Abstract
Hematopoietic stem cells (HSCs) and their development are one of the most widely studied model systems in mammals. In adults, HSCs are predominantly found in the bone marrow, from where they maintain homeostasis. Besides bone marrow and mobilized peripheral blood, cord blood is also being used as an alternate allogenic source of transplantable HSCs. HSCs from both autologous and allogenic sources are being applied for the treatment of various conditions like blood cancers, anemia, etc. HSCs can further differentiate to mature blood cells. Differentiation process of HSCs is being extensively studied so as to obtain a large number of pure populations of various differentiated cells in vitro so that they can be taken up for clinical trials. The ability to generate sufficient quantity of clinical-grade specialized blood cells in vitro would take the field of hematology a step ahead in translational medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The concept of “stem cells” was first introduced by Till and McCulloch in 1961 while studying the hematopoietic system in mice. They defined these cells as multipotent cells, which can self-renew (make copies of their own) as well as differentiate into blood cells. The origin of hematopoietic lineage and its homeostasis and differentiation have been some of the most widely studied systems in mammals. This has expedited the use of hematopoietic cells in clinical translation as well (Kumar and Verfaillie 2012). The present chapter focuses on the differentiation of hematopoietic stem cells (HSCs) into functional blood cells such as dendritic cells (DCs), megakaryocytes (MKs), platelets, red blood cells (RBCs), etc. that could potentially be used in translational medicine. This chapter also focuses on the mechanisms involved in the differentiation of HSCs into various functional blood cells.
2 Hematopoietic Stem Cells
2.1 Origin of HSCs and Their Characterization
HSCs are responsible for maintaining homeostasis and replenishment of the blood cells over the individual’s life span. During embryogenesis, hematopoietic progenitors first emerge in the yolk sac. Gradually, the development site progressively shifts to the aorta-gonad-mesonephros (AGM) region, the placenta, and the fetal liver, where the fetal HSCs mature into adult ones. At birth, the HSCs populate the bone marrow, making it the primary site of adult hematopoiesis (Mikkola and Orkin 2006; Mahony and Bertrand 2019). The bone marrow microenvironment provides a suitable niche, where the HSCs can either self-renew to generate more HSCs, or differentiate and mature into various types of blood cells depending on the physiological demand. The niche comprises of different stromal cells like mesenchymal stem cells (MSCs), osteoblasts, endothelial cells, reticular cells, adipocytes, etc. that play a very important role in providing the developing HSCs with cues and stimuli to facilitate the production of desired types of functional cells (Pinho and Frenette 2019). HSCs are multipotent in nature and are capable of forming the specialized blood lineage cells through asymmetric cell divisions (Till and McCulloch 1961; Fig. 1). HSCs from murine and human sources show different phenotypic characters (Table 1). Murine HSCs are defined as the cells having a long-term reconstituting ability (LT-HSCs), which can give rise to both myeloid and lymphoid lineages and can be isolated using a flow cytometer as the cells that are lineage negative (Lin−), Sca-1 positive (Sca-1+), c-kit positive (c-kit+), and CD34 low/negative (LSK CD34low/neg)(Osawa et al. 1996). On the other hand, very primitive human HSCs are found to be Lin−, CD133+, GPI-80+, and CD34− by marker selection. Interestingly, human HSCs contained in the cord blood source display CD34 positivity as a distinct marker. The ability to home and engraft into the bone marrow of the recipients when injected via intravenous route is the most important characteristic of HSCs and forms the very basis of the experimental and clinical transplantations (Sumide et al. 2018).
2.2 Different Sources of HSCs
In adult individuals, bone marrow (BM) contains the major pool of HSCs. The frequency of CD34-expressing cells in the adult BM typically ranges between 0.5% and 5% (Hordyjewska et al. 2015). There are two methods to harvest the HSCs from this source – the first method consists of isolation of HSCs from BM aspirates, and the second method consists of mobilization of HSCs through apheresis procedure into peripheral blood. BM aspiration is a very invasive and painful procedure for the donor (Chen et al. 2013). On the other hand, apheresis is done by administration of cytokines like G-CSF or pharmacological agents like AMD3100 so that the HSCs mobilize into the bloodstream along with the hematopoietic progenitors, and then these can be separated out based on their CD34 positivity (Domen et al. 2006). Umbilical cord blood (UCB) is yet another rich source of adult HSCs, which has been widely studied and explored (Broxmeyer et al. 2020; Mayani et al. 2020). UCB contains almost 0.02–1.43% of CD34+ cells. The peripheral blood too contains CD34+ cells, but in a very low abundance (<0.01%) (Hordyjewska et al. 2015).
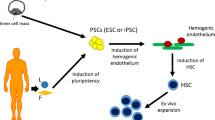
Pluripotent stem cells (PSCs) that comprise of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are also being explored for their differentiation potential to hematopoietic lineage due to their unique ability to differentiate into cells of various lineages. One approach consisting of overexpressing a set of seven transcription factors – ERG, HOXA5, HOXA9, HOXA10, LCOR, RUNX1, and SPI1 – via a lentiviral delivery system into human PSCs converts these cells to HSCs and hematopoietic progenitor cells (HPCs). When these cells were infused in mouse models, these PSC-derived HSCs were found to give rise to both the myeloid and the lymphoid cells and also survive through primary and secondary transplants. However, the molecular signature of these cells is still distinct from UCB-HSCs, suggesting that this approach needs further fine-tuning (Sugimura et al. 2017). The beneficial role of Runx1a overexpression has been identified in the differentiation of PSCs into HPCs (Ran et al. 2013). Use of mouse stromal cell line-OP9 has also been reported to enhance HPC differentiation from PSCs, as this cell line mimics the BM niche and secretes factors that support the development into HPSCs (Demirci et al. 2020). For better clinical utility, attempts have also been initiated to formulate serum-free defined culture media for such differentiation (Chicha et al. 2011, Niwa et al. 2011). However, the HSC-like cells derived from iPSCs in vitro lack important functions like homing and engraftment, and these mostly remain adherent under culture conditions. On further investigation it was found that the overexpression of the epithelial-to-mesenchymal transition inhibiting miRNA in these differentiated progeny was the causative factor of these defects (Risueño et al. 2012; Meader et al. 2018). If the reprogramming genes fail to silence or inactivate after differentiation is induced, they lead to inefficient generation of the differentiated progeny (Ramos-Mejía et al. 2012). Although various groups show differentiation of iPSCs to hematopoietic lineages, there are still several aspects that need improvement before the data can actually be extrapolated to regenerative medicine and the generated cells can be tested in clinical trials.
2.3 Phenotypic Analysis of Different Stages of HSCs’ Differentiation and Maturation
HSCs were identified by Harrison and group as the pool of cells having an ability to repopulate the lymphoid and the myeloid compartments following a transplant (Harrison and Zhong 1992). In humans, HSCs are identified as cells having Lin− CD34+ CD38− CD90+ CD45RA− phenotype. As these cells progress to a state where they form multipotent progenitors, their phenotype changes to Lin− CD34+ CD38− CD90− CD45RA−. These can further form common lymphoid progenitors (CLPs) or common myeloid progenitors (CMPs) depending on the physiological demand for differentiated cells. The CLPs are characterized as Lin− CD34+ CD38+ CD10+, and these progenitors can further form T cells, B cells, natural killer cells (NKs), or DCs based on the cytokine stimulus they are exposed to during maturation. The CMPs display a Lin− CD34+ CD38+ IL3Ralow CD45RA− phenotype. These CMPs can then take up either the megakaryocyte/erythrocyte progenitor (MEP), granulocyte/macrophage progenitor (GMP), or pro-DC fate. The MEPs are Lin− CD34+ CD38+ IL3Ra− CD45RA− which can further specialize into mature platelets or erythroid cells. The GMPs are characterized as Lin− CD34+ CD38+ IL3Ra+ CD45RA−, which can differentiate into either granulocytes or macrophages. Granulocytes comprise of the protein granule-containing cells, viz., the neutrophils, basophils, and eosinophils (Table 1; Seita and Weissman 2010; Sumide et al. 2018; Tajer et al. 2019). The hematopoietic progenitor and HSC states are very tightly regulated by an array of cytokines that help these cells in performing their optimal functions (Ikonomi et al. 2020). Even a small perturbation in this development can cause serious disease conditions like anemia, thrombocytopenia, neutropenia, leukemia, etc. (Tunstall-Pedoe et al. 2008; Roy et al. 2012). The quiescence and the activation of HSCs during homeostasis need to be appropriately controlled; a non-responsive HSC pool could lead to severe deficiencies of mature blood cells, whereas a hyperactive HSC pool could lead to a premature exhaustion of the HSC population (Wilson et al. 2004). Apart from the primary role of BM progenitors in regulating hematopoietic homeostasis, very recently, they have also been identified in the formation of non-hematopoietic decidual cells, aiding in the formation and functioning of decidual stroma during pregnancy (Tal et al. 2019).
3 Challenges Faced While Using In Vitro Generated Differentiated Cells as an Alternative to Those Directly Obtained from UCB or Peripheral Blood (PB): Attempts Made to Improve the Process
For the treatment of certain blood-related disorders, apheresis procedures are conducted to suffice the need for healthy blood cells (Adamski et al. 2018). For such transplantation purposes, cells need to be isolated from suitable donors. This procedure is limited by several factors such as unavailability of HLA-matched donors, risk of transmissible diseases, use of high doses of cytokines, variable efficacy of the yield, etc. (Mcmanus and Mitchell 2014). As an alternative to this, in vitro differentiation of blood cells from the HSCs was proposed to meet the increasing demand for such cells (Devine et al. 2010; Kumar and Geiger 2017). HSCs isolated from BM or UCB can serve as a starting material for obtaining these differentiated cells. However, to obtain them in sufficient quantities required for transplantation, there is a need to first expand the HSCs. Several attempts have been made in this direction. Use of cytokines and small molecules such as insulin-like growth factor-binding protein 2 (IGFBP2), angiopoietin-like proteins, stemregenin-1 (aryl hydrocarbon receptor inhibitor), nicotinamide (SIRT1 inhibitor), UM171 (pyrimidoindole derivative), stem cell factor, thrombopoietin, Fms-related tyrosine kinase 3 ligand, interleukin-6, interleukin-3, resveratrol, etc. has been successful in the expansion of HSCs in vitro (Zhang et al. 2008; Peled et al. 2012; Flores-Guzmán et al. 2013; Fan et al. 2014; Farahbakhshian et al. 2014; Fares et al. 2014; Heinz et al. 2015; Huang et al. 2019). Use of apoptosis pathway modulators like caspase and calpain inhibitors during in vitro HSC expansion has also shown promising results in engraftment of UCB-HSCs when transplanted in mouse models (Sangeetha et al. 2010; Sangeetha et al. 2012). Co-culture of HSCs with human mesenchymal stem/stromal cells (hMSCs) has been shown to result in an efficient expansion of HSCs (Kadekar et al. 2015; Perucca et al. 2017; Papa et al. 2020). But the conditions provided to the HSCs in vitro for proliferation and expansion usually result in pushing the HSCs into a cycling phase. However, for the HSCs to sustain their multipotency during longer duration, maintenance of a quiescent state is very important. Hence, expansion of HSCs without compromising their “stemness” has still remained a huge challenge in the field. In a recent study, HSCs could be held in a steady state in vitro by modulating the culture conditions to hypoxia, low cytokines, and high fatty acid composition in the culture media (Kobayashi et al. 2019).
Cells developed in vitro mostly rely upon 2D culturing methods which acclimatize the cells to specific substrates and culture conditions that result in low transplant efficiencies with limited functionality of the cells. To overcome this, 3D culture methods have been proposed for maintaining and differentiating cells from various stem cells. In one approach, hMSCs entrapped in hydrogels were used as a 3D substrate so as to recapitulate the in vivo niche for the human BM-derived HSCs. The resultant HSCs depicted superior functionality and stem cell attributes, as compared to those cultured under 2D conditions (Sharma et al., 2012). Another study depicts the beneficial effects of 3D fibrin scaffolds in combination with MSCs to expand UCB-HSCs (Ferreira et al. 2012). In a recent report, use of 3D zwitterionic hydrogel cultures was found to be beneficial for the expansion of both UCB- and BM-HSCs (Bai et al. 2019). Although these in vitro expanded cells are closer to the native state in terms of growth conditions and functionality, it is difficult to reproducibly obtain them in large numbers that would be sufficient for transplant purposes (McKee and Chaudhry 2017). When a comparison was performed between HSCs and progenitor cells cultured in vitro from UCB-HSCs and their freshly isolated counterparts, it was observed that although the in vitro cultured cells were immunophenotypically similar to those present in vivo, they lacked the functionality and genetic signature of their in vivo counterparts, i.e., freshly isolated UCB-HSCs. The in vitro cultured cells displayed a distinct myeloid bias, and additionally, the long-term-culture-initiating cells (LTC-IC), which are considered to be the most primitive HSCs during transplantation setting, were also found to be in lower numbers in the in vitro generated cells, as opposed to those present in the fresh UCB. To improve on this, a strategy of co-infusing in vitro expanded HSCs with the native freshly isolated HSCs was used; however, even in this situation, the cells that survived longer during the transplant in the host were the freshly isolated ones, thereby indicating that the in vitro manipulated HSCs lack the longevity and functionality as the fresh ones (Dircio-Maldonado et al. 2018). Despite these challenges, efforts to efficiently expand the UCB units are ongoing so that they can be utilized for clinical applications (Kiernan et al. 2017; Mayani 2019). A few phase I/II clinical trials make use of HSCs that have been expanded in vitro using different compounds like UM171 (Cohen et al. 2020), nicotinamide (Horwitz et al. 2019), StemRegenin-1 (Wagner Jr et al. 2016), and copper chelator-tetraethylenepentamine (De Lima et al. 2008). In these trials, the outcome has been promising as the expanded HSCs did engraft efficiently in the recipient after the transplant.
4 Cord Blood Is a Superior Source of In Vitro Generated HSCs and HPCs
During ex vivo expansion, UCB-HSCs exhibited better expansion ability and yielded a much higher number of progenitor colonies, as compared to BM-HSCs under serum-free conditions with cytokine stimulus (Kim et al. 2005). In a study employing in vitro priming of HSCs on OP9 stroma, the UCB-HSCs displayed better reconstitution of the T-lymphoid fraction, as compared to BM-HSCs, whereas the myeloid fraction remained unchanged (De Smedt et al. 2011). To address the issue of graft-vs-host disease (GvHD), expansion of T regulatory (Tregs) cells in vitro from either UCB or adult PB is an approach before using them in cell therapy. In such cases, Tregs having better immunomodulatory activity could be obtained in higher numbers from UCB samples, as compared to PB sample (Fan et al. 2012). UCB samples have certain advantages like the naïve state of their HSCs, tolerability in terms of HLA mismatches (a 4–6/6 is accepted in the case of UCB donors), ready availability in UCB banks due to easy collection and harvesting procedures, etc. (Schönberger et al. 2004; Beksac 2016). In a pediatric clinical trial assessment, UCB stem cell transplants yielded better engraftment in the recipients with early and committed progenitors, as compared to BM transplant (Frassoni et al. 2003). Recent strategies employ depletion of TCRαβ+/CD19+ in donor cells, which helps reduce GvHD, thus enhancing survivability on UCB transplant in a HLA mismatch recipient (Elfeky et al. 2019). Even in the aspect of obtaining differentiated progeny, specifically the megakaryocyte lineage, the UCB source proves to be superior, as compared to BM (Tao et al. 1999) or even mobilized PB (Bruyn et al. 2005). Domogala and colleagues observed that NK cells differentiated from UCB-CD34 cells in vitro could be obtained in higher numbers and were comparable to those isolated directly from UCB and PB samples for functional and phenotypic characteristics to be used in immunotherapy (Domogala et al. 2017). Furthermore, both sources, PB-MNCs and UCB-MNCs, displayed comparable phenotypic and functional attributes when primed toward the DC lineage (Kumar et al. 2015).
5 In Vitro Differentiated Hematopoietic Cells for Clinical Applications
The ultimate goal of in vitro differentiation studies of stem cells is to utilize the protocols for applications in cell therapy. Here we describe some such studies which have potential clinical applications in the near future.
5.1 T Cells
An approach of modulation of the hematopoietic niche to stimulate Notch signaling, which in turn leads to an enhanced T cell progenitor generation, can be extrapolated for clinical studies (Shukla et al. 2017). Olbrich et al. formulated a retroviral construct in PB- and CB-CD34+ cells that overexpress chimeric antigen receptor (CAR) targeted against the human cytomegalovirus (HCMV) glycoprotein B that is known to be dominant during viral reactivation. On differentiating such transduced CD34+ to T cells, the generated T cells exhibit remarkable on-target specificity and cytotoxicity to HCMV infections under in vitro conditions and in mouse models, thus proving to be a good target for further clinical studies (Olbrich et al. 2020). Use of StemRegenin-1 (SR-1), a purine derivative that antagonizes aryl hydrocarbon receptor, in expansion medium results in >250-fold expansion of HPCs in vitro. Due to this property it is now being tested in clinical trials. Expanded HPCs obtained by this method have further been evaluated for differentiation into pro-T cells in vitro which also display effective T cell functions on being infused in vivo (Singh et al. 2019).
5.2 Dendritic Cells
Since DCs are present in very low numbers in peripheral blood, obtaining them in sufficient numbers for immune therapies poses difficulty. The SR-1 expanded HPCs were differentiated to DCs that resulted in large-scale generation with functional properties comparable to the circulating DCs (Thordardottir et al. 2014). Plantinga et al. proposed a method of generation of conventional DCs from CB-CD34+ cells by selection based on CD115 positivity. These cells generated DCs with 75–95% purity and were functional as a DC vaccine even on maturation and stimulation (Plantinga et al. 2019). The findings were taken a step ahead by generating GMP-grade DCs from UCB-CD34 cells that specifically expressed Wilms’ tumor 1 protein as a potent DC vaccine candidate for the use in pediatric acute myeloid leukemia (AML) patients (Plantinga et al. 2020). In the case of multiple myeloma cancer patient samples, DCs derived from HSCs were found to be a potent source for generation of DC vaccine. The phenotypic characters, antigen uptake, migration, and T cell stimulation were comparable to those derived from healthy donors. These DCs could also raise a tumor-specific cytotoxic T lymphocyte (CTL) reaction, but it was less robust when compared to the healthy donor source due to T cell exhaustion seen in multiple myeloma samples. Therefore, if CTL-4 blocking was used in combination with such DCs, it could serve as a promising candidate for immunotherapy in multiple myeloma (Shinde et al. 2019).
5.3 Megakaryocytes and Platelets
Large-scale platelets could be obtained in vitro from UCB-HSCs using a three-step method, comprising of adherent and liquid cultures. These platelets were similar to PB platelets and the yield obtained was approximately 3.4 units of platelets from 1 unit of CB used (Matsunaga et al. 2006). With an aim to reduce incidences of graft rejection, platelets that lacked β2-microglobulin gene have been differentiated from hiPSCs. The resultant platelets produced in high numbers were also HLA-ABC negative, and hence, such approaches could be explored from the therapeutic aspect (Feng et al. 2014; Norbnop et al. 2020). Improvements have also been tried on the culture method aspects, wherein a gas-permeable silicone membrane was used instead of the regular in vitro 2D culture dishes. The enhanced gas and media exchange resulted in the generation of MKs and platelets from mobilized PB-CD34 cells in higher numbers with functional attributes that made the process more clinically relevant (Martinez and Miller 2019). In another attempt to produce GMP-grade MKs and platelets, a roller bottle culture system was used that generated a large number of MKs from UCB-CD34+ cells that retained all standard characteristics coupled with high purity. The expansion was in the order of 2.5 × 104 MKs from one CD34+ cell, and the optimal temperature for short-term storage to be applied in clinics was observed to be 22 °C when stored in saline supplemented with 10% human serum albumin (Guan et al. 2020).
5.4 Natural Killer Cells
Cryopreserved UCB units were used to purify HSCs, which were then differentiated into NKs in a closed bioreactor system that allowed a clinically relevant scaling up for their use in adoptive immunotherapy. This method yielded almost 2000-fold expansion of the cells with >90% purity while retaining their functional attributes (Spanholtz et al. 2011). To eliminate the cytotoxicity induced due to chemotherapy given for various blood cancer treatment regimes, a strategy was devised for acute lymphoblastic leukemia (ALL) to increase the efficacy of cell-based therapies. UCB-CD34+-derived CD16+ NK cells when used in combination with anti-CD47 antibody were found to be potent in building up an anti-tumor immune response and reducing the load of ALL (Valipour et al. 2020). In another phase-1 clinical trial, NK cells were differentiated from PB mononuclear cells (MNCs) and sufficient expansion was achieved ex vivo on stromal cells expressing IL-21. These cells were then infused to leukemic patients undergoing haploidentical hematopoietic stem cell transplant (HSCT) at different time points from the same MNC donor. The leukemic patients that received NK cell transplant in addition to HSCT showed lower rate of relapse and mortality, lower viral infections, and reduced GvHD (Ciurea et al. 2017).
5.5 Red Blood Cells
To eliminate the batch-to-batch variation in experimental methods, RBCs were differentiated from UCB-MNCs in a xeno-free controlled environment, where not only the yield was high, but the method could also be scaled up for clinical applications (Rallapalli et al. 2019). On similar lines, PB-MNCs (without the need for CD34 isolation step) could be differentiated to erythroid cultures under GMP conditions in vitro, which also could be scaled up in bioreactors for a large-scale RBC production for use in clinics. More than 90% of them achieved enucleation and the functional attributes like oxygen-binding ability were comparable to their in vivo counterparts (Heshusius et al. 2019). Production of clinical-grade RBCs along with their expansion was achieved from CB-CD34+ cells by in vitro stimulus using cytokines followed by expansion on CB-derived MSCs (Baek et al. 2008). The in vitro culture duration of obtaining RBCs from HSCs was accelerated by 3 days on supplementing TGF-β1 during the terminal erythroid maturation step (Kuhikar et al. 2020). Functional erythrocytes on a large scale were obtained from CB-CD34+ cells ex vivo using a bottle-turning device culture method. About 200 million erythrocytes with more than 90% positivity for RBC marker CD235a could be obtained from one CB-CD34+ cell. Safety and efficacy studies were validated on murine and non-human primate model, and therefore, this approach can provide an alternative to the traditional transplants in clinics (Zhang et al. 2017).
6 Efficient Cryopreservation of In Vitro Generated Differentiated Blood Cells Is Crucial for Their Therapeutic Use
There are some reports in the literature that attempt to achieve optimal cryopreservation of specialized blood cells from HSCs that can be taken to clinical trials. Different freezing agents and methods have been employed for the purpose; however, DMSO still remains the most widely used cryoprotectant (Li et al. 2019). A DC-based cellular vaccine derived from CB-CD34+ cells for use as immunotherapy for esophageal cancer displayed effective cryopreservation in 2.5% DMSO, 2.5% glucose, and 10% FCS. The thawed cellular vaccine maintained viability, immunophenotype, T cell activation, and specific cytotoxic T lymphocyte activity (Yu et al. 2016). Balan et al. demonstrated that both fresh and frozen units of UCB could generate DCs with equivalent morphological and functional characteristics (Balan et al. 2010). Similar findings were reported in the context of freezing NK cells derived from CB-CD34+ cells, where the cells did not show diminished morphological or functional attributes upon thawing (Domogala et al. 2016). Surprisingly, Luevano et al. observed that cryopreserved units of CB-CD34+ cells gave rise to a higher number of NK cells without compromising their functionality after differentiation in vitro, when compared to their freshly isolated counterparts from mobilized PB or CB (Luevano et al. 2014). In another preclinical study, a commercial solution, CryoStor, containing 10% DMSO was used to cryopreserve CB-CD34+ cell-derived MKs. The results indicated an efficient MK recovery and platelet production post-thaw (Patel et al. 2019).
Mobilized peripheral blood HSCs demonstrated comparable viability, i.e., > 95%, and functionality when cryopreserved either for 5 weeks or for 10 years in 5% DMSO as the freezing agent (Abbruzzese et al. 2013). For autologous or allogenic transplants, units of BM or PB are usually collected just before a planned infusion; hence, they seldom undergo a long-term storage before use. On the other hand, UCB units need a long-term storage as their use depends on the demand for an allogenic HLA-matched transplant (Watt et al. 2007). Thus, its efficient cryopreservation is of prime importance if these cells need to be explored for clinical applications. In a fresh unit of UCB, cells are viable for a maximum of few days when stored at 4 °C. Cryopreservation increases its availability by providing easy transport and enhancing its shelf life. Dimethyl sulfoxide (DMSO) has been the most commonly used freezing agent for cryopreservation of HSCs (Hornberger et al. 2019). DMSO in the range of 7.5–10% was found to be optimal for cryopreservation of UCB cells when added not more than 1 h before freezing and washed out before 30 min post-thaw (Fry et al. 2015). In addition, 5% DMSO with 6% pentastarch and 25% human albumin were found to cryopreserve UCB-MNCs in a better way, as compared to 10% DMSO alone (Hayakawa et al. 2010). Inclusion of trehalose – a membrane stabilizer and catalase – an antioxidant to the 10% DMSO freezing mixture significantly enhanced recovery of UCB-HSCs post-thaw (Limaye and Kale 2001; Sasnoor et al. 2005). Mitchell et al. monitored the recovery of total nucleated cells post-thaw in UCB units stored for a long time and did not find a difference in cell recovery after a storage period of 10 years (Mitchell et al. 2015). Hence, UCB cells can be effectively stored in DMSO-containing freezing media with a minimal loss of viability for longer duration as well (Jaing et al. 2018). Ice recrystallization during freezing has been one of the major causes of damage to the recovery of UCB progenitors. Use of ice recrystallization inhibitor (IRI)-N-(2-fluorophenyl)-D-gluconamide during freezing improves the percentage of engraftable cells post-thaw (Jahan et al. 2020). In an attempt to find alternatives to DMSO, so as to avoid the toxicity associated with it, compounds like pentaisomaltose – a carbohydrate – were used and found to effectively cryopreserve UCB stem cells, when compared to DMSO (Svalgaard et al. 2016).
7 Future Prospects of Stem Cell-Derived Differentiated Cells in Therapeutics
Blood cells obtained by apheresis (enriched in HSCs) are already applied therapeutically in autologous and allogenic settings. However, use of specialized cells generated in vitro from the HSCs is still at an early stage of research and preclinical studies. The field of regenerative medicine and transplantation is in dire need of alternative and novel sources and methods that can yield a large number of functional blood cells needed for therapeutic purposes. Recent attempts aim at producing a large-scale generation of differentiated cells from HSCs as well as PSCs for their application in regenerative medicine, and immunotherapies have shown promising results and hence can soon lead to their clinical applications (Xue and Milano 2020). However, before these methodologies can be applied clinically, strict ethical regulations and guidelines need to be formulated so as to channelize their use for transplant purposes (Kline and Bertolone 1998).
Abbreviations
- BM:
-
bone marrow
- CLP:
-
common lymphoid progenitor
- CMP:
-
common myeloid progenitor
- DCs:
-
dendritic cells
- DMSO:
-
dimethyl sulfoxide
- ESCs:
-
embryonic stem cells
- FCS:
-
fetal calf serum
- GMP:
-
good manufacturing practice
- GvHD:
-
graft-vs-host disease
- HLA:
-
human leukocyte antigen
- HPCs:
-
hematopoietic progenitor cells
- HSCs:
-
hematopoietic stem cells
- iPSCs:
-
induced pluripotent stem cells
- LT-HSCs:
-
long-term-hematopoietic stem cells
- MKs:
-
megakaryocytes
- MNCs:
-
mononuclear cells
- MSCs:
-
mesenchymal stem cells
- PB:
-
peripheral blood
- PSCs:
-
pluripotent stem cells
- RBCs:
-
red blood cells
- Tregs:
-
T regulatory cells
- UCB:
-
umbilical cord blood
References
Abbruzzese L et al (2013) Long term cryopreservation in 5% DMSO maintains unchanged CD 34+ cells viability and allows satisfactory hematological engraftment after peripheral blood stem cell transplantation. Vox Sang 105:77–80. https://doi.org/10.1111/vox.12012
Adamski J, Ipe TS, Kinard T (2018) Therapeutic and donor apheresis. In: Transfusion medicine, apheresis, and hemostasis. Academic, pp 327–351. https://doi.org/10.1016/B978-0-12-803999-1.00014-6
Baek EJ, Kim HS, Kim S, Jin H, Choi TY, Kim HO (2008) In vitro clinical-grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion 48:2235–2245. https://doi.org/10.1111/j.1537-2995.2008.01828.x
Bai T et al (2019) Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat Med 25:1566–1575. https://doi.org/10.1038/s41591-019-0601-5
Balan S, Kale VP, Limaye LS (2010) A large number of mature and functional dendritic cells can be efficiently generated from umbilical cord blood–derived mononuclear cells by a simple two-step culture method. Transfusion 50:2413–2423. https://doi.org/10.1111/j.1537-2995.2010.02706.x
Beksac M (2016) Is there any reason to prefer cord blood instead of adult donors for hematopoietic stem cell transplants? Front Med 2:95. https://doi.org/10.3389/fmed.2015.00095
Broxmeyer HE, Cooper S, Capitano ML (2020) Enhanced collection of phenotypic and engrafting human cord blood hematopoietic stem cells at 4° C. Stem Cells 38:1326–1331. https://doi.org/10.1002/stem.3243
Bruyn CD, Delforge A, Martiat P, Bron D (2005) Ex vivo expansion of megakaryocyte progenitor cells: cord blood versus mobilized peripheral blood. Stem Cells Dev 14:415–424. https://doi.org/10.1089/scd.2005.14.415
Chen SH, Wang TF, Yang KL (2013) Hematopoietic stem cell donation. Int J Hematol 97:446–455. https://doi.org/10.1007/s12185-013-1298-8
Cheng H, Zheng Z, Cheng T (2020) New paradigms on hematopoietic stem cell differentiation. Protein Cell 11:34–44. https://doi.org/10.1007/s13238-019-0633-0
Chicha L, Feki A, Boni A, Irion O, Hovatta O, Jaconi M (2011) Human pluripotent stem cells differentiated in fully defined medium generate hematopoietic CD34+ and CD34− progenitors with distinct characteristics. PLoS One 6:e14733. https://doi.org/10.1371/journal.pone.0014733
Ciurea SO et al (2017) Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood 130:1857–1868. https://doi.org/10.1182/blood-2017-05-785659
Cohen S et al (2020) Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol 7:134–145. https://doi.org/10.1016/S2352-3026(19)30202-9
De Smedt M, Leclercq G, Vandekerckhove B, Kerre T, Taghon T, Plum J (2011) T-lymphoid differentiation potential measured in vitro is higher in CD34+ CD38−/lo hematopoietic stem cells from umbilical cord blood than from bone marrow and is an intrinsic property of the cells. Haematologica 96:646–654. https://doi.org/10.3324/haematol.2010.036343
Demirci S et al (2020) Definitive hematopoietic stem/progenitor cells from human embryonic stem cells through serum/feeder-free organoid-induced differentiation. Stem Cell Res Ther 11:1–14. https://doi.org/10.1186/s13287-020-02019-5
Devine H, Tierney DK, Schmit-Pokorny K, McDermott K (2010) Mobilization of hematopoietic stem cells for use in autologous transplantation. Clin J Oncol Nurs 14:2. https://doi.org/10.1188/10.CJON.212-222
Dircio-Maldonado R et al (2018) Functional integrity and gene expression profiles of human cord blood-derived hematopoietic stem and progenitor cells generated in vitro. Stem Cells Transl Med 7:602–614. https://doi.org/10.1002/sctm.18-0013
Domen J, Wagers A, Weissman IL (2006) Bone marrow (hematopoietic) stem cells. Regen Med:13–34. https://www.uv.es/~elanuza/Dinamica/Regenerative_Medicine_2006.pdf#page=17
Domogala A, Madrigal JA, Saudemont A (2016) Cryopreservation has no effect on function of natural killer cells differentiated in vitro from umbilical cord blood CD34+ cells. Cytotherapy 18:754–759. https://doi.org/10.1016/j.jcyt.2016.02.008
Domogala A, Blundell M, Thrasher A, Lowdell MW, Madrigal JA, Saudemont A (2017) Natural killer cells differentiated in vitro from cord blood CD34+ cells are more advantageous for use as an immunotherapy than peripheral blood and cord blood natural killer cells. Cytotherapy 19:710–720. https://doi.org/10.1016/j.jcyt.2017.03.068
Elfeky R et al (2019) New graft manipulation strategies improve the outcome of mismatched stem cell transplantation in children with primary immunodeficiencies. J Allergy Clin Immunol 144:280–293. https://doi.org/10.1016/j.jaci.2019.01.030
Fan H et al (2012) Comparative study of regulatory T cells expanded ex vivo from cord blood and adult peripheral blood. Immunology 136:218–230. https://doi.org/10.1111/j.1365-2567.2012.03573.x
Fan X et al (2014) Low-dose insulin-like growth factor binding proteins 1 and 2 and angiopoietin-like protein 3 coordinately stimulate ex vivo expansion of human umbilical cord blood hematopoietic stem cells as assayed in NOD/SCID gamma null mice. Stem Cell Res Ther 5:1–9. https://doi.org/10.1186/scrt460
Farahbakhshian E et al (2014) Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells. PLoS One 9:e105642. https://doi.org/10.1371/journal.pone.0105642
Fares IJ et al (2014) Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345:1509–1512. https://doi.org/10.1126/science.1256337
Feng Q et al (2014) Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep 3:817–831. https://doi.org/10.1016/j.stemcr.2014.09.010
Ferreira MSV et al (2012) Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 33:6987–6997. https://doi.org/10.1016/j.biomaterials.2012.06.029
Flores-Guzmán P, Fernández-Sánchez V, Mayani H (2013) Concise review: ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: basic principles, experimental approaches, and impact in regenerative medicine. Stem Cells Transl Med 2:830–838. https://doi.org/10.5966/sctm.2013-0071
Frassoni F et al (2003) Cord blood transplantation provides better reconstitution of hematopoietic reservoir compared with bone marrow transplantation. Blood 102:1138–1141. https://doi.org/10.1182/blood-2003-03-0720
Fry LJ, Querol S, Gomez SG, McArdle S, Rees R, Madrigal JA (2015) Assessing the toxic effects of DMSO on cord blood to determine exposure time limits and the optimum concentration for cryopreservation. Vox Sang 109:181–190. https://doi.org/10.1111/vox.12267
Guan X, Wang L, Wang H, Wang H, Dai W, Jiang Y (2020) Good manufacturing practice-grade of megakaryocytes produced by a novel ex vivo culturing platform. Clin Transl Sci 13:1115–1126. https://doi.org/10.1111/cts.12788
Harrison DE, Zhong RK (1992) The same exhaustible multilineage precursor produces both myeloid and lymphoid cells as early as 3-4 weeks after marrow transplantation. Proc Natl Acad Sci U S A 89:10134–10138. https://doi.org/10.1073/pnas.89.21.10134
Hayakawa J et al (2010) 5% dimethyl sulfoxide (DMSO) and pentastarch improves cryopreservation of cord blood cells over 10% DMSO. Transfusion 50:2158–2166. https://doi.org/10.1111/j.1537-2995.2010.02684.x
Heinz N, Ehrnström B, Schambach A, Schwarzer A, Modlich U, Schiedlmeier B (2015) Comparison of different cytokine conditions reveals resveratrol as a new molecule for ex vivo cultivation of cord blood-derived hematopoietic stem cells. Stem Cells Transl Med 4:1064–1072. https://doi.org/10.5966/sctm.2014-0284
Heshusius S et al (2019) Large-scale in vitro production of red blood cells from human peripheral blood mononuclear cells. Blood Adv 3:3337–3350. https://doi.org/10.1182/bloodadvances.2019000689
Hordyjewska A, Popiołek Ł, Horecka A (2015) Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology 67:387–396. https://doi.org/10.1007/s10616-014-9796-y
Hornberger K, Yu G, McKenna D, Hubel A (2019) Cryopreservation of hematopoietic stem cells: emerging assays, cryoprotectant agents, and technology to improve outcomes. Transfus Med Hemother 46:188–196. https://doi.org/10.1159/000496068
Horwitz ME et al (2019) Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol 37:367–374. https://doi.org/10.1200/JCO.18.00053
Huang X, Guo B, Capitano M, Broxmeyer HE (2019) Past, present, and future efforts to enhance the efficacy of cord blood hematopoietic cell transplantation. F1000Research 8. https://doi.org/10.12688/f1000research.20002.1
Ichii M, Oritani K, Kanakura Y (2014) Early B lymphocyte development: similarities and differences in human and mouse. World J Stem Cells 6:421–431. https://doi.org/10.4252/wjsc.v6.i4.421
Ikonomi N, Kühlwein SD, Schwab JD, Kestler HA (2020) Awakening the HSC: dynamic modeling of HSC maintenance unravels regulation of the TP53 pathway and quiescence. Front Physiol 11:848. https://doi.org/10.3389/fphys.2020.00848
Jahan S, Adam MK, Manesia JK, Doxtator E, Ben RN, Pineault N (2020) Inhibition of ice recrystallization during cryopreservation of cord blood grafts improves platelet engraftment. Transfusion 60:769–778. https://doi.org/10.1111/trf.15759
Jaing TH, Chen SH, Wen YC, Chang TY, Yang YC, Tsay PK (2018) Effects of cryopreservation duration on the outcome of single-unit cord blood transplantation. Cell Transplant 27:515–519. https://doi.org/10.1177/0963689717753187
Kadekar D, Kale V, Limaye L (2015) Differential ability of MSCs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived CD34+ cells. Stem Cell Res Ther 6:201. https://doi.org/10.1186/s13287-015-0194-y
Kiernan J et al (2017) Clinical studies of ex vivo expansion to accelerate engraftment after umbilical cord blood transplantation: a systematic review. Transfus Med Rev 31:173–182. https://doi.org/10.1016/j.tmrv.2016.12.004
Kim SK, Ghil HY, Song SU, Choi JW, Park SK (2005) Ex vivo expansion and clonal maintenance of CD34+ selected cells from cord blood and peripheral blood. Korean J Pediatr 48:894–900
Kline RM, Bertolone SJ (1998) Umbilical cord blood transplantation: providing a donor for everyone needing a bone marrow transplant? South Med J 91:821–828
Kobayashi H et al (2019) Environmental optimization enables maintenance of quiescent hematopoietic stem cells ex vivo. Cell Rep 28:145–158. https://doi.org/10.1016/j.celrep.2019.06.008
Kuhikar R, Khan N, Philip J, Melinkeri S, Kale V, Limaye L (2020) Transforming growth factor β1 accelerates and enhances in vitro red blood cell formation from hematopoietic stem cells by stimulating mitophagy. Stem Cell Res Ther 11:1–15. https://doi.org/10.1186/s13287-020-01603-z
Kumar S, Geiger H (2017) HSC niche biology and HSC expansion ex vivo. Trends Mol Med 23:799–819. https://doi.org/10.1016/j.molmed.2017.07.003
Kumar A, Verfaillie C (2012) Basic principles of multipotent stem cells. In: Progenitor and stem cell technologies and therapies. Woodhead Publishing Series in Biomaterials, pp 100–117. https://doi.org/10.1533/9780857096074.1.100
Kumar J, Kale V, Limaye L (2015) Umbilical cord blood-derived CD11c+ dendritic cells could serve as an alternative allogeneic source of dendritic cells for cancer immunotherapy. Stem Cell Res Ther 6:1–15. https://doi.org/10.1186/s13287-015-0160-8
Li R, Johnson R, Yu G, McKenna DH, Hubel A (2019) Preservation of cell-based immunotherapies for clinical trials. Cytotherapy 21:943–957. https://doi.org/10.1016/j.jcyt.2019.07.004
Lima D et al (2008) Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant 41:771–778. https://doi.org/10.1038/sj.bmt.1705979
Limaye LS, Kale VP (2001) Cryopreservation of human hematopoietic cells with membrane stabilizers and bioantioxidants as additives in the conventional freezing medium. J Hematother Stem Cell Res 10:709–718. https://doi.org/10.1089/152581601753193931
Luevano M et al (2014) Frozen cord blood hematopoietic stem cells differentiate into higher numbers of functional natural killer cells in vitro than mobilized hematopoietic stem cells or freshly isolated cord blood hematopoietic stem cells. PloS One 9:1. https://doi.org/10.1371/journal.pone.0087086
Mahony CB, Bertrand JY (2019) How HSCs colonize and expand in the fetal niche of the vertebrate embryo: an evolutionary perspective. Front Cell Dev Biol 7:34. https://doi.org/10.3389/fcell.2019.00034
Martinez AF, Miller WM (2019) Enabling large-scale ex vivo production of megakaryocytes from CD34+ cells using gas-permeable surfaces. Stem Cells Transl Med 8:658–670. https://doi.org/10.1002/sctm.18-0160
Matsunaga T et al (2006) Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells 24:2877–2887. https://doi.org/10.1634/stemcells.2006-0309
Mayani H (2019) Human hematopoietic stem cells: concepts and perspectives on the biology and use of fresh versus in vitro–generated cells for therapeutic applications. Curr Stem Cell Rep 5:115–124. https://doi.org/10.1007/s40778-019-00162-1
Mayani H, Wagner JE, Broxmeyer HE (2020) Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant 55:48–61. https://doi.org/10.1038/s41409-019-0546-9
McKee C, Chaudhry GR (2017) Advances and challenges in stem cell culture. Colloids Surf B: Biointerfaces 159:62–77. https://doi.org/10.1016/j.colsurfb.2017.07.051
Mcmanus LM, Mitchell RN (2014) Pathobiology of human disease: a dynamic encyclopedia of disease mechanisms. Elsevier, pp 1800–1808
Meader E et al (2018) Pluripotent stem cell-derived hematopoietic progenitors are unable to downregulate key epithelial-mesenchymal transition-associated miRNAs. Stem Cells 36:55–64. https://doi.org/10.1002/stem.2724
Mikkola HK, Orkin SH (2006) The journey of developing hematopoietic stem cells. Development 133:3733–3744. https://doi.org/10.1242/dev.02568
Mitchell R et al (2015) Impact of long-term cryopreservation on single umbilical cord blood transplantation outcomes. Biol Blood Marrow Transplant 21:50–54. https://doi.org/10.1016/j.bbmt.2014.09.002
Niwa A et al (2011) A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS One 6:e22261. https://doi.org/10.1371/journal.pone.0022261
Norbnop P, Ingrungruanglert P, Israsena N, Suphapeetiporn K, Shotelersuk V (2020) Generation and characterization of HLA-universal platelets derived from induced pluripotent stem cells. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-65577-x
Olbrich H et al (2020) Adult and cord blood-derived high-affinity gB-CAR-T cells effectively react against human Cytomegalovirus infections. Hum Gene Ther 31:423–439. https://doi.org/10.1089/hum.2019.149
Osawa M, Hanada KI, Hamada H, Nakauchi H (1996) Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273:242–245. https://doi.org/10.1126/science.273.5272.242
Papa L, Djedaini M, Hoffman R (2020) Ex vivo HSC expansion challenges the paradigm of unidirectional human hematopoiesis. Ann N Y Acad Sci 1466:39. https://doi.org/10.1111/nyas.14133
Patel A et al (2019) Pre-clinical development of a cryopreservable megakaryocytic cell product capable of sustained platelet production in mice. Transfusion 59:3698–3713. https://doi.org/10.1111/trf.15546
Peled T et al (2012) Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 40:342–355. https://doi.org/10.1016/j.exphem.2011.12.005
Perucca S et al (2017) Mesenchymal stromal cells (MSCs) induce ex vivo proliferation and erythroid commitment of cord blood haematopoietic stem cells (CB-CD34+ cells). PLoS One 12:e0172430. https://doi.org/10.1371/journal.pone.0172430
Pinho S, Frenette PS (2019) Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 20:303–320. https://doi.org/10.1038/s41580-019-0103-9
Plantinga M et al (2019) Cord-blood-stem-cell-derived conventional dendritic cells specifically originate from CD115-expressing precursors. Cancers 11:181. https://doi.org/10.3390/cancers11020181
Plantinga M et al (2020) Clinical grade production of Wilms’ Tumor-1 loaded cord blood-derived dendritic cells to prevent relapse in pediatric AML after cord blood transplantation. Front Immunol 11:2559. https://doi.org/10.3389/fimmu.2020.559152
Rallapalli S, Guhathakurta S, Narayan S, Bishi DK, Balasubramanian V, Korrapati PS (2019) Generation of clinical-grade red blood cells from human umbilical cord blood mononuclear cells. Cell Tissue Res 375(2):437–449. https://doi.org/10.1007/s00441-018-2919-6
Ramos-Mejía V et al (2012) Residual expression of the reprogramming factors prevents differentiation of iPSC generated from human fibroblasts and cord blood CD34+ progenitors. PLoS One 7:e35824. https://doi.org/10.1371/journal.pone.0035824
Ran D et al (2013) RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood 121:2882–2890. https://doi.org/10.1182/blood-2012-08-451641
Risueño RM et al (2012) Inability of human induced pluripotent stem cell-hematopoietic derivatives to downregulate microRNAs in vivo reveals a block in xenograft hematopoietic regeneration. Stem Cells 30:131–139. https://doi.org/10.1002/stem.1684
Roy A et al (2012) Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc Natl Acad Sci U S A 109:17579–17584. https://doi.org/10.1073/pnas.1211405109
Sangeetha VM, Kale VP, Limaye LS (2010) Expansion of cord blood CD34+ cells in presence of zVADfmk and zLLYfmk improved their in vitro functionality and in vivo engraftment in NOD/SCID mouse. PLoS One 5:e12221. https://doi.org/10.1371/journal.pone.0012221
Sangeetha VM, Kadekar D, Kale VP, Limaye LS (2012) Pharmacological inhibition of caspase and calpain proteases: a novel strategy to enhance the homing responses of cord blood HSPCs during expansion. PLoS One 7:e29383. https://doi.org/10.1371/journal.pone.0029383
Sasnoor LM, Kale VP, Limaye LS (2005) A combination of catalase and trehalose as additives to conventional freezing medium results in improved cryoprotection of human hematopoietic cells with reference to in vitro migration and adhesion properties. Transfusion 45:622–633. https://doi.org/10.1111/j.0041-1132.2005.04288.x
Schönberger S et al (2004) Transplantation of hematopoietic stem cells derived from cord blood, bone marrow or peripheral blood: a single centre matched-pair analysis in a heterogeneous risk population. Klin Padiatr 216:356–363. https://doi.org/10.1055/s-2004-832357
Seita J, Weissman IL (2010) Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2:640–653. https://doi.org/10.1002/wsbm.86
Sharma MB, Limaye LS, Kale VP (2012) Mimicking the functional hematopoietic stem cell niche in vitro: recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells. Haematologica 97:651–660. https://doi.org/10.3324/haematol.2011.050500
Shinde P, Melinkeri S, Santra MK, Kale V, Limaye L (2019) Autologous hematopoietic stem cells are a preferred source to generate dendritic cells for immunotherapy in multiple myeloma patients. Front Immunol 10:1079. https://doi.org/10.3389/fimmu.2019.01079
Shukla S et al (2017) Progenitor T-cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1. Nat Methods 14:531–538. https://doi.org/10.1038/nmeth.4258
Singh J, Chen EL, Xing Y, Stefanski HE, Blazar BR, Zúñiga-Pflücker JC (2019) Generation and function of progenitor T cells from StemRegenin-1–expanded CD34+ human hematopoietic progenitor cells. Blood Adv 3:2934–2948. https://doi.org/10.1182/bloodadvances.2018026575
Spanholtz J et al (2011) Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One 6:e20740. https://doi.org/10.1371/journal.pone.0020740
Sugimura R et al (2017) Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 545:432–438. https://doi.org/10.1038/nature22370
Sumide K et al (2018) A revised road map for the commitment of human cord blood CD34-negative hematopoietic stem cells. Nat Commun 9:1–17. https://doi.org/10.1038/s41467-018-04441-z
Svalgaard JD et al (2016) Low-molecular-weight carbohydrate Pentaisomaltose may replace dimethyl sulfoxide as a safer cryoprotectant for cryopreservation of peripheral blood stem cells. Transfusion 56:1088–1095. https://doi.org/10.1111/trf.13543
Tajer P, Pike-Overzet K, Arias S, Havenga M, Staal FJ (2019) Ex vivo expansion of hematopoietic stem cells for therapeutic purposes: lessons from development and the niche. Cell 8:169. https://doi.org/10.3390/cells8020169
Tal R et al (2019) Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol 17:e3000421. https://doi.org/10.1371/journal.pbio.3000421
Tao H, Gaudry L, Rice A, Chong B (1999) Cord blood is better than bone marrow for generating megakaryocytic progenitor cells. Exp Hematol 27:293–301. https://doi.org/10.1016/s0301-472x(98)00050-2
Thordardottir S et al (2014) The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cells Dev 23:955–967. https://doi.org/10.1089/scd.2013.0521
Till JE, McCulloch EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14:213–222. https://doi.org/10.1667/rrav01.1
Tunstall-Pedoe O et al (2008) Abnormalities in the myeloid progenitor compartment in down syndrome fetal liver precede acquisition of GATA1 mutations. Blood 112:4507–4511. https://doi.org/10.1182/blood-2008-04-152967
Valipour B et al (2020) Cord blood stem cell derived CD16+ NK cells eradicated acute lymphoblastic leukemia cells using with anti-CD47 antibody. Life Sci 242:117223. https://doi.org/10.1016/j.lfs.2019.117223
Wagner JE Jr et al (2016) Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell 18:144–155. https://doi.org/10.1016/j.stem.2015.10.004
Watt SM, Austin E, Armitage S (2007) Cryopreservation of hematopoietic stem/progenitor cells for therapeutic use. In: Cryopreservation and freeze-drying protocols. Humana Press, pp 237–259. https://doi.org/10.1007/978-1-59745-362-2_17
Wilson A et al (2004) c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev 18:2747–2763. https://doi.org/10.1101/gad.313104
Xue E, Milano F (2020) Are we underutilizing bone marrow and cord blood? Review of their role and potential in the era of cellular therapies. F1000Research 9:26. https://doi.org/10.12688/f1000research.20605.1
Yu J, Chen LXS, Zhang J, Guo G, Chen B (2016) The effects of a simple method for cryopreservation and thawing procedures on cord blood derived dc-based esophageal carcinoma vaccine. Cryo Letters 37:272–283
Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF (2008) Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood 111:3415–3423. https://doi.org/10.1182/blood-2007-11-122119
Zhang Y et al (2017) Large-scale ex vivo generation of human red blood cells from cord blood CD34+ cells. Stem Cells Transl Med 6:1698–1709. https://doi.org/10.1002/sctm.17-0057
Conflict of Interest
The authors declare no conflict of interests.
Ethical Approval
The authors declare that this article does not contain any direct studies with animals or human participants.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fernandes, S.S., Limaye, L.S., Kale, V.P. (2021). Differentiated Cells Derived from Hematopoietic Stem Cells and Their Applications in Translational Medicine. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 14. Advances in Experimental Medicine and Biology(), vol 1347. Springer, Cham. https://doi.org/10.1007/5584_2021_644
Download citation
DOI: https://doi.org/10.1007/5584_2021_644
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80491-6
Online ISBN: 978-3-030-80492-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)