Abstract
During the 1920s, pyrethrin was studied because of its potential as a precursor for synthetic organic pesticides. The first pyrethroid pesticide, allethrin, was identified in 1949. It is a type I pyrethroid because of a carboxylic ester of cyclopropane. Type II was created with the addition of a cyano group in α position. Some phenylacetic 3-phenoxybenzyl esters missing the cyclopropane but with the cyano group are also considered type II. In the 1970s, pyrethroids transitioned from mere household products to pest control agents in agriculture. Later, pyrethroids have replaced organophosphate pesticides in most of their applications the same way the latter had replaced organochlorinated pesticides before. Works on the optimisation of pyrethroids has granted them better photostability without compromising their biodegradability, as well as selective toxicity, metabolic routes of degradation and more effectivity, translating into the use of smaller amounts. Most pyrethroids present different isomers, each with different biological activity and, therefore, different toxicity. Pyrethroids account for a quarter of the pesticides used nowadays. Pyrethroids’ relative molecular mass is clearly above 300 g mol−1; they are highly hydrophobic, photosensitive and get easily hydrolysed, with degradation times below 60 days. They are not persistent and mammals can metabolise them. However, pyrethroids have been proven to bioaccumulate in marine mammals and humans. Studies in mammals reported carcinogenic, neurotoxic and immunosuppressive properties and potential for reproductive toxicity mainly. Acceptable daily intake values and no observed adverse effect level values have been established at 0.02–0.07 mg (kg body weight)−1 day−1 and 1–7 mg (kg body weight)−1 day−1.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chemical structures
- Metabolisation
- Pest control
- Pesticides
- Physicochemical properties
- Pyrethroids
- Toxicity
1 History and Impact
During the 1920s, pyrethrin was studied because of its potential as a precursor for synthetic organic pesticides. Pyrethrin was extracted from pyrethrum, a plant of the family of chrysanthemums [1]. Research on synthetic organic pesticides increased in the 1930s, and in 1939, dichlorodiphenyltrichloroethane (DDT) was synthesised. It proved to be effective for many plagues. DDT was so effective that other organochlorinated compounds were studied with the aim of obtaining cheap and persistent pesticides.
At first, pesticides were not considered to affect health or the environment. However, in 1962 Rachel Carson published Silent Spring, where she warned about the effects of pesticides on the environment with the image of dead birds in her garden.
This field observation prompted several research studies about environmental and mesocosm models focused on the assessment of pyrethroids and other pesticides [1]. As a consequence, some regulation agencies came into existence. In 1970, the Environmental Protection Agency (EPA) was founded. From that moment on, the use of organochlorinated compounds was restricted or banned as they were considered toxic and contaminant [2, 3]. Nevertheless, they are still allowed to fight malaria [4,5,6].
In the 1940s, it was discovered that many organophosphate compounds had unique properties for the protection of plants – and that the most volatile and toxic could be used as chemical weapons. However, not until the 1960s did organophosphate compounds become popular. At the end of the same decade, there was an increasing interest in carbamate pesticides.
Organophosphates and carbamates had simple structures, and it was easy to synthesise analogous derivatives. They also showed some advantages over organochlorinated pesticides [1]. They were selectively toxic with different effects depending on the species; they affected insects more than mammals [7]; the effects on mammals occurred mostly after intense exposition rather than accumulation; they were more biodegradable, therefore, less persistent, and they allowed the creation of compounds that stay inside the plants for a few weeks and protect them. On the other hand, regulations and bans on the use of organophosphates and carbamates emerged as a consequence of new data on their actual toxicity [8]. Toxicology studies are a key element of the development of new pesticides nowadays.
In the 1970s, pyrethroids stopped being mere household products to become pest control agents in agriculture. Moreover, in the last couple of decades, pyrethroids have replaced organophosphate pesticides in most of their applications the same way the latter had replaced organochlorinated pesticides before [9, 10]. Pyrethroids were very effective.
Works on the optimisation of these derivatives from pyrethrin had been going on for decades, and several improvements were achieved [1]. Their photostability was improved without compromising their biodegradability. They achieved a selective toxicity and metabolic routes of degradation – that were different for cis and trans isomers. They were produced as fumigants as well as soil pesticides. And they were made more powerful so that smaller amounts would need to be used and environmental contamination would be reduced.
The development of pyrethroids included some aspects that helped reduce the impact of pesticides on the environment: higher effectiveness implying smaller amounts of product needed, selective toxicity, concern on the occurrence of pesticides in the environment and replacement of persistent compounds with degradable compounds [1].
2 The Compounds
The first pyrethroid pesticide, allethrin, was identified in 1949 [11]. It is a type I pyrethroid because of the carboxylic ester of cyclopropane. Type II was created with the addition of a cyano group in α position, which increased the pesticide effect of pyrethroids (Figs. 1, 2 and 3).
Additionally, pesticide activity was detected in some phenylacetic 3-phenoxybenzyl esters that missed the cyclopropane but had the cyano group [11]. These esters were still considered type II pyrethroids and originated compounds such as fenvalerate.
Due to the cyclopropane and the cyano group, most pyrethroids present different isomers, each with different biological activity and, therefore, different toxicity. Type I pyrethroids have two chirality centres, hence two diastereoisomers or enantiomeric pairs. Type II pyrethroids present three chirality centres, hence four diastereoisomers. The bonds that are responsible for the existence of enantiomeric pairs are represented with winding lines in Figs. 2 and 3. These diastereoisomers present different properties [12]. More detailed information of pyrethroid stereoselectivity is presented in Chapter “Stereoselectivity and Environmental Behaviour of Pyrethroids”.
Pyrethroids account for a quarter of the pesticides used nowadays [1, 13]. They were believed to be the ideal pesticides because they are not persistent and were thought to be metabolised and not bioaccumulate [14, 15]. Thus they replaced the previously banned pesticides. Total organic pesticide production in the United States increased from about 15 tons per year in 1945 to over 630 tons per year in 1976 [16]. In 2006 over 433 tons of pesticides were used worldwide, 400 tons in 2007 [17]. Pyrethroids account for about 25% of the pesticide use.
Pyrethroids have applications as pesticides in households, in commercial products and in medicine against scabies and lice (Table 1). In tropical countries, mosquito nets are impregnated with solutions of deltamethrin, cyhalothrin or cypermethrin to control malaria [11].
3 Properties
Pyrethroids present somewhat similar physicochemical properties among them (Table 2). Their relative molecular mass (Mr) is clearly above 300 g mol−1. They are highly hydrophobic, with logarithm of the octanol-water partition coefficient (Kow) between 4 and 7, and show low very low solubility in water of a few μg L−1. Pyrethroids are photosensitive and get easily hydrolysed; therefore their degradation time for 50% of the substance (DT50) – indicating persistence – is very low, below 60 days [21].
Organic contaminants include a wide variety of families. Some of them have been considered persistent organic pollutants (POPs). The Stockholm Convention on Persistent Organic Pollutants defined four factors that make a compound dangerous and that qualify it as a POP [22]. These are the requirements a compound needs to meet to be included in the list of the Stockholm Convention:
-
1.
To be persistent in the environment. POPs have half-lives greater than 2 months in water or greater than 6 months in soil and sediment.
-
2.
To bioaccumulate. POPs have bioconcentration factors (BCFs) or bioaccumulation factors (BAFs) in aquatic species greater than 5,000 or, when unknown, their log Kow is greater than 5.
-
3.
To have potential for long-range transport. POPs are detected far from the emission source; data show they have been transported via air (half-live in air over 2 days), water or migratory species.
-
4.
To have adverse effects. POPs are proved to have adverse effects on human health or on the environment.
The original list of the Stockholm Convention included 12 POPs that were banned or restricted. Eight of them were organochlorinated pesticides: aldrin, endrin, dieldrin, chlordane, DDT, heptachlor, mirex and toxaphene. These pesticides were considered safe when they first entered the market, but data proved them to cause long-term adverse effects on human health and on the environment. New compounds have been added to the list throughout the years.
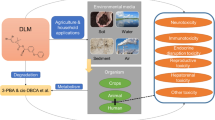
Some other compounds, like pyrethroids, cannot be classified as POPs, but cause concern in the scientific community due to their properties, sometimes close to those of POPs. Pyrethroids have logarithms of Kow on the limit of POPs and affect organisms by design. However, they are not persistent and thus cannot be transported long distances and mammals can metabolise them [14, 23]. Conversely they have been proved to bioaccumulate in marine mammals and humans [24, 25]. More detailed information of bioaccumulation of pyrethroids in wildlife and humans is presented in Chapter “Bioavailability and Bioaccumulation of Pyrethroid Insecticides in Wildlife and Humans”.
The Water Framework Directive (Directive 2000/60/EC) named a group of pesticides that could be toxic, persistent and bioaccumulate. Among them, cypermethrin was listed. Due to their production volume and extensive application, pesticides such as pyrethroids are always present in the environment despite not being persistent and are therefore considered pseudo-persistent organic contaminants [26].
4 Metabolisation
The capacity of mammals of metabolising pyrethroids has been regarded as one of the best qualities of these pesticides. The metabolisation route differs with the organism. However, the routes are equivalent for many mammals, and the mechanism in humans will serve as an example.
The liver is the main organ responsible for disintoxication in humans, although other organs and tissues possess the required enzymes to treat xenobiotics. This disintoxication usually proceeds in two steps [27]. The first step consists in increasing the polarity of the xenobiotic molecular through processes like hydroxylation, deamination or the N-oxidation. In the second step, the metabolite – which is more polar than the original molecule – is combined with endogen products of the cell, such as methyl or acetyl groups, monosaccharides or amino acids. This increases the metabolite solubility making it easier for it to be excreted in urine. This is the reason why exposition of humans to pyrethroids is studied through the analysis of their metabolites in urine [28].
The first step of the metabolisation of pyrethroids in humans can occur through two pathways. One is the breakdown of the ester to produce carboxylic acid and the corresponding alcohol by the action of carboxylesterases [29]. Then, alcohol can be oxidised to a benzoic acid (Fig. 4).
The carboxylesterases required for this metabolisation are found in the plasma of mammals at higher concentrations than in fish or birds [30]. This could be a factor in explaining the lower toxicity of pyrethroids in mammals.
On the other hand, carboxylesterases present isoenzymes that can be found in different proportions in each individual depending on factors such as species, age or gender [30]. Each isoenzyme can have a different activity on different isomers of pyrethroids, thus making the capacity of metabolising these compounds change not only among species, but also among individuals of different age and gender [31].
The second pathway for the first step of the metabolisation of pyrethroids in humans is hydroxylation by monooxygenases. The process usually undergoes transformation via both pathways producing secondary products such as 4-hydroxy-3-phenoxybenzoyl and 4-hydroxy-3-phenoxylbenzaldehyde for permethrin. These compounds can be stronger endocrine disruptors than their non-hydroxylated analogues [32].
However, it is important to note that despite the fact that mammals can metabolise pyrethroids, studies have shown that they can also bioaccumulate these pesticides [25, 33].
5 Toxicity
Exposition of organisms to pyrethroids causes concern due to the toxicity of the pesticides [34]. Recent studies in mammals reported carcinogenic, neurotoxic and immunosuppressive properties and potential for reproductive toxicity [12, 35, 36]. Type I pyrethroids cause tremors and reflex hyperexcitability, while type II cause hyperexcitability, salivation, seizures and choreoathetosis [37].
The main action of pyrethroids is on the sodium channels and chloride channels, which drive the ions through the cell membrane [1, 11, 13]. Pyrethroids lower the threshold of the action potential of nerve cells and muscle cells and cause repeated stimulation [7, 38]. At high concentrations, the entrance of sodium can prevent the generation of the action potential, block conduction and cause paralysis. Small amounts are sufficient to affect the sensitivity of nerve cells.
Type II pyrethroids also decrease the flux of chloride through the chloride channels. Additionally, relatively high concentrations of type II pyrethroids can affect the receptors of γ-aminobutyric acid and cause cataleptic attacks, which have been documented in humans [11, 37].
Pyrethroids are about 2,250 times more toxic for insects than mammals. Insects have more sensitive sodium channels, smaller bodies and low body temperatures. Moreover, the absorption through the skin in mammals is weak, and they can metabolise them into non-toxic compounds fast [11].
Human exposition to pyrethroids has been documented studying their metabolites in urine of German children and teenagers [28], in hair and blood of pregnant women and meconium of babies [39], in plasma of pregnant women from rural areas of South Africa [40] and in human milk [25, 33].
A few studies focused on marine organisms including different tissues of Brazilian dolphins [24, 41], Mediterranean dolphins [42] and wild and edible fish from Spanish rivers [43].
Seafood production has experimented a 3.2% yearly growth since 1961 [44]. Aquaculture is responsible for half of the seafood production worldwide, and the world annual fish consumption per capita is about 20 kg. While concern about the application of pyrethroids in fish farms against fish parasites exists, pyrethroid ingestion has been reported to be below the accepted daily intake (ADI) [45]. More detailed information of effect of salmon industry in the marine environment is presented in Chapter “Environmental Risks of Synthetic Pyrethroids Used by the Salmon Industry in Chile”.
Most of the professional exposure is due to skin absorption. The main effect of dermal exposition is paresthesia, probably caused by the hyperactivity of cutaneous nerves, especially on the face. Paresthesia increases with stimuli such as heat, sunlight, sweat or contact with water [11]. Paresthesia disappears in 12–24 h and no special treatment is required. However, topical administration of vitamin E can reduce its symptoms.
Ingestion of pyrethroids causes sore throat, nausea, vomit and abdominal pain in a few minutes. Mouth ulcers, increased secretion or dysphagia may occur [11]. Inhalation is less important, but it increases when pyrethroids are used in closed spaces. Systemic effects appear 4–48 h after exposition. The effects usually include dizziness, headache and tiredness. Less frequent effects are palpitations, chest oppression and blurry sight.
Regarding long-term exposition to pyrethroids at low concentrations, a study in humans concluded that chronic toxicity of pyrethroids does not cause any specific symptoms. What could be detected were combinations and correlations of symptoms caused by the accumulative effect of pyrethroids in nerve tissue such as brain dysfunction, polyneuropathy, immunosuppression or motor problems due to multiple sclerosis or Parkinson disease [46, 47]. It was also suggested that chronic toxicity of pyrethroids affect fertility. This hypothesis was proved in rats being administered small doses of permethrin for a maximum time period of 2 months [48].
On the other hand, these results have been criticised [49] because of the experimental design [50], because pyrethroids were not believed to cause irreversible effects according to studies on sodium channels [51] or because it was thought that mammals did not bioaccumulate them [52].
Other studies researched the chronic toxicity of cis-bifenthrin in Daphnia magna and its cytotoxicity in ovarian cells of Chinese hamster (Cricetulus griseus) and in human cervical carcinoma cells [53]. The lowest observed effect concentration (LOEC) and the no observed effect concentration (NOEC) for daphnia were 0.02 and 0.01 μg L−1, respectively. The chronic value was 0.014 μg L−1. Half-maximal inhibitory concentration (IC50) for hamster ovarian cells and human carcinoma cells were 3.2 × 10−5 and 4.0 × 10−5 mol L−1, respectively. These data proved the chronic toxicity of cis-bifenthrin in both invertebrates and mammals.
Male Wistar rats were administered for a year a mixture of pyrethroids equivalent to a 5th or a 25th of what is in cereals and vegetables consumed by an average Indian adult [54]. Altered oxidant and antioxidant status; severe anatomical damage in the caput, cauda, kidney, liver, lung, prostate and testis; and increased serum glutamate-pyruvate transaminase, serum glutamic oxaloacetic transaminase and alkaline phosphatase activity were clear for all the groups. Decreased levels of 3β- and 17β-hydroxy steroid dehydrogenase activity, litter size and impaired acrosome reaction were detected in all the groups. Exposure to very low levels of pyrethroids for longer periods may cause damage to important tissues and male reproductive physiology [54]. Cypermethrin has been reported to cause adverse effects on the immune system, fertility, the liver metabolism and cardiovascular and enzyme activity in vertebrates, and a recent study suggests that it reduces the ovarian reserve in mice via apoptosis in granulosa cells by mitochondrial-related pathways [55].
An important toxicological parameter for pyrethroids is their enantiomeric composition as different isomers can present different toxicities [56,57,58].
6 Legislation
No pesticide can be used in the European Union unless it has been proved to be effective against pests and to be safe for the human health and the environment.
The European Union regulates the sustainable use of pesticides in order to regulate their risks and impacts on human health and the environment [59]. Directive 2009/128/EC includes key points about national action plans, education for professional consumers and pesticide distributors, public information and awareness, aerosol regulation, minimisation of use or ban of pesticides, revision of equipment and integral management of pests with limitation of chemical products.
Pesticides leave residues in the treated products. The maximum residue level (MRL) is the highest concentration of a pesticide allowed by the regulation. The European Commission establishes MRLs at concentrations that are safe for the consumers and as low as possible. The MRLs are available at the European Union Pesticides database [60] (Tables 3 and 4).
MRLs have been set for about 1,100 compounds in over 300 fresh products and for the same products after processing in order to take into account dilution or concentration effects. When MRLs for a pesticide are not stated, the accepted default value is 0.01 μg g−1, which usually corresponds to the limit of detection (LOD) [59]. The European Food Safety Authority (EFSA) assesses the safety for every consumer group – adults, kids, vegetarians, etc. – based on the pesticides’ toxicity and the maximum typical concentrations of pesticides in food from the different diets around Europe.
Additionally, acceptable daily intake (ADI) values and no observed adverse effect level (NOAEL) values have been established. ADI values for pyrethroids are between 0.02 and 0.07 mg kg−1 day−1 (mg of pyrethroid per kg of consumer’s body weight per day), and NOAEL values are set between 1 and 7 mg kg−1 day−1 [36] (Table 4).
Current relevant regulation for pyrethroids is:
-
Regulation 283/2013/EU – Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
-
Regulation 284/2013/EU – Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
-
Regulation 1107/2009/EC – Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC (ban on some active substances) and 91/414/EEC (commerce of phytosanitary products).
-
Directive 2009/128/EC – Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for community action to achieve the sustainable use of pesticides.
Abbreviations
- ADI:
-
Acceptable daily intake
- BAF:
-
Bioaccumulation factor
- BCF:
-
Bioconcentration factor
- DDT:
-
Dichlorodiphenyltrichloroethane
- DT50:
-
Degradation time for 50% of the substance
- EFSA:
-
European Food Safety Authority
- EPA:
-
Environmental Protection Agency
- IC50:
-
Half-maximal inhibitory concentration
- K ow :
-
Octanol-water partition coefficient
- LOD:
-
Limit of detection
- LOEC:
-
Lowest observed effect concentration
- M r :
-
Relative molecular mass
- MRL:
-
Maximum residue level
- NOAEL:
-
No observed adverse effect level
- NOEC:
-
No observed effect concentration
- POP:
-
Persistent organic pollutant
References
Casida JE, Quistad GB (1998) Golden age of insecticide research: past, present, or future? Annu Rev Entomol 43:1–16. https://doi.org/10.1146/annurev.ento.43.1.1
Hellou J, Lebeuf M, Rudi M (2013) Review on DDT and metabolites in birds and mammals of aquatic ecosystems. Environ Rev 21:53–69. https://doi.org/10.1139/er-2012-0054
Rabitto I d S, Bastos WR, Almeida R et al (2011) Mercury and DDT exposure risk to fish-eating human populations in Amazon. Environ Int 37:56–65. https://doi.org/10.1016/j.envint.2010.07.001
Resnik DB (2009) Human health and the environment: in harmony or in conflict? Health Care Anal 17:261–276. https://doi.org/10.1007/s10728-008-0104-x
Richardson M (1998) Pesticides – friend or foe? Water Sci Technol 37:19–25. https://doi.org/10.1016/s0273-1223(98)00257-1
Roberts DR, Manguin S, Mouchet J (2000) DDT house spraying and re-emerging malaria. Lancet 356:330–332. https://doi.org/10.1016/s0140-6736(00)02516-2
Narahashi T, Ginsburg KS, Nagata K et al (1998) Ion channels as targets for insecticides. Neurotoxicology 19:581–590
Kozawa K, Aoyama Y, Mashimo S et al (2009) Toxicity and actual regulation of organophosphate pesticides. Toxin Rev 28:245–254. https://doi.org/10.3109/15569540903297808
Amweg EL, Weston DP, You J et al (2006) Pyrethroid insecticides and sediment toxicity in urban creeks from California and Tennessee. Environ Sci Technol 40:1700–1706. https://doi.org/10.1021/es051407c
Zhan Y, Zhang MH (2014) Spatial and temporal patterns of pesticide use on California almonds and associated risks to the surrounding environment. Sci Total Environ 472:517–529. https://doi.org/10.1016/j.scitotenv.2013.11.022
Bradberry SM, Cage SA, Proudfoot AT et al (2005) Poisoning due to pyrethroids. Toxicol Rev 24:93–106. https://doi.org/10.2165/00139709-200524020-00003
Jin YX, Liu JW, Wang LG et al (2012) Permethrin exposure during puberty has the potential to enantioselectively induce reproductive toxicity in mice. Environ Int 42:144–151. https://doi.org/10.1016/j.envint.2011.05.020
Shafer TJ, Meyer DA, Crofton KM (2005) Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect 113:123–136. https://doi.org/10.1289/ehp.7254
Casida JE, Ueda K, Gaughan LC et al (1975) Structure-biodegradability relationships in pyrethroid insecticides. Arch Environ Contam Toxicol 3:491–500
Leng G, Leng A, Kuhn KH et al (1997) Human dose-excretion studies with the pyrethroid insecticide cyfluthrin: urinary metabolite profile following inhalation. Xenobiotica 27:1273–1283
Ridgway RL, Tinney JC, Macgregor JT et al (1978) Pesticide use in agriculture. Environ Health Perspect 27:103–112. https://doi.org/10.2307/3428869
Grube A, Donaldson D, Kiely T et al (2011) Pesticides industry sales and usage – 2006 and 2007 market estimates [en línia]. U.S. Environmental Protection Agency (EPA), Washington. http://www.epa.gov/pesticides/pestsales/. Accessed Aug 2015
HSDB (2001) Hazardous Substances Data Bank (HSDB). TOXNET Toxicology Data Network, United States National Library of Medicine. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Accessed Nov 2019
Metcalf RL (1995) Insect control technology. In: Kirk-Othmer encyclopedia of chemical technology. Wiley, New York
NAWQA (2001) U.S. Geological Survey (U.S. Department of the Interior). Pesticide National Synthesis Project (National Water-Quality Assessment (NAWQA) Program). https://water.usgs.gov/nawqa/pnsp. Accessed Nov 2019
AERU – Agriculture & Environment Research Unit (University of Hertfordshire) (2007) PPDB: Pesticide Properties DataBase. http://sitem.herts.ac.uk/aeru/footprint/index2.htm. Accessed Nov 2019
SC (2008) Stockholm Convention on Persistent Organic Pollutants. http://chm.pops.int/. Accessed Aug 2017
Abernath C, Ueda K, Engel JL et al (1973) Substrate specificity and toxicological significance of pyrethroyd-hydrolyzing esterases of mouse liver-microsomes. Pestic Biochem Physiol 3:300–311. https://doi.org/10.1016/0048-3575(73)90028-x
Alonso MB, Feo ML, Corcellas C et al (2012) Pyrethroids: a new threat to marine mammals? Environ Int 47:99–106. https://doi.org/10.1016/j.envint.2012.06.010
Corcellas C, Feo ML, Torres JP et al (2012) Pyrethroids in human breast milk: occurrence and nursing daily intake estimation. Environ Int 47:17–22. https://doi.org/10.1016/j.envint.2012.05.007
Daughton CG (2004) Non-regulated water contaminants: emerging research. Environ Impact Assess Rev 24:711–732. https://doi.org/10.1016/j.eiar.2004.06.003
Corcellas C (2017) Estudi dels insecticides piretroides en mostres biològiques i humanes. Universitat de Barcelona, Barcelona, 234 pp
Heudorf U, Angerer J, Drexler H (2004) Current internal exposure to pesticides in children and adolescents in Germany: urinary levels of metabolites of pyrethroid and organophosphorus insecticides. Int Arch Occup Environ Health 77:67–72. https://doi.org/10.1007/s00420-003-0470-5
Satoh T, Hosokawa M (1998) The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol 38:257–288. https://doi.org/10.1146/annurev.pharmtox.38.1.257
Hosokawa M, Maki T, Satoh T (1990) Characterization of molecular species of liver microsomal carboxylesterases of several animal species and humans. Arch Biochem Biophys 277:219–227
Huang H, Fleming CD, Nishi K et al (2005) Stereoselective hydrolysis of pyrethroid-like fluorescent substrates by human and other mammalian liver carboxylesterases. Chem Res Toxicol 18:1371–1377. https://doi.org/10.1021/tx050072+
Tange S, Fujimoto N, Uramaru N et al (2014) In vitro metabolism of cis- and trans-permethrin by rat liver microsomes, and its effect on estrogenic and anti-androgenic activities. Environ Toxicol Pharmacol 37:996–1005. https://doi.org/10.1016/j.etap.2014.03.009
Zehringer M, Herrmann A (2001) Analysis of polychlorinated biphenyls, pyrethroid insecticides and fragrances in human milk using a laminar cup liner in the GC injector. Eur Food Res Technol 212:247–251. https://doi.org/10.1007/s002170000223
Mauck WL, Olson LE (1976) Toxicity of natural pyrethrins and five pyrethroids to fish. Arch Environ Contam Toxicol 4:18–29
Shafer T, Rijal S, Gross G (2008) Complete inhibition of spontaneous activity in neuronal networks in vitro by deltamethrin and permethrin. Neurotoxicology 29:203–212
WHO (2005) Safety of pyrethroids for public health use. World Health Organization, Geneva
Ray DE, Forshaw PJ (2000) Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. J Toxicol Clin Toxicol 38:95–101
Pollack RJ, Kiszewski A, Armstrong P et al (1999) Differential permethrin susceptibility of head lice sampled in the United States and Borneo. Arch Pediatr Adolesc Med 153:969–973
Ostrea EM, Bielawski DM, Posecion NC et al (2009) Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environ Res 109:116–122. https://doi.org/10.1016/j.envres.2008.09.004
Channa KR, Rollin HB, Wilson KS et al (2012) Regional variation in pesticide concentrations in plasma of delivering women residing in rural Indian Ocean coastal regions of South Africa. J Environ Monit 14:2952–2960. https://doi.org/10.1039/c2em30264k
Alonso MB, Feo ML, Corcellas C et al (2015) Toxic heritage: maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ Pollut 207:391–402. https://doi.org/10.1016/j.envpol.2015.09.039
Aznar-Alemany Ò, Giménez J, de Stephanis R et al (2017b) Insecticide pyrethroids in liver of striped dolphin from the Mediterranean Sea. Environ Pollut 225:346–353. https://doi.org/10.1016/j.envpol.2017.02.060
Corcellas C, Eljarrat E, Barceló D (2015) First report of pyrethroid bioaccumulation in wild river fish: a case study in Iberian river basins (Spain). Environ Int 75:110–116. https://doi.org/10.1016/j.envint.2014.11.007
FAO – Food and Agriculture Organization of the United Nations (2016) The State of World Fisheries and Aquaculture 2016. Contributing to food security and nutrition for all [en línia]. http://reliefweb.int/sites/reliefweb.int/files/resources/a-i5555e.pdf. Accessed Sept 2017
Aznar-Alemany Ò, Eljarrat E, Barceló D (2017a) Effect of pyrethroid treatment against sea lice in salmon farming regarding consumers’ health. Food Chem Toxicol 105:347–354. https://doi.org/10.1016/j.fct.2017.04.036
Kolaczinski JH, Curtis CF (2004) Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem Toxicol 42:697–706. https://doi.org/10.1016/j.fct.2003.12.008
Muller-Mohnssen H, Hahn K (1995) A new method for early detection of neurotoxic diseases (exemplified by pyrethroid poisoning). Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 57:214–222
Issam C, Zohra H, Monia Z et al (2011) Effects of dermal sub-chronic exposure of pubescent male rats to permethrin (PRMT) on the histological structures of genital tract, testosterone and lipoperoxidation. Exp Toxicol Pathol 63:393–400. https://doi.org/10.1016/j.etp.2010.02.016
Moshammer H (1996) Comment on Muller-Mohnssen, H., K. Hahn. A method for early detection of neurotoxic diseases. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 58:47–49
Nasterlack M, Chr Dietz M (1996) Comment on Muller-Mohnssen, H., K. Hahn. A method for early detection of neurotoxic diseases. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 58:49–50
Narahashi T (1992) Nerve membrane Na+ channels as targets of insecticides. Trends Pharmacol Sci 13:236–241
Woollen BH, Marsh JR, Laird WJD et al (1992) The metabolism of cypermethrin in man – differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica 22:983–991
Wang C, Chen F, Zhang Q et al (2009) Chronic toxicity and cytotoxicity of synthetic pyrethroid insecticide cis-bifenthrin. J Environ Sci (China) 21:1710–1715. https://doi.org/10.1016/s1001-0742(08)62477-8
Ravula AR, Yenugu S (2019) Long term oral administration of a mixture of pyrethroids affects reproductive function in rats. Reprod Toxicol 89:1–12. https://doi.org/10.1016/j.reprotox.2019.06.007
Wang H, He Y, Cheng D et al (2019) Cypermethrin exposure reduces the ovarian reserve by causing mitochondrial dysfunction in granulosa cells. Toxicol Appl Pharmacol 379:114693. https://doi.org/10.1016/j.taap.2019.114693
Sun D, Pang J, Zhou Z et al (2016) Enantioselective environmental behavior and cytotoxicity of chiral acaricide cyflumetofen. Chemosphere 161:167–173. https://doi.org/10.1016/j.chemosphere.2016.06.087
Wang F, Liu D, Qu H et al (2016) A full evaluation for the enantiomeric impacts of lactofen and its metabolites on aquatic macrophyte Lemna minor. Water Res 101:55–63. https://doi.org/10.1016/j.watres.2016.05.064
Zhao M, Chen F, Wang C et al (2010) Integrative assessment of enantioselectivity in endocrine disruption and immunotoxicity of synthetic pyrethroids. Environ Pollut 158:1968–1973. https://doi.org/10.1016/j.envpol.2009.10.027
EC – European Commission (2012) Health and consumers > plants > pesticides. http://ec.europa.eu/food/plant/pesticides/index_en.htm. Accessed Mar 2017
DG SANCO – Directorate General for Health and Consumers (2008) EU Pesticides database. https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN. Accessed Nov 2019
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Aznar-Alemany, Ò., Eljarrat, E. (2020). Introduction to Pyrethroid Insecticides: Chemical Structures, Properties, Mode of Action and Use. In: Eljarrat, E. (eds) Pyrethroid Insecticides. The Handbook of Environmental Chemistry, vol 92. Springer, Cham. https://doi.org/10.1007/698_2019_435
Download citation
DOI: https://doi.org/10.1007/698_2019_435
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55695-2
Online ISBN: 978-3-030-55696-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)