Abstract
A xeno-free method for ex vivo generation of red blood cells (RBCs) is attempted in order to replicate for large-scale production and clinical applications. An efficient milieu was formulated using injectable drugs substituting the animal-derived components in the culture medium. Unfractionated mononuclear cells isolated from human umbilical cord blood were used hypothesizing that the heterogeneous cell population could effectively contribute to erythroid cell generation. The strategy adopted includes a combination of erythropoietin and other injectable drugs under low oxygen levels, which resulted in an increase in the number of mature RBCs produced in vitro. The novelty in this study is the addition of supplements to the medium in a stage-specific manner for the differentiation of unfractionated umbilical cord blood mononuclear cells (MNCs) into erythropoietic lineage. The erythropoietic lineage was well established by day 21, wherein the mean cell count of RBCs was found to be 21.36 ± 0.9 × 108 and further confirmed by an upregulated expression of CD235a+ specific to RBCs. The rationale was to have a simple method to produce erythroid cells from umbilical cord blood isolates in vitro by mitigating the effects of multiple erythroid-activating agents and batch to batch variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The safest blood transfusion is of great medical concern in most developing countries as it is dependent on healthy human volunteers (Ramesh and Guhathakurta 2013). Considering the insufficient numbers of blood donors, the prospect of manufactured RBCs for routine clinical use has stimulated much excitement in the medical community (Chandrashekar and Kantharaj 2014). However, the large-scale production of human functional RBCs for clinical application has not yet reached the platform beyond research (Giarratana et al. 2011). The feasibility has been envisaged by many groups; however, the reproducibility, cost of production and biosafety aspects have not been standardized (Lim et al. 2011). Most of the conventional methods for culturing human erythroblasts utilized a variety of cellular progenitors, i.e., somatic stem cells (Giarratana et al. 2005), human embryonic stem cells (Lu et al. 2008) and induced pluripotent stem cells (iPSCs) (Lapillonne et al. 2010). Unfortunately, existing methods for the ex vivo production of RBCs are extremely costly and their efficacy has not been extensively tested (Xie et al. 2014).
Umbilical cord blood (UCB) is considered to be an excellent source of hematopoietic stem cells (HSCs) than adult bone marrow or peripheral blood and often shows increased sensitivity to added factors (Kögler et al. 2004; de Lima et al. 2008). UCB has several advantages over other sources of HSCs, including that it is easily accessible, banked with non-invasive collection procedures, has lower probability of allogenic graft versus host disease and more plasticity for multi-lineage differentiation (Hiroyama et al. 2011). UCB has the least possibility of viral contamination with common pathogens compared with other stem cell sources and hence, many researchers had attempted to expand RBCs from cord blood-derived HSCs (Glen et al. 2013; Jin et al. 2014). Given that UCB transplants are able to support lifelong hematopoiesis in recipients, it seemed probable that the limited ex vivo yields of RBCs achieved to date are a consequence of sub-optimal performance rather than biological capacity (Timmins and Nielsen 2011).
Biosafety is important for the application of RBCs generated ex vivo along with good manufacturing practices to produce stem cell-derived RBCs (Mountford and Turner 2011). Hence, the choice of source of stem cells, reagents and animal-free culture components forms an essential decisive factors for clinical application (Xie et al. 2014).The successful large-scale production of RBCs in the laboratory depends on several factors such as selection of potential hematopoietic cell source; optimized cell culture; bio-physiological parameters; clinically applicable erythropoietic differentiation inducers; scalability; contamination-free, non-reactive, non-tumorigenic end product; stability; and functionality of cultured RBCs (Giarratana et al. 2013). Earlier reports suggested that the sequential application of specific growth factor combinations in serum-free culture medium could result in the maturation of erythroid precursors when transplanted into immunodeficient mice (Neildez-Nguyen et al. 2002). Hence, a combinatorial approach was adapted by systematically exploring the renewal and differentiation requirements of erythroid progenitors from UCB under hypoxic conditions (Vlaski et al. 2009). We hypothesized that multiple factors regulate erythropoiesis in vitro, through integration of a range of intrinsic and extrinsic cellular cues. An efficient milieu was formulated considering the physiological factors, which favors the long-term differentiation of hematopoietic progenitor cells in vivo.
The goals of the present study were (a) to develop a simple and cost-effective method for ex vivo production of RBCs using unfractionated MNCs from human UCB and (b) to formulate a culture medium free of animal-derived products that would improve robustness of the process and eliminate the risk of disease transmission during clinical application.
Material and methods
The present study was conducted after due approval by the Institutional Research and Ethics Committee of the Indian Institute of Technology Madras, Chennai, India. Human UCB was collected with informed consent from healthy pregnant women after delivery (n = 6). The mononuclear cell fraction was isolated using hydroxy ethyl starch density gradient centrifugation method as previously described (Rubinstein et al. 1995). An average of 50 mL total UCB was collected per donor, yielding approximately 132 × 106 MNCs to 245 × 106 MNCs. MNC recovery and purity was determined on samples obtained from the UCB before and after volume reduction (Supplementary Fig. 1).
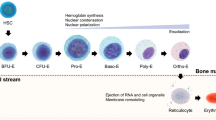
The expansion procedure comprised of several steps that are typical for stress erythropoiesis (Fig. 1). Supplements were added to the medium in a stage-specific manner to induce MNCs to stream into erythroid lineage. In the first step (days 1–3), MNCs (1 ± 0.4 × 106/mL) were cultured in erythroid essential medium (a base medium composed of IMDM and 20% AB+ve plasma pooled, supplemented with 125 μg/mL gentamycin sulphate and 2.5 μg/mL amphotericin B (Table 1, column A). The plates were maintained under hypoxia (low oxygen tension of 3% O2) at 37 °C in a humidified incubator with 5% CO2. This procedure allowed the ex vivo expansion of CD34+ HSCs. In the second step (day 4–7), the cells obtained by day 4 were resuspended in erythroid expansion medium (Table 1, column B; erythroid expansion medium = erythroid essential medium + 4 units/mL human recombinant erythropoietin). The plates were maintained under hypoxia (low oxygen tension of 3% O2) at 37 °C in a humidified incubator with 5% CO2. The medium was changed every 3 days and the cells were resuspended in a fresh medium supplemented with 4 IU/mL of human recombinant erythropoietin (EPO).
In the third step (day 8–14), the cells obtained on day 8 were resuspended in erythroid differentiation medium (Table 1, column C). This procedure allowed the differentiation of progenitor cells into erythroid precursor population and their subsequent rapid expansion into immature erythrocytes. In the fourth step, for terminal maturation (day 15–21) cells were not passaged but grown to high cell densities (5 × 106 cells/mL) in erythroid maturation medium (Table 1, coloumn D) on an agitated rocker (with rocking motion) for 1 week, at an agitation rate of 15 rpm. For control experiments, cultures were maintained for 21 days in erythroid expansion medium. The medium was changed twice per week and the cells were maintained for 4 weeks in 5% CO2 and 37 °C humidified atmosphere.
Cell counting
Live cell concentration and viability was determined using trypan blue dye exclusion method. The number of total viable cells was determined by diluting the cells 1:2 with trypan blue and differentially counting viable and dead cells using a hemocytometer under a microscope at × 100 magnification. For morphological analyses; cells were air-dried and fixed in 100% methanol for 10 min. After phosphate-buffered saline (PBS) wash, cells were stained with Wright-Giemsa (van den Akker et al. 2010).
Clonogenic assays
At various time points, aliquots of non-adherent cells were grown in methylcellulose for progenitor cell evaluation. Triplicate cultures were set up with a final concentration of 105 mononuclear cells/mL in MethoCult medium 4434 (StemCell Technologies) in standard 35-mm well tissue-culture plates incubated in a humidified, 37 °C, 5% CO2 incubator. Colonies were scored on day 14 and growth was reported as the mean colony count per 105 nucleated cells.
Flow cytometric analyses of erythroid markers
Cultured cells were harvested on days 4, 8, 14, 21 and immunophenotypes were determined. Staining was performed on raw culture supernatant (no lyse, no wash blood preparation), then diluted before flow cytometry (FACS) analysis. Antibodies to CD235a-PE (glycophorin A), CD71-FITC, CD45-FITC and CD34-PE (all from Biolegend, CA, USA) and fetal hemoglobin-PE (BD Pharmingen) with the appropriate isotype controls were stained in accordance with the manufacturer’s suggestion. The gating strategy was based on the isotype controls. The presence or absence of DAPI fluorescence was used to analyze nucleated and enucleated erythroid cells in the culture. Analyses were done on FACS Calibur flow cytometer (Becton Dickinson) using FACS diva (version 6.1.3).
Functional analysis
Routine clinical assays were performed according to standard protocols. Hematological parameters, i.e., total RBCs count, WBC count, hemoglobin concentration, mean cell volume, nucleated RBC count, were assessed by using a quantitative automated hematology analyzer (Sysmex XE 2100). Oxygen equilibrium curves were determined using a hemox analyzer. Gas-phase gradients were obtained using nitrogen and room air and the curves were run in both directions.
Long-term storage of RBCs
To assess the hemolysis during cold storage, free hemoglobin in the RBC supernatant was determined by an automated analyzer and the percentage of hemolysis was calculated accordingly. The total viable RBCs at different storage durations were counted on a hemocytometer using trypan blue dye exclusion and the morphology of the stored blood cell types were visualized under light microscope.
Statistics
The experiments were conducted at least in triplicate and all data are expressed as mean ± standard error of the mean. The statistical significance of the data obtained was analyzed by Student’s paired t test. A p value less than 0.05 was considered significant. Statistical analysis and graphical representations of data were performed using GraphPad Prism (version 5.03).
Results
In the present study, inducers were selected and identified from the literature based on their impact on erythrocyte differentiation in culture. First, cell proliferation was induced in a humanized medium supplemented without erythropoietin for 3 days. Secondly, cells were induced to pre-expansion by culturing in the presence of erythropoietin for additional 4 days. The cell population obtained at this stage potentially has production benefits associated with enhanced progenitor expansion. In the third stage, culture-expanded progenitor cell populations were exposed to a range of different cytokine conditions to induce erythroid differentiation. In the fourth step, cells were again exposed to a range of different cytokines along with modifying the physical conditions (i.e., cells were gently agitated up to 7 days). By day 21, the mean cell amplification of UCB MNCs reached a plateau of 21.36 ± 0.9 × 108 (Table 2). The control samples yielded a cell count of 3.01 ± 0.7 × 108 and the humanized medium contributed to 7-fold higher expansion on day 21 (Table 2).
The morphology of the cultured RBC was observed by light microscopy and the chronologic appearance of ex vivo-generated RBCs is shown in Fig. 2. By days 3 ± 1 of the culture, several colonies of erythroid progenitor cells mimicking the erythroblast islands were observed (data not shown). Colonies of tightly clustered cells that contained approximately 50 cells, not clearly displaying a red color were observed by 6 ± 1 days. Most of these colonies were relatively smaller with an uneven distribution of hemoglobin and had not differentiated into morphologically distinguishable colony types (Fig. 2a). On day 8, cells were transferred from the erythroid expansion medium to the erythroid differentiation medium (supplemented with dexamethasone, insulin, thyroxine and hydrocortisone). The erythroid expansion medium augmented the growth of distinguishable erythroid colony-type burst-forming units (BFUs) and hemoglobinization of the colonies had progressively increased. BFUs with single or multiple-centered red colonies composed of very tightly clustered cells acquired the proliferative thrust and colonies started to increase and peaked at 10 ± 1 days of culture (Fig. 2b). This was followed by a gradual decrease in BFU frequency over the next few days of culture while the erythroid colony-forming units (CFU-E) with loosely grouped red segments, reached maximal concentrations by days 14 ± 1 days (Fig. 2c).
Cytological observations: Upper panel, representative photomicrographs of erythroid colonies obtained from methyl cellulose assays arranged as (a) day 7, (b) day 10 and (c) day 14 of culture (× 100 magnification). Arrow heads point to (a) early BFUs, (b) BFUs and (c) CFU-e; scale bar—10 μm. Lower panel: representative Wright-Giemsa stain of cells for (d) day 4, (e) day 7 and (f) day 14 of culture. Arrowhead points to (d) early erythroblasts, (e) intermediate erythroblasts and (h) late normoblast; scale bar—10 μm
Cytological observations (Wright-Giemsa stain) confirmed that over the course, proerythroblasts matured to polychromatic erythroblasts characterized by nuclear condensation. Erythroid progenitors at day 4 were large cells, bright blue round nucleus, scant cytoplasm and pale perinuclear zones consistent with early erythroblast morphology (Fig. 2d). Intermediate-stage cells at day 8 displayed typical features of basophilic-erythroblastic cells, including a slightly paler blue cytoplasm and a smaller nucleus-cytoplasmic ratio compared to day 4 cells (Fig. 2e). Cells from day 14 cultures displayed features of late normoblastic stage, namely homogenous nucleus and hemoglobinization that imparts a paler blue or gray color to the cytoplasm (Fig. 2f).
Clonogenic assays performed on semisolid media using culture-expanded cells indicated that less than 10% of the colonies were granulocyte-macrophage colony-forming units (CFU-GM) on day 4 and the number of myeloid colonies diminished rapidly thereafter. The presence of primitive erythroid progenitor cells in cultures was assessed at various time points. It was observed that the progenitor cell frequency peaked by day 8 and our culture expansion procedure favored the proliferation and maintenance of erythroid progenitors (Table 3). BFUs were predominant in early stages of culture, constituted the majority of the colonies observed, persisting up to day 8. CFU-E progenitors proliferated extensively after day 8 of culture and became the predominant colonies observed by day 15 and the other progenitor cell types were markedly reduced (Table 3).
MNC recovery was determined on samples obtained from the UCB before and after volume reduction and HES-based separation method gave MNC recovery at 81.79% ± 5.18. These results were determined using differential cell counts coupled with flow cytometric analysis of CD45+ cells (Supplementary Fig. 1). UCB MNCs expressed high levels of CD45+ (~ 92%), which is usually expressed on almost all hematopoietic cells except for mature erythrocytes and low levels of hematopoietic progenitor marker CD34+ (~ 3.5%) (Fig. 3d). The expression of erythroid lineage-specific markers CD71+ (~ 2%) and CD235a+ (~ 2%) were very low or undetectable in the MNC fraction (Fig. 3c). The differentiation profile of the cultured cells was further examined by flow cytometry after each phase in culture and a representative micrograph of these stained populations is presented (Fig. 4 and Table 2). During the first days of culture, CD34+ population increased with time, starting from low levels similar to values reported for freshly prepared UCB MNCs and the percentage of CD34+ cells increased 10-fold up to 21.4 ± 3.4% (Fig. 4i and Table 2) by day 4 (Pranke et al. 2006). This substantiates the persistence of multipotent progenitors during the first 3 days, which then gradually developed into progenitors of the erythroid lineage. The percentage of CD45+ cells decreased and the expression of CD235a+ marker too remained low during the step. Analysis by flow cytometry revealed an onset of expression of CD235a+ and upregulated CD71+ on day 8 of culture as the erythropoietin-sensitive BFU cell population changed to a colony-forming unit erythroid cell population (Fig. 4m–p). By day 14, CD235a+ was expressed on the majority of the cells, showing that a significant fraction of cells had undergone erythroid differentiation (Fig. 4t).The increased number of cells expressing CD235a+, with a reduction of CD71+ and CD45+ cells, was consistent with erythroid cell maturation, a phenotype most pronounced up to day 21 (Fig. 4u–x). Cells exhibited decreased growth rate when maintained in control media and a cessation of growth combined with restricted immunophenotypes were found in erythroid expansion media (data not shown).
Flow cytometric analyses of ex vivo-generated RBCs: Cell surface expressions of specific markers as percentage of positive cells against culture time in days (n = 6). Representative FACS histograms arranged as rows isotype controls, MNCs fractionate, day 4, day 8, day 14 and day 21 of culture. The percentages of marker expressions for CD34, CD45, CD71 and CD235a are given in the representative histograms. Marker expression indicates gradual establishment of cells committed to the erythroid lineage. Images were corrected for color uniformity using Adobe Photoshop software
We determined the degree of maturity of cultured RBCs by assessing the proportion of erythroid progenitors expressing CD235a+ and/or CD71+ markers (Fig. 5). The erythroid progenitor cells were only just detectable in early stages and on day 8 of culture, double-positive cells for CD71 and CD235a had increased approximately to 30% (Table 2). The culture-expanded cells had progressively acquired CD235a+ (Fig. 5d) and at day 14, 75.1 ± 4.8% of the events were double-positive for CD71 and CD235a signifying the presence of progenitor cells (Fig. 5e). The percentage of mature RBCs (CD71− and CD235a+) gradually increased from days 14 to 21 with a concomitant increase in CD235a+ expression and a decrease in CD71+ expression and the percentages of CD71 and CD235a double-positive events (17.5 ± 3.7%) had significantly decreased (Fig. 5f). To compare maturation enhancement, the number of cells with different maturation statuses were counted at the end of culture and the mean values of immunophenotypes of cultured RBCs were (1) CD71− CD235a+ mature RBCs (80.8 ± 4.7%); (2) CD71+ CD235a+ immature RBCs (17.5 ± 3.7%); and (3) CD71+ CD235a− erythroid progenitors (3.4 ± 1.2%), which was significantly less frequent than the other two populations.
FACS dot plot of CD235a+ versus CD71+: Representative FACS dot plots for (a) isotype controls, (b) MNCs fractionate, (c) day 4, (d) day 8, (e) day 14 and (f) day 21 of culture. The proportion of cells (% of total events acquired) expressing CD235a+ and/or CD71+ in different phases of culture is indicated in each quadrant (n = 3). The proportion of committed erythroid progenitors (CD235a+ CD71+) is indicated in the upper right quadrant of the dot plot
In order to elucidate the effects of culture conditions on enucleation efficiency, we subjected cells at various stages of culture to flow cytometry analysis using CD235a+ and DAPI staining (Table 2; n = 3). Figure 6 depicts the dot plot of CD235a+ versus DAPI used to quantify two distinct cell populations, i.e., CD235a+DAPI− (enucleated RBCs) and CD235a+ DAPI+ (nucleated RBCs). The percentage of enucleated RBCs slowly increased from days 8 to 14 as demonstrated by the increased CD235a+ DAPI− cell population (Fig. 6b, c). By day 21, i.e., during terminal maturation of cultures, the percentages of CD235a+ and DAPI−events (85.1 ± 3.7%) had significantly increased (Fig. 6d).
Analysis of enucleation: Representative FACS dot plots of CD235a+versus DAPI for (a) day 4, (b) day 8, (c) day 14 and (e) day 21 of culture (n = 3). The percentage of cell populations in each quadrant is indicated; enucleated cells (CD235a+DAPI−) fractionate in the upper left quadrant, nucleated RBCs (CD235a+ DAPI+) in the upper right quadrant
The culture-derived RBCs displayed a mean cell volume (MCV) of 113.23 ± 6.11 fl/cell, mean cell hemoglobin (MCH) of 33.6 ± 1.9 pg/cell and a mean cell hemoglobin concentration (MCHC) of 30.8 ± 2.6 g/dL (Fig. 7). The cells displayed a significant increase in MCV (132.2 ± 2.12 fl/cell), MCHC (41.5 ± 3.7 g/dL) and MCH (39.6 ± 3.9 pg/cell) compared with the reference range of the routinely used red cell indices by the 21st day. At the final stage of maturation (day 21), the number of nucleated RBCs and other cell forms were significantly reduced (Fig. 7b, c). The RBCs expanded from cord blood expressed fetal hemoglobin (HbF) as confirmed by FACS analysis (Supplementary Fig. 2). The high HbF content of cultured RBCs (~ 80%) with bright fluorescence, corresponding to HbF, allowed discrimination between ex vivo produced RBCs and adult peripheral blood RBCs.
Oxygen equilibrium measurements were carried out on RBCs derived from UCB MNCs to assess affinity and cooperativity (Fig. 8a). The oxygen equilibrium curves of the cultured RBCs (day 21 cultures) were similar to that of normal adult RBCs (n = 6). Oxygen equilibrium curves showed that a suspension of ex vivo-expanded RBCs binds O2 reversibly in the same manner as a suspension of native RBCs. The P50 value was 24.4 for cultured RBC hemoglobin as compared to 26.3 for control RBC hemoglobin (a decrease in P50 of about 10%). The Hill coefficient was also similar to that of the normal control (2.2 versus 2.3). In fact, the RBC samples showed the expected decrease in P50 upon exposure to carbon dioxide (Fig. 8b). Carbon dioxide influences intracellular pH and low levels of CO2 have the effect of shifting the curve to the right, while higher levels cause a leftward shift.
Hemox analysis: (a) Oxygen equilibrium curves of cultured RBCs (day 21) and normal human adult RBCs (control). The P50 value was 24.4 for cultured RBC hemoglobin as compared to 26.3 for control RBC hemoglobin. b Culture-expanded RBCs when exposed to CO2 displayed the expected decrease in P50 (n = 6). Low levels of C02 have the effect of shifting the curve to the right, while higher levels cause a leftward shift
The feasibility of storing in vitro produced RBCs at 4 °C as the way peripheral blood RBCs are stored in blood banks was also tested. The osmotic fragility of RBCs determines their susceptibility to hemolysis and may be used as an index of stability. The ex vivo-expanded RBCs were stored at 4 °C up to 8 weeks in a SAG-M preservative-based solution (saline adenine glucose mannitol) and successfully survived over 28 days as the natural RBCs (n = 6).The deformability of culture-expanded RBCs stored for 4 weeks was close to that of fresh RBCs (Table 4).
Discussion
There are two aspects of cord blood cell therapy: transplantation and transfusion. While cord blood transplantation for treating hemato-oncologic diseases is common, there are also reports describing the use of umbilical cord blood for auto-transfusion in very low birth weight neonates. Several authors have investigated whether autologous placental blood could be used as an alternative for allogeneic blood transfusion (Taguchi et al. 2003). Only few case reports concerning the transfusion of such packed red cells derived from autologous placental blood have been published to date (Brune et al. 2002; Khodabux et al. 2008). Cord blood might be beneficial as a supplemental source for transfusions; however, the number of RBCs within 150–200 mL of cord blood unit (approximately 20 mL) would not be enough as a true blood transfusion substitute. There is also a concern of high bacterial contamination rates reported in collected cord blood (Garritsen et al. 2003). Therefore, the strategy to overcome the low cell content in a single UCB unit was to increase the infused cell dose using ex vivo expansion.
Previous studies focused upon designing various models of in vitro erythropoiesis for large-scale production of mature RBCs (Glen et al. 2013; Xi et al. 2013). In such models, two- to three-phase cultures using various growth factor combinations of steroids and cytokines, with or without serum for specific phases have been developed (Leberbauer et al. 2005; Cheung et al. 2007). Multiple signaling pathways have been implicated in guiding hematopoietic stem cell differentiation, suggesting the potential value of subpopulation cells in enhancing ex vivo expansion (Glen et al. 2013). Emile van den Akker et al. previously demonstrated that the majority of the in vitro erythroid expansion potential resides in CD34− cells, a fraction that has until now been largely ignored when culturing erythroblasts (van den Akker et al. 2010). Indeed, CD34− cells isolated from UCB have long-term repopulation abilities in irradiated NOD/SCID mice demonstrating their hematopoietic potential (Wang et al. 2003). Indumathi S et al. (2015) suggested that the non-sorted MNCs and lineage-depleted fractions of UCB to be rich in progenitor cell populations.
In the present study, unfractionated mononuclear cells were used, hypothesizing that the heterogeneous mononuclear cell population from UCB could effectively contribute to erythroid cell generation. MNCs had been grown for 3 days in a basal medium and were switched to medium present with erythropoietin and cell numbers were measured during the culture period. The data revealed that the initial population of UCB MNCs used to initiate erythroblast cultures contained very low levels of erythroid progenitors. At this stage, stimulation with EPO selectively promoted the proliferation of erythroid progenitors. A possible approach to overcome limited lineage yield would be to increase the proliferation of the early-stage progenitors (Crauste et al. 2008; Glen et al. 2013). Hence, erythroid differentiation medium with a different cytokine combination was formulated by adding insulin, dexamethasone and thyroxine to promote differentiation of BFUs. We took clues from the fact that the combination of dexamethasone, insulin interacts synergistically with EPO to stimulate the amplification of erythroid progenitor cells under hypoxic conditions (Sawada et al. 1989; England et al. 2011). In addition to cytokine supplements, mineral elements such as iron, copper, cobalt, (Lahoti et al. 2012) vitamin A (Saraiva et al. 2014), vitamin D (Icardi et al. 2013), vitamin E (Drake and Fitch 1980), riboflavin, folic acid pantothenic acid, and cyanocobalamin (Haiden et al. 2006) were added to promote erythroid progenitor proliferation. Reports support the complex interplay between vitamin D effects on production of RBCs in the bone marrow, as well as its ability to maintain a pool of uncommitted precursor cells, as a reservoir for future needs (Moore et al. 1991). Vitamin E has an important role in maintaining cell membrane integrity by reducing membrane fragility of erythrocytes (Drake and Fitch 1980). Adenosine nucleotides are capable of stimulating erythropoietic proliferation and erythroblasts are unable to maintain in vitro the same nucleotide content found in vivo (Stocchi et al. 1987). Hence, nucleotides along with essential amino acids were used in the culture medium. Although often overlooked, fat is an integral part of the bone marrow microenvironment and plays a fundamental role by providing local or systemic factors (Rosen et al. 2009). The erythroid differentiation medium supplemented with essential fatty acids and phospholipids enhanced the erythrocyte membrane integrity and greatly improved maintenance of cell function and morphology.
For terminal maturation, low-density cultures under static conditions were transferred to agitated culture phase. Low agitation had a significant impact on erythroid cell yields while agitation with increased speed accelerated the erythroid differentiation. Dexamethasone and insulin were removed during the maturation phase; however, high concentrations of EPO were maintained as EPO is crucial for survival and terminal differentiation of erythrocytes in vitro (von Lindern et al. 1999). These methods are of critical importance in the stem cell differentiation process where cell populations and their bio-manufacture demands change rapidly (Boehm et al. 2010; Glen et al. 2013).
The growth conditions enhanced CD235a+ expression and nuclear condensation before enucleation occurred. The bimodal histogram of CD235a+-positive cells showed the distribution of two cell populations with variable fluorescence signal and was skewed to the right (Fig. 4t). The subpopulations of reticulocytes produce a secondary peak and contribute to the right skew of the graph (Constantino 2011). This pattern may be explained by the heterogeneity within the initial cell population composed of progenitors committed to the erythroid lineage (resulting in the first peak of RBCs) and immature cells that require longer time in culture before giving rise to the second erythroid differentiation peak. One week later, mature RBCs became the predominant cells and the histogram for CD235a+ started to skew to the left and a normal single-peaked distributional shape of the histogram was observed (Fig. 4x). The differentiation and enucleation status of cultured cells at different days was examined by flow cytometric analysis using combinations of CD71-CD235a and DAPI-CD235a, respectively (Figs. 5 and 6). Progenitor erythroid cells have been reported to be CD71+ CD235a−, further developing into CD71+ CD235a+ and finally into mature CD71−CD235a+ cells (Giarratana et al. 2011). In the present study, the presence of a certain number of erythroid cells in the cultures at day zero could not be ruled out. However, CD71− events (initial stages), as well as high proportions of double-positive CD235a+ CD71+ events, in later stages of the cultures suggests that immature erythroid progenitors developed in the culture and the culture conditions allowed selective survival of progenitors. Thus, some of the observed erythroid growth may be due to the maintenance and differentiation of erythroid precursors present at day zero. However, this observation is interesting bearing in mind that the culture conditions supported the proliferation and differentiation of highly heterogenous unfractionated MNCs. A concomitant decrease of CD235a+ DAPI+ immunophenotype was observed, a sign of terminal maturation to erythrocytes and the patterns of changes in marker distribution were reproducible for several samples from individual UCB donors.
The hematological aspects of ex vivo-produced RBCs constitute an interesting area. We observed that hematological parameters such as MCH, MCV and MCHC showed higher mean values compared to the normal adult RBCs in the present study (Fig. 7). The slight variation observed between cultured RBCs from UCB and peripheral blood is not unexpected as the starting material (UCB MNCs) is comprised of cells at different stages of maturation. The variation could also be attributed to the behavior of red cells in vitro, where the cells become enlarged (Xi et al. 2013). Furthermore, the hemoglobin content of cells stored for 4 weeks was similar to that of adult peripheral blood, indicating that the cultured RBCs were stable and functional. Similar erythrocytes indices are observed in prematurely delivered neonates (≤ 25 weeks) (Christensen et al. 2008). A high MCHC can be an indication that the erythrocytes have a shape intermediate between a sphere and a biconcave disk. Overall, hemoglobin values in these populations reached about 40 pg of the level in 21 days (Fig. 7f) and we speculate that during the process of in vitro erythropoiesis, the larger erythrocytes, loaded with HbF, exhibit more affinity for oxygen. The lower values of P50 compared to control further demonstrate that the in vitro-generated RBCs have oxygen-carrying properties that are comparable with those of adult functional erythrocytes.
To improve process robustness and eliminate the risk of disease transmission, fully defined culture medium that is free from animal-derived products should be ideally employed (Duran et al. 2012). Although serum free, previously described erythroid culture media contain animal-derived albumin/plasma. Hence, the possibility of directly substituting these components for commercially available injectable drugs was investigated and the ingredients of nutrition and minerals in their therapeutic form were used as supplements in the cell culture medium. These substituted ingredients enhanced batch to batch reproducibility, with no further modifications required either to the medium or the culture protocol. Hence, the concentrations of supplements used are of significant value for reducing material costs and improving performance of cell culture medium. Over and above expensive growth factors were not used in the present process and the only growth factor used was recombinant human erythropoietin.
In conclusion, we used a multi-step process including the use of human plasma, injectable drugs and optimization of culture parameters (low oxygen tension, agitation culture) for enhanced ex vivo production of RBCs. The rationale was to have a simple method to produce erythroid cells from UCB isolates in vitro by reducing the effects of multiple erythroid activating agents and batch to batch variability. We believe our approach a platform for the production of clinical-grade RBCs and envisage further that this could be a potential solution for availability of rare blood type units.
Abbreviations
- EPO:
-
Erythropoietin
- UCB:
-
Umbilical cord blood
- MNCs:
-
Mononuclear cells
References
Boehm D, Murphy WG, Al-Rubeai M (2010) The effect of mild agitation on in vitro erythroid development. J Immunol Methods 360:20–29. https://doi.org/10.1016/j.jim.2010.05.007
Brune T, Garritsen H, Witteler R et al (2002) Autologous placental blood transfusion for the therapy of anaemic neonates. Biol Neonate 81:236–243. https://doi.org/10.1159/000056754
Chandrashekar S, Kantharaj A (2014) Legal and ethical issues in safe blood transfusion. Indian J Anaesth 58:558–564. https://doi.org/10.4103/0019-5049.144654
Cheung JOP, Casals-Pascual C, Roberts DJ, Watt SM (2007) A small-scale serum-free liquid cell culture model of erythropoiesis to assess the effects of exogenous factors. J Immunol Methods 319:104–117. https://doi.org/10.1016/j.jim.2006.11.002
Christensen RD, Jopling J, Henry E, Wiedmeier SE (2008) The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J Perinatol 28:24–28. https://doi.org/10.1038/sj.jp.7211852
Constantino BT (2011) The red cell histogram and the dimorphic red cell population. Lab Med 42:300–308. https://doi.org/10.1309/LMF1UY85HEKBMIWO
Crauste F, Pujo-Menjouet L, Génieys S et al (2008) Adding self-renewal in committed erythroid progenitors improves the biological relevance of a mathematical model of erythropoiesis. J Theor Biol 250:322–338. https://doi.org/10.1016/j.jtbi.2007.09.041
de Lima M, McMannis J, Gee A et al (2008) Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant 41:771–778. https://doi.org/10.1038/sj.bmt.1705979
Drake JR, Fitch CD (1980) Status of vitamin E as an erythropoietic factor. Am J Clin Nutr 33:2386–2393
Duran JM, Taghavi S, George JC (2012) The need for standardized protocols for future clinical trials of cell therapy. Transl Res 160:399–410. https://doi.org/10.1016/j.trsl.2012.07.004
England SJ, McGrath KE, Frame JM, Palis J (2011) Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood 117:2708–2717. https://doi.org/10.1182/blood-2010-07-299743
Garritsen HSP, Brune T, Louwen F et al (2003) Autologous red cells derived from cord blood: collection, preparation, storage and quality controls with optimal additive storage medium (Sag-mannitol). Transfus Med 13:303–310. https://doi.org/10.1046/j.1365-3148.2003.00457.x
Giarratana M-C, Kobari L, Lapillonne H et al (2005) Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol 23:69–74. https://doi.org/10.1038/nbt1047
Giarratana M-C, Rouard H, Dumont A et al (2011) Proof of principle for transfusion of in vitro-generated red blood cells. Blood 118:5071–5079. https://doi.org/10.1182/blood-2011-06-362038
Giarratana MC, Marie T, Darghouth D, Douay L (2013) Biological validation of bio-engineered red blood cell productions. Blood Cells Mol Dis 50:69–79. https://doi.org/10.1016/j.bcmd.2012.09.003
Glen KE, Workman VL, Ahmed F et al (2013) Production of erythrocytes from directly isolated or Delta1 Notch ligand expanded CD34+ hematopoietic progenitor cells: process characterization, monitoring and implications for manufacture. Cytotherapy 15:1106–1117. https://doi.org/10.1016/j.jcyt.2013.04.008
Haiden N, Klebermass K, Cardona F et al (2006) A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics 118
Hiroyama T, Miharada K, Kurita R, Nakamura Y (2011) Plasticity of cells and ex vivo production of red blood cells. Stem Cells Int 2011:195780. https://doi.org/10.4061/2011/195780
Icardi A, Paoletti E, De Nicola L et al (2013) Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transplant 28:1672–1679
Indumathi S, Harikrishnan R, Rajkumar JS, Dhanasekaran M (2015) Immunophenotypic comparison of heterogenous non-sorted versus sorted mononuclear cells from human umbilical cord blood: a novel cell enrichment approach. Cytotechnology 67:107–114. https://doi.org/10.1007/s10616-013-9663-2
Jin H, Kim H-S, Kim S, Kim HO (2014) Erythropoietic potential of CD34+ hematopoietic stem cells from human cord blood and G-CSF-mobilized peripheral blood. Biomed Res Int 2014:435215. https://doi.org/10.1155/2014/435215
Khodabux CM, von Lindern JS, van Hilten JA et al (2008) A clinical study on the feasibility of autologous cord blood transfusion for anemia of prematurity. Transfusion 48:1634–1643. https://doi.org/10.1111/j.1537-2995.2008.01747.x
Kögler G, Sensken S, Airey JA et al (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200:123–135. https://doi.org/10.1084/jem.20040440
Lahoti V, Murphy W, Al-Rubeai M (2012) Mathematical approach for the optimal expansion of erythroid progenitors in monolayer culture. J Biotechnol 161:308–319. https://doi.org/10.1016/j.jbiotec.2012.06.003
Lapillonne H, Kobari L, Mazurier C et al (2010) Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica 95:1651–1659. https://doi.org/10.3324/haematol.2010.023556
Leberbauer C, Boulmé F, Unfried G et al (2005) Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 105:85–94. https://doi.org/10.1182/blood-2004-03-1002
Lim M, Panoskaltsis N, Ye H, Mantalaris A (2011) Optimization of in vitro erythropoiesis from CD34+ cord blood cells using design of experiments (DOE). Biochem Eng J 55:154–161. https://doi.org/10.1016/j.bej.2011.03.014
Lu S-J, Feng Q, Park JS et al (2008) Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood 112:4475–4484. https://doi.org/10.1182/blood-2008-05-157198
Moore DC, Carter DL, Bhandal AK, Studzinski GP (1991) Inhibition by 1,25 dihydroxyvitamin D3 of chemically induced erythroid differentiation of K562 leukemia cells. Blood 77:1452–1461
Mountford JC, Turner M (2011) In vitro production of red blood cells. Transfus Apher Sci 45:85–89. https://doi.org/10.1016/j.transci.2011.06.007
Neildez-Nguyen TMA, Wajcman H, Marden MC et al (2002) Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol 20:467–472. https://doi.org/10.1038/nbt0502-467
Pranke P, Hendrikx J, Alespeiti G et al (2006) Comparative quantification of umbilical cord blood CD34+ and CD34+ bright cells using the ProCount-BD and ISHAGE protocols. Braz J Med Biol Res 39:901–906. https://doi.org/10.1590/S0100-879X2006000700008
Ramesh B, Guhathakurta S (2013) Large-scale in-vitro expansion of RBCs from hematopoietic stem cells. Artif Cells Nanomed Biotechnol 41:42–51. https://doi.org/10.3109/10731199.2012.702315
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19:109–124. https://doi.org/10.1016/j.bbi.2008.05.010
Rubinstein P, Dobrila L, Rosenfield RE et al (1995) Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A 92:10119–10122. https://doi.org/10.1073/pnas.92.22.10119
Saraiva BCA, Soares MCC, Dos Santos LC et al (2014) Iron deficiency and anemia are associated with low retinol levels in children aged 1 to 5 years. J Pediatr 90:593–599. https://doi.org/10.1016/j.jped.2014.03.003
Sawada K, Krantz SB, Dessypris EN et al (1989) Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest 83:1701–1709. https://doi.org/10.1172/JCI114070
Stocchi V, Cucchiarini L, Magnani M, Fornaini G (1987) Adenine and pyridine nucleotides in the erythrocyte of different mammalian species. Biochem Int 14:1043–1053
Taguchi T, Suita S, Nakamura M et al (2003) The efficacy of autologous cord-blood transfusions in neonatal surgical patients. J Pediatr Surg 38:604–607. https://doi.org/10.1053/JPSU.2003.50131
Timmins NE, Nielsen LK (2011) Manufactured RBC - rivers of blood, or an oasis in the desert? Biotechnol Adv 29:661–666. https://doi.org/10.1016/j.biotechadv.2011.05.002
van den Akker E, Satchwell TJ, Pellegrin S et al (2010) The majority of the in vitro erythroid expansion potential resides in CD34(−) cells, outweighing the contribution of CD34(+) cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica 95:1594–1598. https://doi.org/10.3324/haematol.2009.019828
Vlaski M, Lafarge X, Chevaleyre J et al (2009) Low oxygen concentration as a general physiologic regulator of erythropoiesis beyond the EPO-related downstream tuning and a tool for the optimization of red blood cell production ex vivo. Exp Hematol 37:573–584. https://doi.org/10.1016/j.exphem.2009.01.007
von Lindern M, Zauner W, Mellitzer G et al (1999) The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood 94:550–559
Wang J, Kimura T, Asada R et al (2003) SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood 101:2924–2931
Xi J, Li Y, Wang R et al (2013) In vitro large scale production of human mature red blood cells from hematopoietic stem cells by coculturing with human fetal liver stromal cells. Biomed Res Int 2013:807863. https://doi.org/10.1155/2013/807863
Xie X, Li Y, Pei X (2014) From stem cells to red blood cells: how far away from the clinical application? Sci China Life Sci 57:581–585. https://doi.org/10.1007/s11427-014-4667-5
Acknowledgments
The authors would like to acknowledge Dr.Asra, Dr. Chandra and Mr. Fida Hussain for their kind help.
Funding
A part of this work is funded by the Department of Biotechnology (DBT), Govt. of India (Ref. No. BT/PR 5729/PID/6/675/2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig 1
Cell recovery and purity was determined on samples obtained from the UCB before (pre) and after volume reduction (post) using differential cell counts coupled with flow cytometric analysis of specimens stained with 7-amino-actinomycin D (7AAD) and CD45+ cells. Top panel: FACS plots of unfractionated cord blood (a, b and c). A gate was placed around the population of interest (a) and the events selected were then sent to a 7-AAD /SSC plot to discriminate the live and dead cells (b). The selected even were then sent to a CD45 + /SSC plot, where viable CD45 + cells were counted (c). Bottom panel: FACS plots of MNCs obtained after density gradient centrifugation. The same gating strategy was applied for viable CD45+ cell counting in MNCs fraction (d, e and f). (JPG 436 kb)
Supplementary Fig 2
Flow cytometry results using anti– HbF antibody. The high HbF content of cultured RBCs (~80%) with bright fluorescence, corresponding to HbF, allowed discrimination between day 21 ex vivo generated RBCs (a) and essentially HbF negative adult peripheral blood RBCs (b) . (JPG 41 kb)
Rights and permissions
About this article
Cite this article
Rallapalli, S., Guhathakurta, S., Narayan, S. et al. Generation of clinical-grade red blood cells from human umbilical cord blood mononuclear cells. Cell Tissue Res 375, 437–449 (2019). https://doi.org/10.1007/s00441-018-2919-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2919-6