Abstract
Microbial source tracking (MST) tools are used to identify sources of faecal pollution to accurately assess public health risks and implement best management practices. Many different viruses are excreted by humans and animals and are frequently detected in water contaminated with faeces or/and urine. Because of the large degree of host specificity of each virus and the substantial stability of many excreted viruses in the environment, some viral groups are considered to be accurate MST indicators. The Laboratory of Virus Contaminants of Water and Food at the University of Barcelona has proposed the use of viral indicators as well as cost-effective methods for the concentration of viruses from water. The developed procedures have been used to determine the levels of faecal pollution in environmental samples as well as for tracing the origin of faecal contamination. Such tools were recently used by the Catalan Water Agency to identify nitrate contamination sources in groundwater.
Human adenoviruses, human polyomavirus JC, porcine adenoviruses, bovine polyomaviruses, chicken/turkey parvoviruses, and ovine polyomaviruses can be quantified in samples using molecular methods (qPCR). The selected DNA viruses specifically infect their hosts and are persistently excreted in faeces and/or urine throughout the year in all geographical areas studied. The procedures that have been developed to quantify these viruses have been applied to bathing, coastal, surface and groundwater. In this study, the source of nitrate contamination in groundwater was identified by analysing viral markers, thereby demonstrating the usefulness of the selected viruses for the identification of sources of contamination in water. This methodology can be used to provide information to guide the proper application of measures in place to protect water from pollution caused by nitrates from several sources and thus to facilitate the accurate application of the 91/676/EEC Directive, which is mainly focused on agricultural sources of water contamination.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adenovirus
- Faecal contamination
- Microbial source tracking
- Nitrate contamination
- Polyomavirus
- Viral markers
1 Faecal Contamination in Groundwater
Humans, as well as farmed animals, play an important role in the microbial contamination of water, crops and food and introduce large quantities of pathogens into the environment through their excretions.
Although most pathogens could be removed if sewage, manure and slurry were appropriately treated, many are discharged into receiving waters or may be disposed of in biosolids on land. Pollutants enter the water environment from two main types of sources: point sources, which are single and identifiable sources of contamination, and nonpoint sources, which are more diffuse sources of contamination. Nonpoint sources of contamination may release pollutants intermittently and may be attributable to infiltration from farmland treated with pesticides and fertilisers. Examples of point sources are landfills, leaking gasoline storage tanks, leaking septic tanks and accidental spills. Both point and nonpoint sources of contamination may affect groundwater, and several waterborne disease outbreaks that are believed to have had viral aetiologies have been attributed to the consumption of polluted groundwater [1, 2]. Viruses (23–80 nm) are much smaller than bacteria (0.5–3 μm) and protozoa (4–15 μm) and thus move more easily through soil pores. They are highly stable at low temperatures in the darkness and survive for long periods in groundwater environments. However, relatively limited data on the level of viral contamination in groundwater are available compared with other environmental water matrices [3].
Detailed knowledge about sources of contamination is needed to develop efficient and cost-effective waste management strategies to minimise faecal contamination in watersheds and food, to evaluate the effectiveness of best management practices and to conduct system and risk assessments as part of water- and food-safety plans, as recommended by the World Health Organisation. Faecal sources of contamination have high nitrogen content, and both pathogens and nitrates present in groundwater polluted with faeces may pose a risk to human health when such groundwater is used as a source of drinking water.
Nitrate is the most widespread groundwater quality problem in many countries, and it is the most frequent cause of a groundwater body failing to meet good status under the WFD in some EU countries (http://ec.europa.eu/environment/water/water-nitrates/reports.html), including Catalonia [4]. The principal nitrogen inputs into groundwater are derived from manure, fertilisers, sewage sludge and crop residues from agricultural areas [5]. In the environment, several forms of nitrogen (NO2, NH4, NH3) can potentially be transformed into nitrate (NO3). Various activities may cause nitrate groundwater pollution in agricultural areas. The use of synthetic nitrogen fertilisers as well as the use of organic fertilisers, such as manure and slurries, is the main cause of this pollution. In some areas, high levels of nitrates in groundwater used as a source of drinking water are a consequence of the increase in livestock production that has occurred in recent years. Moreover, an absence of slurry, manure tanks or storage facilities may also contribute to this problem. The disposal of municipal or industrial effluents by spreading sludge on fields may also be a diffuse source of nitrate pollution in groundwater.
Other sources of nitrate pollution in groundwater include the following: interactions between groundwater and surface water, nitrogen-rich effluents, poorly constructed wells that allow water to be exchanged between polluted and nonpolluted aquifer layers, old and badly designed landfills, septic tanks and leaking sewerage systems (http://www.who.int/water_sanitation_health/dwq/chemicals/en/nitrateschap1.pdf).
The intensification of livestock production results in an increase in the amount of animal waste that must be managed. Catalonia, with a population of nearly 7.5 million people, has an important meat industry, with 6.8 million pigs, 0.5 million cattle and 0.6 million sheep [6]. A total of 19 out of 53 (36%) groundwater bodies in Catalonia have been classified as being of poor chemical quality as a result of high nitrate levels. Most of the affected groundwater bodies are located in agricultural areas, although not all stresses on groundwater result from agricultural activities. In some cases, urban wastewater leakage may also contribute to this problem. However, to date, agricultural sources and manure applications on fields, in particular, have been the main causes of pressure on groundwater. Together with nitrogen compounds, faecal microorganisms are released into the environment in manure in holding ponds or storage areas or are applied to pastures to fertilise crops. Most livestock manure is disposed of on the ground, depending on the crop type, and annual quantities of nitrogen that are applied per hectare are specifically restricted in vulnerable areas [7, 8]. However, microorganisms and especially viruses can still, in some cases, infiltrate groundwater. The survival, fate and transport properties of viruses in the environment vary based on the type of virus, viral inactivation kinetics at high temperatures, UV exposure, filtration or adsorption in porous media or sediments and deposition and resuspension of sediments [9, 10].Survival is likely shorter in surface water than in groundwater because of UV exposure, higher temperatures (depending on the time of year and the location) and the opportunity for more interactions with other organisms that can inactivate viruses [11] in superficial water. Tracing and identifying the sources (human and/or animal) of faecal contamination in water are therefore essential, both to improve waste management and to assess risks to human health.
2 Development of Microbial Source Tracking (MST) Techniques
Faecal pollution is a primary health concern in the environment, in water and in food; for this reason, bacterial faecal indicators have been analysed widely to assess the microbiological quality of water, and such assessments are required by water safety regulations. The use of index microorganisms, whose presence points to the possible occurrence of a similar pathogenic organism, and indicator microorganisms, whose presence represents a failure affecting the final product, to assess the microbiological quality of water or food is well-established and has been practised for almost a century.
Classic microbiological indicators, such as faecal coliform bacteria, Escherichia coli and enterococci, are most commonly analysed to evaluate the level of faecal contamination. However, whether these bacteria are suitable indicators of the occurrence and concentration of pathogens such as viruses and protozoan cysts has been questioned for the following reasons: (1) indicator bacteria are more sensitive to inactivation by treatment processes and sunlight than are viral or protozoan pathogens; (2) indicator bacteria may not originate exclusively in faecal sources; (3) indicator bacteria may have an ability to multiply in some environments of interest; (4) it may not be possible to identify the source of faecal contamination; and (5) the presence of indicator bacteria may be poorly correlated with the presence of other pathogens. Thus, various authors have concluded that these indicators could fail to predict the risk of contamination with waterborne pathogens, including viruses [12–16]. Therefore, the team at the Laboratory of Virus Contaminants of Water and Food at the University of Barcelona has proposed that quantitative tests of specific viruses be used as complementary indicators of faecal contamination in water.
Methods for detecting and identifying the source of faecal pollution in the environment are known as microbial source tracking (MST) tools [17, 18]. These methods mainly focus on detecting a microorganism that is intrinsically related to faeces and that thus indicates the presence of contamination and hence of potentially excreted pathogens, such as bacteria, viruses and parasites. MST can assist health and environmental agencies with the identification of sources of faecal contamination. MST tools can also be employed to help make decisions related to the management of drinking water sources, shellfish-growing waters and recreational waters.
A large body of work has been developed in the MST field over the past several years. The first reviews listing the available methods for identifying indicators of faecal pollution in water were published in 2002 [19, 20]. Three years later, the US Environmental Protection Agency published the first guide document [21], and since then, several authors have published newer methods and have compared their applicability with existing methods [18, 22–26]. MST tools can be classified into several broad categories: genotypic versus phenotypic analyses of either cultivated target organisms or indicators or cultivation-independent approaches in which samples from the environment are analysed directly.
Some of the MST methods proposed in the literature lack environmental stability, host specificity and/or global prevalence. Moreover, some MST methods are laborious; they require large and suitable databases for each context and good statistical tools to allow meaningful interpretation of the results [18]. These limitations can be overcome using molecular methods to detect and quantify host-specific viral faecal indicators in water and food. Molecular techniques, specifically nucleic-acid amplification-based assays, provide sensitive, rapid and quantitative analytical tools for studying pathogens, newly emergent strains and indicators that are examined for microbial source tracking. Such methods are used to evaluate the microbiological quality of water [27], the efficiency of virus removal in drinking water and wastewater treatment plants [28–30].
3 Viruses Used for Tracing the Sources of Contamination in Water
Considering the limitations of current standard bacterial faecal indicators, selected viral groups have been proposed as alternative or complementary indicators to improve control of the microbiological quality of water and to reduce microbiological risk. Viruses are more stable than common bacterial indicators in the environment and are usually highly host-specific; because they are host-specific, their detection helps to trace the origin of faecal contamination. The viruses most commonly used for MST to detect faecal pollution are bacteriophages and DNA viruses (Table 1).
These viruses are recognised as important waterborne pathogens that are present in faeces, and new viruses that produce both symptomatic and asymptomatic infections are currently being described by metagenomic techniques [60]. Many orally transmitted viruses produce subclinical infections, and symptoms due to these viruses are only observed in a small proportion of the population. However, some viruses may give rise to life-threatening conditions, such as acute hepatitis in adults, as well as severe gastroenteritis in small children and the elderly. Some of the most important faecal viral pathogens are noroviruses, enteroviruses, adenoviruses, rotaviruses and the hepatitis A and E viruses. Human and animal viruses, such as adenoviruses [41, 42, 52], polyomaviruses [44, 55, 61] and parvoviruses [59], are frequently asymptomatic in immunocompetent hosts and often cause persistent infections. Moreover, they are highly host-specific, highly stable in the environment and resistant to disinfection [42, 62, 63]. Thus, the identification and quantification of specific viruses using molecular assays can be used for MST [42, 44].
3.1 Adenovirus
The Adenoviridae family has a double-stranded DNA genome of approximately 35,000 base pairs (bp) surrounded by a 90–100 nm, non-enveloped, icosahedral capsid with fibrelike projections from each vertex. Adenovirus infection may be caused by consumption of contaminated water or food or by inhalation of aerosols from contaminated waters, such as those used for recreational purposes. HAdV comprises 7 species with 57 types, which are responsible for enteric and respiratory illnesses and eye infections [64–66]. Among animal adenoviruses, porcine adenovirus (PAdV) may cause gastroenteritis symptoms such as diarrhoea, anorexia or dehydration in piglets, while sows can suffer multifactorial respiratory diseases and even abortion [67].
-
Excretion pattern: HAdV particles may be excreted in faeces for months or even years [49, 68]. Fifty per cent of the population has asymptomatic AdV infections at some time, and gastroenteritis occurs in 60% of children under 4 years of age [69]. HAdV40 and 41 serotypes of HAdV can be excreted at high concentrations in faeces (1011 viral particles per gram) and transmitted via the faecal-oral route. Other adenoviruses, such as HAdV-1, HAdV-2, HAdV-5, HAdV-7, HAdV-12 and HAdV-31, are related to respiratory diseases and have also been detected in contaminated water and shellfish [70, 71]. PAdV infections can also be asymptomatic and are detected in nearly 70% of swine faeces, with most isolates being closely related to serotype 3 [49].
-
Prevalence: Human and porcine adenovirus (HAdV) have been detected in contaminated water samples throughout the year in all geographical areas studied [29, 44, 49, 55, 72]. HAdV has been found in nearly 100% of urban wastewater samples tested, including those from cities in Africa, the USA, Central and South America and Europe. Adenoviruses are also frequently detected in shellfish, including samples that met current safety standards based on levels of faecal bacteria [73].
-
Stability: Adenovirus is inactivated only after 2 h at 85°C [74]. With moist heat, the time and temperature of inactivation are slightly reduced; exposure to 65°C for 30 min is then sufficient to inactivate adenovirus particles [75]. Chlorine treatment, which is very commonly used to disinfect and purify water, oxidises viral protein shells and nucleic acids [76]. Nevertheless, infectious HAdV can still be detected after chlorine treatment for 30 min (2.5 mg/L), although its concentration drops by approximately 2.7 log10 [77, 78].
3.2 Polyomavirus
Polyomaviruses are small, icosahedral viruses that have circular, double-stranded DNA genomes approximately 5,000 bp in length and that infect several species of vertebrates. The first human polyomaviruses, JC and BK (JCPyV and BKPyV), were identified in clinical samples from immunocompromised patients [79, 80]. The pathogenicity of JCPyV is commonly associated with progressive multifocal leukoencephalopathy (PML) in immunocompromised states, and infections with this virus have attracted new attention because of JCPyV reactivation and pathogenesis in some patients with autoimmune diseases who are being treated with immunomodulators [81, 82]. Among the known animal polyomaviruses, bovine polyomaviruses (BPyV) does not cause significant pathogenicity in cattle, and no disease has as yet been ascribed to this agent.
-
Excretion pattern: Both human and animal PyVs are excreted in urine by healthy individuals [52, 83, 84]. JCPyVs have been detected in 40–80% of the population, and BPyV has been detected in 30% of the bovine urine samples analysed [52, 61]. Polyomaviruses are transmitted by an unknown mechanism, although it is speculated that respiratory, cutaneous and faecal-oral routes could be involved in their transmission.
-
Prevalence: Human JCPyV is distributed worldwide, and specific antibodies have been detected in over 80% of humans [85]. JCPyV and BKPyV were first described in environmental samples in 2000 [44]. JCPyV is frequently detected in river water, seawater, reclaimed water [72], drinking water [86] and shellfish grown in waters affected by sewage [61]. These viruses are present in nearly 100% of all sewage samples from different geographical areas [72]. BPyV has been identified as the cause of a widely disseminated infection in bovines, and it is a frequent contaminant of commercial bovine serum. BPyV has been detected in river water samples near slaughterhouses, farms and grazing areas [72].
-
Stability: Polyomaviruses, such as SV40, are only significantly affected by exposure to a temperature of 95°C for 1 h [74]. Numbers of JC polyomavirus Mad4 viral particles were reduced by 1.5 to 1.1 log10 GC, as measured by qPCR after 30 min of contact (2.5 mg/L), although no infectivity assays were conducted for this virus in these studies [77].
3.3 Parvovirus
The Parvoviridae family comprises small animal viruses with 5 kb linear, single-stranded DNA genomes with two large open reading frames. This family of viruses is divided in two subfamilies: the Parvoviridae, which mainly infect vertebrates, and the Densoviridae, which infect arthropod hosts.
-
Excretion pattern: Human parvoviruses have been detected in stool samples, but their transmission pathways remain unclear [87, 88].
-
Stability: These viruses have shown high resistance to temperature and low pH [89, 90] and have been found in commercial meat samples [91]. Bovine parvovirus was not significantly affected by exposure to 95°C for 2 h [74].
-
Prevalence: When sewage water, as a representative matrix that can be used to test large populations, was monitored, a high prevalence (81%) of parvovirus was observed [92]. Avian parvoviruses are excreted in poultry faeces and have been reported in studies from different countries [59].
4 Methods for the Use of Viral Markers for MST in Groundwater
Viruses are present in the environment in low concentrations and are distributed unevenly. To detect viruses in the environment, it is essential to collect a significant volume of sample and to concentrate the viral particles before employing a detection assay. Detection of viruses in minimally or moderately polluted waters requires that viruses from at least several litres of water be concentrated into a much smaller volume (Fig. 1).
There are several concentration methods available, and many of them include two concentration steps in series, which will affect the recovery efficiency of the whole process. The development of cost-effective methods for the concentration of viruses from water and of cost-effective molecular assays, as well, facilitates the use of viruses as indicators of faecal contamination and as MST tools. The first methods used were based on the detection of viral indicators by PCR [41, 42, 44, 49]; more recently, quantitative PCR techniques have been developed that allow not only the detection but also the quantification of these viruses in environmental samples [29, 52, 58, 59] (Fig. 2).
It has been proposed that HAdVs and JCPyVs be quantified to trace human faecal contamination. HAdVs are present in sewage samples from all geographical areas that have been studied, while JCPyV is a less abundant but highly human-specific virus [93]. For this reason, the analysis of both viruses to determine the extent of human faecal pollution of environmental samples is a good approach that has a specificity of 100%. Both viruses have been evaluated in various studies in different water matrices, and their utility in MST has been demonstrated (Tables 2 and 3).
Porcine adenoviruses (PAdVs) and bovine polyomaviruses (BPyVs) have been proposed as porcine and bovine faecal indicators [49, 51], and several studies have shown that these viruses are widely disseminated in swine and bovine populations, respectively, without producing clinically severe disease (Table 4) and are thus useful MST tools.
More recently, the quantification of ovine polyomaviruses and chicken/turkey polyomavirus has been suggested for tracing ovine and poultry faecal pollution, respectively [58, 59]. Quantification of each of these viruses has been used to trace the origins of nitrate pollution in groundwater in some areas of Catalonia, as described in the next section.
5 Case Study: Identification of the Sources of Nitrate Contamination in Catalonian Groundwater
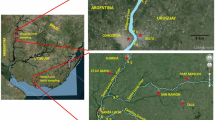
Virus-detection assays have been used to detect viruses in groundwater samples from diverse areas in which nitrate levels exceeded >50 mg/L [137, 138] to trace the origins of nitrate pollution, as a collaborative study with the Catalan Water Agency and the Laboratory of Virus Contaminants of Water and Food from the University of Barcelona.
To ensure the designation of vulnerable zones according to the Directive against pollution caused by nitrates from agricultural sources [138], a total of 14 different monitoring stations were evaluated (Table 5). This study aimed to determine whether the pollution sources in these areas were manure, urban wastewater sludge or chemical fertilisers applied for agricultural uses. From three to five samples were taken per well for later analysis for the presence of different human and animal viruses. Samples were assayed for the presence of human adenovirus (HAdV) and human polyomavirus (JCPyV) to detect human pollution sources (from urban wastewater sludge used in agriculture or from sewage leaks); samples were assayed for the presence of porcine adenovirus (PAdV) to detect porcine sources of pollution (from the application of pig manure); and samples were assayed for the presence of bovine polyomavirus (BPyV) to detect bovine sources of pollution (from cow manure applications; Table 5).
Viruses were concentrated using the procedure described by Calgua and coworkers [131], based on flocculation with skimmed milk. After viruses were concentrated from 10 L water samples, viral nucleic acids were extracted. Then, qPCR assays specific for human adenoviruses (HAdV), JC polyomavirus (JCPyV), porcine adenoviruses (PAdV), bovine polyomaviruses (BPyV), ovine polyomaviruses (OPyV) and chicken/turkey parvoviruses (Ch/TuPV) were used to determine the relative quantities of each of these viruses in the samples and hence to determine the source of faecal contamination. The source of faecal contamination determined in this way is then indicative of the source of nitrates in the groundwater from which samples were taken [43, 45, 52, 55].
The results obtained by qPCR were further confirmed by nested PCR and sequencing, as previously described [44, 49, 51]. The results obtained are summarised in Table 5.
The results show that in one area (Olius), faecal/urine contamination of porcine origin is clearly present (4/4 replicates tested positive), strongly suggesting that the application of swine slurries could be a significant source of nitrate contamination in the groundwater at that location. In the Pinós area, for which low levels of nitrate were measured, sporadic bovine contamination was detected (1/4 replicates tested positive), and diffuse contamination or the application of bovine manure was considered to be the potential source of the viruses that were detected. Finally human faecal pollution was detected as the main source of contamination in 4 other studied areas; further investigation is needed to identify the sources of contamination in these areas. This methodology has been tested in areas where nitrate concentrations are above the statutory limit (i.e. >50 mg/L) and thus where the use of groundwater as drinking water is compromised. A greater number of samples would be required to determine whether a relationship exists between the concentration of nitrates and the presence of the virus.
This study determines the origins of contamination of nitrates in groundwater, so that their sources (urban, animal or inorganic fertiliser use, in the case that viruses were not detected) could be established. The conclusions of this study could have implications for the future management of water in the region.
6 Conclusions and Future Trends
Groundwater is a vital source of water that provides, in Europe alone, drinking water for 300 million inhabitants. In Catalonia, over 587 hm3/year of groundwater is used, and this amount represents close to 20% of the total water used in the region. Today, high nitrate levels in groundwater remain an important target for pollution reduction worldwide, with implications for human and environmental health [139]. Nitrate and pesticide pollution from agricultural sources are major, well-known problems with groundwater quality, and increases in water demand and population density will increase the probability of faecal contamination of groundwater. Moreover, falling groundwater levels will further endanger the quality of groundwater and its ability to clean itself. In addition to these problems, overabstraction has already begun to induce saltwater intrusion along most stretches of the Mediterranean coast, rendering the groundwater in those areas useless for drinking and most other purposes. Appropriate management of water resources and more specifically of groundwater resources requires the reliable evaluation of water quality and the identification of sources of contamination. These measures are needed to prevent further contamination, to implement remediation measures and especially to provide information that can be used to institute measures to protect waters from pollution caused by nitrates from agricultural sources [138].
Currently, microbiological quality assessments of environmental waters largely rely on detecting faecal indicator bacteria. Although this approach has clearly reduced health risks in many countries, the faecal indicator approach may be combined with monitoring of more environmentally stable viral indicators specific to human and animal sources of contamination [27, 93]. The viral MST tools developed in this study can be used to track faecal contamination of human, bovine, ovine, porcine and avian origins using specific individual assays or, in the near future, using multiplex assays. Multiplex diagnostic tools are already available, and multiplex quantitative PCR assays for MST have been described previously in a study that examined diverse human and animal viruses [133].
Human (HAdV, JCPyV), bovine (BPyV), porcine (PAdV) and ovine (OPyV) viral markers have been shown to be useful for identifying the origin of faecal contamination in river water and seawater in Brazil, Sweden, Spain, Hungary, Greece and New Zealand [58, 72]. However, more information on the environmental stability and distribution of viruses in diverse geographical areas and water matrices will be needed to validate some animal viral markers, including new viral MST tools that may be developed in the future for other animals representing other sources of contamination.
Routine quantitative PCR assays for viral indicators may also be improved and standardised, in light of new methods that have been developed that allow the absolute quantification of genome copies without requiring that independent calibration curves be generated [140]. Other technical improvements that can be expected include advances in microfluidics and nanobiotechnology, as a result of which miniaturised systems for the detection of viral indicators could be developed that are based on microchips. Several such approaches have been described [141, 142]. New technologies, such as high-throughput mass sequencing, have been used to analyse urban sewage from diverse geographical areas and have produced a wealth of data about the viruses present in wastewater [60]. However, further development of NGS techniques are still needed to provide more sensitive and affordable assays that could potentially be used for routine analyses.
Cost-effective methods for using specific DNA viruses as markers of the source of faecal (or nitrate) contamination in water have been developed and validated. These methods may be standardised to acceptable levels of cost, feasibility, sensitivity and repeatability, especially in the case of the DNA viruses selected in our MST studies. The sampling strategies should also be considered carefully to obtain samples that best represent the water in question. Ideal sampling strategies could involve the use of hydrological and physicochemical sensors and time- and flow-integrating automated sampling devices.
Abbreviations
- ACA:
-
Catalan Water Agency (in Catalan)
- CRBD:
-
Catalan River Basin District
- WFD:
-
Water Framework Directive
References
Hunt RJ, Borchardt MA, Richards KD, Spencer SK (2010) Assessment of sewer source contamination of drinking water wells using tracers and human enteric viruses. Environ Sci Technol 44:7956–7963. doi:10.1021/es100698m
Borchardt MA, Spencer SK, Kieke BA, Lambertini E, Loge FJ (2012) Viruses in nondisinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ Health Perspect 120:1272–1279. doi:10.1289/ehp.1104499
Abbaszadegan BYM, Lechevallier M, Gerba C (2003) Occurrence of viruses in ground waters. Am Water Works Assoc J 95:107–120
ACA (2012) Agència Catalana de l’Aigua, Generalitat de Catalunya. http://www.gencat.cat/aca/
DEFRA (2006) Department for Environment, Food and Rural Affairs and the Forestry Commission, 2006 Report. http://archive.defra.gov.uk/corporate/about/reports/documents/2006deptreport.pdf. Accessed 17 Dec 2014
IDESCAT (2012) Anuari estadístic de Ramaderia a Catalunya. http://www.idescat.cat/pub/?id=aec&n=48. Accessed 21 Mar 2014
Decret 136/2009 (2009) Diari Oficial de la Generalitat de Catalunya, no5457, d’1 de setembre, d'aprovació del programa d’actuació aplicable a les zones vulnerables en relació amb la contaminació de nitrats que procedeixen de fonts agràries i de gestió de les dejeccions ra. 65858–65902
Decret 220/2001 (2001) Diari Oficial de la Generalitat de Catalunya, no3447, d’1 d'agost, de gestió de les dejeccions ramaderes. 2001:1–8
Rzezutka A, Cook N (2004) Survival of human enteric viruses in the environment and food. FEMS Microbiol Rev 28:441–453. doi:10.1016/j.femsre.2004.02.001
John DE, Rose JB (2005) Review of factors affecting microbial survival in groundwater. Environ Sci Technol 39:7345–7356
Meixell BW, Borchardt MA, Spencer SK (2013) Accumulation and inactivation of avian influenza virus by the filter-feeding invertebrate Daphnia magna. Appl Environ Microbiol 79:7249–7255. doi:10.1128/AEM.02439-13
Gerba CP, Goyal SM, LaBelle RL, Cech I, Bodgan GF (1979) Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am J Public Health 69:1116–1119
Solo-gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ (2000) Sources of Escherichia coli in a Coastal Subtropical Environment. Appl Environ Microbiol 66:230–237
Lipp EK, Farrah SA, Rose JB (2001) Assessment and impact of microbial fecal pollution and human enteric pathogens in a coastal community. Mar Pollut Bull 42:286–293
Byappanahalli MN, Shively DA, Nevers MB, Sadowsky MJ, Whitman RL (2003) Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol Ecol 46:203–211. doi:10.1016/S0168-6496(03)00214-9
Pote J, Haller L, Kottelat R, Sastre V, Arpagaus P, Wildi W (2009) Persistence and growth of faecal culturable bacterial indicators in water column and sediments of Vidy Bay, Lake Geneva, Switzerland. J Environ Sci (China) 21:62–69
Field KG, Samadpour M, Wuertz S, Field J (2007) Fecal source tracking, the indicator paradigm, and managing water quality. Water Res 41:3517–3538. doi:10.1016/j.watres.2007.06.056
Stoeckel DM, Harwood VJ (2007) Microbial source tracking studies MINIREVIEW. Appl Environ Microbiol. doi:10.1128/AEM.02473-06
Scott TM, Rose JB, Jenkins TM, Samuel R, Lukasik J, Farrah SR (2002) Microbial source tracking : current methodology and future directions microbial source tracking : current methodology and future directions. Appl Environ Microbiol 68:5796–5803. doi:10.1128/AEM.68.12.5796
Simpson J, Santo Domingo J, Reasoner D (2002) Microbial source tracking: state of the science. Environ Sci Technol 36:5279–5288
EPA (2005) Microbial source tracking guide document. U.S. Environmental Protection Agency, Cincinnati
Savichtcheva O, Okabe S (2006) Alternative indicators of fecal pollution: relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res 40:2463–2476
Roslev P, Bukh A (2011) State of the art molecular markers for fecal pollution source tracking in water. Appl Microbiol Biotechnol 89:3080. doi:10.1007/s00253-010-3080-7
Wu J, Long SC, Das D, Dorner SM (2011) Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health 9(2):265–278. doi:10.2166/wh.2011.117
Boehm AB, Van De Werfhorst LC, Griffith JF, Holden PA, Jay JA, Shanks OC, Wang D, Weisberg SB (2013) Performance of forty-one microbial source tracking methods: a twenty-seven lab evaluation study. Water Res 47:6812–6828. doi:10.1016/j.watres.2012.12.046
Harwood VJ, Staley C, Badgley BD, Borges K, Korajkic A (2013) Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol Rev. doi:10.1111/1574-6976.12031
Girones R, Ferrús M, Alonso J, Rodriguez-Manzano J, Calgua B, Corrêa A, Hundesa A, Carratala A, Bofill-Mas S (2010) Molecular detection of pathogens in water–the pros and cons of molecular techniques. Water Res 44:4325–4339. doi:10.1016/j.watres.2010.06.030
Albinana-Gimenez N, Miagostovich MP, Calgua B, Huguet JM, Matia L, Girones R (2009) Analysis of adenoviruses and polyomaviruses quantified by qPCR as indicators of water quality in source and drinking-water treatment plants. Water Res 43:2011–2019. doi:10.1016/j.watres.2009.01.025
Bofill-Mas S, Albinana-Gimenez N, Clemente-Casares P, Hundesa A, Rodriguez-Manzano J, Allard A, Calvo M, Girones R (2006) Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol 72:7894–7896. doi:10.1128/AEM.00965-06
Brady AMG, Gellner TM, Spencer SK, Williston AG, Borchardt MA, Bushon RN, Francy DS, Riddell KR, Stelzer EA (2012) Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal waste waters. Water Res 46:4164–4178. doi: 10.1016/j.watres.2012.04.044
Havelaar AH, Furuse K, Hogeboom WM (1986) Bacteriophages and indicator bacteria in human and animal faeces. J Appl Bacteriol 60:255–262
Hsu FC, Shieh YS, van Duin J, Beekwilder MJ, Sobsey MD (1995) Genotyping male-specific RNA coliphages by hybridization with oligonucleotide probes. Appl Environ Microbiol 61:3960–3966
Schaper M, Jofre J, Uys M, Grabow WOK (2002) Distribution of genotypes of F-specific RNA bacteriophages in human and non-human sources of faecal pollution in South Africa and Spain. J Appl Microbiol 92:657–667
Kirs M, Smith DC (2007) Multiplex quantitative real-time reverse transcriptase PCR for F+-specific RNA coliphages: a method for use in microbial source tracking. Appl Environ Microbiol 73:808–814. doi:10.1128/AEM.00399-06
Kim S-H, Cheon D-S, Kim J-H, Lee D-H, Jheong W-H, Heo Y-J, Chung H-M, Jee Y, Lee J-S (2005) Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of norovirus. J Clin Microbiol 43:4836–4839. doi:10.1128/JCM.43.9.4836
Chung H, Sobsey MD (1993) Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water Sci Tech 27:425–428
Doré WJ, Henshilwood K, Lees DN (2000) Evaluation of F-specific RNA bacteriophage as a candidate human enteric virus indicator for bivalve molluscan shellfish. Appl Environ Microbiol 66:1280–1285
Tartera C, Jofre J (1987) Bacteriophages active against Bacteroides fragilis in sewage-polluted waters. Appl Environ Microbiol 53:1632–1637
Gómez-Doñate M, Payán A, Cortés I, Blanch AR, Lucena F, Jofre J, Muniesa M (2011) Isolation of bacteriophage host strains of Bacteroides species suitable for tracking sources of animal faecal pollution in water. Environ Microbiol 13:1622–1631. doi:10.1111/j.1462-2920.2011.02474.x
Tartera C, Lucena F, Jofre J (1989) Human origin of Bacteroides fragilis bacteriophages present in the environment. Appl Environ Microbiol 55:2696–2701
Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R (1994) Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol 60:2963–2970
Pina S, Lucena F, Girones R (1998) Viral pollution in the environment and in Shellfish : human adenovirus detection by PCR as an index of human viruses viral pollution in the environment and in Shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol 64:3376
Hernroth B, Conden-Hansson A, Rehnstam-Holm A, Girones R, Allard A (2002) Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: the first Scandinavian report. Appl Environ Microbiol 68:4523–4533. doi:10.1128/AEM.68.9.4523
Bofill-Mas S, Pina S, Girones R (2000) Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol 66:238–245
Pal A, Sirota L, Maudru T, Peden K, Lewis AM (2006) Real-time, quantitative PCR assays for the detection of virus-specific DNA in samples with mixed populations of polyomaviruses. J Virol Methods 135:32–42. doi:10.1016/j.jviromet.2006.01.018
Fong T-T, Griffin DW, Lipp EK (2005) Molecular assays for targeting human and bovine enteric viruses in coastal waters and their application for library-independent source tracking. Appl Environ Microbiol 71:2070–2078. doi:10.1128/AEM.71.4.2070-2078.2005
Wong K, Fong T-T, Bibby K, Molina M (2012) Application of enteric viruses for fecal pollution source tracking in environmental waters. Environ Int 45:151–164. doi:10.1016/j.envint.2012.02.009
Rosario K, Symonds EM, Sinigalliano C, Stewart J, Breitbart M (2009) Pepper mild mottle virus as an indicator of fecal pollution. Appl Environ Microbiol 75:7261–7267. doi:10.1128/AEM.00410-09
Maluquer de Motes C, Clemente-Casares P, Hundesa A, Martín M, Girones R (2004) Detection of bovine and porcine adenoviruses for tracing the source of fecal contamination. Appl Environ Microbiol 70:1448–1454. doi:10.1128/AEM.70.3.1448
Wong K, Xagoraraki I (2011) A perspective on the prevalence of DNA enteric virus genomes in anaerobic-digested biological wastes. Environ Monit Assess. doi:10.1007/s10661-011-2316-z
Hundesa A, Maluquer de Motes C, Bofill-Mas S, Albinana-Gimenez N, Girones R (2006) Identification of human and animal adenoviruses and polyomaviruses for determination of sources of fecal contamination in the environment. Appl Environ Microbiol 72:7886–7893. doi:10.1128/AEM.01090-06
Hundesa A, Bofill-Mas S, Maluquer de Motes C, Rodriguez-Manzano J, Bach A, Casas M, Girones R (2010) Development of a quantitative PCR assay for the quantitation of bovine polyomavirus as a microbial source-tracking tool. J Virol Methods 163:385–389. doi:10.1016/j.jviromet.2009.10.029
Ley V, Higgins J, Fayer R (2002) Bovine enteroviruses as indicators of fecal contamination. Appl Environ Microbiol 68:3455–3461
Jiménez-Clavero MA, Escribano-Romero E, Mansilla C, Gómez N, Córdoba L, Roblas N, Ponz F, Ley V, Sáiz J-C (2005) Survey of bovine enterovirus in biological and environmental samples by a highly sensitive real-time reverse transcription-PCR. Appl Environ Microbiol 71:3536–3543. doi:10.1128/AEM.71.7.3536-3543.2005
Hundesa A, Maluquer de Motes C, Albinana-Gimenez N, Rodriguez-Manzano J, Bofill-Mas S, Suñen E, Rosina Girones R (2009) Development of a qPCR assay for the quantification of porcine adenoviruses as an MST tool for swine fecal contamination in the environment. J Virol Methods 158:130–135. doi:10.1016/j.jviromet.2009.02.006
Viancelli A, Garcia LAT, Kunz A, Steinmetz R, Esteves PA, Barardi CRM (2012) Research in Veterinary Science Detection of circoviruses and porcine adenoviruses in water samples collected from swine manure treatment systems. Res Vet Sci 93:538–543. doi:10.1016/j.rvsc.2011.07.022
Jiménez-Clavero MA, Fernández C, Ortiz JA, Pro J, Carbonell G, Tarazona JV, Roblas N, Ley V (2003) Teschoviruses as indicators of porcine fecal contamination of surface water. Appl Environ Microbiol 69:6311–6315. doi:10.1128/AEM.69.10.6311
Rusiñol M, Carratalà A, Hundesa A, Bach A, Kern A, Vantarakis A, Girones R, Bofill-Mas S (2013) Description of a novel viral tool to identify and quantify ovine faecal pollution in the environment. Sci Total Environ 458–460:355–360. doi:10.1016/j.scitotenv.2013.04.028
Carratalà A, Rusiñol M, Hundesa A, Biarnes M, Rodriguez-Manzano J, Vantarakis A, Kern A, Sunen E, Girones R, Bofill-Mas S (2012) A novel tool for specific detection and quantification of chicken/Turkey parvoviruses to trace poultry fecal contamination in the environment. Appl Environ Microbiol 78:7496–7499. doi:10.1128/AEM.01283-12
Cantalupo PG, Calgua B, Zhao G, Hundesa A, Wier AD, Katz JP, Grabe M, Hendrix RW, Girones R, Wang D, Pipas JM (2011) Raw sewage harbors diverse viral populations. mBio 2:e00180–11–e00180–11. doi:10.1128/mBio.00180-11.Editor
Bofill-Mas S, Formiga-cruz M, Clemente-casares P, Calafell F, Girones R (2001) Potential Transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol 75:10290–10299. doi:10.1128/JVI.75.21.10290
Calgua B, Carratalà A, Guerrero-Latorre L, de Abreu CA, Kohn T, Sommer R, Girones R (2014) UVC inactivation of dsDNA and ssRNA viruses in water: UV fluences and a qPCR-based approach to evaluate decay on viral infectivity. Food Environ Virol. doi:10.1007/s12560-014-9157-1
Wyn-jones AP, Carducci A, Cook N, D’Agostino M, Divizia M, Fleischer J, Gantzer C, Gawler A, Girones R, Höller C, de Roda Husman AM, Kay D, Kozyra I, López-Pila J, Muscillo M, Nascimento MSJ, Papageorgiou G, Rutjes S, Sellwood J, Szewzyk R, Wyer M, Agostino MD, Ho C, Maria A, Husman DR, Sa M, Lo J (2011) Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res 5:1025–1038. doi:10.1016/j.watres.2010.10.015
Jones MS, Harrach B, Ganac RD, Gozum MMA, Dela Cruz WP, Riedel B, Pan C, Delwart EL, Schnurr DP (2007) New adenovirus species found in a patient presenting with gastroenteritis. J Virol 81:5978–5984. doi:10.1128/JVI.02650-06
Robinson CM, Singh G, Henquell C, Walsh MP, Peigue-Lafeuille H, Seto D, Jones MS, Dyer DW, Chodosh J (2011) Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology 409:141–147. doi:10.1016/j.virol.2010.10.020
Walsh MP, Seto J, Liu EB, Dehghan S, Hudson NR, Lukashev AN, Ivanova O, Chodosh J, Dyer DW, Jones MS, Seto D (2011) Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J Clin Microbiol 49:3482–3490. doi:10.1128/JCM.00156-11
Buller CR, Moxley RA (1988) Natural infection of porcine ileal dome M cells with rotavirus and enteric adenovirus. Vet Pathol 25:516–517
Adrian T, Schäfer G, Cooney MK, Fox JP, Wigand R (1988) Persistent enteral infections with adenovirus types 1 and 2 in infants: no evidence of reinfection. Epidemiol Infect 101:503–509
Sharp IR, Wadell G (1995) Adenoviruses. In: Zuckerman AJ, Banatvala JE, Pattison JR (eds) Principles and practice of clinical virology, 3rd edn. Wiley, New York, pp 287–308
Formiga-Cruz M, Tofiño-Quesada G, Bofill-Mas S, Lees DN, Henshilwood K, Allard a K, Conden-Hansson A-C, Hernroth BE, Vantarakis A, Tsibouxi A, Papapetropoulou M, Furones MD, Girones R (2002) Distribution of human virus contamination in shellfish from different growing areas in Greece, Spain, Sweden, and the United Kingdom. Appl Environ Microbiol 68:5990–5998. doi:10.1128/AEM.68.12.5990
Bofill-Mas S, Rodriguez-Manzano J, Calgua B, Carratala A, Girones R (2010) Newly described human polyomaviruses Merkel cell, KI and WU are present in urban sewage and may represent potential environmental contaminants. Virol J 7:141. doi:10.1186/1743-422X-7-141
Rusiñol M, Fernandez-Cassi X, Hundesa A, Vieira C, Kern A, Eriksson I, Ziros P, Kay D, Miagostovich M, Vargha M, Allard A, Vantarakis A, Wyn-Jones P, Bofill-Mas S, Girones R (2014) Application of human and animal viral microbial source tracking tools in fresh and marine waters from five different geographical areas. Water Res 59:119–129. doi:10.1016/j.watres.2014.04.013
Rodriguez-Manzano J, Hundesa A, Calgua B, Carratala A, Maluquer de Motes C, Rusiñol M, Moresco V, Ramos AP, Martínez-Marca F, Calvo M, Monte Barardi CR, Girones R, Bofill-Mas S (2013) Adenovirus and norovirus contaminants in commercially distributed shellfish. Food Environ Virol. doi:10.1007/s12560-013-9133-1
Sauerbrei A, Wutzler P (2009) Testing thermal resistance of viruses. Arch Virol 154:115–119. doi:10.1007/s00705-008-0264-x
Brodsky I, Rowe W, Hartley J, Lane W (1959) Studies of mouse polyoma virus infection II Virus stability. J Exp Med 109:439–447
Page MA, Shisler JL, Mariñas BJ (2010) Mechanistic aspects of adenovirus serotype 2 inactivation with free chlorine. Appl Environ Microbiol 76:2946–2954. doi:10.1128/AEM.02267-09
deAbreu CA, Carratalà A, Calvo M, Barardi CRM, Bofill-Mas S, Girones R, de Abreu CA (2012) Comparative inactivation of murine norovirus, human adenovirus, and human JC polyomavirus by chlorine in seawater. Appl Environ Microbiol 78:6450–6457. doi:10.1128/AEM.01059-12
Girones R, Carratalà A, Calgua B, Calvo M, Rodriguez-Manzano J, Emerson S (2014) Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. J Water Health 12:436–442. doi:10.2166/wh.2014.027
Gardner SD, Field AM, Coleman DV, Hulme B (1971) New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1:1253–1257
Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH (1971) Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1:1257–1260
Berger JR, Houff SA, Major EO (2009) Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs 1:583–589
Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue E-W, Jäger HR, Clifford DB (2006) Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 354:924–933. doi:10.1056/NEJMoa054693
Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y (1990) High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J Infect Dis 161:1128–1133
Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL (1999) JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 52:253–260
Weber T (1997) Molecular epidemiology and emerging infectious diseases of the nervous system. J Neurovirol 3(Suppl 1):S46–S49
Albinana-Gimenez N, Clemente-casares P (2006) Distribution of human polyoma-viruses, adenoviruses, and hepatitis E virus in the environment and in a drinking-water treatment plant. Environ Sci Technol 40:7416–7422
Norja P, Hedman L, Kantola K, Kemppainen K, Suvilehto J, Pitkäranta A, Aaltonen L-M, Seppänen M, Hedman K, Söderlund-Venermo M (2012) Occurrence of human bocaviruses and parvovirus 4 in solid tissues. J Med Virol 84:1267–1273. doi:10.1002/jmv.23335
Väisänen E, Kuisma I, Phan TG, Delwart E, Lappalainen M, Tarkka E, Hedman K, Söderlund-Venermo M (2014) Bufavirus in feces of patients with gastroenteritis, Finland. Emerg Infect Dis 20:1077–1080. doi:10.3201/eid2006.131674
Nath Srivastava R, Lund E (1980) The stability of bovine parvovirus and its possible use as an indicator for the persistence of enteric viruses. Water Res 14:1017–1021. doi:10.1016/0043-1354(80)90146-3
Baylis SA, Tuke PW, Miyagawa E, Blümel J (2013) Studies on the inactivation of human parvovirus 4. Transfusion 53:2585–2592. doi:10.1111/trf.12372
Zhang W, Li L, Deng X, Kapusinszky B, Delwart E (2014) What is for dinner? Viral metagenomics of US store bought beef, pork, and chicken. Virology 468–470:303–310. doi:10.1016/j.virol.2014.08.025
Blinkova O, Rosario K, Li L, Kapoor A, Slikas B, Bernardin F, Breitbart M, Delwart E (2009) Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J Clin Microbiol 47:3507–3513. doi:10.1128/JCM.01062-09
Bofill-Mas S, Rusiñol M, Fernandez-Cassi X, Carratalà A, Hundesa A, Girones R (2013) Quantification of human and animal viruses to differentiate the origin of the fecal contamination present in environmental samples. BioMed Res Int 2013:192089. doi:10.1155/2013/192089
Choi S, Jiang SC (2005) Real-time PCR quantification of human adenoviruses in urban rivers indicates genome prevalence but low infectivity. Appl Environ Microbiol 71:7426–7433. doi:10.1128/AEM.71.11.7426
He J-W, Jiang S (2005) Quantification of enterococci and human adenoviruses in environmental samples by real-time PCR. Appl Environ Microbiol 71:2250–2255. doi:10.1128/AEM.71.5.2250
Haramoto E, Katayama H, Oguma K, Ohgaki S (2005) Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Appl Environ Microbiol 71:2403–2411. doi:10.1128/AEM.71.5.2403
Dong Y, Kim J, Lewis GD (2010) Evaluation of methodology for detection of human adenoviruses in wastewater, drinking water, stream water and recreational waters. J Appl Microbiol 108:800–809. doi:10.1111/j.1365-2672.2009.04477.x
Heim A, Ebnet C, Harste G, Pring-Akerblom P (2003) Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol 70:228–239. doi:10.1002/jmv.10382
Hamza IA, Jurzik L, Stang A, Sure K, Uberla K, Wilhelm M (2009) Detection of human viruses in rivers of a densly-populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res 43:2657–2668. doi:10.1016/j.watres.2009.03.020
Ogorzaly L, Tissier A, Bertrand I, Maul A, Gantzer C (2009) Relationship between F-specific RNA phage genogroups, faecal pollution indicators and human adenoviruses in river water. Water Res 43:1257–1264. doi:10.1016/j.watres.2008.12.011
Bofill-Mas S, Hundesa A, Calgua B, Rusiñol M, Maluquer de Motes C, Girones R (2011) Cost-effective method for microbial source tracking using specific human and animal viruses. J Vis Exp 58:2820. doi:10.3791/2820
Haramoto E, Kitajima M, Katayama H, Ohgaki S (2010) Real-time PCR detection of adenoviruses, polyomaviruses, and torque teno viruses in river water in Japan. Water Res 44:1747–1752. doi:10.1016/j.watres.2009.11.043
Jurzik L, Hamza IA, Puchert W, Uberla K, Wilhelm M (2010) Chemical and microbiological parameters as possible indicators for human enteric viruses in surface water. Int J Hyg Environ Health 213:210–216. doi:10.1016/j.ijheh.2010.05.005
Rigotto C, Victoria M, Moresco V, Kolesnikovas CK, Corre AA, Miagostovich MP (2010) Assessment of adenovirus, hepatitis A virus and rotavirus presence in environmental samples in Florianopolis, South. J Appl Microbiol. doi:10.1111/j.1365-2672.2010.04827.x
Schlindwein A, Rigotto C, Cm S, Cr B (2010) Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Sci Technol 61:2166. doi:10.2166/wst.2010.845.Detection
Calgua B, Barardi C, Bofill-Mas S, Rodriguez-Manzano J, Girones R (2011) Detection and quantitation of infectious human adenoviruses and JC polyomaviruses in water by immunofluorescence assay. J Virol Methods 171:1–7. doi:10.1016/j.jviromet.2010.09.013
Gibson KE, Opryszko MC, Schissler JT, Guo Y, Schwab KJ (2011) Evaluation of human enteric viruses in surface water and drinking water resources in southern Ghana. Am J Trop Med Hyg 84:20–29. doi:10.4269/ajtmh.2011.10-0389
Guerrero-Latorre L, Carratala A, Rodriguez-Manzano J, Calgua B, Hundesa A, Girones R (2011) Occurrence of water-borne enteric viruses in two settlements based in Eastern Chad: analysis of hepatitis E virus, hepatitis A virus and human adenovirus in water sources. J Water Health 9:515. doi:10.2166/wh.2011.126.Occurrence
Hamza IA, Jurzik L, Überla K, Wilhelm M (2011) Methods to detect infectious human enteric viruses in environmental water samples. Int J Hyg Environ Health 214:424–436. doi:10.1016/j.ijheh.2011.07.014
Kokkinos PA, Ziros PG, Mpalasopoulou A, Galanis A, Vantarakis A (2011) Molecular detection of multiple viral targets in untreated urban sewage from Greece. Virol J 8:195. doi:10.1186/1743-422X-8-195
Barardi CRM, Viancelli A, Rigotto C, Pilotto MR, Garcia LA. T, Kunz A, Esteves PA (2012) Surveillance of human and swine adenovirus, human norovirus and swine circovirus in water samples in Santa Catarina, Brazil. J Water Health 10:445–52. doi:10.2166/wh.2012.190
Fongaro G, Nascimento MA, Viancelli A, Tonetta D, Petrucio MM, Barardi CRM (2012) Surveillance of human viral contamination and physicochemical profiles in a surface water lagoon. Water Sci Technol 66:2682–2687. doi:10.2166/wst.2012.504
Rodriguez-Manzano J, Alonso JL, Ferrús MA, Moreno Y, Amorós I, Calgua B, Hundesa A, Guerrero-Latorre L, Carratala A, Rusiñol M, Girones R (2012) Standard and new faecal indicators and pathogens in sewage treatment plants, microbiological parameters for improving the control of reclaimed water. Water Sci Technol 66:2517–2523. doi:10.2166/wst.2012.233
Fumian TM, Vieira CB, Leite JPG, Miagostovich MP (2013) Assessment of burden of virus agents in an urban sewage treatment plant in Rio de Janeiro, Brazil. J Water Health 11:110–119. doi:10.2166/wh.2012.123
Hewitt J, Greening GE, Leonard M, Lewis GD (2013) Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Res 47:6750–6761. doi:10.1016/j.watres.2013.09.001
Katukiza AY, Temanu H, Chung JW, Foppen JWA, Lens PNL (2013) Genomic copy concentrations of selected waterborne viruses in a slum environment in Kampala, Uganda. J Water Health 11:358–370. doi:10.2166/wh.2013.184
Sidhu JPS, Ahmed W, Gernjak W, Aryal R, McCarthy D, Palmer A, Kolotelo P, Toze S (2013) Sewage pollution in urban stormwater runoff as evident from the widespread presence of multiple microbial and chemical source tracking markers. Sci Total Environ 463–464:488–496. doi:10.1016/j.scitotenv.2013.06.020
Sidhu JPS, Ahmed W, Toze S (2013) Sensitive detection of human adenovirus from small volume of primary wastewater samples by quantitative PCR. J Virol Methods 187:395–400. doi:10.1016/j.jviromet.2012.11.002
Ye XXY, Ming X, Zhang YLY, Xiao WQW, Huang XN, Cao YG, Gu KD (2012) Real-time PCR detection of enteric viruses in source water and treated drinking water in Wuhan, China. Curr Microbiol 65:244–253. doi:10.1007/s00284-012-0152-1
Lee CS, Lee C, Marion J, Wang Q, Saif L, Lee J (2014) Occurrence of human enteric viruses at freshwater beaches during swimming season and its link to water inflow. Sci Total Environ 472:757–766. doi:10.1016/j.scitotenv.2013.11.088
McQuaig SM, Scott TM, Lukasik JO, Paul JH, Harwood VJ (2009) Quantification of human polyomaviruses JC Virus and BK Virus by TaqMan quantitative PCR and comparison to other water quality indicators in water and fecal samples. Appl Environ Microbiol 75:3379–3388. doi:10.1128/AEM.02302-08
Biel SS, Held TK, Landt O, Niedrig M, Gelderblom HR, Siegert W, Nitsche A, Koch-institut R (2000) Rapid quantification and differentiation of human polyomavirus DNA in undiluted urine from patients after bone marrow transplantation. J Clin Microbiol 38:3689–3695
Abdelzaher AM, Wright ME, Ortega C, Solo-Gabriele HM, Miller G, Elmir S, Newman X, Shih P, Bonilla JA, Bonilla TD, Palmer CJ, Scott T, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano C, Gidley M, Plano LRW, Zhu X, Wang JD, Fleming LE (2010) Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl Environ Microbiol 76:724–732. doi:10.1128/AEM.02127-09
Ahmed W, Wan C, Goonetilleke A, Gardner T (2010) Evaluating sewage-associated JCV and BKV polyomaviruses for sourcing human fecal pollution in a Coastal River in Southeast Queensland, Australia. J Environ Qual 39:1743. doi:10.2134/jeq2010.0062
Fumian TM, Guimarães FR, Pereira Vaz BJ, da Silva MTT, Muylaert FF, Bofill-Mas S, Gironés R, Leite JPG, Miagostovich MP (2010) Molecular detection, quantification and characterization of human polyomavirus JC from waste water in Rio De Janeiro, Brazil. J Water Health 8:438–445. doi:10.2166/wh.2010.090
Hellein K, Battie C (2011) Culture-based indicators of fecal contamination and molecular microbial indicators rarely correlate with Campylobacter spp. in recreational waters. J Water Health 9:1–14. doi:10.2166/wh.2011.154
Chase E, Hunting J, Staley C, Harwood VJ (2012) Microbial source tracking to identify human and ruminant sources of faecal pollution in an ephemeral Florida river. J Appl Microbiol 113:1396–1406. doi:10.1111/jam.12007
Gordon KV, Mott J, Wang S, Brownell M, Lepo JE, Nathaniel R, Hellein KN, Harwood VJ, Kilgen M, Kennedy E (2012) Relationship of human-associated microbial source tracking markers with enterococci in Gulf of Mexico waters. Water Res 47:996–1004. doi:10.1016/j.watres.2012.10.032
McQuaig S, Griffith J, Harwood VJ (2012) Association of fecal indicator bacteria with human viruses and microbial source tracking markers at coastal beaches impacted by nonpoint source pollution. Appl Environ Microbiol 78:6423–6432. doi:10.1128/AEM.00024-12
Staley C, Gordon KV, Schoen ME, Harwood VJ (2012) Methods for microbial source tracking of performance of two quantitative PCR methods for microbial source tracking of human sewage and implications for microbial risk assessment in recreational waters. Appl Environ Microbiol 78:7317–7326. doi:10.1128/AEM.01430-12
Calgua B, Fumian T, Rusiñol M, Rodriguez-Manzano J, Mbayed V, Bofill-Mas S, Miagostovich M, Girones R (2013) Detection and quantification of classic and emerging viruses by skimmed-milk flocculation and PCR in river water from two geographical areas. Water Res 47:2797–2810. doi:10.1016/j.watres.2013.02.043
Bofill-Mas S, Hundesa A, Calgua B, Rusiñol M, Maluquer de Motes C, Girones R (2011) Cost-effective method for microbial source tracking using specific human and animal viruses. J Vis Exp 5:5–9. doi:10.3791/2820
Wolf S, Hewitt J, Greening G (2010) Viral multiplex quantitative PCR assays for tracking sources of fecal contamination. Appl Environ Microbiol 76:1388–1394. doi:10.1128/AEM.02249-09
Wong K, Xagoraraki I (2011) Evaluating the prevalence and genetic diversity of adenovirus and polyomavirus in bovine waste for microbial source tracking. Appl Microbiol Biotechnol 90:1521–1526. doi:10.1007/s00253-011-3156-z
Viancelli A, Kunz A, Steinmetz RLR, Kich JD, Souza CK, Canal CW, Coldebella A, Esteves PA, Barardi CRM (2013) Chemosphere performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere 90:1539–1544. doi:10.1016/j.chemosphere.2012.08.055
Corsi SR, Borchardt MA, Spencer SK, Hughes PE, Baldwin AK (2014) Human and bovine viruses in the Milwaukee River watershed: hydrologically relevant representation and relations with environmental variables. Sci Total Environ 490:849–860. doi:10.1016/j.scitotenv.2014.05.072
Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy. EU Water Framework Directive
European Commission DG XI (1997) The implementation of council directive 91/676/EEC concerning the protection of waters against pollution caused by nitrates from agricultural sources. Report COM (97)473
Skeffington R (2002) European nitrogen policies, nitrate in rivers and the use of the INCA model. Hydrol Earth Syst Sci 6:315–324
Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S, Emslie KR (2012) Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem 84:1003–1011
Gilbride KA, Lee D-Y, Beaudette LA (2006) Molecular techniques in wastewater: understanding microbial communities, detecting pathogens, and real-time process control. J Microbiol Methods 66:1–20. doi:10.1016/j.mimet.2006.02.016
Ivnitski D, O’Neil DJ, Gattuso A, Schlicht R, Calidonna M, Fisher R (2003) Nucleic acid approaches for detection and identification of biological warfare and infectious disease agents. BioTechniques 35:862–869
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bofill-Mas, S., Rusiñol, M., Fraile, J., Garrido, T., Munné, A., Girones, R. (2015). Specific Viruses Present in Polluted Groundwater Are Indicative of the Source of Nitrates and Faecal Contamination in Agricultural Areas. In: Munné, A., Ginebreda, A., Prat, N. (eds) Experiences from Ground, Coastal and Transitional Water Quality Monitoring. The Handbook of Environmental Chemistry, vol 43. Springer, Cham. https://doi.org/10.1007/698_2015_426
Download citation
DOI: https://doi.org/10.1007/698_2015_426
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23903-3
Online ISBN: 978-3-319-23904-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)