Abstract
Lin28 is a highly conserved RNA binding protein that regulates stemness whose molecular role has been widely studied in vitro. However, the regulation and the molecular role of Lin28 during the development of the vertebrate central nervous system (CNS) in vivo are not completely understood. Here, the expression and the putative role of Lin28 in the development of the mammalian CNS are reviewed in the context of recent results showing the progressive cellular and molecular changes in neural progenitor cells. Downstream genes that may play a role during CNS development and the effect of misregulated expression of Lin28 are discussed. Evidence suggests that Lin28 promotes symmetric divisions over asymmetric divisions, increasing the number of progenitors during early neurogenesis. Future quantitative analysis of Lin28 isoforms levels and stabilities together with single cell transcriptomics data, cell cycle dynamics and cell fate analysis in Lin28 gain- and loss-of-function experiments will provide a better understanding of the molecular role of Lin28 during development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Central nervous system
- Cerebral cortex
- Developmental timing
- Differentiation
- Heterochronic genes
- Let-7
- Lin28
- Neural progenitor cells
- Proliferation
1 Introduction
Lin28 was one the heterochronic genes identified in analyses of mutants in the nematode Caenorhabditis elegans (Ambros and Horvitz 1984). The term “heterochrony” refers to any difference in the timing or duration of a developmental process in an organism relative to other organism (for example, in a mutant relative to the wildtype or in an animal relative to its ancestors). Heterochronic genes in C. elegans control the timing and pattern of cell divisions during larval development (Rougvie 2001). Mutants for these genes show defects in proliferation and differentiation in several tissues because progenitor cells skip, repeat, or delay specific division patterns. For example, lin28 loss-of-function mutants show precocious differentiation in some lineages because symmetric divisions of progenitor cells do not occur in one of the specific larval stages (Ambros and Horvitz 1984). In contrast, lin28 gain-of-function induce excess of proliferation due to repetitions of symmetric divisions of progenitor cells (Moss et al. 1997).
Lin28 is highly conserved in animals and two Lin28 orthologues have been identified in vertebrates, Lin28a and Lin28b. Lin28a and Lin28b promote proliferation of stem cells by regulating the expression of genes related to metabolism and cell cycle (Shyh-Chang and Daley 2013; Tsialikas and Romer-Seibert 2015). Accordingly, Lin28a and Lin28b are aberrantly expressed in several tumors (Viswanathan et al. 2009) and enhance the reprogramming of differentiated cells to generate induced pluripotent stem cells (iPSCs) (Yu et al. 2007; Zhang et al. 2016).
Lin28a and Lin28b expression in vivo has been studied in different tissues of several vertebrates (Faas et al. 2013; Faunes et al. 2017; Gundermann et al. 2019; La Torre et al. 2013; Moss and Tang 2003; Ouchi et al. 2014; Yang et al. 2015). The general trend is that Lin28a and Lin28b are expressed at higher levels during early stages of embryogenesis or larval development compared to adults, with the highest expression in undifferentiated cells (Moss and Tang 2003; Shyh-Chang and Daley 2013; Yang and Moss 2003). Consistent with the role of Lin28 in mammal progenitor cells and developmental timing in C. elegans, gain- and loss-of-function experiments in vertebrates show that Lin28 is involved in growth (Shinoda et al. 2013; Zhu et al. 2010), metabolism (Zhu et al. 2011), regeneration (Shyh-Chang et al. 2013), puberty (Zhu et al. 2010) and metamorphosis (Faunes et al. 2017; Gonzalez-Itier et al. 2018).
Lin28a and Lin28b are RNA binding proteins that regulate the translation of several mRNAs and the biogenesis of specific microRNAs (Shyh-Chang and Daley 2013; Tsialikas and Romer-Seibert 2015). Lin28a and Lin28b regulate the levels of proteins involved in cell cycle, cell growth, protein synthesis and metabolism by direct binding to mRNAs or indirectly through inhibition of the biogenesis of the micro-RNA let-7. In addition, Lin28a controls transcription by binding to promoters (Zeng et al. 2016). Therefore, Lin28a and Lin28b are master regulators of gene expression. Although several target genes have been identified in stem cells in vitro, the repertoire of genes controlled by Lin28a and Lin28b during embryogenesis is still incomplete.
In this work, recent evidence about the role of Lin28a and Lin28b in vivo is reviewed, specifically, in the development of the vertebrate central nervous system (CNS). The focus is on the cerebral cortex of mice because several studies have characterized the types of cell divisions (symmetric versus asymmetric divisions), the cell cycle length, and the cell fate of the progeny of neural progenitors during embryogenesis. In addition, single cells analyses of neural progenitors have revealed how gene expression changes during neurogenesis. These works are reviewed to discuss the expression of Lin28 and the effect of gain- and loss-of-function experiments on the CNS.
2 Overview of the CNS Development in Mice
Nervous system (NS) originates from the definitive ectoderm, which is committed into neuroectoderm around embryonic day 7.5 (E7.5) by the underlying notochord. Rapid cell divisions thicken the neuroectoderm (neural plate), which then begins to fold to form the neural tube. The neural tube is closed around E9/E10. The most anterior (rostral) part of the neural tube -the forebrain- is divided into telencephalon (from which the cerebral cortex is derived) and diencephalon.
Neurogenesis in vertebrates has been extensively reviewed (Cardenas and Borrell 2020; Florio and Huttner 2014; Paridaen and Huttner 2014). Here, only some of the processes that occur in mice with a focus on the cerebral cortex development are summarized. Neuroepithelium cells (NECs) expressing Sox1, Sox2, Sox3, and Lin28 undergo symmetric amplifying divisions that expand the number of NECs (Fig. 1a). Around E10, some of these NECs are converted into radial glia cells (RGCs) and express Pax6, Nestin and Vimentin. This population of NECs and RGCs is heterogenous and contains cells with different potential. Some of these NECs and RGCs self-renew (i.e. one daughter cell is a new NEC or RGC and the other daughter cell is a different cell type) and can generate neurons, astrocytes, and oligodendrocytes and, therefore, they are neural stem cells. However, other cells can generate only neurons and, therefore, they are neural progenitors and have a more limited proliferation capacity. At present, there are no expression markers to clearly distinguish between neural stem cells (that can generate all the cell types in the NS) and neural progenitor cells (that can generate only one cell type). In this review, the term “neural progenitor cells” is used, but it is important to consider that some of these cells may have a greater potential. NECs and RGCs are also known as apical progenitors (APs) and have apical and basal processes in contact with the ventricle (apical side) and the pia (basal side) of the neuroepithelium, respectively (Fig. 1a).
Overview of cerebral cortex development. (a) Scheme of neurogenesis in mice showing the types of divisions of neural progenitors and the progeny during embryogenesis (E8 to E21, embryonic days are approximate). Neuroepithelial cell (NECs) and radial glial cells (RGCs) undergo symmetric divisions that expand the pool of progenitors at the beginning of neurogenesis (dark green). Asymmetric divisions of RGCs (light green) can generate a neuron (direct neurogenesis) or an intermediate progenitor cell (IPC), which divides into two neurons (indirect neurogenesis). Glial progenitors (blue) are not shown as generated from neurogenic RGCs to indicate that the population of progenitors is heterogenous. Different layers of the epithelium are shown at approximate times of generation. VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; CP, cortical plate; MZ, marginal zone. (b) Cellular changes during neurogenesis. Lin28 levels and Wnt/β-catenin signaling decreases in progenitor cells during neurogenesis. Membrane potential and cell cycle length are only shown in the temporal window described in the text
After the expansion of progenitors through symmetric divisions at the beginning of neurogenesis, RGCs switch from symmetric to asymmetric divisions. Studies using 3H and BrdU incorporation have estimated that 11 cell cycles occur between E11 and E17 with an increase in the cell cycle length from 8 h at E11 to 18 h at E16-17 and an increase in the proportion of asymmetric divisions in the neuroepithelium (Takahashi et al. 1995, 1996) (Fig. 1a, b).
Different types of asymmetric divisions have been described: (1) RGCs can self-renew into one RGC daughter cell and one neuron (direct neurogenesis); (2) RGCs can self-renew into one RGC daughter cell and one intermediate progenitor cell (IPC) that later divides into two neurons (indirect neurogenesis); and (3) RGCs can divide into two neurons (Florio and Huttner 2014). The contribution of these types of divisions to the final set of cells of the NS is not completely clear and may vary in time and regions, but it is thought that direct neurogenesis occurs early, and indirect neurogenesis predominates at later stages. During indirect neurogenesis, IPCs switch from Pax6 to Tbr2/Eomes expression and migrate to the basal side of the VZ, forming the sub-ventricular zone (SVZ). IPCs are considered one of the two types of basal progenitors (the other type, the basal glia radial bRG is highly abundant in primates and is not further discussed here (Cardenas and Borrell 2020; Florio and Huttner 2014)). In the SVZ, IPCs lose the expression of markers of RGCs and most of the IPCs divide once, generating two neurons.
Recent in vivo single cell analysis using a pulse-label method in VZ-born cells at different times show that APs change their identity from E12 to E15 (Telley et al. 2019). Gene expression in APs change from “internally driven” at E12 to a more “environmentally driven” at E15 as revealed for transcriptomic analyses. Intrinsic genetic programs -including cell cycle regulation- are predominant in early APs and environment sensing programs -including ion transport-related processes- predominate at later stages. This temporal change in APs is correlated with an increase in the duration of the cell cycle, consistent with a previous work (Takahashi et al. 1995). Cell cycle length has been proposed to be important for differentiation and this aspect is later discussed considering the role of Lin28 on proliferation. Importantly, the transcriptional program underlying differentiation is conserved among temporally different APs, suggesting that the diversity of cell types of the progeny is mainly generated from the temporal progression of molecular programs in APs. Interestingly, the transcriptional changes from early APs to late APs occur independently of cell division, as observed after simultaneous blocking of cell division and differentiation of E11–12 APs by overexpressing the cell cycle inhibitor p18 and the Notch-intracellular domain (NICD), respectively (Okamoto et al. 2016). These results show that the cell division program can be decoupled from the genetic network that regulates AP identity.
These gene expression transitions in APs are also linked to membrane properties. Membrane potential increases from E12.5 (around −40 mV) to E15.5 (around −80 mV), as measured in cortical slices using patch clamp (Vitali et al. 2018). Strikingly, by modifying the membrane potential specifically in APs through in utero electroporation of a potassium channel to hyperpolarize and electroporation of a DREADD (Designer Receptor Exclusively Activated by Designer Drug) to restrict hyperpolarization, the fate of the progeny is altered. At E12.5, low membrane potential (−40 mV) promotes direct neurogenesis and the generation of early fate neurons. Progressive hyperpolarization represses Wnt/β-catenin signaling and promotes indirect neurogenesis and the birth of late fate neurons. Hyperpolarization may also regulate the ability of APs to respond to extracellular signals, consistent with the evidence of the predominant extracellular control at later stages (Telley et al. 2019). How hyperpolarization, Wnt/β-catenin signaling, and AP identity are linked at the molecular levels requires further investigation.
After E15, RGCs produce GFAP+ astrocytes and O4+ oligodendrocytes (gliogenesis). As Lin28 is not expressed in neural progenitors at these later stages, gliogenesis is not described here. In conclusion, cell proliferation, expression of self-renewal and differentiation factors, signaling pathways and membrane potential must be tightly coordinated in time to generate the high cell diversity during CNS development.
3 Expression of Lin28a and Lin28b during CNS Development in Mice
3.1 Endogenous Expression During Embryogenesis and Post-natal Development
To understand the role of Lin28 in the CNS, the endogenous expression of Lin28a and Lin28b during neurogenesis is first described. The focus is on Lin28a and Lin28b expression during mice development. Different splice isoforms, post-transcriptional and post-translational mechanisms described for Lin28 in other biological contexts are discussed.
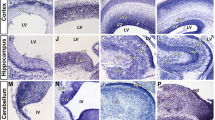
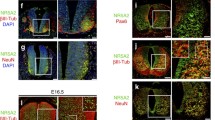
During mice embryogenesis, Lin28a protein is detected in embryonic and extraembryonic ectoderm and endoderm at E6.5 (Yang and Moss 2003). Lin28a is expressed in several tissues derived from the three germ layers at E8.5 but then the expression is mainly restricted to some epithelia, including neuroepithelia (Yang and Moss 2003). Between E9.5 and E12, in situ hybridization and immunofluorescence show that Lin28a is detected in the neural tube (Balzer et al. 2010), mainly in the ventricular zone (VZ) of the developing cerebral cortex (Yang et al. 2015). During these stages, Lin28a colocalizes with Sox2+ and Nestin+ cells and with the most apical subset of Pax6+ cells, indicating that Lin28 is mainly expressed in APs (Balzer et al. 2010; Herrlinger et al. 2019; Yang et al. 2015). Interestingly, at E11.5, Lin28a expression is higher in the forebrain compared to midbrain and hindbrain, indicating that expression may be spatially controlled (Herrlinger et al. 2019). In contrast to Lin28a, spatial expression of Lin28b has not been characterized in detail.
Western blots in developing cerebral cortex extracts show that Lin28a and Lin28b protein levels decrease from E10.5 to birth (Yang et al. 2015). High levels of Lin28a protein are observed at E9.5 compared to E12.5 and no Lin28a expression is detected after E16.5. Similarly, Lin28b levels are higher at E9.5 compared to E18.5. These results are consistent with the progressive increase of differentiated cells compared to undifferentiated cells. Interestingly, Lin28a has been detected by western blot in the cerebral cortex at post-natal day 1 and 3 (P1 and P3) and not detected at later stages P7 to P42 (Nathan et al. 2020). Different antibodies and procedures used by these studies can explain this post-natal detection, but it is also probable that different regions of the cortex may have different levels of Lin28a. Notably, Lin28a expression colocalizes at P1 and P3 with the terminal differentiation marker Neu by immunofluorescence, indicating that Lin28a expression is compatible with the expression of neuronal markers (Nathan et al. 2020).
Different protein isoforms have been described for Lin28a in mouse hippocampal cells (Amen et al. 2017) and for Lin28b in human hepatocarcinoma cells (Guo et al. 2006) and brain tadpoles in Xenopus (Faunes et al. 2017) (Fig. 2a, b). Importantly, the expression of the isoforms correlates with the differentiation of cells. The most characterized Lin28a isoform (25 kDa) is typically detected in undifferentiated cells but a different specific band around 37 kDa is observed in differentiated cells (Amen et al. 2017). Similarly, full length Lin28b (around 35 kDa), which is associated with undifferentiated cells, promotes proliferation and inhibits the biogenesis of let-7 (Guo et al. 2006; Mizuno et al. 2018). In contrast, a short form of Lin28b (Lin28b-S, around 22 kDa), which is detected in differentiated cells, is not able to promote proliferation or to regulate let-7 biogenesis (Guo et al. 2006; Mizuno et al. 2018). Lin28b-S is generated by alternative splicing and starts with an open reading frame with a different start codon keeping the same downstream protein sequence (Fig. 2b, black “ATG” and blue “ATG”). These results indicate that alternative splicing controls the balance of isoforms during differentiation and that these isoforms may play different roles in progenitor and differentiated cells. In addition to alternative splicing, different lin28b transcripts can be generated from alternative promoter usage in hepatocarcinoma and medulloblastoma cells (Guo et al. 2018; Hovestadt et al. 2014). How different promoters of Lin28 are used and how splicing is regulated during embryogenesis has not been studied. These works suggest that proper levels of Lin28 isoforms may be crucial to control proliferation and differentiation. Thus, if Lin28a detected by immunofluorescence at post-natal stages corresponds to the 25 kDa or the 37 KDa isoform or both is unknown. The use of antibodies to detect specific segments of each isoform by immunofluorescence would complement the study of the differential expression of isoforms. In addition, it would be interesting to determine the stability and the molecular targets of each isoform of Lin28a and Lin28b in neural progenitor cells and neurons.
In the retina, another structure of the CNS, both Lin28a and Lin28b RNAs decrease from E12 to P3 (Xia et al. 2018). However, Lin28a shows a small but significant increase in adults compared to E18. A similar pattern is observed for Lin28b protein and no expression is detected at P1 and P3 (La Torre et al. 2013). Interestingly, Lin28a is also detected in neurons Neu+, similar to the cerebral cortex (Nathan et al. 2020). In summary, Lin28a and Lin28b are highly expressed in neural progenitors at early stages of embryogenesis, their expression decreases during development of the CNS, low levels are detected after birth (including expression in neurons) and no expression is observed after P7.
3.2 Regulation of Lin28a and Lin28b in Neural Stem and Progenitor Cells
The decrease of Lin28a and Lin28b levels during development has been described in several tissues. However, the specific transcription factors and signaling pathways that regulate their expression in vivo have not been characterized.
Some factors that directly regulate Lin28 expression in vitro have been identified and are described here to compare with the expression and role of Lin28 in vivo. In mouse embryonic stem cells (ESCs), Lin28 expression is controlled by the core pluripotency network, including the transcription factors Oct4 and Nanog, which directly bind to Lin28 promoter (Marson et al. 2008). ESCs exist in functionally distinct states: ground, naïve and primed pluripotency, which are maintained in specific culture conditions, exhibit distinct properties, and represent different stages of their counterparts in vivo (Nichols and Smith 2009; Ying et al. 2008). Lin28 increases in the presence of serum (naïve state) and in the presence of FGF/activin (primed state) compared to ground state (Ghimire et al. 2018; Kalkan et al. 2017; Kumar et al. 2014; Marks et al. 2012; Parisi et al. 2017). This increase has been interpreted as a reinforcement of self-renewal under differentiation stimuli (serum or FGF/activin). However, it is also probable that this increase in Lin28 expression is required to change cell cycle parameters and accelerate proliferation as a first step in the differentiation program. Accordingly, Lin28a and Lin28b double KO (lin28a−/−;lin28b−/−) cells maintain a ground state morphology in the presence of FGF/Activin (Zhang et al. 2016) and high expression of nanog (Li et al. 2017), suggesting that the increase of Lin28 may be involved in the exit of ground pluripotency of ESCs in vitro. Consistent with this idea, Lin28 is expressed in proliferating cells and not in the basal stem cells in the adult intestine (Yang and Moss 2003). Furthermore, Lin28a expression is undetectable in muscle satellite stem cells, but it is expressed during myoblast differentiation in vitro (Polesskaya et al. 2007). Finally, Lin28a and Lin28b increase during reprogramming of fibroblasts to transit to a state similar to primed and naive states and then decrease at the ground state (Zhang et al. 2016). Altogether, these results indicate that Lin28a and Lin28b promote cell proliferation during commitment of ESCs, regeneration in adult tissues and reprogramming in vitro. Similarly, Lin28 may accelerate cell division in vivo to increase the number of neural progenitor cells, as discussed below.
The transcription factors Otx2 and HMGA2 also bind to promoters and increase the expression of Lin28a and Lin28b during the transition to the primed state (Parisi et al. 2017). In human neural stem cells, Sox2 binds to and controls the activation of the lin28a promoter (Cimadamore et al. 2013). In neuroblastoma cell lines and other human and mouse tumors, cMyc directly regulates Lin28b expression (Beckers et al. 2015; Chang et al. 2009). Otx2, HMGA2, Sox2 and cMyc are expressed during CNS development and, therefore, they may control the expression of Lin28a and Lin28b in vivo (Fig. 2).
The interplay of these transcription factors with signaling pathways in the control of Lin28 in vivo has not been studied. In adult mammalian retina, Wnt/β-catenin pathway regulates Lin28a and Lin28b expression after injury (Yao et al. 2016). In Xenopus, Lin28a and Lin28b are downregulated after FGF inhibition during early development (Faas et al. 2013). During Xenopus metamorphosis, Lin28b decreases in the brain while thyroid hormones (THs) increase (Faunes et al. 2017). However, if THs directly inhibit Lin28b expression has not been studied. Considering the important role of THs in nervous system development and the increase of THs that occur during late embryogenesis and the peri-natal period in mice (Barez-Lopez and Guadano-Ferraz 2017), it would be interesting to determine if THs regulate the expression of Lin28. THs directly inhibit the transcription of cMyc in neuroblastoma cells (Perez-Juste et al. 2000) and, therefore, THs may indirectly decrease the levels of Lin28 through downregulation of cMyc. In summary, Wnt/β-catenin, FGF and THs may control the transcription of Lin28 genes, but further research is required to determine if these signaling pathways directly regulate Lin28 expression during neurogenesis.
Lin28 translation is repressed by the miRNA let-7 in hepatocellular carcinoma and P19 cells (Guo et al. 2006; Wu and Belasco 2005). Consistent with this mutual inhibition between Lin28 and let-7 in cell lines, let-7 expression is detected in APs and increases during the development of the CNS in mice (Fairchild et al. 2019; Zhao et al. 2010) and in the brain and spinal cord during Xenopus metamorphosis (Faunes et al. 2017). Interestingly, in the neuroblast Ad12 HER10 cell line, the levels of let-7 oscillate during the cell cycle with higher levels in G2/M compared to G1 (Fairchild et al. 2019). At E13.5, let-7 expression is mainly detected at the basal side of the VZ and lower expression is detected at the apical side, indicating that let-7 levels are dynamically controlled during the cell cycle, consistent with the interkinetic nuclear migration of neural progenitors in the VZ. However, this dynamic expression in the VZ is no longer observed at later stages and let-7 is expressed at higher levels (Fairchild et al. 2019).
In addition to transcriptional and post-transcriptional regulation, Lin28 is regulated at the post-translational level (Fig. 2a). The brain-derived neurotrophic factor (BDNF) increases Lin28a expression through protein stabilization in primary murine hippocampal neurons prepared from P0 mice (Amen et al. 2017). This work suggests that Lin28a half-life is low or that Lin28a is synthesized at low levels at post-natal stages but that Lin28 levels can be modulated by extracellular signals. In P19 cells, Lin28a is phosphorylated at the serine 200 and cells expressing a Lin28a-S200A mutant form (phospho-deficient) show higher proliferation and reduced differentiation upon retinoic acid treatment, indicating that phosphorylation of Lin28a promotes differentiation (Liu et al. 2017). Furthermore, the cellular localization of Lin28 may also be modulated by other proteins such as Musashi1 in differentiation of ESCs (Kawahara et al. 2011). Interestingly, before E13.5, Lin28 localizes to the nucleolus and cytoplasm in the cerebral cortex and, after E13.5, the nucleolar localization is not detected (Herrlinger et al. 2019). These results indicate that Lin28a level and function are modulated by several post-translational mechanisms. It would be interesting to study how phosphorylation and subcellular localization together with transcriptional and splicing regulation are coordinated during neurogenesis in vivo. Detailed immunofluorescence analysis of Lin28 isoforms with specific antibodies and gene expression studies in specific cell populations isolated at different time points would give us insights into these mechanisms.
In summary, the complex regulation of splicing, translation, and post-translational modifications of Lin28 suggests that the balance of Lin28 (compared to other regulators such as let-7) is crucial for normal development. This balance may regulate the dynamics and type of cell division and the levels of regulators of cell cycle and factors that control commitment and differentiation.
4 Role of Lin28 in Proliferation and Cell Fate Decisions in the CNS
Several approaches have been used to study the role of Lin28a and Lin28b during CNS development. In this section, gain- and loss-of-function experiments are reviewed with emphasis on the developmental time when Lin28a or Lin28b are affected, the strategy used, and the read-out analyzed.
By using the Cre-lox system activated under the control of nestin promoter (i.e. active in AP cells), Lin28a was continuously expressed in the progeny of Nestin+ cells (Yang et al. 2015). In these lin28a Tg; Nestin-Cre animals, brain size and the cerebral cortex thickness increase at E18.5 compared to controls, consistent with an increase of proliferation detected at E15.5. This overexpression of Lin28a increases the number of Pax6+ cells and decreases the number of Tbr2+ cells compared to controls, indicating an imbalance between APs (Pax6+) and IPCs (Tbr2+). Interestingly, this phenotype is identical to the obtained after the overexpression of a stabilized form of β-catenin under the control of an enhancer of nestin (Wrobel et al. 2007), suggesting that Lin28a may be one the downstream genes of Wnt/β-catenin in APs, similar to the control in the mammalian adult retina, as mentioned above (Yao et al. 2016).
Overexpression of Lin28a has also been done by in utero electroporation at E14.5. In this case, plasmids carrying Lin28a-GFP under the control of the constitutive promoter CAG were injected in the right ventricle and cortical cells were harvested 2 days after electroporation (at E16.5) and cultured in vitro (Bhuiyan et al. 2013). At day in vitro (DIV) 0, the number of proliferating cells (Ki67+) is higher in Lin28a overexpressing cells compared to controls. In addition, Lin28a overexpression prevents apoptosis after 13 DIV. It is important to mention that in these conditions, Lin28a-GFP colocalizes with the neuronal marker Neu+, indicating that Lin28a overexpression is also compatible with the expression of neuronal markers in vitro. Consistent with this effect, overexpression of Lin28a in Sox2-knockdown neural stem cells rescues the defects in proliferation and the expression of the neuronal differentiation, Tuj1 (Cimadamore et al. 2013). Interestingly, Lin28 overexpression does not rescue the expression of the neuronal marker MAP2, suggesting that some functions of Sox2 are independent of Lin28a or, alternatively, overexpression of Lin28a prevents full terminal differentiation. Accordingly, morphology and action potential analysis of neurons of post-natal animals that overexpress Lin28a from E14.5 are altered compared to controls, indicating that continuous presence of Lin28a affects terminal differentiation (Jang et al. 2019). Considering the changes in membrane potential during development, it would be interesting to study the molecular link between Lin28 and its targets and membrane potential in progenitors. Furthermore, Lin28a overexpressing animals show memory deficits determined in water maze tests (Jang et al. 2019). These results indicate that it is important to consider functional analyses in addition to the study of expression of progenitor and neuronal markers.
Altogether these experiments show that Lin28 induces neurogenesis in vivo probably by expansion of early APs when Lin28a and Lin28b are expressed at higher levels. In P19 cells that stably overexpress Lin28a or Lin28b, RA-induced differentiation towards neurons is preferred over glia, based on Tuj1 and GFAP expression, respectively (Balzer et al. 2010). Although a direct neurogenesis-inducer role of Lin28a or Lin28b cannot be ruled-out, another possibility is that Lin28a and Lin28b control cell cycle parameters to favor rapid divisions like those seen in early APs that produce neurons (see below).
In contrast to the effect on proliferation induced by overexpression of Lin28a during embryonic neurogenesis, Lin28a overexpression during post-natal stages by electroporation of plasmids into the lateral ventricle at day P0 reduced the number of neurons in the olfactory bulb and has no effect on proliferation studied by the Ki67 marker 3 days after electroporation (Romer-Seibert et al. 2019). In this experimental setup, Lin28a overexpression decreases the number of Sox2+ cells in the SVZ and increases the number of Doublecortin (DCX) + cells, suggesting that Lin28a is converting APs into neuroblasts and decreasing the pool of progenitors. The reason why Lin28a in these conditions has no effect on proliferation is not clear. One possibility is that APs at postnatal stages are intrinsically different to APs present at early stages of embryogenesis. In addition, the extracellular environment is different and antiproliferative signals present at post-natal stages probably prevent amplifying divisions of APs. Interestingly, despite the increase of DCX+ cells, the final number of neurons and astrocytes in the olfactory bulb in Lin28a-overexpressing animals is reduced compared to controls and the proportion of neuronal subtypes is altered. If this fate depends on cell cycle length and if Lin28a plays a role in this parameter is unknown. Lin28a overexpression may also affect migration, similar to the effect of gain-of-function of Wnt3 (Wrobel et al. 2007).
The absence of effect of Lin28 overexpression on proliferation in post-natal or adult stages has also been described in other biological contexts. In the retina, transfection of Lin28 at E16 increases the number of Brn3+ cells (early fate) compared to controls but the transfection of Lin28 at P1 has no effect on the number of Brn3+ cells. Similarly, Lin28b upregulation has no effect in proliferation, ganglion size, and let-7 expression during early postnatal development (Hennchen et al. 2015). Consistent with a possible anti-proliferative environment at post-natal and adult stages, damage in the nervous system induces the expression of Lin28 to promote regeneration or repair in some contexts (see below). These experiments suggest that Lin28 accelerates proliferation when extracellular proliferative signals are present (or anti-proliferative signals are reduced), but Lin28 is not sufficient to induce proliferation. This ability of Lin28 to promote rapid cell divisions is thought to be important for reprogramming of fibroblast to iPSCs (Hanna et al. 2009; Zhang et al. 2016). Altogether, these results indicate that the role of Lin28 in vivo is incompletely understood and future studies on the differences among embryonic and post-natal progenitors, the effect on proliferation, cell fate decision, migration, maturation and neuronal function may reveal new aspects of the function of Lin28.
Consistent with the effect of gain-of-function of Lin28 on early neurogenesis, Lin28a/b double knockout (dKO) mice show reduced proliferation in the neuroepithelium at E9.5 and in the VZ of the cerebral cortex at E11.5 compared to wild-type animals (Herrlinger et al. 2019). Importantly, the number of Tuj1+ cells is higher in dKO animals compared to controls, suggesting precocious differentiation, and indicating that Lin28a or Lin28b are not required to initiate the neuronal differentiation program. dKO animals present neural tube closure defects and die before birth. In contrast to dKO animals, no defects are observed in single Lin28b KO mutants. In Lin28a KO mutants, brain and other organs are smaller compared to controls at P1 (Yang et al. 2015). In Lin28a KO animals, proliferation in the cerebral cortex is reduced compared to controls at E15.5. Interestingly, the number of Pax6+ cells and Tbr2+ cells is significantly lower in Lin28a KO animals compared to controls at E17.5 and P1 in the cerebral cortex but no difference is detected at E15.5. These defects are more severe when one allele of Lin28b is lost in Lin28a KO mutants (lin28a−/−; lin28b+/−), suggesting that Lin28a and Lin28b play overlapping roles in CNS development. Thus, Lin28a and Lin28b are required for the maintenance and expansion of APs during the development of the CNS (Herrlinger et al. 2019; Yang et al. 2015).
In summary, Lin28a overexpression during embryogenesis increases the proliferation of APs but does not prevent the expression of neuronal markers. However, terminal differentiation seems to be affected by the continuous presence of Lin28a. Considering the normal morphology of animals that overexpress Lin28a, these results show that the differentiation program starts normally and overcomes the ectopic expression of Lin28a, probably due to post-translational modifications that inhibit the function of Lin28a. Consistently, Lin28a and Lin28b are required for normal proliferation during development, as shown by single Lin28a KO and dKO mutants. In the absence of Lin28a and Lin28b, neuronal differentiation occurs preciously but the neural tube is not closed, and animals die during embryogenesis probably due to defects in several tissues.
5 Molecular Mechanisms Underlying the Role of Lin28 in the CNS
Lin28a and Lin28b regulate the translation of mRNAs in the cytosol and inhibit the processing of the let-7 precursor in the nucleus and cytosol (Tsialikas and Romer-Seibert 2015). Several genes are known to be directly regulated by Lin28 in stem cells, tumors and cell lines in vitro, including cyclins, cyclin-dependent kinases (cdks), insulin growth factor 2 (igf2), ribosomal proteins, glycolytic enzymes and mitochondrial enzymes (Balzeau et al. 2017; Hafner et al. 2013; Peng et al. 2011; Polesskaya et al. 2007; Shyh-Chang et al. 2013; Xu et al. 2009; Zhu et al. 2011). In addition, by inhibiting the biogenesis of let-7, Lin28 indirectly controls the levels of the let-7 target genes Myc, Ras, Cyclin D1, cyclin D2, Hmga2, among others (Shyh-Chang and Daley 2013). Therefore, the module Lin28/let-7 is a global regulator of genes related to metabolism, protein synthesis and cell cycle and the balance between Lin28 and let-7 is crucial for proliferation and differentiation in progenitor cells. In this section, genes and processes regulated by Lin28 in the CNS and neural stem cell lines are briefly described.
In the neural stem cell line NE-4C (established from cerebral vesicle of E9 mice), Lin28a binds mRNAs of Imp1 (IGF2 mRNA-binding protein 1, also known as Igf2bp1), IGF2 and Hmga2 (high-mobility group AT-hook 2) (Yang et al. 2015). Accordingly, Lin28a overexpression up-regulates IGF2 and HMGA2 in culture neurons after in utero electroporation (Bhuiyan et al. 2013; Jang et al. 2019) and knockdown using RNAi for Lin28 decrease the levels of the receptor of IGF1 (IGF1R) and HMGA2 (Jang et al. 2019). In vivo, IGF1R and HMGA2 protein levels decrease in progenitor cells isolated from cerebral cortex of lin28a−/−;lin28b+/− animals compared to wild-type animals (Yang et al. 2015). This effect of Lin28 on Imp1, IGF2, IGF1R indicates that Lin28 regulates IGF2-mTOR signaling. Consistently, the activation of this pathway induced by IGF2 is decreased in cerebral cortex dissected at E12.5 of Lin28a mutant animals compared to wild-type animals (Yang et al. 2015). In addition, phosphorylation of the S6 ribosome protein, a readout of Igf2-mTOR signaling, is reduced in progenitor cells of Lin28a KO animals compared to controls at P1. This reduction in Igf2-mTOR signaling is also observed in the brain of lin28a−/−b+/− animals at E14.5 (Yang et al. 2015). These results confirm that mTOR signaling is directly regulated by Lin28 in the CNS.
HMGA2 is also directly regulated by Lin28. HMGA2 is a chromatin-associated protein that controls transcription and is widely expressed in undifferentiated cells. HMGA2 is enriched in early neural progenitors (Telley et al. 2019) and promotes self-renewal by decreasing the levels of the tumor suppressors p16Ink4a and p19Arf (Nishino et al. 2008). Therefore, in addition to directly promote cell proliferation through regulation of cyclins, Lin28 favors proliferation through regulation of HMGA2 and the levels of tumor suppressors. However, it has also been described that Lin28a represses the translation of HMGA2 in primed ESCs and HMGA2 cooperates with Otx2 to increase the expression of Lin28 (Parisi et al. 2017). Considering that both Lin28 and HMGA2 are targets of let-7, this evidence indicates that the levels of Lin28, HMGA2 and let-7 are tightly balanced during proliferation and differentiation in the CNS.
Consistent with the proliferation induced by Lin28 overexpression, mis-expression of let-7 reduces proliferation of neural stem cells in vitro (Cimadamore et al. 2013). Overexpression of let-7 decreases the number of cells in the S phase in Ad12 HER10 cells, in cortical stem cells isolated from E14.5 brain and in primary cultures of E11.5 cortex and lengthens the cell cycle in Ad12 HER10 cells (Fairchild et al. 2019). Accordingly, knockdown of let-7 with antagomirR shortens the cell cycle in these cultures, indicating that let-7 controls proliferation and cell cycle exit in neural progenitors. These results indicate that Lin28 and let-7 form a regulatory loop that mutually controls their levels and the levels of their target genes and, consequently, the dynamic of the cell cycle. It would be interesting to determine if the levels of Lin28 and HMGA2 change during the cell cycle.
Lin28a modulates translation both positively and negatively (Cho et al. 2012). To determine which of these activities is predominant during the development of the CNS, a genetic analysis using a mouse containing a hypomorphic allele of the ribosomal protein L24 (Rpl24Bst/+) was performed. Rpl24Bst/+ mice show reduced global protein synthesis. Whereas Lin28a−/− and Rpl24Bst/+ animals do not show neural closure defects at E11.5, lin28a−/−;Rpl24Bst/+ animals present open neural tubes, similar to Lin28 dKO embryos (Herrlinger et al. 2019), suggesting that the predominant activity of Lin28 during CNS development is to promote translation. Consistent with this role, Rpl24Bst/+ rescues the increase in brain size and the defect in the ratio of Pax6+/Tbr2+ cells observed in animals that overexpress Lin28a. Altogether, these results indicate that Lin28a mainly promotes translation in neural progenitors.
Transcriptome analysis between E11.5 neuroepithelium from wild-type and Lin28 dKO animals show that only 15 genes decrease and only 19 increase in mutants. In contrast, the analysis of mRNAs associated with polysomes show that 368 genes decrease, and 187 genes increase in the mutants compared to wild-type animals (Herrlinger et al. 2019). Gene Ontology (GO) analysis indicated that categories related to ribosome biogenesis and protein synthesis decreased and categories related to neurotransmitter complexes and the post-synapse increased in mutants. All these results are consistent with the role of Lin28 in promoting translation of genes associated to protein synthesis and a role in blocking translation of some genes associated to differentiation during CNS development.
The cellular and molecular effects of gain- and loss-of-functions experiments described here indicate that the role of Lin28 in CNS is directly linked to cell division and global protein synthesis (Fig. 3a). Lin28 may contribute to the control the dynamics of the cell cycle of early APs (when is highly expressed during neurogenesis), promoting symmetric over asymmetric cell divisions. Consequently, overexpression of Lin28 alters the timing of differentiation without preventing the continuous activation of commitment and differentiation factors (Fig. 3b). This alteration in timing has also been observed for overexpression of cdk4/cyclin D1 (Lange et al. 2009) and loss of p27 (Durand et al. 1998).
Model of action of Lin28 in neural progenitors. (a) Lin28 regulates the levels of metabolic enzymes, proteins involved in translation and regulators of the cell cycle that must be are coordinated with other factors to set a threshold that switch from symmetric to asymmetric division in a single progenitor cell. (b) The genetic network that controls the expression of commitment and neurogenic factors works independently of cell cycle and Lin28, and the level of these factors continuously increases. When the threshold is reached, the progenitor cell will divide asymmetrically to generate a neuron or an intermediate progenitor. In this scheme, the wild type threshold is proposed to decrease during development as Lin28 decreases. However, it is also possible that other levels keep the threshold at the same level during embryogenesis. In gain-of-function of Lin28 (GOF Lin28, green), the threshold is higher than the normal level, so the switch occurs later. In loss-of-function of Lin28 (LOF Lin28, blue), the threshold is lower than the normal level, so the switch occurs earlier. (c) The consequence of these changes is an excess of progenitor cells in GOF Lin28 and precocious differentiation with lower number of progenitor cells in LOF Lin28 relative to the wild type
Based on estimations of cell cycle length and type of divisions (Takahashi et al. 1995, 1996), it can be proposed that Lin28 is part of a module that determines a threshold, which must be reached to trigger the transition to symmetric to asymmetric divisions (Fig. 3b). This threshold probably decreases during neurogenesis as Lin28 levels- or activity- decreases. Increasing the levels of Lin28 in APs (for example, using the Nestin promoter) would increase the threshold and may induce a couple of one or two rounds of symmetric divisions instead of asymmetric divisions between E11 and E14 increasing the number of progenitor cells. If commitment and differentiation factors levels or activity continuously increase, consistent with the idea that transcriptional programs are independent of cell cycle (Okamoto et al. 2016), this threshold is reached, but a couple of divisions after the normal timing (Fig. 3b). After this threshold is reached, an asymmetric division is produced (to generate a neuron in direct neurogenesis or an IPC in indirect neurogenesis) and the excess of Lin28 would not affect the program of expression of neuronal markers, consistent with increase in brain size and the higher proportion of Pax6+ cells over Tbr2+ cells observed in animals that overexpress Lin28 (Yang et al. 2015). This proposal is consistent with the delay observed in other biological contexts after Lin28 overexpression, probably due to the effect on crucial tissues (Faunes et al. 2017; Moss et al. 1997; Zhu et al. 2010).
This idea of Lin28 being part of a module that determines a threshold for asymmetric divisions in APs is consistent with the “cell cycle length hypothesis”, which proposes that a certain period of time is required for the neurogenic factors to trigger differentiation (Calegari and Huttner 2003; Götz and Huttner 2005). In this cell cycle length hypothesis, lengthening the cell cycle, mainly in G1, favors the exit of the cell cycle because there is enough time for differentiation factors to act and the threshold can be reached. Shorter cell cycles prevent that the threshold is reached causing progenitor cells to proliferate. Under this scenario, Lin28 overexpression may favor short cell cycles, preventing reaching of the threshold at the normal timing (consistent with the opposite effect of let-7 overexpression). However, in this case, it is also necessary that commitment or differentiation factors continuously increase even after cell divisions, so the threshold be reached, but at later divisions compared to the normal development. This idea is also consistent with the decreased proliferation and precocious differentiation observed in Lin28 KO animals. In this case, the threshold to trigger asymmetric division is set below the normal level and the number of progenitor cells is low to allow the normal closure of the neural tube and differentiation begins earlier. How this threshold works at the molecular level, how is linked to cell cycle dynamics, signaling and metabolic pathways and how the rate of these processes is affected in the absence or excess of Lin28 is unknown. Future quantitative measurements of Lin28 levels in single cells and during cell cycle together with studies on cell cycle length, division patterns and the fate of the progeny after Lin28 overexpression with different promoters or during specific temporal windows would be useful to address these questions.
6 Reactivation of Lin28 in NS Regeneration and Misregulation in Diseases
Lin28 expression is not detected in most adult tissues (Yang and Moss 2003). As mentioned above, Lin28a is not expressed in intestinal basal stem cells but is expressed in proliferating cells (transit amplifying cells). Lin28 is also expressed in skeletal muscle and in vitro studies in myoblasts have shown that Lin28a is required for muscle differentiation (Polesskaya et al. 2007). This evidence suggests that Lin28 expression is required in tissues undergoing normal proliferation and repair, such as the intestine and the muscle.
Lin28a also increases after damage in the NS. In the peripheral NS of mice, Lin28a and Lin28b increase in the L4/5 dorsal root ganglia (DRG) after sciatic nerve axotomy (Wang et al. 2018). Importantly, overexpression of Lin28a specifically in the L4/5 DRG and ubiquitous and inducible overexpression of Lin28b promote axon regeneration in this experimental model. Consistent with a role of Lin28 in axon regeneration, simultaneous knock-down of Lin28a and Lin28b decreases axon regeneration compared to controls (Wang et al. 2018).
Overexpression of Lin28a or Lin28b also promotes regeneration in the CNS after optic nerve crush (Nathan et al. 2020; Wang et al. 2018), retinal injury (Yao et al. 2016; Zhang et al. 2019) and spinal cord injury (Nathan et al. 2020). In the retina, Lin28 promotes proliferation of Müller glia (Yao et al. 2016). This role of Lin28 in the regeneration of the retina has been also described in zebrafish (Gorsuch et al. 2017; Mitra et al. 2019; Ramachandran et al. 2010). Altogether, all these works indicate that Lin28 is re-activated after damage and promotes regeneration in the mammalian NS.
In contrast to the regulated re-activation of Lin28 under repair and regeneration, Lin28 is aberrantly upregulated in cancer and other diseases (Balzeau et al. 2017; Thornton and Gregory 2012; Viswanathan et al. 2009). Specifically in the NS, misregulated expression of Lin28 is observed in neuroblastoma (Beckers et al. 2015; Hennchen et al. 2015; Molenaar et al. 2012) and medulloblastoma (Hovestadt et al. 2014). In addition to tumors, increased expression of Lin28 is detected in Rett Syndrome due to downregulation of MECP2 (Kim et al. 2019). In contrast, conditional knockout of Lin28a in mice results in degeneration of midbrain-type dopamine neurons (Chang et al. 2019). These works indicate that the regulation of Lin28 levels is critical for normal homeostasis of the NS in adults. Understanding the mechanisms underlying the control of expression and activity of Lin28 and its downstream genes may provide strategies to restore normal levels in these contexts.
7 Perspectives
The detailed analysis of the endogenous expression and the role of Lin28 in vivo are still incomplete. The balance of Lin28 with commitment and differentiation factors seems to be crucial for the decision between proliferate and differentiate, as described in other contexts (Radzisheuskaya et al. 2013; Sansom et al. 2009). Different splice isoforms of Lin28 and post-translational regulation add new layers of complexity to this process. Progressive changes in intrinsic molecular programs in progenitors must be coordinated with Lin28-regulated genes and with the factors that control the duration of cell cycle, which it has been proposed to regulate these decisions. To determine the dynamics of these changes during the cell cycle and neurogenesis through single cell analyses, real time reporters and pulse-label approaches together with a detailed quantitative analysis of different isoforms of Lin28 will give us new insights into the cellular and molecular role of Lin28 during embryogenesis.
Abbreviations
- AP:
-
Apical progenitor
- CNS:
-
Central nervous system
- CP:
-
Cortical plate
- ESC:
-
Embryonic stem cell
- IPC:
-
Intermediate progenitor cell
- IZ:
-
Intermediate zone
- MZ:
-
Marginal zone
- NEC:
-
Neuroepithelium cell
- NS:
-
Nervous system
- SVZ:
-
Subventricular zone
- VZ:
-
Ventricular zone
References
Ambros V, Horvitz HR (1984) Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226:409–416
Amen AM, Ruiz-Garzon CR, Shi J, Subramanian M, Pham DL, Meffert MK (2017) A rapid induction mechanism for Lin28a in trophic responses. Mol Cell 65(490–503):e497
Balzeau J, Menezes MR, Cao S, Hagan JP (2017) The LIN28/let-7 pathway in Cancer. Front Genet 8:31
Balzer E, Heine C, Jiang Q, Lee VM, Moss EG (2010) LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development 137:891–900
Barez-Lopez S, Guadano-Ferraz A (2017) Thyroid hormone availability and action during brain development in rodents. Front Cell Neurosci 11:240
Beckers A, Van Peer G, Carter DR, Gartlgruber M, Herrmann C, Agarwal S, Helsmoortel HH, Althoff K, Molenaar JJ, Cheung BB, Schulte JH, Benoit Y, Shohet JM, Westermann F, Marshall GM, Vandesompele J, De Preter K, Speleman F (2015) MYCN-driven regulatory mechanisms controlling LIN28B in neuroblastoma. Cancer Lett 366:123–132
Bhuiyan MI, Lee JH, Kim SY, Cho KO (2013) Expression of exogenous LIN28 contributes to proliferation and survival of mouse primary cortical neurons in vitro. Neuroscience 248:448–458
Calegari F, Huttner WB (2003) An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci 116:4947–4955
Cardenas A, Borrell V (2020) Molecular and cellular evolution of corticogenesis in amniotes. Cell Mol Life Sci 77:1435–1460
Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA, Thomas-Tikhonenko A, Mendell JT (2009) Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A 106:3384–3389
Chang MY, Oh B, Choi JE, Sulistio YA, Woo HJ, Jo A, Kim J, Kim EH, Kim SW, Hwang J, Park J, Song JJ, Kwon OC, Henry Kim H, Kim YH, Ko JY, Heo JY, Lee MJ, Lee M, Choi M, Chung SJ, Lee HS, Lee SH (2019) LIN28A loss of function is associated with Parkinson's disease pathogenesis. EMBO J 38:e101196
Cho J, Chang H, Kwon SC, Kim B, Kim Y, Choe J, Ha M, Kim YK, Kim VN (2012) LIN28A is a suppressor of ER-associated translation in embryonic stem cells. Cell 151:765–777
Cimadamore F, Amador-arjona A, Chen C, Huang C-T, Terskikh AV (2013) SOX2–LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proceedings of the National Academy of Sciences 2013
Durand B, Fero ML, Roberts JM, Raff MC (1998) p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr Biol 8:431–440
Faas, L., Warrander, F.C., Maguire, R., Ramsbottom, S.A., Quinn, D., Genever, P., Isaacs, H.V., 2013. Lin28 proteins are required for germ layer specification in Xenopus. Development (Cambridge, England) 140, 976–986
Fairchild CLA, Cheema SK, Wong J, Hino K, Simo S, La Torre A (2019) Let-7 regulates cell cycle dynamics in the developing cerebral cortex and retina. Sci Rep 9:15336
Faunes F, Gundermann DG, Munoz R, Bruno R, Larrain J (2017) The heterochronic gene Lin28 regulates amphibian metamorphosis through disturbance of thyroid hormone function. Dev Biol 425:142–151
Florio M, Huttner WB (2014) Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141:2182–2194
Ghimire S, Van der Jeught M, Neupane J, Roost MS, Anckaert J, Popovic M, Van Nieuwerburgh F, Mestdagh P, Vandesompele J, Deforce D, Menten B, Chuva de Sousa Lopes S, De Sutter P, Heindryckx B (2018) Comparative analysis of naive, primed and ground state pluripotency in mouse embryonic stem cells originating from the same genetic background. Sci Rep 8:5884
Gonzalez-Itier S, Contreras EG, Larrain J, Glavic A, Faunes F (2018) A role for Lin-28 in growth and metamorphosis in Drosophila melanogaster. Mech Dev 154:107–115
Gorsuch RA, Lahne M, Yarka CE, Petravick ME, Li J, Hyde DR (2017) Sox2 regulates Muller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a. Exp Eye Res 161:174–192
Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788
Gundermann DG, Martinez J, De Kervor G, Gonzalez-Pinto K, Larrain J, Faunes F (2019) Overexpression of Lin28a delays Xenopus metamorphosis and down-regulates albumin independently of its translational regulation domain. Dev Dynamics 248:969–978
Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, Aburatani H (2006) Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 384:51–61
Guo W, Hu Z, Bao Y, Li Y, Li S, Zheng Q, Lyu D, Chen D, Yu T, Li Y, Zhu X, Ding J, Zhao Y, He X, Huang S (2018) A LIN28B tumor-specific transcript in cancer. Cell Rep 22:2016–2025
Hafner M, Max KE, Bandaru P, Morozov P, Gerstberger S, Brown M, Molina H, Tuschl T (2013) Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA 19:613–626
Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R (2009) Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462:595–601
Hennchen M, Stubbusch J, Abarchan-El Makhfi I, Kramer M, Deller T, Pierre-Eugene C, Janoueix-Lerosey I, Delattre O, Ernsberger U, Schulte JB, Rohrer H (2015) Lin28B and Let-7 in the control of sympathetic neurogenesis and neuroblastoma development. J Neurosci 35:16531–16544
Herrlinger S, Shao Q, Yang M, Chang Q, Liu Y, Pan X, Yin H, Xie LW, Chen JF (2019) Lin28-mediated temporal promotion of protein synthesis is crucial for neural progenitor cell maintenance and brain development in mice. Development 146
Hovestadt V, Jones DT, Picelli S, Wang W, Kool M, Northcott PA, Sultan M, Stachurski K, Ryzhova M, Warnatz HJ, Ralser M, Brun S, Bunt J, Jager N, Kleinheinz K, Erkek S, Weber UD, Bartholomae CC, von Kalle C, Lawerenz C, Eils J, Koster J, Versteeg R, Milde T, Witt O, Schmidt S, Wolf S, Pietsch T, Rutkowski S, Scheurlen W, Taylor MD, Brors B, Felsberg J, Reifenberger G, Borkhardt A, Lehrach H, Wechsler-Reya RJ, Eils R, Yaspo ML, Landgraf P, Korshunov A, Zapatka M, Radlwimmer B, Pfister SM, Lichter P (2014) Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature 510:537–541
Jang HJ, Kim JY, Kim SY, Cho KO (2019) Persistent Lin28 expression impairs neurite outgrowth and cognitive function in the developing mouse neocortex. Mol Neurobiol 56:3780–3795
Kalkan T, Olova N, Roode M, Mulas C, Lee HJ, Nett I, Marks H, Walker R, Stunnenberg HG, Lilley KS, Nichols J, Reik W, Bertone P, Smith A (2017) Tracking the embryonic stem cell transition from ground state pluripotency. Development 144:1221–1234
Kawahara H, Okada Y, Imai T, Iwanami A, Mischel PS, Okano H (2011) Musashi1 cooperates in abnormal cell lineage protein 28 (Lin28)-mediated let-7 family microRNA biogenesis in early neural differentiation. J Biol Chem 286:16121–16130
Kim JJ, Savas JN, Miller MT, Hu X, Carromeu C, Lavallee-Adam M, Freitas BCG, Muotri AR, Yates JR 3rd, Ghosh A (2019) Proteomic analyses reveal misregulation of LIN28 expression and delayed timing of glial differentiation in human iPS cells with MECP2 loss-of-function. PLoS One 14:e0212553
Kumar RM, Cahan P, Shalek AK, Satija R, DaleyKeyser A, Li H, Zhang J, Pardee K, Gennert D, Trombetta JJ, Ferrante TC, Regev A, Daley GQ, Collins JJ (2014) Deconstructing transcriptional heterogeneity in pluripotent stem cells. Nature 516:56–61
La Torre A, Georgi S, Reh TA (2013) Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci U S A 110:E2362–E2370
Lange C, Huttner WB, Calegari F (2009) Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5:320–331
Li MA, Amaral PP, Cheung P, Bergmann JH, Kinoshita M, Kalkan T, Ralser M, Robson S, von Meyenn F, Paramor M, Yang F, Chen C, Nichols J, Spector DL, Kouzarides T, He L, Smith A (2017) A lncRNA fine tunes the dynamics of a cell state transition involving Lin28, let-7 and de novo DNA methylation. elife 6
Liu X, Chen M, Li L, Gong L, Zhou H, Gao D (2017) Extracellular signal-regulated kinases (ERKs) phosphorylate Lin28a protein to modulate P19 cell proliferation and differentiation. J Biol Chem 292:3970–3976
Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Stewart AF, Smith A, Stunnenberg HG (2012) The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149:590–604
Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young Ra, Jaenisch R (2008) Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3:132–135
Mitra S, Sharma P, Kaur S, Khursheed MA, Gupta S, Chaudhary M, Kurup AJ, Ramachandran R (2019) Dual regulation of lin28a by Myc is necessary during zebrafish retina regeneration. J Cell Biol 218:489–507
Mizuno R, Chatterji P, Andres S, Hamilton K, Simon L, Foley SW, Jeganathan A, Gregory BD, Madison B, Rustgi AK (2018) Differential regulation of LET-7 by LIN28B isoform-specific functions. Mol Cancer Res: MCR 16:403–416
Molenaar JJ, Domingo-Fernandez R, Ebus ME, Lindner S, Koster J, Drabek K, Mestdagh P, van Sluis P, Valentijn LJ, van Nes J, Broekmans M, Haneveld F, Volckmann R, Bray I, Heukamp L, Sprussel A, Thor T, Kieckbusch K, Klein-Hitpass L, Fischer M, Vandesompele J, Schramm A, van Noesel MM, Varesio L, Speleman F, Eggert A, Stallings RL, Caron HN, Versteeg R, Schulte JH (2012) LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet 44:1199–1206
Moss EG, Tang L (2003) Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol 258:432–442
Moss EG, Lee RC, Ambros V (1997) The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88:637–646
Nathan FM, Ohtake Y, Wang S, Jiang X, Sami A, Guo H, Zhou FQ, Li S (2020) Upregulating Lin28a promotes axon regeneration in adult mice with optic nerve and spinal cord injury. Mol Ther 28:1902–1917
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4:487–492
Nishino J, Kim I, Chada K, Morrison SJ (2008) Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell 135:227–239
Okamoto M, Miyata T, Konno D, Ueda HR, Kasukawa T, Hashimoto M, Matsuzaki F, Kawaguchi A (2016) Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat Commun 7:11349
Ouchi Y, Yamamoto J, Iwamoto T (2014) The heterochronic genes lin-28a and lin-28b play an essential and evolutionarily conserved role in early zebrafish development. PLoS One 9:e88086
Paridaen JT, Huttner WB (2014) Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 15:351–364
Parisi S, Passaro F, Russo L, Musto A, Navarra A, Romano S, Petrosino G, Russo T (2017) Lin28 is induced in primed embryonic stem cells and regulates let-7-independent events. FASEB J 31:1046–1058
Peng S, Chen LL, Lei XX, Yang L, Lin H, Carmichael GG, Huang Y (2011) Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 29:496–504
Perez-Juste G, Garcia-Silva S, Aranda A (2000) An element in the region responsible for premature termination of transcription mediates repression of c-myc gene expression by thyroid hormone in neuroblastoma cells. J Biol Chem 275:1307–1314
Polesskaya A, Cuvellier S, Naguibneva I, Duquet A, Moss EG, Harel-Bellan A (2007) Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev 21:1125–1138
Radzisheuskaya A, Chia Gle B, dos Santos RL, Theunissen TW, Castro LF, Nichols J, Silva JC (2013) A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat Cell Biol 15:579–590
Ramachandran R, Fausett BV, Goldman D (2010) Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol 12:1101–1107
Romer-Seibert JS, Hartman NW, Moss EG (2019) The RNA-binding protein LIN28 controls progenitor and neuronal cell fate during postnatal neurogenesis. FASEB J 33:3291–3303
Rougvie AE (2001) Control of developmental timing in animals. Nat Rev Genet 2:690–701
Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ (2009) The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet 5:e1000511
Shinoda G, Shyh-Chang N, Soysa TY, Zhu H, Seligson MT, Shah SP, Abo-Sido N, Yabuuchi A, Hagan JP, Gregory RI, Asara JM, Cantley LC, Moss EG, Daley GQ (2013) Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells 31:1563–1573
Shyh-Chang N, Daley GQ (2013) Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12:395–406
Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ (2013) Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155:778–792
Takahashi T, Nowakowski RS, Caviness VS Jr (1995) The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci 15:6046–6057
Takahashi T, Nowakowski RS, Caviness VS Jr (1996) The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci 16:6183–6196
Telley L, Agirman G, Prados J, Amberg N, Fievre S, Oberst P, Bartolini G, Vitali I, Cadilhac C, Hippenmeyer S, Nguyen L, Dayer A, Jabaudon D (2019) Temporal patterning of apical progenitors and their daughter neurons in the developing neocortex. Science 364:eaav2522
Thornton JE, Gregory RI (2012) How does Lin28 let-7 control development and disease? Trends Cell Biol 22:474–482
Tsialikas J, Romer-Seibert J (2015) LIN28: roles and regulation in development and beyond. Development 142:2397–2404
Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, Mermel CH, Beroukhim R, Azam M, Teixeira J, Meyerson M, Hughes TP, Llovet JM, Radich J, Mullighan CG, Golub TR, Sorensen PH, Daley GQ (2009) Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 41:843–848
Vitali I, Fievre S, Telley L, Oberst P, Bariselli S, Frangeul L, Baumann N, McMahon JJ, Klingler E, Bocchi R, Kiss JZ, Bellone C, Silver DL, Jabaudon D (2018) Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell 174(1264–1276):e1215
Wang XW, Li Q, Liu CM, Hall PA, Jiang JJ, Katchis CD, Kang S, Dong BC, Li S, Zhou FQ (2018) Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell Rep 24(2540–2552):e2546
Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A (2007) Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol 309:285–297
Wu L, Belasco JG (2005) Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol Cell Biol 25:9198–9208
Xia X, Teotia P, Ahmad I (2018) Lin28a regulates neurogliogenesis in mammalian retina through the Igf signaling. Dev Biol 440:113–128
Xu B, Zhang K, Huang Y (2009) Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA 15:357–361
Yang DH, Moss EG (2003) Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns 3:719–726
Yang M, Yang SL, Herrlinger S, Liang C, Dzieciatkowska M, Hansen KC, Desai R, Nagy A, Niswander L, Moss EG, Chen JF (2015) Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development 142:1616–1627
Yao K, Qiu S, Tian L, Snider WD, Flannery JG, Schaffer DV, Chen B (2016) Wnt regulates proliferation and neurogenic potential of Muller glial cells via a Lin28/let-7 miRNA-dependent pathway in adult mammalian retinas. Cell Rep 17:165–178
Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A (2008) The ground state of embryonic stem cell self-renewal. Nature 453:519–523
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zeng Y, Yao B, Shin J, Lin L, Kim N, Song Q, Liu S, Su Y, Guo JU, Huang L, Wan J, Wu H, Qian J, Cheng X, Zhu H, Ming GL, Jin P, Song H (2016) Lin28A binds active promoters and recruits Tet1 to regulate gene expression. Mol Cell
Zhang J, Ratanasirintrawoot S, Chandrasekaran S, Wu Z, Ficarro SB, Yu C, Ross CA, Cacchiarelli D, Xia Q, Seligson M, Shinoda G, Xie W, Cahan P, Wang L, Ng SC, Tintara S, Trapnell C, Onder T, Loh YH, Mikkelsen T, Sliz P, Teitell MA, Asara JM, Marto JA, Li H, Collins JJ, Daley GQ (2016) LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell 19:66–80
Zhang Y, Williams PR, Jacobi A, Wang C, Goel A, Hirano AA, Brecha NC, Kerschensteiner D, He Z (2019) Elevating growth factor responsiveness and axon regeneration by modulating presynaptic inputs. Neuron 103(39–51):e35
Zhao C, Sun G, Li S, Lang M-F, Yang S, Li W, Shi Y (2010) MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci U S A 107:1876–1881
Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ (2010) Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet 42:626–630
Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147:81–94
Acknowledgements
I thank Dr. Carlos Oliva and Dr. Gonzalo Cordova for critical reading of the manuscript. Images were adapted from Illustration Toolkits Biology and Molecular Cell Biology by Motifolio.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faunes, F. (2020). Role and Regulation of Lin28 in Progenitor Cells During Central Nervous System Development. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 12. Advances in Experimental Medicine and Biology(), vol 1326. Springer, Cham. https://doi.org/10.1007/5584_2020_607
Download citation
DOI: https://doi.org/10.1007/5584_2020_607
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71932-6
Online ISBN: 978-3-030-71933-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)