Abstract
The water sources of drinking water generally contain natural organic matter (NOM) as a result of the interactions between the hydrologic cycle and the environment. The amount, character, and properties of NOM vary considerably according to the origins of the waters and depend on the biogeochemical cycles of their surrounding environments. NOM can negatively influence water quality in drinking water supply systems, and it can significantly influence the performance of drinking water treatment processes. Hence, NOM removal is an important issue in order to optimize drinking water treatment operation and to reduce the risks of water alteration in the distribution systems. Several treatment processes can be applied for NOM removal depending on water quality, the nature of NOM, and the treatments already existing in the supply system. Among the most effective conventional solutions coagulation/flocculation, filtration, and carbon adsorption are available. An interest has recently increased toward nonconventional solutions based on membrane filtration and advanced oxidation processes (AOPs). An overview on the AOPs will be presented and discussed. Moreover, the AOP with ozone and UV radiation, with two low pressure UV lamps, at 254 and 185 nm wavelength, was experimented on a surface water in order to study the removal of odorous and pesticide, organic compounds (UV absorbance and THMs precursors) and bromate formation. Different batch tests were performed with ozone concentration up to 10 mg L−1, UV dose up to 14,000 J m−2, and a maximum contact time of 10 min. The main results show that metolachlor can be efficiently removed with ozone alone while for geosmin and MIB a complete removal can be obtained with the advanced oxidation of ozone, with concentration of 1.5–3 mg L−1 and contact time of 2–3 min, with UV radiation (with doses of 5,000–6,000 J m−2). As concerns the influence of the organic precursors, all the experimented processes show a medium removal of about 20–40% for UV absorbance and 15–30% for THMFP (trihalomethane formation potential).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Natural organic matter (NOM) is ubiquitous in water as it comes from the degradation of plants, bacteria, algae, and vegetal organisms in general. NOM is defined as a complex matrix of organic materials, present in all natural water. Water systems often have multiple sources of NOM, thus different organic carbon fractions [1]. The amount, character, and properties of NOM vary considerably according to the origins of the waters and depend on the biogeochemical cycles of their surrounding environments [2]. Concentrations of TOC in the ground water are typically in the range of 0.1–2.0 mg L−1 and 1–20 mg L−1 in surface water [3, 4] although several dozens of mg L−1 of TOC are not uncommon [5]. The factors that determine the composition of NOM are location dependent and include the source of organic matter, the water chemistry, temperature, pH, and biological processes. Moreover, the range of organic components in NOM can vary seasonally at the same location [6], for example, due to rainfall, snowmelt runoff, floods, or droughts. NOM found in natural waters consists of both hydrophobic and hydrophilic components, of which the largest fraction is generally hydrophobic acids, making up approximately 50% of the total organic carbon (TOC) in water [7].
NOM has a significant impact on many aspects of water treatment, including the performance of unit processes, necessity for and application of water treatment chemicals, and the biological stability of the water. Once the composition and quantity of NOM in the water source has been examined, suitable methods for efficient NOM removal can be applied. No single process alone can be used to treat NOM due to its high variability. Among the suitable solutions, the following treatment processes can be efficiently applied for NOM removal: adsorption, biological treatments, coagulation/flocculation, electrochemical methods, ion exchange, membrane technology, and AOP [8]. An increasing interest in drinking water treatment has recently been shown for the AOPs due to several advantages: low chemical consumption in some AOPs, complete mineralization of pollutant, oxidation of disinfection by-product (DBP) rapid reaction, unselective oxidants, harmful to microorganisms, and easily implemented in existing water treatment plants.
This chapter presents an overview on AOPs applied for the NOM removal during drinking water treatments. Moreover, the main results of an experimental study on the O3/H2O2 process for NOM and micropollutant removal will be presented.
2 NOM Characteristics and Characterization
NOM compounds are complex mixtures possessing unique combinations of various functional groups, including esteric, phenolic, quinine, carboxylic, hydroxyl, amino, and nitroso, which are usually negatively charged at neutral pH. NOM found in natural waters consists of both hydrophobic and hydrophilic components, of which the largest fraction is generally hydrophobic acids, making up approximately 50% of the total organic carbon (TOC) in water [7]. These hydrophobic acids can be described as humic substances comprising (1) humic acids (HAs), which are soluble in alkali, but insoluble in acid; (2) fulvic acids (FA), which are soluble in both alkali and acid; and (3) humins, which are insoluble in both alkali and acid. FAs constitute a major fraction of these humic substances, which vary in molecular size and functional group content [9]. Hydrophobic NOM is rich in aromatic carbon, phenolic structures, and conjugated double bonds, while hydrophilic NOM contains more aliphatic carbon and nitrogenous compounds, such as carbohydrates, sugars, and amino acids.
The amount of NOM in water can be predicted with parameters including ultraviolet and visible (UV–Vis) detected compounds, TOC, and SUVA. TOC and dissolved organic carbon (DOC) are the most convenient parameters for analyzing the NOM removal of treatment processes. DOC is the organic carbon in a water sample filtered through a 0.45 μm filter [10]. TOC is the sum of the particulate and DOC when existing inorganic carbon is removed by acidification.
UV–Vis absorption spectroscopy is a semiquantitative method to determine humic substances in natural waters. Any wavelength from 220 to 280 nm is appropriate for NOM measurements: absorbance at 220 nm is associated with both the carboxylic and aromatic chromophores, whereas absorbance at 254 nm is typical for aromatic groups, and it has been identified as a potential surrogate measure for DOC [11].
Specific UV absorbance (SUVA) is calculated as the ratio of a UV absorbance at a specific wavelength (e.g., UV254 absorbance) and the TOC concentration. A high SUVA value indicates that the organic matter is largely composed of hydrophobic, high-MM organic material. A low SUVA value indicates that the water contains organic compounds that are mainly hydrophilic, with a low MM and charge density [6].
Several methods can be used for the characterization of NOM including resin adsorption, size exclusion chromatography, nuclear magnetic resonance (NMR) spectroscopy, and fluorescence spectroscopy. More precise methods for determining NOM structures have been developed recently: pyrolysis gas chromatography mass spectrometry, multidimensional NMR techniques, and Fourier transform ion cyclotron resonance mass spectrometry.
A detailed description of the methods used to characterize various features of natural organic matter (NOM) is reported in literature [12].
3 Impact of NOM on Drinking Water Supply Systems
Significance of NOM for drinking water quality and stability is represented by the impact on several aspects of its treatment [13]:
-
NOM greatly affect organoleptic properties of the water (taste, color, and odor) [4, 14].
-
NOM influence chemical properties in terms of mostly negative reactions and interferences with other chemicals used for oxidation and disinfection by lowering their effectiveness and thus increasing their consumption to achieve the treatment goal [4, 14].
-
NOM can form complexes with the toxic heavy metals and synthetic organic chemicals, making them more soluble and more difficult to remove by the treatment [14].
-
NOM are involved in formation of undesired and detrimental DBPs with an increase in bioavailability of organic matter for microorganisms in the water supply systems, enabling their proliferation.
NOM can cause several problems in drinking water treatment and distribution systems. It can affect the performance of unit processes, the necessity for application of water treatment chemicals, and the biological stability of the water. NOM increases the reagent dose necessary during water treatment, especially for coagulant and disinfectant. NOM can also interfere with the performance of unit operations, such as biofilm growth on media, causing rapid filter clogging and fast saturation of activated carbon beds. NOM is also responsible for the fouling of membranes.
During water treatment, NOM has generally been considered the main precursor to DBPs, especially hydrophobic and high molar mass (HMM) NOM, with its high aromatic carbon content [15, 16]. It has also been observed that hydrophilic and low molar mass (LMM) NOM play a significant role in DBP formation [15]. Bromine and iodine appear more reactive with hydrophilic and LMM fractions of NOM in the formation of THMs and HAAs (haloacetic acids). Chlorine has been shown to react more readily with HMM and hydrophobic NOM compounds [15].
NOM can impact also the water behavior in the distribution system as it affects corrosion, is a source of nutrients for heterotrophic bacteria, and acts as a substrate for bacterial growth in the pipes [17].
4 AOPs for the Removal of NOM
AOPs are obtained from the combination of several oxidants, radiation, and catalysts: O3/H2O2, UV/H2O2, UV/O3, UV/TiO2, Fe2+/H2O2, Fe2+/H2O2 + hν, vacuum ultraviolet (VUV) radiation, or ionizing radiation. All these processes involve the generation of highly reactive radical intermediates, especially the OH• radical [18] that is one of the most powerful known oxidants. The reaction rate of a compound in OH• radical-mediated oxidation is usually several orders of magnitude higher than that achieved by molecular ozone under the same conditions.
The reaction rate constants between OH• radicals and organic species are in the range of 108–1010 M−1 s−1 [19].
OH• radicals are highly nonselective oxidants, enabling a very large number of reactions. Once free radical reaction has been initiated (following photolysis, ozone, hydrogen peroxide, or heat), a series of simple reactions will occur. The reactions of OH• radicals with NOM proceed in three different ways:
-
By the addition of OH• radicals to double bonds
-
By H-atom abstraction, which yields carbon-centered radicals
-
By the OH• radical gaining an electron from an organic substituent
4.1 Ozone-Based Treatments (O3/H2O2, O3/UV, and O3/H2O2/UV)
Ozone reacts with NOM by an electrophilic addition to double bonds. This reaction is very selective. In addition to the direct reaction of ozone with NOM, a nonselective and fast reaction occurs with the OH• radicals formed when ozone decomposes in water. The OH• radical formation potential is much lower during ozonation than in AOPs. The combination of ozone with other systems, like UV light or hydrogen peroxide, can promote OH• formation.
The influence of ozone on THM precursors depends on the kind and structures of the organic material that can have different reactivities toward ozone and chlorine. Some authors observed that ozonation produces a transformation of natural organic matter from more reactive hydrophobic DOC, that reacts easily with chlorine to produce THM, to a hydrophilic fraction, with a consequent lower THM formation [20]. This finding is in agreement with other authors [21, 22].
The combination of O3 with H2O2 increases total THM concentrations than in ozonated samples [23]. Similarly, other authors [24] did not observe any significant gains in THM reduction after adding H2O2 or TiO2 to the ozone treatment.
Other authors [25] observed that TOC, THMFP, and TOX decreased with O3/UV in comparison with ozone alone. Moreover, O3/UV results in significant mineralization of DOC, reduction of trihalomethane formation potential (THMFP), and haloacetic acid formation potential (HAAFP), according to [26].
The combined system O3/H2O2/UV was not remarkable more efficient than O3/UV in HAA decomposition [27].
4.2 UV Light-Based Treatments
Much research has focused on developing applications for UV/H2O2; little attempt has been made to evaluate the impact of UV/H2O2 on NOM. Past studies have demonstrated that substantial reduction of DBP formation potential (DBPFP) could be achieved using UV/H2O2 [28,29,30]. But all these studies mainly focused on strong advanced oxidation conditions made possible by very long UV exposures (i.e., fluence) and/or high UV/H2O2 concentration. Under such conditions NOM is mineralized leading to a reduction in the concentration of NOM [31].
Although the combination of UV irradiation at 254 nm and H2O2 treatment increases the NOM reduction and promotes the OH• radical formation, a combination of high UV fluence and high peroxide concentration is required in order to generate significant reduction of NOM [30]. Moreover, under fluence and H2O2 concentrations typically applied in drinking water treatment applications, UV standard dose for disinfection is about 400 mJ cm−2 [26], and NOM is not removed.
4.3 Photocatalytic Oxidation
The photocatalytic oxidation combines the UV light with a heterogeneous photocatalysis in which several different semiconductors are employed (e.g., TiO2, ZnO, ZnS, WO3, SrTiO3) for catalysis.
The TiO2/UV process was applied for controlling membrane fouling by NOM [32]; even though the rate of TOC removal was relatively low, membrane fouling of both an MF and a UF was completely eliminated after 20 min of treatment due to the changes in NOM molecular characteristics.
The presence of hardness (Ca2+, Mg2+, Fe2+/Fe3+) and the accompanying common anions (e.g., Cl−, NO3 −, SO4 2−, and HCO3 −/CO3 2−) have considerable effects on the degradation kinetics of NOM expressed as DOC or UV254 [33].
Little is known about the by-products formed during photocatalytic oxidation of NOM. Some authors [34] reported that aldehydes and ketones were typical intermediate products formed during photocatalytic oxidation of NOM in surface waters and these compounds would be oxidized to form carboxylic acids as the reaction proceeded. Photocatalytic oxidation may also form by-products that have different reactivity with chlorine disinfectants [35].
NOM plays an important role as inhibitor of oxidation of target micropollutants during AOPs; the degradation rates can decrease by one order of magnitude or more in the presence of backgrounds such as NOM. Authors [36] provided a novel analytical approach to select strategies to enhance the performance of AOPs, even in systems with high levels of NOM or other background constituents.
4.4 Fenton’s Process
Fenton’s reagent is a catalytic oxidative mixture that contains iron ions and hydrogen peroxide. In this process hydroxyl radicals are produced during the decomposition of hydrogen peroxide in the presence of ferrous salts.
Fenton’s reagent, H2O2 with Fe(II)/Fe(III) ions, in water produces OH radicals. Some authors [16] observed that UV/Fe(III) treatment was ineffective in NOM removal, while the UV/Fe(III)/H2O2 system has the potential to remove organics with a broad range of MMs. Even though high NOM removal rates were detected, the remaining organic compounds appear fairly reactive to chlorine, thus resulting in low reduction of THMFP.
Fenton and photo-Fenton’s processes were compared in order to assess their potential to remove NOM from organic-rich waters [19]. The performance of both processes was dependent on pH, Fe:H2O2 ratio, as well as Fe2+ dose. Under optimum conditions both processes achieved greater than 90% removal of DOC and UV254 absorbance. This removal leads to the THMFP of the water being reduced from 140 to below 10 μg L−1.
A comparison of UV/H2O2, Fenton, and photo-Fenton for treating an upland catchment reservoir (DOC = 7.5 mg L−1) showed how both Fenton’s processes could remove the excess of 90% of DOC and UV-absorbing species and are significantly quicker than UV/H2O2 [37].
5 Case Study: Effect of the O3/UV Process on Organic Matter Removal and Influence on DBPs Formation
This experimental research was addressed to compare the ozone conventional oxidation with the advanced oxidation with ozone and UV radiation, as concerns geosmin, MIB and metolachlor removal, the influence on DBPs organic precursors, and bromate formation [38].
5.1 Raw Water
Raw water, collected from the river Secchia at the treatment plant of Tressano, managed by AGAC of Reggio Emilia, in the North of Italy, showed the characteristics indicated in Table 1: TOC = 1.8 mg L−1; UV absorbance = 0.36 cm−1; THMFP = 70–80 μg L−1; bromide = 30 μg L−1.
During the experimental tests, water was artificially contaminated with 0.5 μg L−1 geosmin (trans-1,10-dimethyl-trans-9-decalol), 0.2–0.4 μg L−1 MIB (2-methylisoborneol), and 7–10 μg L−1 metolachlor [2-chloro-N-(2-ethyl-6-methylphenyl)-N-(2-methoxy-1-methylethyl)-acetamide].
5.2 Experimental Plant
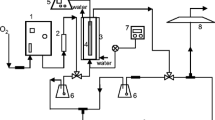
The experimental tests were performed in batch conditions on a laboratory-scale plant (Fig. 1) (Q = 10 L min−1) formed by a stainless steel reactor (volume of 20 L), a pump, and an in-line ozone injection followed by a static mixer and a low pressure mercury-vapor ultraviolet (UV) lamp. Ozone was generated by means of “Ozonia Triogen Compact Ozone Generator” (Model TOGC2), with a capacity of 8 gO3 h−1. Two UV lamps (Model TR-65) were applied separately with various nominal wavelengths of maximum light intensity of 254 and 185 nm. Each lamp had about 80% of the radiation around the maximum wavelength, and the intensity was about 25 W m−2.
The experimental conditions tested on water samples were ozone concentrations =0–15 mg L−1, ozone contact time = 0–14 min, and UV dose = 0–14,000 J m−2. Water samples were collected in an outlet from the UV chamber after 2, 4, 6, 8, and 10 min from the beginning of the test (the time required for one complete cycle in the system is 2 min).
5.3 Analytical Methods
Geosmin, MIB, and metolachlor were analyzed with a capillary column gas chromatography-mass spectrometry (GC/MS). Trihalomethane formation potential (THMFP) was determined following [39]; THMs were determined with a gas chromatograph (GC Perkin-Elmer 8600) with the static headspace method. TOC was analyzed with a total carbon monitor (Model 1010), the UV absorbance with an UV–visible spectrophotometer with a 1 cm quartz cell and turbidity with a 2100 AN Hach turbidimeter. Ozone concentration was measured by means of an ozone analyzer (BMT 961) that analyzes the flow of ozone production and that of ozone outlet from the reactor; the difference between the second and the first term gives the amount of ozone effectively transferred to water.
5.4 Results: Removal of Organic Matter
As concerns the influence on organic matter of the experimented treatments, ozone alone and combined with UV radiation reduces the absorption of radiation at the wavelength of 254 nm of about 10–20% with ozone/UV 254 nm and 10% with ozone/UV 185 nm (Fig. 2). This is due to a removal of aromatic structures and double bonds of natural organic matter.
As shown in Fig. 3, the removal of THM precursors with ozone and ozone/UV is very different (from 0 to 40%), and no improvements are observed with increasing ozone and UV dose and contact time. The partial THMFP reduction (10–30%) observed in most of the trials is due to the degradation of humic substances into low molecular weight compounds that are less reactive toward chlorine. However, at the same time, bromide, whose concentration in raw water varies from 5 to 90 μg L−1, is oxidized to hypobromite which further leads to brominated compounds [40], with a consequent higher THM formation.
The combination of ozone with UV does not improve THM precursor removal with respect to ozone alone, according to the results of other authors [25] that found a significant reduction of total organic halide while no differences were shown for chloroform.
5.5 Results: Removal of Geosmin, MIB, and Metolachlor
Molecular ozone has very different reaction rates with organic compounds (Fig. 4): it reacts very fast with metolachlor, while the odorous compounds (MIB and geosmin) are more persistent, and their complete removal can be obtained only with ozone combined with UV radiation (Fig. 5).
The improvement in removal rates for taste and odor compounds obtained by the advanced oxidation process (O3/UV) can be explained by the action of initiators (UV rays) to introduce the decomposition of the ozone in water, thus generating hydroxyl radicals that are very reactive when the water has a low alkalinity (that means low concentration of scavengers like HCO3 −, CO3 2−, etc.).
The main results show that metolachlor can be efficiently removed with ozone alone with C × t = 8–10 mg min L−1 (ozone concentration = 1 mg L−1 and 8–10 min contact time); the same removal can be obtained with C × t = 4 mg min L−1 for ozone combined with UV (UV dose = 4,000–6,000 J m−2). This means that, for the same contact time, ozone concentration can be reduced from 1 mg L−1 to 0.2–0.4 mg L−1, whereas, for geosmin and MIB, a complete removal can be obtained only with the combination of ozone (with concentration of 1.5–3 mg L−1 and contact time of 2–3 min) with UV radiation, with doses of 5,000–6,000 J m−2.
5.6 Results: Bromate Formation
As concerns bromate formation (Fig. 6), a significant reduction is shown in the AOPs with ozone/UV with respect to conventional oxidation with ozone. UV radiation, in the wavelength range of 180–300 nm, provides energy to reduce bromate to hypobromite ion as intermediate and to bromide and oxygen as end products [41] via complex reactions generated by the primary reaction of photolysis. Ozone combined with UV lamp at 185 nm wavelength is about 10–20% lower than ozone alone; the highest bromate destruction is obtained with ozone combined with UV at 254 nm wavelength, for which bromate is about 40–50% lower than ozone alone and its final concentration is generally lower than the 31/01 Italian Legislative Decree Limit of 10 μg L−1.
5.7 Conclusions
The conventional and advanced oxidation tests performed on water contaminated with geosmin, MIB and metolachlor show that the combination of ozone with UV radiation, both at 254 nm and 185 nm wavelengths, improves the process efficiency and offers a complete removal for all the analyzed contaminants with C × t of 4–10 mg min L−1 and UV doses of 4,000–6,000 J m−2. All the experimented treatments offer a good removal of organic precursors, while a significant reduction (below the limit of 10 μg L−1 of 31/01 Italian Legislative Decree) of bromate is obtained only with ozone combined with UV 254 nm.
6 General Considerations About the Application of AOPs for NOM Removal in Drinking Water Treatment Plants
AOPs are among the most studied and promising technologies for drinking water purification and disinfection. Although total reduction of NOM has not been achieved with AOPs, several studies have shown efficient NOM reduction and mitigation of DBP formation.
The results of various studies dealing with NOM removal by AOPs are always study specific depending on the water characteristics, such as the amount of organic matter. Therefore, the characterization of NOM in water should be made before the design and optimization of the AOP treatment. Furthermore, in order to assess its influence on the downstream processes, it is important to determine the organic characteristics of the treated water.
The implementation of an AOP process in a drinking water treatment plant can be an interesting solution for the removal of NOM and DBP minimization in drinking water systems. First, the objective of the AOP treatment needs to be defined, whether it is to enhance biodegradability of organic matter or mineralize organic compounds. Therefore, the optimal conditions for each case must be determined according to the type and amount of organic compounds and the interfering background compounds present in the water.
AOPs generally are not applied alone but may find better application in combination with other treatments, thus enhancing their efficiency for NOM removal. For example, coagulation prior to oxidation removes most of the HMM NOM, thus impacting on subsequent AOP treatment. The combination of AOP and BAC treatment has been suggested to offer a more viable option for the reduction of harmful DBPs than the AOP alone.
It should be emphasized that AOPs have site-specific effects, so pilot-scale and full-scale tests must be conducted to define the optimal conditions for the process. Experimental and pilot-scale studies are often conducted under conditions that are not economically feasible in commercial applications. The full-scale application of AOPs in drinking water treatment plants is still limited because of high cost, high level of pretreatment required, a lack of experience, and operational difficulties. Nevertheless, there is an increasing interest for AOPs that will take to an increase of full-scale installation of these processes.
Abbreviations
- AOPs:
-
Advanced oxidation processes
- DBPFP:
-
Disinfection by-products formation potential
- DBPs:
-
Disinfection by-products
- DOC:
-
Dissolved organic carbon
- FAs:
-
Fulvic acids
- GC:
-
Gas chromatography
- HAAFP:
-
Haloacetic acid formation potential
- HAAs:
-
Haloacetic acids
- HAs:
-
Humic acids
- HMM:
-
High molar mass
- LMM:
-
Low molecular mass
- NMR:
-
Nuclear magnetic resonance
- NOM:
-
Natural organic matter
- SUVA:
-
Specific UV absorbance
- THMFP:
-
Trihalomethane formation potential
- THMs:
-
Trihalomethanes
- TOC:
-
Total organic carbon
- TOX:
-
Total organic halide
- UV–Vis:
-
Ultraviolet and visible
- VUV:
-
Vacuum ultraviolet
References
Rigobello E, Dantas A, Di Bernardo L, Vieira E (2011) Influence of the apparent molecular size of aquatic humic substances on colour removal by coagulation and filtration. Environ Technol 32:1767–1777
Fabris R, Chow C, Drikas M, Eikebrokk B (2008) Comparison of NOM character in selected Australian and Norwegian drinking waters. Water Res 42:4188–4196
Rodrigues A, Brito P, Janknecht MF, Proença R, Nogueira R (2009) Quantification of humic acids in surface water: effects of divalent cations, pH, and filtration. J Environ Monit 11:377–382
Crittenden JC (2012) MWH’s water treatment: principles and design. Wiley, Hoboken
Kokorite M, Klavins V, Rodinov G (2012) Trends of natural organic matter concentrations in river waters of Latvia. Environ Monit Assess 184:4999–5008
Sharp E, Jarvis P, Parsons S, Jefferson B (2006) Impact of fractional character on the coagulation of NOM. Colloids Surf A 286:104–111
Thurman E (1985) Organic geochemistry of natural waters. Martinus Nijhoff/Dr W. Junk Publishers, Dordrecht
Shestakova M, Sillanpää M (2013) Removal of dichloromethane from ground and wastewater: a review. Chemosphere 93:1258–1267
Snoeyink VL, Jenkins D (1980) Water chemistry. Wiley, New York
Danielsson L (1982) On the use of filters for distinguishing between dissolved and particulate fractions in natural waters. Water Res 16:179–182
Korshin G, Chow C, Fabris R, Drikas M (2009) Absorbance spectroscopy-based examination of effects of coagulation on the reactivity of fractions of natural organic matter with varying apparent molecular weights. Water Res 43:1541–1548
Sillanpää M (2015) Natural organic matter in water. Characterization and treatment methods. Elsevier, New York
Cehovin M, Medic A, Scheideler J, Mielcke J, Ried A, Kompare B, Gotvajn AZ (2017) Hydrodynamic cavitation in combination with the ozone, hydrogen peroxide and the UV-based advanced oxidation processes for the removal of natural organic matter from drinking water. Ultrason Sonochem 37:394–404
Matilainen A, Sillanpää M (2010) Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 80:351–365
Hua G, Reckhow D (2007) Characterization of disinfection byproduct precursors based on hydrophobicity and molecular size. Environ Sci Technol 41:3309–3315
Sanly, Lim M, Chiang K, Amal R, Fabris R, Chow C, Drikas M (2007) A study on the removal of humic acid using advanced oxidation process. Sep Sci Technol 42:1391–1404
Jacangelo J, DeMarco J, Owen D, Randtke S (1995) Selected processes for removing NOM: an overview. J Am Water Works Assoc 87(1):64–77
Glaze W, Kang J, Chapin D (1987) The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng 9:335–352
Murray CA, Parsons SA (2004) Removal of NOM from drinking water: Fenton’s and photo-Fenton’s processes. Chemosphere 54(7):1017–1023
Galapate R, Baes A, Okada M (2001) Transformation of dissolved organic matter during ozonation: effects on trihalomethane formation potential. Water Res 35:2201–2206
Treguer R, Tatin R, Couvert A, Wolbert D, Tazi-Pain A (2010) Ozonation effect on natural organic matter adsorption and biodegradation – application to a membrane bioreactor containing activated carbon for drinking water production. Water Res 44:781–788
Molnar J, Agbaba J, Dalmacija B, Tubić A, Krčmar D, Maletić S, Tomašević D (2013) The effects of matrices and ozone dose on changes in the characteristics of natural organic matter. Chem Eng J 222:435–443
Irabelli A, Jasim S, Biswas N (2008) Pilot-scale evaluation of ozone vs. peroxone for trihalomethane formation. Ozone Sci Eng 30:356–366
Mosteo R, Miguel N, Martin-Muniesa S, Ormad M, Ovelleiro J (2009) Evaluation of trihalomethane formation potential in function of oxidation processes used during the drinking water production process. J Hazard Mater 172:661–666
Kusakabe K, Aso S, Hayashi J, Isomura K, Morooka S (1990) Decomposition of humic acid and reduction of trihalomethane formation potential in water by ozone with UV irradiation. Water Res 24:781–785
Chin A, Bérube PR (2005) Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res 39(10):2136–2144
Wang GS, Liao CH, Chen HW, Yang HC (2006) Characteristics of natural organic matter degradation in water by UV/H2O2 treatment. Environ Technol 27(3):277–287
Kleiser G, Frimmel F (2000) Removal of precursors for disinfection by-products (DBPs) – differences between ozone- and OH-radical-induced oxidation. Sci Total Environ 256:1–9
Liu W, Andrews SA, Sharpless C, Stefan M, Linden KG, Bolton JR (2002) Bench-scale investigations into comparative evaluation of DBP formation from different UV/H2O2 technologies. In: Proceedings of the AWWA water quality technology conference, Seattle
Toor R, Mohseni M (2007) UV/H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water. Chemosphere 66:2087–2095
Sarathy SR, Mohseni M (2009) UV/H2O2 treatment of drinking water: impacts on NOM characteristics, vol 11. IUVA News
Huang X, Leal M, Li Q (2008) Degradation of natural organic matter by TiO2 photocatalytic oxidation and its effect on fouling of low-pressure membranes. Water Res 42(4–5):1142–1150
Uyguner CS, Bekbolet M (2009) Application of photocatalysis for the removal of natural organic matter in simulated surface and ground waters. J Adv Oxid Technol 12(1):2371–1175
Liu S, Lim M, Fabris R, Chow C, Drikas M, Amal R (2008) TiO2 Photocatalysis of natural organic matter in surface water: impact on trihalomethane and haloacetic acid formation potential. Environ Sci Technol 42:6218–6223
Pichat P (2013) In: Pichat P (ed) Photocatalysis and water purification: from fundamentals to recent applications. Wiley, New York
Brame J, Long M, Li Q, Alvarez P (2015) Inhibitory effect of natural organic matter or other background constituents on photocatalytic advanced oxidation processes: mechanistic model development and validation. Water Res 84:1–10
Parsons S, Byrne A (2004) Water treatment applications. In: Parsons S (ed) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London, pp 329–34638
Collivignarelli C, Sorlini S (2004) AOPs with ozone and UV radiation in drinking water: contaminants removal and effects on disinfection byproducts formation. Water Sci Technol 48(4):51–56
Standard methods for the examination of water and wastewater (1998) Front cover, 20th edn. APHA – American Public Health Association
Camel V, Bermond A (1998) The use of ozone and associated oxidation processes in drinking water treatment. Water Res 32(11):3208–3222
Siddiqui MS, Amy GL, McCollum LJ (1996) Bromate destruction by UV irradiation and electric arc discharge. Ozone Sci Eng 18:271–290
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Sorlini, S. (2017). Natural Organic Matter: Characterization and Removal by AOPs to Assist Drinking Water Facilities. In: Gil, A., Galeano, L., Vicente, M. (eds) Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment. The Handbook of Environmental Chemistry, vol 67. Springer, Cham. https://doi.org/10.1007/698_2017_159
Download citation
DOI: https://doi.org/10.1007/698_2017_159
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76881-6
Online ISBN: 978-3-319-76882-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)