 years ago, were all bio-based as there were no petrochemical raw materials available at that time. These so-called old economy bioplastics were based on the renewable plant-based raw materials cellulose and natural latex or on animal proteins. With increasing industrialization at the end of the nineteenth century, the availability of the raw materials was of great concern to produce these materials. The natural raw materials available at that time were modified as part of the material manufacturing process in such a way that they resulted in the first really durable polymer materials with a completely new property profile for that time, without being explicitly described as bioplastics. These old economy bioplastics, therefore, belong to the group of bio-based, non-degradable bioplastics. Of the old economy bioplastics, the only ones still of economic significance on the plastics market are natural rubber, regenerated cellulose, and cellulose derivatives (cellophane, viscose, celluloid, cellulose acetate) as well as linoleum in smaller volumes (Fig. 3).
Old and New Economy Bioplastics ([2], modified)
The renewed development of novel bioplastics (new economy bioplastics), which began around 30 years ago, was at first driven by excess supply of food in Europe, such as “butter or tomato mountains” and faced with the waste problem as it existed at the time as well as the unsatisfactory disposal situation with regard to conventional plastics. The objective of these developments was biobased, degradable bioplastics as a solution to the agricultural surpluses and waste problem. There are currently increasing applications in medicine, landscaping and gardening, wastewater treatment, etc., for which degradability permits an additional function under the respective environmental conditions, such as films for use in agriculture which can be plowed in after use, bioresorbable implants or suture materials for use in surgery, or the targeted release of active substances (fertilizers, medical substances).
2.6 “Drop-in” Bioplastics
In the context of the latest developments in bioplastics, the use of sustainable, bio-based raw materials is once again becoming of more concern. Within the group of bio-based and durable bioplastics, the development of the so-called drop-in solutions has made significant technological advances over the past 15 years. The aim is to produce established plastic structures with a maximized bio-based feedstock. To put it simply, the attempt is made to replace the petrochemical raw material with biogenic raw materials, while the established synthesizing processes are retained and ending up in the same chemical structures. That means the goal here is to create bio-based plastics that are similar to familiar conventional plastics. Due to the identical chemical structure, the drop-ins have completely the same property profiles as their petrochemical equivalents. This means that when conventional plastics are replaced by the respective drop-ins, no changes in processing, use and, in particular, recovery and recycling are to be expected. In this context, for example, polyvalent biogenic alcohols or bio-based carboxylic acids are being used to produce a fully bio-based polyethylene (bio-PE) and fully or partly bio-based polyamides (bio-PA), polyurethanes (bio-PUR), acryl butadiene styrene (bio-ABS), polyethylene terephthalates (bio-PET), and various other polyesters. Currently, work is being intensified on these non-degradable bio-based drop-in solutions by large chemical companies, such as BASF, Bayer, Braskem (Brazil), Dow Chemical, DSM, Evonik, or Solvay.
This means at the end that the new economy bioplastics are made up of two basic groups:
-
1.
The chemically-novel biopolymers, that is, unknown in the field of plastics from a chemical point of view until a few years ago (e.g., novel bio-based polyesters such as PLA (polylactic acid), PBAT (polybutylene adipate terephthalate), PEF (polyethylene furanoate), or PTT (polytrimethylene terephthalate)) and
-
2.
Drop-ins which are identical in chemical structure but partially or completely bio-based plastics. Currently, regarding the commercial market share, the most prominent examples of these are bio-PET and bio-PE. Alongside these, work is currently being carried out on further drop-ins, including that in the field of other thermoplastic materials like an also bio-based PP, thermosets (e.g., bio-based EP resins), or elastomer polymer materials (e.g., bio-based EPDM or bio-based polyurethanes).
Depending on the perspective, this means that there are several different types of bioplastics. To avoid misunderstandings, bioplastics should, therefore, generally not be mentioned without further specifying, through additional information, which group is meant.
3 Degradation
3.1 Primary and Ultimate Degradation
As regards material dissociation, it is necessary to differentiate more precisely between a primary degradation (splitting of the macromolecules) and an ultimate degradation of the fission products into water, carbon dioxide, methane, and biomass [3, 4] (Fig. 4).
The metabolic potential of the (macro-)molecular fission products formed as part of the primary degradation defines here whether the process is simply a macroscopic disintegration process of a component or material or whether it is in fact a complete ultimate degradation [5, 6]. When the ultimate degradation of fission products is not assured, the decomposition products can accumulate, for example, in compost or in groundwater. Therefore, in this case the term “biological degradability” should not be used. One prominent example of a product exclusively coming from primary degradation of (macro-)plastics is microplastic (called as secondary microplastic), which is increasingly accumulating in all the different water systems on earth like the oceans or groundwater and consequently also in living beings. The respective test standards for certifying degradability, therefore, usually include as a significant core element the quantification of the decomposition products formed in the ultimate degradation and/or a record of the oxygen required for this. The oxygen requirement or the amount of CO2 produced is then compared to the amount theoretically expected in a complete chemical conversion of the material/product to be degraded.
Primary and ultimate degradation ([1], modified)
3.2 Oxo-Degradability
During molecular degradation, primary degradation is initiated not only by biologically induced decomposition reactions, but also by other mechanisms. One of these mechanisms is macromolecule scission due to radiation. The most important natural radiation in this respect is the UV content in sunlight. The exposure to sunlight can result in direct polymer chain scission, particularly in polymers with chromophore groups in their molecular structure, e.g. aromatic polyesters or polyamides (photodegradable polymers) [5, 7].
Catalyst residue, contamination, peroxides, as well as other oxygenic components can also absorb sunlight and initiate degradation. Indirect chain scission processes use host molecules, such as aldehydes or conjugated double bond systems; they are excited by radiation and in a second step, the energy required to split the bond is transferred to the actual polymer molecule.
Besides this pure photodegradation, sunlight in combination with oxygen also causes photo-oxidative degradation. Heat or the effect of light can initiate an oxo-degradation sequence by radical formation. Next, alkyl radicals can form and once they react with oxygen, light-sensitive hydroperoxides can form as an intermediate step of photo-oxidative degradation. Continued exposure to light and elevated temperatures together with the previously formed hydroperoxides cause continued radical formation (alkoxy, peroxide, and alkyl radicals), until the polymer chains ultimately degrade. Reaction products such as carboxyl acids or alcohols are subject to further final degradation.
Another method for the initiation of the primary degradation is a chemical dissolving process, for example, in water with subsequent or parallel hydrolysis for water-soluble polymers.
These different reaction mechanisms (Fig. 5) have in common that they can initiate to macroscopic primary degradation, without ensuring final degradation of the products of decomposition. Therefore, total biological degradability or compostability of materials cannot automatically be presumed, even when there is macroscopic disintegration or macrobiological damage and a reduction and/or loss of mechanical properties, surface change, or odor development.
Degradation mechanisms in degradable polymers ([1], modified)
It is, therefore, particularly important in the case of macroscopic disintegration or macrobiological damage, that a complete biological degradability or compostability of the materials is not automatically assumed. The respective additive-enhanced plastics, whose macroscopic decay or primary degradation is initiated solely through oxo-degradation, may therefore not be designated as being degradable or even compostable bioplastics, as ultimate degradation is the crucial process as regards ensuring degradability. From a scientific perspective, there is no further need for discussion as regards oxo-induced or solution-induced primary degradation at all because here, in accordance with the testing standards, the quantification of the resultant final degradation products and the oxygen or possibly hydrogen demand necessary for the metabolization forms the basis for an accurate statement concerning complete degradability.
Current research is concentrating on the oxo-degradability of polyolefins, especially PE, by incorporating special metal ions to initiate a radical oxidation mechanism. However, this oxo-degradation method is very controversial as well as those described before. According to most experts, total microbiological final degradation generally has not been reached in oligomer decomposition products until they are reduced to less than 20 to 25 C atoms (4). The decomposition products of polymer oxo-degradation are usually much larger. In order to obtain smaller, completely, or ultimately degradable oligomers for total degradation, high doping levels are required, which in turn cause a considerable and generally unacceptable decrease in materials properties. The additives for the initiation of the primary degradation once again do not ensure the ultimate degradation. Instead of that, they can, however, lead to a reduction in the stability of the primary materials and also to a contamination of recycled products and thereby, for example, to a reduction in the stability of secondary polyolefins. Additives which solely initiate a primary oxo-degradation present a potential problem for the established recycling of polyolefins.
3.3 Biological Degradability
In most cases, biodegradation of the plastic items occurs on the surface, i.e. at the solid-liquid interface. Microbes and enzymes cannot penetrate the solid plastic, so only the exposed surface is available for biodegradation processes. The physical effect of biodegradation on a solid plastic object is mainly chemical and mechanical erosion as well as light exposure, which leads to thinning and weakening of the object. This process results in the object losing mass, physical properties, such as the reduction of the mechanical values, the visual appearance (surface structure, coloration, etc.), the development of an odor and ultimately the loss of physical integrity through fragmentation into biodegradable particles, the ultimate fate of which is to biodegrade. The term disintegration is used just when the degradation process is prolonged until the original object is completely fragmented into particles below a defined size. This definition does not include the degradation to lower-molecular parts, so that the following definition describes the biological degradability of polymers better: Biological degradation is a process caused by biological activities that leads to a change of the polymer chains to naturally occurring metabolic products. A plastic product is biodegradable, when all its material components end up in microbial metabolic products like water, carbon dioxide, hydrogen or methane and biomass [8]. When microorganisms cause degradation processes, biodegradation, biofragmentation and biological disintegration are the right terms [9].
In the course of total biological degradation, microorganisms in fact require extracellular enzymes to digest plastics and/or their molecular decomposition products. These enzymes essentially use oxidation and hydrolytic processes to break the material down into even smaller components, which can then be absorbed by the cell, where they become metabolized [10,11,12]. In dependence from the initial starting point of degradation, an exo- and endodegradation mechanism of polymer chain can be distinguished [13]. In case of exodegradation monomers or small oligomers are cleaved from the chain end while endodegradation describes enzymatic chain cleavages which take place statically distributed over the chain. This initial enzymatic degradation step often determinates the degradation rate.
However, the enzymes are too voluminous to penetrate the degrading material efficiently. Therefore, this process can function only as surface erosion, or as a diffusion-controlled sequence in liquid carrier media, especially water. In the other case, the enzymes are adsorptively or covalently bound to the cell wall. The microorganisms have to adhere closely to the polymer surface in order to bring the immobilized enzymes into contact with the substrate so that they can react [14]. Since the enzymes cannot penetrate the plastic due to their size, biodegradation is an interface process, and the material is removed from the surface.
Given that, biodegradation can take place under a wide variety of ambient conditions (ground, water, seawater, clarification plants, compost, human body, etc.) covering a wide range of biological degradation scenarios (Fig. 6).
Finally, biologically degradable plastics consist of natural (renewable) or petrochemical raw materials and, as polymer materials, are amenable to biological degradation reactions – that is, they break down ultimately under the influence of microorganisms and/or enzymes.
3.4 Compostability
Even total biodegradation of a material still does not automatically mean that the material or parts produced from it are also compostable. A material is considered biodegradable if all its organic components generally and regardless of any time factor are subject to primary and ultimate degradation by biological activity [15, 16].
A material or material mix and parts produced from it is considered compostable, when, under defined conditions in a composting system, it is entirely transformed into CO2, H2O, CH4, and biomass within a specified length of time, i.e. mostly during a composting cycle ranging from a few weeks to months [17]. In this context, a tree trunk, for example, is biodegradable, but not compostable.
That means, while biodegradability covers a lot of different biological degradation scenarios without any information on the surrounding conditions, temporal course or the duration of the degradation process, compostability describes a degradation process under specified environmental conditions like a domestic compost or industrial composting facility. There are several national, European, and international standards defining compostability of degradable materials and/or products made from these materials (e.g., packaging). At this point it should be noted that the first standard of its kind, German standard DIN V 54900, has been replaced by European standard EN 13432.
Even so, DIN V 54900 represents an important basis for other standards in this field. It consists of five parts and describes succinctly and in detail the individual test/procedures and evaluation criteria (Fig. 7).
Steps in the test for compostability according to DIN 54900 ([1], modified)
Its first part, DIN V 54900-1 describes the data required regarding the chemical composition of a material. This forces material manufacturers to reveal materials composition. An IR spectrum is created and stored for the precise identification of the particular material.
DIN V 54900-2 describes the test procedures for total biodegradability under clearly defined reproducible laboratory conditions. Two test variations are available, one in aqueous environment and one in compost. During each test, biopolymer metabolization is measured, i.e. the resulting amount of CO2 or the oxygen consumed during the process. So that a material can be certified as compostable, at least one test version has to indicate total biodegradability of the material, i.e. at least 80% of the theoretical value of total final degradation has to be achieved.
In order to more precisely evaluate degradation behavior of the materials, in the next step screening tests are performed using an aerobically driven, aquatic respirometer testing system. Here, the quantity of oxygen is measured in BOD units (Biochemical oxygen demand) that would have to be produced to maintain pressure in the closed system without changing the volume at a constant temperature. Biochemical oxygen demand (BODm) determines the quantity of oxygen in milligrams consumed in m days by the biochemically oxidizable substances contained in 1 L of test water. In order to keep the specific surface of the materials investigated virtually constant, the materials are generally ground to a powder with a specified surface. A fraction of the powder with a particular grain size is sifted out. Subsequently, analogous to DIN 53739, a certain amount of it is added to a potassium phosphate buffered medium (pH value >7) composed as follows (for 1 L):
KH2PO4 | 0.7 g |
K2PO4 | 0.7 g |
MgSO4 • 7H2O | 0.7 g |
NH4NO3 | 1.0 g |
NaCl | 5.0 mg |
FeSO4 • 7H2O | 2.0 mg |
ZnSO2 • 7H2O | 2.0 mg |
MnSO4 • 7H2O | 1.0 mg |
To inoculate the test substance (medium + powdered material) with microbes, an inoculum of conventional fresh compost can be used that represents a wide variety of microorganisms specific to compost.
In a reaction vessel, the samples thus inoculated are thoroughly mixed by a bar magnet throughout the duration of the test so that they can continuously absorb oxygen until saturation. During substrate oxidation, oxygen is consumed and carbon dioxide is formed. The CO2 generated is absorbed by soda lye. This procedure creates an under-pressure in the reaction vessel that causes an increase in the electrolyte solution (0.5% H2SO4) in a precision manometer. Pressure changes cause the contact between the two electrodes to close, triggering the control and regulation unit that generates oxygen electrolytically. The oxygen thus produced causes system pressure to rise again, thereby breaking the contact.
In a second vessel, copper sulfate and sulfuric acid are the electrolyte used to ensure that no further gas can form besides oxygen. The electrolytic current is then kept constant until enough oxygen is produced to recompensate pressure. For the entire duration of the test, current flow is recorded, and the units are added and converted into the corresponding amount of oxygen. The sum of currents serves as a measure for oxygen consumption.
By comparing the measured oxygen requirement ΔO2 (BOD) with the theoretical, i.e., the chemical amount of oxygen (COD or ThOD) demanded at the start of the test for total oxidation of the test compound, the so-called degree of degradation (DoD) is determined by the following formula:
The third part of standard DIN V 54900-3, in contrast to laboratory tests, describes the test under realistic conditions. It determines the maximum material thickness that can be degraded within a realistic degradation time. One possible measure is the amount of material that can be found after a certain time in compost (sieving).
Subsequently, quality testing regarding recycling properties (DIN V 54900-4) and ecotoxicity (DIN V 54900-5) is performed on the generated compost [15].
The European standard DIN EN 13432 [16] was conceived specifically for packaging and defines requirements and methods for establishing compostability and anaerobic treatment of packaging and packing materials. DIN EN 13432 has replaced German standard DIN 54900, yet its content is based essentially on the German standard. DIN EN 13432, similar to DIN V 54900, is divided into four technical steps apart from general information about the material from the supplier:
-
Characterization of materials composition
-
Biological degradability
-
Disintegration during biological treatment (no polymer constituent must be visible following composting)
-
Effect on the quality of the compost created
In the end, a two-step process is required to certify compostability of a product (Fig. 8), first a positive evaluation for the material(s) used and secondly for the package that was made from the certified material(s). Materials that fulfill the requirements according to DIN EN 13432 can be registered as compostable. The processing industry can use this registration to its advantage when using certified materials. When seeking certification for a product made from certified materials (semi-finished products), the material, but also the articular layer- or wall-thickness, and the specific accessible surface are important characteristics. In Germany DIN CERTCO verifies whether a product with a certain thickness can be certified as being compostable according to the particular standard. If this is the case, the product receives the composting symbol and a certificate stating the maximum permissible layer or wall thickness.
Two-step evaluation of compostability of a product ([1], modified)
In addition to DIN CERTCO, there are of course further certification organizations worldwide like Vincotte (Belgium) or BPI (USA) employing their own procedures and symbols to certify compostable products. Furthermore, a lot of more international standards are available and used regarding compostability such as DIN EN 14995, ISO 17088, or ASTM 6400. These various standards can be subdivided into two basic groups: Framing standards for product requirements and general descriptions of testing procedures and specific test standards describing in detail how the various investigations are to be performed, including special standards for packaging, aerobic and anaerobic as well as aquatic and terrestrial biodegradation in which the standards for degradability in marine environment and soil are very limited.
3.5 Aquatic Degradability
Pollution of the natural environment, especially the oceans, with conventional plastic becoming more and more a serious environmental issue. Due to the persistence of plastics and their increasing production volume at the same time, plastics in both visible and not directly visible form as micro- and nanoplastics can now be found in almost all ecosystems across the globe. While compostability of plastics has been well studied, there are currently no reliable field test methods and equivalents to these biodegradability standards for unmanaged natural environments. So far, the standards for assessment and certification of biodegradation for aquatic environments, such as wastewater, unmanaged freshwater, or marine habitats have been very incomplete. Freshwater habitats include environments such as rivers, streams, lakes, and wetlands. Marine environments cover a wide variety of habitats, including beaches, ocean surface, open ocean and coastal ecosystems, and deep-sea environments.
In view of the legislative framework and characterization of marine degradability, it is therefore necessary to adapt or adjust existing standards and/or develop new regulations/standards to deliver more results that better match real marine conditions, due to the broad and complex range of physical and chemical conditions as well as microbiological parameters encountered within these natural ecosystems. In addition, the existing standards and test methods for biodegradability in aqueous systems do not contain any toxicity tests and do not take into account the potentially disadvantageous ecological effects of polymer degradation products as well as dissolved additives or small (microscopic) plastic particles that could result from fragmentation of the plastic material [1, 8, 18].
There are various methods for studying aquatic degradability, performed in laboratory systems or in real field experiments. To give an overview, the existing standards and test methods for evaluating biodegradability of plastics in aquatic environments are presented in Table 1 and summarized briefly below, regarding the scale (laboratory or field tests), the inoculum resp. test conditions, the sample, measuring principle, the applied temperatures, and the test duration as well as the way of assessing the results.
The general principle of all laboratory methods is the exact measurement of the end products of degradation under defined environmental conditions, reflecting only a small part of the environment, such as temperature, salinity, nutrition, oxygen availability, exposure, etc. The test conditions on the one hand should represent the various aquatic systems as good as possible, but on the other hand the conditions are selected in a way to achieve a high degradation rate and shorten the examination time. Therefore, the investigations were performed in a laboratory scale with a view preselected inocula such as digested or activated sludge, marine sediments, seawater with indigenous microorganisms and mesophilic temperatures in the range of 15–35°C.
In addition to these laboratory tests, there are also some standards which are carried out under real field conditions to overcome these drawbacks. Given that, the samples are exposed for the degradation studies in the various marine eulittoral or benthic habitats by fixing them in special constructions in order to minimize the physical influence on the material by the experimental design. Afterwards or in the meantime, the remaining material will be evaluated according to specified periods.
The degree of degradation can be assessed either directly by analyzing the remaining material using surfaces’ modifications, changes in mechanical or physical properties, weight loss or chemical-molecular polymer analysis such as GC-FID or GC-MS, LC-MS, GPC, HPLC, NMR or FT-IR [37,38,39,40,41,42,43,44,45,46,47] or indirectly by analyzing the biogas evolved (carbon dioxide and methane or stable isotopes) [19, 22, 27, 28, 31, 32, 35] or oxygen demand for the degradation and conversion process of plastics [23, 26, 30, 36] in relationship to the theoretical values, required to degrade the material completely. The CO2 or biogas generation and the oxygen demand can only be measured by tests which run on a laboratory scale, while direct methods are preferably applied in field tests.
But so far, there are just three standards which are performed under or close to natural conditions [21, 29, 33] whereas all the other standards focus on laboratory methods to characterize biodegradability in aqueous systems. Of course, field tests are much closer to reality. They can represent various marine habitats as well as various freshwater systems if they are carried out within these environments.
However, it is disadvantageous that these field tests only show the material loss as a measured variable. If necessary, the remaining material can also be examined for possible material changes, but no statement can be made about degradation products or the mechanism of the material loss. It is, therefore, not possible to distinguish between a primary and an ultimate degradation. In contrast, laboratory tests provide information about the rate of degradation and, through measurement of the metabolism of the test material to CO2 in relationship to the theoretical amount of carbon dioxide evolved after completely oxidizing the material, calculated from the molecular formula, also information about primary and final degradability. Further advantages of lab tests are controllable conditions such as temperature, exposure to light or water flow and the additional possibility of analyzing the applied aqueous systems with regard to possible degradation products such as dissolved plastic additives or microplastics.
On the other hand, laboratory tests are closed systems that are not subject to natural fluctuations or a continuous supply of nutrients. The laboratory test systems used can only try to represent a certain environmental condition in the start phase of the test by the applied inocula and therefore deliver results that only reflect the degradation behavior in complex real marine ecosystems to a very limited extent. In addition, they are usually operated at elevated temperatures not occurring in reality to accelerate the degradation behavior and thus reduce the test duration.
3.6 Degradability in Soil
Degradation in soil is a disposal option in particular for products in agricultural applications, e.g. mulch films or flowerpots. Degradation in terrestrial systems eliminates the expense of collecting and cleaning products as well as of disposing of the product itself. At the same time, there is the possibility for controlled release of active ingredients such as fertilizer or herbicides. The effects of degradation products on soil quality play a decisive role in degradation behavior. A sufficiently short degradation time is also important. If the degradation is incomplete or if environmentally related, or rather harmful substances are formed, this leads to deterioration of soil quality.
Focusing on characterizing the terrestrial degradability, just a few test standards are available [48,49,50,51,52,53]. Table 2 gives an overview on these standards once again regarding the scale (laboratory or field tests), the inoculum resp. test conditions, the sample, measuring principle, the applied temperatures, and the duration as well as the final way of assessing the results. Must standards focus on degradation of organic chemicals and do not consider the degradation of biolastics in soil. Positive exceptions are the OENORM EN ISO 15985 [52], that describes a method for anaerobic degradation of plastics by measuring the released gaseous carbon under high-solids conditions which are typical for digestion facilities and DIN EN 17033 [53], addressing biodegradability of mulch films by measuring the released CO2 and resulting ecotoxicity of used soils with reproduction rate of earthworms, microbial nitrification effect, and growth of plants as indicators. Other parameters for investigation of degradability in soil are the loss of parent compounds, oxygen consumption, CH4 production, volatile compounds (C14-labeled), and extractable as well as non-extractable residues. The temperatures vary from 10 to 52°C. Whereas in all the standards the temperatures have a mesophilic level in the range from 10 to 35°C, OENORM EN ISO 15985 recommends a thermophilic temperature of 52°C that is representative for anaerobic digestion. The inoculum respective test environment in all standards consists of soil in a lab scale, partly with knowledge of its physical, chemical, and biological properties. The test duration is between 20 days and 24 months. Just the specific measurement of the resistance of cellulose containing textiles to microorganisms in EN ISO 11721-2 [49] is carried out under real field conditions as soil burial test, whereas all other standards for degradability in soil run on a laboratory scale.
3.7 Anaerobic Digestion (Biogas Generation)
Anaerobic digestion is also known as biogasification. Metabolization to biogas (mainly methane, carbon dioxide, water) is an additional option for degradation of biopolymers that has scarcely been considered so far. The available data are mostly limited to organic waste with high moisture contents, such as mixed green biowaste, kitchen waste, or food waste.
The conversion of organic compounds such as proteins, fats, carbohydrates, or degradable polymers into biogas can generally be subdivided into the following four anaerobic process steps (Fig. 9) [54]:
-
1.
Hydrolysis
-
Solid substances are broken down (hydrolyzed) by bacterial enzymes into water-soluble monomers (e.g., amino acids, glucose, fatty acids).
-
2.
Bacterial acidification
-
The dissolved substances are degraded to organic acids (acetic acid, propionic acid, and butyric acid), low alcohols, aldehydes, hydrogen, carbon dioxide, and other gases, such as ammonia and hydrogen sulfide. This process continues until the bacteria are inhibited by their own degradation products (low pH-values).
-
3.
Acetogenesis
-
In their acetogenic phase, the substances are converted further to acetic acid by acid-forming bacteria.
-
4.
Methanogenesis
-
Methane bacteria form methane by splitting acetic acid or by reduction of CO2 with hydrogen in a strongly anaerobic milieu (pH 6.7–8.0) (Fig. 9).
Steps of biogas formation ([1], modified)
Anaerobic digestion processes can be distinguished as mesophilic and thermophilic processes, 1-phase or 2-phase processes, or as dry and wet processes. In a 2-phase digestion process, hydrolysis and acidification and then subsequently methanogenesis are run in separate tanks. In a 1-phase digestion process, complete digestion is taking place in one unit. Dry digestion processes run at a moisture content <85%, while in wet systems the process is run at a moisture content >85% [54, 55].
In general, all commercial anaerobic digestion systems consist of a first step of anaerobic fermentation in a wet system, followed by an aerobic composting step. This second step is needed to stabilize the anaerobic sludge. For most biogas facilities, the digestion step runs on a mesophilic level. Whether biodegradation occurs during the first anaerobic phase or during the second aerobic phase impacts only biogas production; however, it does not impact the quality of final compost [56]. Here again, whether or not bioplastics are based on renewable resources is not relevant. The key element is the fact that the material is biodegradable and compatible with the anaerobic digestion process. Correct recovery is assured as long as it is eventually biodegraded and no residues are left after the process is completed.
There are little published data regarding conversion of biopolymers to biogas in a biogas plant, such as temperature, pH-value, microorganisms present, anaerobic/aquatic conditions, etc., or regarding the precise optimum parameters, such as materials flow density, dwell time, gas composition, and gas output. Further research will have to assess potential biogas (energy) production due to bioplastics. Also, the discussion and standardization of requirements for anaerobic biodegradation or anaerobic treatability is still in an early, initial phase [57].
Similar to marine degradability the biogasification rate can be calculated by the ratio of theoretical biogas yield and the real biogas yield. Basically, if the stoichiometric composition is known, the theoretical biogas yield can be calculated approximately according to Buswell:
With c, h, o, n and s = molar ratios x = 1/8 (4c + h − 2o − 3n − 2 s) and y = 1/4 (4c − h − 2o + 3n + 2 s)
Examples:
The investigations on anaerobic digestion of bioplastics often show low conversation rates [1, 58,59,60]. One underlying reason for these differences may be due to the influence of fungi. Fungi are abundantly available and very active in aerobic composting, while in anaerobic fermentation no fungi are active. Some polymers are mainly (or even only) degraded by fungi and not by bacteria and will therefore biodegrade by aerobic composting and not, or only much slower, by anaerobic digestion. Another reason for the partly insufficient material degradation is the reduced temperature compared to industrial composting processes. The anaerobic mesophilic degradation of PCL, PVAL, PBS, and PLA with its high glass transition temperature is particularly difficult.
First experiments were carried out using a thermal pretreatment of biopolymers [58,59,60], however, the digestion rate could not be improved. Only PCL with its low melting temperature showed improved digestion rates. For all other biopolymers investigated the digestion rate and the resulting biogas yield decreased. In particular for PLA and other biopolyesters, the thermal pretreatment led to post-processing re-crystallization, which reduced their anaerobic digestibility.
3.8 Decomposition/Degradation in Organisms
These are typically medical applications, either for controlled drug release or to support the healing process following surgery. The best-known applications in this field are absorbable suture materials made from PLA, which can remain inside the body. For the same reason, various temporary implants, such as bone screws for fixating fractures, etc., were developed in recent years using PLA.
Besides these applications, there have also been various attempts to establish biopolymers as edible packaging. However, these attempts have failed mostly, because, among other things, such edible packaging itself requires secondary packaging in order to meet food hygiene and food safety requirements.
4 Key Factors for Biodegradation
Microbiological degradation depends on a couple of parameters. These parameters can be assigned to the three pillars: microbiology/microorganism, the environmental surrounding conditions, and material related factors (Fig. 10). Not only does the variety of these parameters reflect the complexity of this system – the factors are also not necessarily independent of each other.
4.1 Microstructure of Material
While the primary chemical structure determines biodegradability of plastics in principle, the polymer degradation rate is determined by other properties, such as the melting point and degree of crystallization, which in turn depend on the primary structure. Table 3 gives an overview of significant material related parameters that influence the degradation behavior.
In general, high-molecular engineering polymers whose backbones contain exclusively carbon atoms, such as polyethylene, polypropylene or polystyrene, are inert toward biological degradation [61]. Polyvinyl alcohol is an exception despite its exclusively carbonic backbone [62]. Here, degradation takes place via primary oxidation of the numerous OH-groups with subsequent backbone cleavage – similar to fatty acid degradation [63, 64]. Primarily an enzymatic cleavage of the carbon backbone is caused by either a dehydrogenase or oxidase and subsequently by hydrolase or aldolase reaction [65].
Natural rubber (cis-1,4-polyisoprene) is also biodegradable, although only carbon atoms are present in its polymer backbone [66]. Here, the primary degradation step, backbone cleavage, is initiated by a specific dioxygenase or peroxidase [67].
Other degradable, natural, and synthetic plastic materials usually contain heteroatoms in their backbones, such as oxygen or nitrogen, that represent points of attack for enzymatic catalyzed cleavage products (Fig. 11).
Examples of heteroatoms enabling biodegradability ([1], modified)
Given that, degradability increases in general as the ratio of heteroatoms to carbon increases, in particular in the main chain, i.e. the biodegradability increases in the following order: PVOH < PCL < PLA < Starch/Cellulose [1].
Chemically unchanged natural polymers, such as cellulose or starch, offer therefore a good biodegradability. However, chemical modifications, e.g. esterification to cellulose triacetate, create plastics that can no longer be cleaved by enzymes.
Polymers with aromatic components or branched structures tend to be more resistant to microbial attack than linear, aliphatic components [56, 61]. For enzymatic hydrolysis, the polymer chain must be flexible enough to fit into the active center of the degrading enzyme. This is the explanation for the easy biological degradation of flexible aliphatic polyester, whereas rigid aromatic polyesters resist biological degradation [68]. An analogous effect can be observed with polyamides. Here, the crystals limit chain flexibility by intermolecular interaction [14].
Crystallinity is an additional factor discussed with regard to the degradation rate in polyesters [68]. In addition to degradation rate dependence on crystallinity, it turns out that the crystalline zones within a plastic are enzymatically hydrolyzed more slowly than amorphous zones. References [69,70,71] show that crystallinity in PCL films increases during degradation, i.e. the amorphous phases are reduced.
Crosslinking in plastics reduces water concentration via reduced swelling and with it the accessibility of the plastic for the enzyme, also resulting in a reduced degradation rate [67].
A direct connection between the degradation rate and melting temperature was introduced by [72, 73]. They observed that the degradation rate in aliphatic–aromatic copolyester and aliphatic homopolyester decreases with increasing melting temperature [67]. Comprehensive and systematic investigations of the role of chain mobility for polyester degradation show that, for polyesters of similar crystallinity, degradation is controlled exclusively by polymer chain mobility, where mobility is mainly determined by the difference between the ambient temperature and melting temperature [74].
Overall, biodegradability and compostability of biopolymers and/or products made from them increase with certain factors due to the resulting simplified access for microorganisms to the molecules, thus enhancing metabolizability.
Table 4 summarizes the impact of different microstructural material parameters on its degradability.
Another impact factor influencing biodegradability is the finishing process, i.e. the type of modification from biopolymers as virgin polymers to bioplastics as tailor-made “ready to use” materials (Fig. 12). During the finishing process the polymers are modified by some additives such as stabilizers, plasticizer, coloring agents, or reinforcements. Alongside biodegradability of themselves, these additives influence the microstructure and by this biodegradability of the resulting bioplastic.
Finishing process from virgin biopolymers to tailor-made “ready to use” bioplastics ([2], modified)
If additives change the crystallinity or are not themselves degradable, the degradability decreases and if vice versa biodegradable fillers such as starch are used, the rate of degradation can be increased significantly. First, the easily biodegradable fillers are broken down. This enlarges the surface area accessible for microorganisms and accelerates the degradation of the remaining matrix.
The most important biodegradable plastics include starch-based polymer materials and various polyesters such as polyhydroxyalkanoates, e.g. PHB (poly-hydroxybutyrate), PLA (polylactic acid), PBAT (polybutylene adipate terephthalate), and PBS (Polybutylene succinate).
4.2 Physico-Chemical Environmental Conditions
As well as differentiating between macroscopic decomposition of the material (primary degradation) and microscopic ultimate degradation, information about the respective environmental conditions and the time span is also essential for a complete description of the degradation process. Biological decomposition can vary enormously under a variety of environmental conditions (soil, water, salt water, compost, human body, etc.) as, apart from the material itself, the degradation process depends on a variety of other (environmental) factors such as microorganisms present, humidity, temperature, available oxygen, pH-value, time, etc. [75]. The following Table 5 summarizes the main factors for biodegradability related to surrounding physico-chemical environmental conditions.
Microorganism development (biodiversity, concentration, activity, adaptation) is also determined by specific environmental influencing factors. They include the presence and absence of oxygen, water content, temperature, pH-value, available nutrients as well as available alternative carbon sources [67]. At the same time, polymer decomposition into small particles or polymer metabolism into water-soluble products, for instance, influences the structure, pH-value, and nutrient content of the environment – not to mention the potential danger that plastics represent to the ecology caused by the accumulation of long-lived, often potentially toxic metabolites. Microorganisms in turn can affect changes in ambient conditions either directly, by excreting metabolism products (e.g., acids) or indirectly, by secreting enzymes that catalyze the formation of reactive reagents in the environment [76].
As an example, biological decomposition of the material in an industrial composting plant with continuous irrigation and turning of the heap takes place more quickly than under a domestic composting process (Fig. 13). Due to the higher temperatures and better oxygen availability, the industrial composting process is much faster than domestic composting and digestion in landfills will take a long time vice versa.
Conditions in industrial and domestic composting and biogas plants ([1], modified)
Industrial composting can be defined as “the controlled biological decomposition of organic waste under managed conditions that are predominantly aerobic and that allow the development of thermophilic conditions as a result of biologically produced exothermic heat” [77]. In the course of industrial composting operations, biomass is mixed more frequently, and moisture and oxygen content and temperatures rise up to 50–70°C. When the temperature of the composting pile increases, the microbes adapted to the ambient temperature (mesophiles) stop activity and are replaced by microbes adapted to high temperatures (thermophiles). The rate of activity of microbes is higher at thermophilic temperatures. Due to this shift in microbial populations and the additional treatment of the composting pile, a faster and better degradation of the biopolymers can be ensured. Under these conditions composting is a controlled biotechnological process and therefore the term “industrial” (or municipal) composting is used to distinguish it from “home composting” [78].
Experience has shown that biopolymers certified as compostable under industrial composting conditions are degraded and metabolized well. However, not all biopolymers certified as compostable under industrial composting conditions also degrade under home composting conditions [1, 8, 45].
Municipal composting and home composting share the same designation; however, the conditions for these technologies are quite different [56, 78]. Certified industrial compostability states that products consisting of a certain material and with specified wall thicknesses degrade biologically during a certain time under industrial composting conditions (sufficient oxygen and moisture, regular turning of the pile, temperature development, presence of corresponding microorganisms, pH, carbon/nitrogen ratio, material structure, and size of particles). Industrial compostability of biopolymer materials certified according to legal standards must never be equated with total degradability in domestic compost, also defined as cold composting, i.e. degradation through aerobic biodegradation at ambient temperature (between 21 and 28°C). Certification according to the standards for municipal composting (e.g., EN 13432 or EN 14995) does not imply good in-home composting properties or shortened rotting cycles.
Neither ISO nor ASTM defines home composting rules. The Belgian certification organization AIB Vinçotte issues a specific “home compostability” certification program and an “OK Compost Home” label. Materials degrading to a sufficient level in private composting systems, i.e. home compost, can be labeled additionally or exclusively with this symbol. The certification program for home composting is based only on DIN EN 13432 (see Sect. 3.4). In home composting, 90% biological degradability at ambient temperatures of 20–30°C (in contrast, composting temperatures in industrial plants run approx. 50–70°C) and/or in aquatic surroundings is required (test method according to DIN EN ISO 14851 [26]; cf. Sect. 3.5).
Particularly in Asia, where there is a lack of room and logistics for disposing and industrial composting of biowaste, efforts are being made to support composting in domestic surroundings with heated waste containers to enable domestic composting [79].
However, composting makes sense only when degradability simultaneously offers an additional functional advantage. For example, votive candle holders on graves that can be cleared away with flowers/wreaths etc., films for agriculture that do not have to be collected and disposed of following use, but can be plowed under, laundry bags that dissolve in the washing machine, grocery bags that can be used to collect organic material for composting, or resorbable implants that are metabolized according to the regenerative loop in the human body, etc. In all these applications, degradability and/or compostability results in an additional benefit. By contrast, enforced “composting by decree” requiring separation, collection, and transport to an industrial composting plant represents only additional expense and with it the amount of CO2 generated by composting equals the amount of CO2 released by incineration, but composting does not provide an additional energetic benefit.
There are a lot more examples showing the significant influence of the environmental conditions on biodegradability, such as under “normal” ground or anaerobic conditions in a biogas plant, or even on the ocean floor at temperatures of approx. 4°C with no light and a completely different microflora. The impact of the environmental conditions is also very evident for a product such as wood. Under dry conditions, wooden furniture in a house, for example, has an almost unlimited lifespan, whilst in the forest, biological degradation progresses relatively rapidly. Another example is fossilization. Here, too, environmental conditions have prevented a complete degradation of the organic mass.
4.3 Microbiological Conditions
Microbiological degradation is influenced by various factors that can be subdivided into three categories, i.e. the environmental surrounding conditions, material related factors, and microbiological parameters (see Fig. 10). After considering the first two topics in the previous chapters, the following Table 6 gives an overview of the most important microbiological parameters that influence the degradation behavior of plastics.
Biodegradation first requires suitable microorganisms. Certain organisms can usually only degrade a specific group of plastics. For instance, several degrading microorganisms for polyhydroxybutyric acid [67, 80], synthetic aliphatic–aromatic copolyester [14, 81], and synthetic aliphatic homopolyester [82] have been isolated and identified.
Plastics molecules are too large to pass the cell walls of microorganisms. So that bacteria and fungi can use such substances as nutrients, they must produce enzymes that – after being transported through the cell wall – can act outside the cell. The enzymes break down the insoluble macromolecules layer by layer from the surface into short-chain fragments. Here, the organism producing the polymer cleaving enzyme is not necessarily the immediate consumer of these cleavage products. Other organisms in the population can enter the degradation process, absorb low-molecular compounds in the cell and convert them into carbon dioxide, water, and biomass. Under certain circumstances, they can in turn supply other microorganisms with nutrients by excreting metabolites (e.g., acids) that they cannot use. Many degradation sequences take place according to this type of cooperation between different microorganisms. However, the result can also be accumulation of cleavage products which are not further degradable and due to their potentially toxic effects (inhibition, elimination), can pose a potential danger to the microorganisms [8, 14].
In addition, various microorganisms can be responsible for degradation in all types of degradation media. For example, fungi prefer solid surfaces for growth, which is why they are rarely present for degradation in aqueous systems. In contrast, a variety of fungi are involved in degradation in compost [67].
Fungi and other microorganisms, such as bacteria or actinobacteria (actinomycetes), play an important role in the destruction of organic materials [83]. Microorganisms only proliferate in the presence of moisture (relative moisture 63–99%). They will find optimum growth conditions in the temperature range from 10 to 40°C.
For optimum growth, fungi need oxygen and a pH-value of 4.5–5.0. They proliferate over a wide temperature range up to 45°C, with an optimum between 30 and 37°C. Actinobacteria proliferate under aerobic conditions at a pH-value of 5–7 and optimal temperatures between 30 and 37°C. Bacteria can proliferate under both aerobic and anaerobic conditions. Here, too, the optimum is a pH-value of 5–7 and temperatures between 30 and 37°C [84].
In addition to the mesophilic microorganisms, whose optimum growth temperature is between 30 and 37°C, thermophilic microorganisms can proliferate over a much wider temperature range (up to 70°C). These thermophilic microorganisms are mainly applied for the controlled biological degradation of plastics in industrial composting processes.
5 Conclusions
When it comes to bioplastics, it is important to use a clear wording and by this to differentiate between biopolymers as a macromolecules and bioplastics as materials ready-to-use as well as the distinction between bio-based and biodegradable plastics. Biodegradability is a system feature of the material microstructure, physico-chemical and microbiological surrounding conditions. Because in nature are a variety of environmental conditions, from industrial composting facilities to sewage treatment plants, soils in a variety of climatic regions, rivers, the beach, sea surface and the seabed, or even the human body, it is important to provide also clear information about the environmental conditions under which the degradation takes place. On the other side standards are needed to reflect biodegradability under the various conditions. In case of compostability, some test standards cover the environmental conditions well, while test standards in other areas, such as degradability in soil and particularly in marine systems, are only available in small numbers and do not reflect the complex environmental conditions well. For future material development, in addition to the establishment of appropriate standards, an extensive research is required to work out a better understanding of the relationships between the environmental conditions of various habitats and microbiology as well as material parameters on the one hand and the resulting degradation mechanisms and kinetics on the other hand.
References
Endres H-J, Siebert-Raths A (2011) Engineering biopolymers, München. 978-3-446-42403-6
Endres H-J (2017) Bioplastics. Advances in biochemical engineering/biotechnology. Springer, Cham
Haldenwanger HHM, Göttner GH (1970) Biologische Zerstörung der makromolekularen Werkstoffe (Chemie, Physik und Technologie der Kunststoffe in Einzeldarstellungen, Band 15). Springer, Berlin
[Online] Empfehlung zur Behandlung von kompostierbaren Verpackungen. http://www.nova-institut.de/news-images/20050425-04/IBAW_VVO_Handlungsempfehlung.pdf [date: 2–24, 2011]
N.N. (1989) Mikrobiologische Materialzerstörung und Materialschutz; DECHEMA Arbeitsausschuss. Frankfurt
Owen S, Kawamura R, Sakota N (1995) Biodegradation of poly-D,L-lactic acid polyurethanes. J Macromol Sci Pure Appl Chem 32(4):843–850
Imam SH, Greene RV, Zaidi BR. Biopolymers. Utilizing nature’s advanced materials. ACS symposium series, 723
Ehrenstein GW, Pongratz S (2013) Resistance and stability of polymers. Carl Hanser Verlag, München, pp 833–884
N.N., ISO/DIS:2020 (E), Plastics – Test methods for determination of degradation rate and disintegration degree of plastic materials exposed to marine environmental matrices under laboratory conditions
Hocking P et al (2003) Enzymatic degradability of poly(beta-hydroxybutyrate) as a function of tacticity. Macromol Raprid Commun 15(6):447–452
Kawai F (1995) Proposed mechanism for microbial degradation of polyacrylate. J Macromol Sci Pure Appl Chem 32, 4, 835–838
N.N. (1987) Degradable plastics. Proceedings of the SPI symposium on degradable plastics. Society of the Plastic Industry, Inc., [Hrsg.], Washington
Tänzer W (2000) Biologisch abbaubare Polymere, Deutscher Verlag für die Grundstoffindustrie, Stuttgart
Kleeberg I (1999) Untersuchungen zum mikrobiellen Abbau von aliphatisch-aromatischen Copolyestern sowie Isolierung und Charakterisierung eines polyesterspaltenden Enzyms, Dissertation, Technische Universität Braunschweig
N.N. (1996) DIN V 54900 (1–5); Prüfung der Kompostierbarkeit von polymeren Werkstoffen, Beuth Verlag, Berlin
N.N. (2000) EN 13432, Anforderungen an die Verwertung von Verpackungen durch Kompostierung und biologischen Abbau, Beuth Verlag, Berlin
N.N. (2006) DINCERTCO. Zertifizierungsprogramm – Produkte aus kompostierbaren Werkstoffen. Berlin: s.n.
Harrison JP, Boardman C, O’Callaghan K, Delort A-M, Song J (2018) Biodegradability standards for carrier bags and plastic films in aquatic environments: a critical review. R Soc Open Sci 5:171792. https://doi.org/10.1098/rsos.171792
N.N. ASTM D 6691 – 2017, Standard test method for determining aerobic biodegradation of plastic materials in the marine environment by a defined microbial consortium or natural sea water inoculum
N.N., ASTM D 6691:2017, Determining aerobic biodegradation of plastic materials in the marine environment by a defined microbial consortium or natural sea water inoculum
N.N. ASTM D 7473 2012, Standard Test Method for Weight Attrition of Plastic Materials in the Marine Environment by Open System Aquarium Incubations
N.N. ASTM D 7991 – 2015, Standard test method for determining aerobic biodegradation of plastics buried in sandy marine sediment under controlled laboratory conditions
N.N. DIN EN ISO 9408, Bestimmung der vollständigen aeroben biologischen Abbaubarkeit organischer Stoffe im wäßrigen Medium über die Bestimmung des Sauerstoffbedarfs in einem geschlossenen Respirometer
N.N., EN ISO 9439:2000, Evaluation of ultimate aerobic biodegradability of organic Compounds in aqueous medium – Carbon dioxide evolution test
EN ISO 11733:2004, Determination of the elimination and biodegradability of organic compounds in an aqueous medium – Activated sludge simulation test
N.N. DIN EN ISO 14851, Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium – method by measuring the oxygen demand in a closed respirometer
N.N. DIN EN ISO 14852, Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium – method by analysis of evolved carbon dioxide
N.N. DIN EN ISO 14853, Plastics – determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system – method by measurement of biogas production
N.N. DIN EN ISO 15314, Plastics – methods for marine exposure
N.N. DIN EN ISO 18830, Plastics – determination of aerobic biodegradation of non-floating plastic materials in a seawater/sandy sediment interface – method by measuring the oxygen demand in closed respirometer
N.N. DIN EN ISO 19679, Plastics – determination of aerobic biodegradation of non-floating plastic materials in a seawater/sediment interface – method by analysis of evolved carbon dioxide
N.N. ISO 22404, Plastics – determination of the aerobic biodegradation of non-floating materials exposed to marine sediment – method by analysis of evolved carbon dioxide
N.N. ISO 22766, Plastics – determination of the degree of disintegration of plastic materials in marine habitats under real field conditions
N.N., ISO-DIS 23832 – 2020, Test methods for determination of degradation rate and disintegration degree of plastic materials exposed to marine environmental matrices under laboratory conditions
N.N. ISO/CDIS 23977-1, Plastics – determination of the aerobic biodegradation of plastic materials exposed to seawater – part 1: method by analysis of evolved carbon dioxide
N.N. ISO/CDIS 23977-2, Plastics – determination of the aerobic biodegradation of plastic materials exposed to seawater – part 2: method by measuring the oxygen demand in closed respirometer
Nakajima-Kambe T, Shigeno-Akutsu Y, Nomura N, Onuma F, Nakahara T (1999) Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl Microbiol Biotechnol 51:134–140
Molitoris HP, Moss ST, de Koning GJM, Jendrossek D (1996) Scanning electron microscopy of polyhydroxyalkanoate degradation by bacteria. Appl Microbiol Biotechnol 46:570–579
Dřímal P, Hrnčiřík J, Hoffmann J (2006) Assessing aerobic biodegradability of plastics in aqueous environment by GC-analyzing composition of equilibrium gaseous phase. J Polym Environ 14:309
Dřímal P, Hoffmann J, Družbík M (2007) Evaluating the aerobic biodegradability of plastics in soil environments through GC and IR analysis of gaseous phase. Polym Test 26:729–741
Eyheraguibel B et al (2017) Characterization of oxidized oligomers from polyethylene films by mass spectrometry and NMR spectroscopy before and after biodegradation by a Rhodococcus rhodochrous strain. Chemosphere 184:366–374
Trimpin S, Eichhorn P, Räder HJ, Müllen K, Knepper TP (2001) Recalcitrance of poly(vinylpyrrolidone): evidence through matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. J Chromatogr A 938:67–77
Zgoła-Grześkowiak A, Grześkowiak T, Zembrzuska J, Łukaszewski Z (2006) Comparison of biodegradation of poly(ethylene glycol)s and poly(propylene glycol)s. Chemosphere 64:803–809
Fontanella S et al (2010) Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives. Polym Degrad Stab 95:1011–1021
Sikorska W, Janeczek H (2014) Techniques useful in biodegradation tracking and biodegradable polymers characterization. See http://www.plastice.org/fileadmin/files/EN_Analyses.pdf. Accessed 19 Oct 2017
Koutny M, Lemaire J, Delort A-M (2006) Biodegradation of polyethylene films with prooxidant additives. Chemosphere 64:1243–1252
N.N. International Organization for Standardization (ISO), ISO 10640:2011 Plastics – methodology for assessing polymer photoageing by FTIR and UV/visible spectroscopy. ISO, Geneva, Switzerland, pp 1–29
N.N. BS EN ISO 11266, Guidance on laboratory testing for biodegradation of organic chemicals in soil under aerobic conditions
N.N. EN ISO 11721-2, Determination of the resistance of cellulose containing textiles to microorganisms – soil burial test
N.N. DIN ISO 14239, Soil quality – Laboratory incubation systems for measuring the mineralization of organic chemicals in soil under aerobic conditions
N.N. DIN EN ISO 15473 – Entwurf, Guidance on laboratory testing for biodegradation of organic chemicals in soil under anaerobic conditions
N.N. OENORM EN ISO 15985, Determination of the ultimate anaerobic biodegradation under high-solids anaerobic-digestion conditions – method by analysis of released biogas
N.N. DIN EN 17033, Biodegradable mulch films for use in agriculture and horticulture – requirements and test methods
Endres H-J, Helmke G (2004) Situation der Biogaserzeugung und Potenzial der Biogasproduktion in Niedersachsen. In: Grabkowsky B (ed) Weiße Reihe, vol 23, Vechtaer Druckerei und Verlag
Braun R (1982) Biogas – Methangärung organischer Abfallstoffe. Springer, New York
Huang SJ (1989) Biodegradation. Compr Polymer Sci 6:597–606
[Online] http://www.european-bioplastics.org/media/files/docs/en-pub/FactSheets/FactSheet_Anaerobic_Digestion.pdf. [date: 5–24, 2011]
Kitzler A-S, Endres H-J, Nelles M (2012) Recyclingfähigkeit von Biokunststoffen. In: Tagungsband 15. Dialog Abfallwirtschaft MV Rostock, pp 121–132
Kitzler A-S, Endres H-J, Nelles M (2011) Vergärung von Biopolymer-Verpackungen – Eine sinnvolle Verwertungsoption? In: Tagungsband – 8. Biogastagung Dresden, Band 81, pp 117–122
Endres H-J, Siebert A, Kitzler A-S, Biopolymers – a discussion on end of life options. In: Bioplastics Magazine 01/08, pp 22–25
Shimao M (2001) Biodegradation of plastics. Curr Opin Biotechnol 12(3):242–247
Sakazawa C, Shimao M et al (1981) Symbiotic utilization of polyvinyl alcohol by mixed cultures. Appl Environ Microbiol 41(1):261–267
Sakai K, Hamada N, Watanabe Y (1986) Degradation mechanism of poly(vinylalcohol) by successive reactions of secondary alcohol oxidase and betadiketone hydrolase from Pseudomonas sp. Agric Biol Chem 50(4):989–996
Matsumura S, Tomizawa N et al (1999) Novel poly(vinyl alcohol)-degrading enzyme and the degradation mechanism. Macromolecules 32:7753–7761
Arenskötter M, Baumeister D et al (2002) Mikrobieller Abbau von Natur- und Synthesekautschuk. BIOforum 3:124–126
Kawai F (1995) Breakdown of plastics and polymers by microorganisms. Adv Biochem Eng 52:151–194
Welzel K (2003) Einfluss der chemischen Struktur auf die enzymatische Hydrolyse von Polyester-Nanopartikeln, Dissertation, Technische Universität Braunschweig
Huang SJ (1985) Biodegradable polymers. Enzyclopedie Polymer Sci Eng 2:220–241
Mochizuki M, Hirami M et al (1995) Hydrolysis of polycaprolactone fibers by lipase: effect of draw ratio on enzymatic degradation. J Appl Polym Sci 55:289–296
Gan Z et al (1997) Enzymatic degradation of poly(e-caprolactone) film in phosphate puffer solution containing lipases. Polym Degrad Stab 56:209–213
Eldstäter C, Erlandsson B et al (2000) The biodegradation of amorphous and crystalline regions in film-blown poly(e-caprolactone). Polymer 41:1297–1304
Witt U, Müllera R-J, Deckwer W-D (1995) New biodegradable polyester-copolymers from commodity chemicals with favorable use properties. J Environ Polymer Degrad 3(4):215–223
Tokiwa Y, Suzuki T (1981) Hydrolysis of copolyesters containing aromatic and aliphatic ester blocks by lipase. J Appl Polym Sci 26(2):441–448
Marten E (2000) Korrelation zwischen der Struktur und der enzymatischen Hydrolyse von Polyestern, Dissertation, Technische Universität Braunschweig
Palmisano AC, Pettigrew CA (1992) Biodegradability of plastics. Bioscience 42(9):680–685
Lenz RW (1993) Biodegradable polymers. Adv Polym Sci 107:1–40
[Online] Specification for Composted Materials. http://www.wrap.org.uk/downloads/PAS_100.52b60645.6584.pdf [date: 3–31, 2005
[Online] http://www.european-bioplastics.org/media/files/docs/en-pub/FactSheets/FactSheet_Industrial_Composting.pdf. [date: 11–29, 2009]
Lefebvre F, Daro A, David C (1995) Biodegradation of polycaprolactone by microorganisms from an industrial compost of household refuse. Part II. J Macromol Sci Pure Applchem 32(4):867–873
Jendrossek D (1998) Microbial degradation of polyesters: a review on extracellular poly(hydroxyalkanoic acid)depolymerases. Polym Degrad Stab 59:317–325
Kleeberg I, Hetz C et al (1995) Biodegradation of aliphatic-aromatic copolyesters by Thermomonospora fusca and other thermophilic compost isolates. Appl Environ Polymer Degrad A 64(5):1731–1735
Tokiwa Y, Suzuki T (1977) Purification and some properties of polyethylene adipatedegradation enzyme produced by Penicillium sp. Strain 14 – 3. Agric Biol Chem 41(2):265–274
Schnabel W (1981) Polymer degradation – principles and practical applications. Carl Hanser Verlag, München
Gugumus F, Gächter R, Müller H (Hrsg) (1989) Kapitel antioxidantien. In: Kunststoff-additive, 3. Auflage, Carl Hanser Verlag, München
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hans-Josef, E. (2021). Biodegradable Plastics: End of Life Scenarios. In: Stock, F., Reifferscheid, G., Brennholt, N., Kostianaia, E. (eds) Plastics in the Aquatic Environment - Part I. The Handbook of Environmental Chemistry, vol 111. Springer, Cham. https://doi.org/10.1007/698_2021_745
Download citation
DOI: https://doi.org/10.1007/698_2021_745
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84117-1
Online ISBN: 978-3-030-84118-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)