Abstract
Two-third of patients with chronic hepatitis C show extrahepatic manifestations due to HCV infection of B lymphocytes, such as mixed cryoglobulinemia and non-Hodgkin B-cell lymphoma, or develop a chronic inflammatory status that may favor the development of adverse cardiovascular events, kidney diseases or metabolic abnormalities.
DAAs treatments induce HCV eradication in 95% of treated patients, which also improves the clinical course of extrahepatic manifestations, but with some limitations. After HCV eradication a good compensation of T2DM has been observed, but doubts persist about the possibility of obtaining a stable reduction in fasting glucose and HbA1c levels.
Chronic HCV infection is associated with low total and LDL cholesterol serum levels, which however increase significantly after HCV elimination, possibly due to the disruption of HCV/lipid metabolism interaction. Despite this adverse effect, HCV eradication exerts a favorable action on cardiovascular system, possibly by eliminating numerous other harmful effects exerted by HCV on this system.
DAA treatment is also indicated for the treatment of patients with mixed cryoglobulinemia syndrome, since HCV eradication results in symptom reduction and, in particular, is effective in cryoglobulinemic vasculitis. Furthermore, HCV eradication exerts a favorable action on HCV-related lymphoproliferative disorders, with frequent remission or reduction of clinical manifestations.
There is also evidence that HCV clearance may improve impaired renal functions, but same conflicting data persist on the effect of some DAAs on eGFR.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
1 Introduction
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide. Chronic HCV infection tends to progress towards liver fibrosis and cirrhosis and subsequently to hepatocellular carcinoma (HCC) in the context of bridging fibrosis or liver cirrhosis (Stroffolini et al. 2018; Sagnelli et al. 2013, 2019).
In 2015, 71 million people were living with chronic HCV infection worldwide (Global Hepatitis Report 2017). Exposure to infected blood or blood products (intravenous drug use, iatrogenic exposure, tattooing, piercing) and risky sexual contact (multiple partners, anal sex, presence of genital lesions) were the risk factors most frequently associated with the transmission of this infection (Santantonio et al. 2006; Corey et al. 2006; Daniels et al. 2009; Esteban et al. 2008).
After becoming infected with HCV, almost 35% of the subjects eliminate the virus spontaneously or after asymptomatic acute self-limiting hepatitis, while the remaining 65% progress to chronicity, identifiable by the persistence of HCV RNA in serum for at least 6 months (Alter et al. 1992). The natural history of chronic HCV infection is extremely variable, from a long-term absence of liver lesions to the persistence of minimal liver changes with a slow indolent progression to fibrosis or a rapid progression to liver cirrhosis and its serious complications, such as portal hypertension, liver failure and HCC development (Poynard et al. 1997, 2000). Several factors can accelerate the progression of liver disease, including an older age at the time of infection, concomitant alcohol abuse, presence of diabetes and coinfection with HIV and/or HBV (Alter et al. 1992; Poynard et al. 1997, 2000; Alter and Seeff. 2000; Coppola et al. 2012, 2014, 2015; Webster et al. 2015; Bagaglio et al. 2020; Sagnelli et al. 2020).
Nearly two-thirds of patients with chronic HCV infection (CHC) show extrahepatic manifestations due to HCV infection of B lymphocytes, such as mixed cryoglobulinemia and non-Hodgkin B-cell lymphoma, or to a chronic inflammatory status that may favor the development of adverse cardiovascular events (stroke, coronary artery disease), kidney diseases and metabolic abnormalities (Cacoub et al. 2014; Calogero et al. 2019).
Interferon (INF) alfa was the cornerstone of chronic HCV therapy until 2014, but was later replaced by classes of direct acting antiviral agents (DAA) which, combined with each other, give much better therapeutic results and a marked reduction in treatment times and adverse reactions. Today, thanks to the high and rapid effect of DAA regimens, the sustained virological response rate at the twelfth post-treatment week (SVR12) is about 95%, even in the presence of advanced liver diseases (EASL Recommendations on Treatment of Hepatitis C 2018).
Although the effectiveness of DAA combinations on the eradication of HCV infection is proven by numerous randomized clinical trials and by a day to day worldwide clinical practice, their efficacy on the associated metabolic disorders and related extrahepatic manifestations need further clarification.
2 Chronic Inflammatory Status and Extra-Hepatic Damage
The pathogenesis of extra-hepatic manifestations of HCV infection has not been fully investigated at present (Fletcher and McKeating 2012; Zignego et al. 2007). HCV infection determines the clonal expansion of B lymphocytes (Carbonari et al. 2005; Charles et al. 2008) with the production of rheumatoid factor M immunoglobulin (Ig), which in sensitive subjects causes the deposition of immune complexes in small vessels, with consequent vasculitis. The mechanisms of other manifestations seem multifactorial, including a direct interaction between viral proteins and intracellular signaling pathways, viral replication in extra-hepatic cells and an intensified immune reaction with systemic effects. The activation of the immune system may induce chronic inflammation as occurs in human immunodeficiency virus (HIV) infection (Kuller et al. 2008; Petta et al. 2014; Negro 2014; Negro et al. 2015; Bedimo and Abodunde 2016).
Patients with chronic HCV infection are at risk of developing type 2 diabetes mellitus (T2DM), the most common extra-hepatic manifestation in this infection (Mehta et al. 2000; Wang et al. 2007; Vanni et al. 2016; Mehta et al. 2000). In addition, compared with uninfected controls, HCV infected patients more frequently show high levels of insulin resistance (IR) (Moucari et al. 2008; Younossi et al. 2013). On the other hand, compared with untreated subjects, HCV-infected patients more frequently show a cardioprotective lipid profile, characterized by significantly lower levels of serum total cholesterol, low-density lipoprotein, and triglycerides (TC) and higher serum levels of high-density lipoprotein (Vassalle et al. 2004; Dai et al. 2008). Nonetheless, data from several studies show an association between HCV infection and atherosclerotic damage (Vassalle et al. 2004; Marzouk et al. 2007; Targher et al. 2007; Dai et al. 2008; Alyan et al. 2008; Petta et al. 2012; Hsu et al. 2015b) with increased mortality by circulatory diseases (Lee et al. 2012). These conflicting data deserve careful and thorough investigation.
It has also been observed that, compared to the general population, HCV-positive subjects more frequently develop chronic kidney disease (CKD) (Latt et al. 2012).
Studies have shown that therapy to eradicate HCV infection improves some extra-hepatic manifestations associated with HCV infection, regardless of the severity of liver disease. The evidence is stronger for mixed cryoglobulinemia, which often resolves entirely with viral clearance (Mazzaro et al. 1996; Cacoub et al. 2005; Dammacco and Sansonno 2013), but it remains unclear for extra-hepatic manifestations due to the chronic inflammatory state which have often been considered contraindications to INF-based treatment for the possibility of their exacerbation, for possible drug interaction with drugs used to treat them or for fear of additional toxicity (Massoumy et al. 2013; Kanwal et al. 2014). The directly acting antiviral (DAA) regimens are more effective and better tolerated than interferon-based therapy and therefore more frequently usable in the presence of extra-hepatic manifestations.
The purpose of this narrative review is to provide an overview of the knowledge available on the action exerted by DAA therapy on the extra-hepatic manifestations of chronic HCV infection. The article is addressed to all young doctors, to all doctors working in infectious diseases, gastroenterology, and internal medicine wards and to general practitioners who aid patients with chronic HCV infection.
3 Methods
We conducted a computerized bibliographic search using MEDLINE and EMBASE involving both medical title terminology (MeSH) and relevant keywords for search strings to locate studies that analyzed until April 2020 the outcome of HCV patients after DAA treatment. The following items were used for research in the studies: “hepatitis c”, “HCV”, “direct active antivirals”, “DAA” and “kidney”, “eGFR”, “kidney function”, “diabetes”, “glycemic control”, “blood sugar”, “lipids”, “triglycerides”, “cardiovascular”, “median intimal thickness”, “hypertension”, “lymphoma”, “B-NHL”, “DLBCL”, “CHL”, “Mixed Cryoglobulinemia Syndrome”, “vasculitis”. Based on the main research objectives, the articles were classified into one of the following research topics: kidney function, glycemic control, lipid control and cardiovascular events.
4 DAAs and Glycemic Homeostasis
The correlation between alterations in the glycid balance and CHC is made evident by the frequent development (30–70%) of insulin resistance in CHC patients, by the 3.8 times higher rate of chronic HCV infection in patients with T2DM than in those without (Vanni et al. 2016), by the more frequent progression of fibrosis in patients with CHC and IR (Hui et al. 2003) and by a more frequent development of HCC (Desbois and Cacoub 2017) in patients with CHC and T2DM than in those with CHC alone. The eradication of HCV infection with IFN-alfa therapy induces a substantial improvement in the markers of glucose metabolism as shown in a meta-analysis (Cacoub et al. 2018a) on 7,000 CHC patients from 40 studies; after IFN treatment, the incidence of IR and T2DM was significantly reduced during a long-term post-treatment follow-up in those who achieved a sustained virological response (SVR). In a retrospective study performed in Japan on 2,842 CHC patients treated with IFN the rate of T2DM was 3.6% at the 5th year, 8.0% at the 10th year and 17.0% at the 15th year of post-treatment observation, predictive factors for T2DM development being advanced liver disease, failure to achieve SVR after treatment and age of 50 or more years (Arase et al. 2009). In a retrospective study from Spain on 234 IFN treated CHC or liver cirrhosis patients, glucose abnormalities occurred less frequently in those who obtained SVR than in those who did not (14.6% versus 34.1%) (Simó et al. 2006).
Contrasting data comes from an Italian retrospective study (Giordanino et al. 2008) where no association was found between SVR and a lower risk of developing T2DM during an 8-year follow-up.
A substantial improvement in fasting glucose and glycosylated hemoglobin levels were observed in HCV patients who achieved SVR with DAA treatment (Bose and Ray 2014; Meissner et al. 2015; Pavone et al. 2016; Morales et al. 2016; Hum et al. 2017; Ikeda et al. 2017; Fabrizio et al. 2017; Abdel Alem et al. 2017; Dawood et al. 2017; Ciancio et al. 2018; El Sagheer et al. 2018), regardless of the DAA regimen used (Meissner et al. 2015; Pavone et al. 2016; Morales et al. 2016; Abdel Alem et al. 2017; Fabrizio et al. 2017; Ikeda et al. 2017; Dawood et al. 2017; Ciancio et al. 2018; El Sagheer et al. 2018) (Table 1). In a National Veterans Health System study on 2435 HCV patients treated with different DAA regimens, a significantly higher reduction in mean hemoglobin A1c (HbA1c) was observed in the 2180 who achieved SVR compared to the 275 non-SVR-patients (Hum et al. 2017).
In a study on 91 HCV-positive liver transplant patients, 96% achieved SVR and HbA1c dropped from 35.5 ± 4.3 mmol/L to 33.3 ± 3.6 mmol/L at the 44th week after treatment; in patients not treated with anti-diabetic agents, a fasting glucose level decreased from 6.8 ± 1.7 mmol/L before antiviral therapy to 5.7 ± 1.1 mmol/L at the 24th week after treatment discontinuation (Beig et al. 2018).
The association between SVR induced by DAA regimens and glycaemic control is further supported by the behaviour of other parameters, like IR development, T2DM development and type and doses of anti-diabetic drugs. A prospective study showed that HCV eradication produced a clearance or reduction of IR in 76% of 133 non-diabetic HCV-genotype 1 patients with advanced liver disease who achieved SVR12 (Adinolfi et al. 2018a, b). The DAA-induced SVR also correlates with a reduced risk of developing TDM2 (Adinolfi et al. 2018a) and with an improvement in glycemic control in T2DM patients (Adinolfi et al. 2018a). In addition, HCV patients with T2DM receiving oral anti-diabetic or insulin treatment needed a dose reduction during DAA therapy (Soriano et al. 2016; Hum et al. 2017; Ikeda et al. 2017; Ciancio et al. 2018; Teegen et al. 2019). Compared to the baseline values, a significant improvement in beta-cell function was observed after DAA treatment in a prospective, open-label, multi-center study (107.7 ± 86.8 vs. 86.7 ± 44.5, p = 0.05), an improvement more evident in patients with high baseline IR (Huang et al. 2017). A post hoc analysis of six studies on CHC genotype-1 patients with advanced fibrosis showed a significant reduction in fasting glucose levels in patients treated with paritaprevir/ritonavir/ombitasvir/dasabuvir, compared to those in the placebo group (Tran et al. 2017): the most significant reduction being observed in T2DM patients (22.1 mg/dL less at the 12th week compared to the baseline), followed by those in a pre-diabetic status (−5.78 mg/dL by week 12) (Tran et al. 2017). As for the HbA1c, a substantial reduction was observed only in responders with a high baseline HbA1c level (Hum et al. 2017). In addition, in an Egyptian study on CHC patients with HCV genotype-4 and T2DM who achieved SVR, the independent prognostic factors for a drop in blood glucose levels >20 mg/dl or a drop in HbA1c levels >0.5% were a T2DM duration less than 7 years, a T2D negative family history and a liver disease severity up to cirrhosis Child-Pugh A (Dawood et al. 2017). Pavone et al. retrospectively evaluated 21 HCV-positive patients with T2DM treated with different interferon-free DAA regimens; fasting glucose serum levels significantly decreased during treatment (mean value −52.86 mg/dL, p = 0.007); also glycated haemoglobin values (detected in 10 patients at weeks 4, 8 and/or 12) significantly decreased during treatment (−1.95%, p = 0.021). The Authors concluded that diabetes could be considered as an element to prioritize treatment in CHC patients (Pavone et al. 2016). Soriano et al. described a significant decrease in fasting serum glucose level during DAA treatment in a CHC patient with T2DM, an event forcing a reduction in insulin dosage (Soriano et al. 2016). Also, in a Teegen’s study on liver transplant patients a significant decline in the daily average insulin dose was required to keep stable HbA1c after DAAs therapy (55.3 vs. 38.2 U/d; p = 0.009) (Teegen et al. 2019).
To be noticed, however, that a consistent number of studies did not show a long-term persistence in glycemic control, or showed no significant decline in HbA1c, or no effect of DAA treatment in patients with a severe CHC, or no effect at all, or an increase in TC and LDL values. A retrospective study by Weidner and colleagues showed that HCV eradication through DAA treatment was associated to with a significant reduction in fasting plasma glucose level and in the rate of patients with impaired fasting plasma glucose. In some CHC patients, however, the reduction of FPG levels was only transitory and no significant improvement in glycemic values was observed in cirrhotic patients up to 12 months after therapy (Weidner et al. 2018). In a retrospective study on 122 diabetic subjects with HCV infection published in 2019, Gilad et al. reported favorable HbA1c changes after DAA treatment in 42 (34%) of the 122; among these 42, only 20 of the 28 (71%) with available follow-up showed this effect sustained over 1.5 year (Gilad et al. 2019). Chaudury et al. prospectively examined for a median period of 28 months 251 HCV CHC patients, of whom 31% with HIV coinfection, before and after DAA therapy and only minimal changes in HbA1c and glucose were observed, independently of the achievement of SVR, HIV status, diabetes, or stage of liver disease. To be noticed that TC and LDL increased significantly after treatment (Chaudhury et al. 2017). Beig et al. performed a retrospective single-center study on 91 HCV-related liver transplant recipients with recurrent HCV infection who received DAA treatment, of whom 87 (96%) reached HCV eradication. HCV clearance was associated with a reduction in treatment doses for diabetes by 38% and from a decline of HbA1c levels from 35.5 ± 4.3 to 33.3 ± 3.6 mmol/mol 44 weeks post-treatment (p = 0.03); however, TC and LDL levels significantly increased posttreatment (Beig et al. 2018).
Some studies evaluated the changes in the incidence of T2DM after effective antiviral therapy. Butt and colleagues analyzed the data from patients of the U.S. Veterans Administration and recorded a more significant reduction in T2DM incidence rate in patients treated with DAAs: the incidence rates per 1000 person-years were 9.89 (95% confidence interval [CI] = 8.7–11.1) in DAA-treated patients, 19.8 (95% CI = 18.3–21.4) in those treated with pegylated interferon (Peg-IFN)/ ribavirin (RBV) treatment and 20.6 (95% CI = 19.6–21.6) in those left untreated (p < 0.001) (Butt et al. 2019). A cohort of 5127 nondiabetic patients treated with DAAs was analyzed by Li and coworkers; during an average follow up of 3.7 years they recorded an incidence of T2DM of 6.2% in the SVR group and of 21.7% in the non-SVR group (HR = 0.79; 95% CI = 0.65–0.96) (Li et al. 2019). Similarly, El Serag and colleagues analyzed 45,260 patients treated with DAAs, but the incidence of DM between the SVR and the non-SVR group did not significantly differ (21.04/1000 patients per year versus 23.11/1000 patients per year; hazard ratio (HR) = 0.98, 95% CI = 0.81–1.19, p = 0.86) (El Serag et al. 2019).
Concluding on this topic, there is still some disputes and the available data do not allow us to conclude on whether the achievement of SVR induces a persistent reduction in fasting glucose and HbA1c; despite this, the data from most studies strongly indicate good compensation of T2DM in HCV patients treated with DAA. Long-term prospective follow-up studies on the evolution of glycolic metabolism of diabetic and non-diabetic HCV patients who achieved SVR with DAA treatment are still needed to resolve the remaining disputes.
5 DAAs and Lipid Homeostasis
HCV infection has been associated with lipid and lipoprotein metabolism disorders, such as hypobetalipoproteinemia, hypocholesterolemia, and hepatic steatosis (Felmlee et al. 2013). HCV production is dependent on the very-low-density lipoprotein (VLDL) biosynthetic pathway, and circulating virions are associated with VLDL in lipoviral particles containing host apolipoproteins (APOs), including APOB, APOE, and APOC3 (Dai et al. 2008); the interaction between HCV virions, VLDL and low-density lipoprotein (LDL) particles is responsible for increased viral infectivity. In addition, HCV infection activates the sterol-regulatory-element-binding-protein (SREBP) 1c, which is involved in lipogenesis and HCV-related liver steatosis, partially due to ß mitochondrial-oxidation (Nielsen et al. 2006; Waris et al. 2007; Merz et al. 2011). It has also been shown that HCV core protein inhibits the activity of microsomal triglyceride transfer protein (MTP), resulting in liver steatosis and hypolipidemia. Because of the mechanisms mentioned above, spontaneous or treatment-related HCV eradication, down-regulating SREBP 1c and up-regulating both MTP and CPT-1 may reduce liver lipogenesis and increase VLDL secretion. Other data on lipid homeostasis in anti-HCV treatment is shown in Table 2. Eradication of HCV infection with IFN-based treatment has been found associated with normalization of hypolipidemia (Tada et al. 2009) and with an increased risk of cardiovascular events. HCV eradication has also been found associated with a decrease in CAP and LDL-C values, the parameter used as a measure of liver steatosis, which, however, correlates with elevated values of small-dense LDL-C (sdLDL-C), which has been shown to predict atherogenesis and dyslipidemia in patients with SVR24 (Kawagishi et al. 2018).
A rapid increase in serum LDL-C and total cholesterol (CT) values from the baseline to the 28th week of DAA treatment has also been described, the hyper lipid effect being stronger with ledipasvir/sofosbuvir than with daclatasvir/asunaprevir combination (Hashimoto et al. 2016). These Authors also observed a close correlation between the decrease in the HCV core antigen serum titers and the increase in LDL-L values, especially in patients treated with sofosbuvir + ledipasvir, suggesting a direct influence of the HCV clearance on serum cholesterol levels (Hashimoto et al. 2016).
In a prospective multicenter study on HCV infected and HIV-HCV coinfected patients treated with several DAA combinations, a significant increase in LDL cholesterol serum values was observed (Mauss et al. 2017), while HDL cholesterol remained stable. However, contrasting data were reported by Carvalho et al. (2018) who evaluated lipid homeostasis in chronically HCV-infected patients at baseline and 1 year after the achievement of SVR in 105 patients treated with sofosbuvir + ledipasvir ± RBV and 73 with IFN ± RBV: they found a significant increase in TC and LDL levels after treatment with both regimens, while serum triglyceride levels decreased only in the DAA group (p = 0.015) (Carvalho et al. 2018).
Another marker of lipid homeostasis is the ApoB/ApoA1 ratio, a better predictor of cardiovascular diseases than LDL alone: ApoB is the best direct marker of low atherogenic density LDL-19 and ApoA1 provides a good estimate of HDL. An Italian real-life study (Gitto et al. 2018) analyzed the metabolic changes in 100 HCV patients during a 24-week follow-up period after DAA treatment discontinuation and observed a significant reduction in ApoA1 blood levels and an increase in the ApoB/ApoA1 ratio and Lp (a) (Gitto et al. 2018). Supporting evidence is given by Younossi et al. who evaluated lipoproteins in genotype-1 patients who achieved SVR after ledipasvir/sofosbuvir ± RBV treatment, with an increase in ApoB and LDL and a decline in ApoA1 and apolipoprotein (Younossi et al. 2015).
Concluding on this point, the effect of DAA regimens, especially the sofosbuvir-based, on lipidic homeostasis is characterized by an increase in TC, LDL and the ApoB/ApoA1 ratio. As a consequence of this, a higher incidence of cardiovascular events could have been expected after HCV eradication, eventuality not occurred probably because the adverse effect possibly induced by this increase could have been balanced or overcame by the favorable effect of the DAA-induced HCV eradication on cardiovascular system.
6 DAAs and Cardiovascular Diseases
HCV infection has also been described as an independent non-traditional risk factor for cardiovascular (CV) diseases (Domont and Cacoub 2016) since it induces an increased overall CV mortality (Goossens and Negro 2017; Cacoub et al. 2018a, b), a dysmetabolic syndrome (Loria et al. 2014) and a cytokine remodeling towards chronic systemic inflammation, which triggers endothelial dysfunction in response to recombinant HCV envelope protein (Urbaczek et al. 2014; Katsi et al. 2015; Gonzalez-Reimers et al. 2016; Cammarota et al. 2019; Sigon et al. 2019). Other mechanisms responsible for CV diseases in HCV patients have been identified in the procoagulant imbalance and IR/T2DM, which may directly cause vascular and cardiac damage (Domont and Cacoub 2016; Vassalle et al. 2018; Petta et al. 2018). Apart from some indirect mechanisms, HCV has also been proven to be a direct cardiotropic virus and a causative agent in structural cardiomyopathies, such as dilated, hypertrophic, right ventricular arrhythmogenicity (Matsumori et al. 1998; Matsumori 2006) and an inducer of cardiac fibrosis (Pepe et al. 2015) and myocarditis (Okabe et al. 1997; Goossens and Negro 2017) rarely secondarily to mixed cryoglobulinemia (Terrier et al. 2013). HCV-core protein elicits immune-mediated oxidative damage in myocardial tissue (Sanchez and Bergasa 2008; Frustaci et al. 2002), where HCV-RNA is also detectable (Matsumori et al. 1996; Okabe et al. 1997; Matsumori 2006). Also, genetic HLA and non-HLA susceptibility correlated to cardiomyopathy development has been described (Sanchez and Bergasa 2008). In addition, myocardial scintigraphy showed increased perfusion defects in 87% of 217 HCV-positive patients (Maruyama et al. 2013). This evidence has determined a partial shift in the practical interpretation of cardiovascular dysfunction in the management of HCV patients, CV disease increasingly representing a reason for prompt treatment, rather than an exclusion criterion.
Epidemiological studies showed an association between carotid atherosclerosis (Tomiyama et al. 2003; Perticone et al. 2015), carotid intima-media thickness (cIMT) and β-stiffness (Novo et al. 2018; Negro 2014) and the circulating of HCV-core protein (Ishizaka et al. 2003). However, the role of HCV in atherogenesis is still unclear as HCV might only be a “bystander” when detected within atherosclerotic plaques, rather than their cause (Goossens and Negro 2017; Vassalle et al. 2018; Romano et al. 2018), and the HCV-induced protective lipid profile constitutes a confounding factor on this point (Maggi et al. 1996; Oliveira et al. 2013; Novo et al. 2018). (Table 3).
Other studies reported an increased risk of acute coronary syndrome (ACS) (Tsai et al. 2015) and acute myocardial infarction (AMI) (Butt et al. 2017; Vassalle et al. 2018), with an association between the number of affected vessels and HCV viral load (Vassalle et al. 2004, 2018). Left ventricular dysfunction and congestive heart failure (CHF) may also occur, events predictable by N-terminal-pro-natriuretic peptide plasma values (NT-pro-BNP) (Vassalle et al. 2018). The hepatic damage (necroinflammation, steatosis and fibrosis) appears to promote T2DM and CV diseases in HCV patients (Lonardo et al. 2016). The “Heart and Soul” study analyzed 981 patients with CV diseases, of whom 8.6% was HCV-infected. The latter showed increased TNF-α levels and an augmented risk of heart failure and death (Tsui et al. 2009). The NHANES cohort examined 16,668 HCV patients and showed that CHC is an independent risk factor for impaired glucose metabolism (IR/T2DM), hypertension and congestive heart failure (Katsi et al. 2015). (Table 3).
HCV eradication has been associated with an improvement in CV and metabolic syndromes, creating a reduction in all-cause mortality both in patients receiving IFN-based therapy and in those treated with DAA (Goossens and Negro 2017; Adinolfi et al. 2018c; Mohanty et al. 2019; Revuelto Artigas et al. 2019b).
In the prospective CirVir cohort, including 878 HCV cirrhotic patients from 35 clinical centers, the achievement of SVR by IFN-based regimens was associated with a decrease in CV mortality (Cacoub et al. 2018b). In 2018, Tran et al. studied a cohort of 1554 HCV patients with CHC without cirrhosis enrolled in two phase-3 clinical trials to evaluate the tolerability and efficacy of glecaprevir/pibrentasvir combination therapy and found a statistically significant reduction in CV diseases and in metabolic syndromes in those who achieved SVR (Tran et al. 2018); a post-hoc analysis on 5963 HCV patients undergoing ombitasvir/paritaprevir/ritonavir/dasabuvir+RBV combination therapy obtained similar results (Mehta et al. 2017). Thirty-nine Italian HCV-cirrhotic patients showed no major CV adverse events following HCV eradication with different DAAs regimens and a decrease in subclinical cardiovascular alterations as detected by PWV and β-stiffness index (Novo et al. 2018).
Butt et al. investigated the risk of CV events in USA veterans with CHC, 4436 treated with Peg-IFN and 12,667 with DAAs, matched with untreated controls according to potential confounding factors: CV events were observed in 7.2% of treated patients and in 13.8% of controls, indicating a significantly lower risk after SVR achievement (Butt et al. 2018). An Italian study on 182 HCV patients with severe liver fibrosis and SVR to DAA evaluated the dynamics of carotid atherosclerosis by measuring the carotid intima-media thickness (cIMT): there was a significant decrease in cIMT during a long-term post-treatment follow-up (from 0.94 ± 0.29 mm at the baseline to 0.81 ± 0.27 at the end of observation, p < 0.001) and a reduction of the number of patients with an increased carotid thickening (from 42.8 to 17%, p < 0.001) (Petta et al. 2018). Instead, a Spanish prospective study on 85 HCV-cirrhotic patients did not find cIMT reduction within the 12th month after DAA-mediated HCV eradication (Revuelto Artigas et al. 2019a). In addition, Ichikawa et al. analyzed 48 CHC patients 1 year after they achieved SVR to DAA treatment and observed an increase of cIMT associated with an unfavorable lipidic profile (increase in LDL, HDL, sdLDL) (Ichikawa et al. 2019), suggesting that we cannot conclude on this point and further studies are required to untangle the remaining controversies (Osibogun et al. 2017). Concluding on this point, despite some undesirable effects eventually due to the increase in Total and LDL cholesterol, an overall evaluation of the available data suggests that HCV eradication with DAA therapy, by eliminating numerous other harmful effects of HCV, exerts a favorable action on cardiovascular system, which results in a reduction of adverse events and reduced mortality.
7 DAAs and Renal Function
HCV infection and chronic kidney diseases (CKD) are joined by two main links, the first being the frequent exposure to HCV of patients with advanced CKD in dialysis units, and the second that HCV infection is able to directly induce kidney disease. Epidemiological investigations have underlined that HCV chronic infection increases the incidence of CKD and accelerates CKD progression to end-stage renal disease (Henson and Sise 2019). A strong correlation between anti-HCV positivity and the incidence of CKD has been demonstrated in a meta-analysis published in 2017 (Fabrizi et al. 2017), where anti-HCV positivity was identified as an independent predictor of death for dialysis patients (Fabrizi et al. 2020). HCV infection may contribute to tissue damage by directly infecting the endothelium, tubular epithelial cells, renal infiltrating leukocytes and other types of renal cells, such as mesangial cells, and is associated with three different kidney lesions: mixed cryoglobulinemic nephropathy, membranous-proliferative glomerulonephritis and membranous nephropathy.
Interferon-based treatment induced HCV eradication in nearly half of the treated patients, an event associated with a decreased rate of patients with renal disease and with a reduced progression to an end-stage renal disease (Arase et al. 2011; Feng et al. 2012; Hsu et al. 2015a; Montero et al. 2018; Fabrizi et al. 2020).
With the introduction of DAA therapies for chronic hepatitis C, the SVR rate also increased significantly in patients with CKD (Perazzo et al. 2020). A meta-analysis evaluating 11 studies on HCV patients with CKD-4/5 and treated with DAA-based therapy showed an SVR12 rate of 93.2%, an incidence of serious adverse events of 12.1% and treatment withdrawal of 2.2%, suggesting that DAA-based therapy is safe and efficient in eradicating HCV infection even in this patient setting (Li et al. 2017). It has also been shown that DAA therapy reduces the risk of kidney disease in CHC patients (Fabrizi et al. 2020).
The favorable effect of DAA treatments on glomerular filtration rate (eGFR) has also been proven, especially in patients with mild or moderate CKD (Calleja et al. 2017; Coppola et al. 2019; Sise et al. 2020; D’Ambrosio et al. 2020) (Table 4). In a cohort of 3,264 patients (9.5% in stage CKD 3 and 0.7% in stage 4/5) eGFR improved more significantly in those in CKD-3a stage (p < 0.0001) and CKD-3b (p = 0.0007) than in those in stage CKD-4/5 (p = 0.024) (D’Ambrosio et al. 2020). Half of 38 Spanish patients with baseline eGFR <60 ml/min/1.73 m2 showed a remarkable improvement in these values after HCV eradication with DAA treatment (Calleja et al. 2017). Sise et al. in a follow-up of 573 days (SD = 337) after the discontinuation of DAA observed in patients with baseline eGFR <60 ml/min 1.73 m2 the persistence of a substantial improvement induced by DAA treatment (Sise et al. 2020).
Instead, the results may be different if sofosbuvir-based regimens are used, as observed by Shin et al. in 4 of 28 patients with stage 3 CKD who showed a reduction of eGFR of more than 30% (Shin et al. 2017) (Table 4). In addition, in a cohort of 3264 patients, of whom 89.8% had baseline eGFR >60 ml/min, this index significantly decreased in those with CKD-1 (p < 0.0001) and CKD-2 (p = 0.0002) under a sofosbuvir- RBV combination treatment (D’Ambrosio et al. 2020). Similar results were found in a Spanish cohort of 1567 patients treated with ombisartan/paritaprenvir/ ritonavir + dasabuvir ± RBV and in 1,758 treated with ledipasvir/sofosbuvir ± RBV, where a mean eGFR reduction of −1.6 (SD = 12.4) ml/min/1.73 m2 was observed in patients with baseline normal renal function (Calleja et al. 2017). In a cohort of 17,624 patients treated with sofosbuvir + ledipasvir ± RBV or with paritaprevir/ritonavir/ombitasvir ± RBV, Butt et al. observed that 30% and 38% of patients in the two different therapeutic regimens, respectively, showed a reduction in eGFR by at least 10 ml/min/1.73 m2 compared to the baseline normal value. However, it is useful to underline that these possible reductions in eGFR generally disappear within 12 weeks from the suspension of therapy (Butt et al. 2018).
In a Spanish/Portuguese cohort of 1131 patients, including 658 (58%) HIV/HCV-coinfected patients, the eGFR slightly declined during DAA treatments in patients with normal to moderately impaired renal function (Álvarez-Ossorio et al. 2018). A similar decrease in eGFR was observed in 273 HIV/HCV coinfected patients, more pronounced in those receiving tenofovir, in those treated with DAA for 24 weeks (p = 0.009) and in cirrhotic patients (p = 0.036) (Álvarez-Ossorio et al. 2018). Similar results were observed in a study on 144 HIV/HCV coinfected patients; a strong eGFR decline was observed in those concomitantly treated with tenofovir (p = 0.0001), ribavirin (p = 0.0001) or integrase inhibitors (p < 0.0001), in those with a longer duration of HIV (p = 0.0002) or HCV infection (p = 0.035), in those with a lower baseline HCV RNA (p < 0.0001), or with a previous HCV treatment (p < 0.0001), and in the elderly (p < 0.0001) (Taramasso et al. 2018).
In conclusion on this point, the HCV eradication obtained with DAA therapy in CHC patients exerts a beneficial effect even in those with impaired renal function and only some conflicting data persist on the effect of some DAA regimens on eGFR. Similar beneficial effects of DAA therapy are also observed in patients with HCV/HIV co-infection and even here doubts persist only on the use of drugs which may lead to a transient reduction in eGFR.
8 DAA Treatment of Cryoglobulinemia
Cryoglobulinemia is a condition characterized by the presence of cryoglobulins in the blood, which reversibly precipitate and form a gel at less than 37 °C and dissolve over 37 °C (Roccatello et al. 2018). Brouet’s classification defines three types of cryoglobulinemia: Type I with single monoclonal immunoglobulins; type II, a mixed cryoglobulinemia with monoclonal and polyclonal immunoglobulins; Type III, a mixed cryoglobulinemia with IgM and IgG, both polyclonal. (Brouet 1983). Cryoglobulins could be detected in 25–30% of HCV-positive patients (Dammacco and Sansonno 2013) and 80–90% of cases with type II and type III mixed cryoglobulinemia carry HCV infection (Minopetrou et al. 2013; Roccatello et al. 2018) associated with a high incidence of severe liver fibrosis and cirrhosis (Roccatello et al. 2018). Arthralgia, asthenia and palpable purpura are the most common clinical manifestations of HCV related mixed cryoglobulinemic syndrome (MCS) and skin the most frequently organ involved; however, hematological disease and severe organ disfunction or failure (kidney, hearth, central nervous system, etc.) may occur (Dammacco and Sansonno 2013; Minopetrou et al. 2013). Treatment with Standard IFN provides HCV eradication in only a quarter of treated cases, with a substantial improvement in both liver function and CMS; in cases of temporary viral response, however, CMS usually relapses (Dammacco and Sansonno 2013). The introduction of treatment with Peg-IFN α-2a or 2b and RBV induced SVR in about half of treated patients. In a cohort study published by Gragnani et al. in 2015, the persistence of cryoglobulinemia was linked to a higher probability of Peg-IFN/ RBV treatment failure (HR 2.03, 95% CI = 1.12–3.68, p = 0.0204), while the 63 HCV patients with MCS who reached SVR showed a clear improvement in clinical and laboratory MCS manifestation throughout a mean follow up period of 92.5 months (Gragnani et al. 2015).
The combination of a first generation DAA (telaprevir or boceprevir) with Peg-INF and RBV achieved HCV eradication in 65–75% of treated patients with remission or reduction of sign and symptoms of MCS (Humphries et al. 2014; Gragnani et al. 2014; Saadoun et al. 2014, 2015; Cornella et al. 2015).
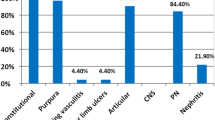
IFN-based regimes have become obsolete once the second generation DAAs have been introduced, because of their good safety profile and higher effectiveness (SVR in 95% of treated patients). Same studies published in 2015 and in 2016 have shown a frequent remission of symptoms and signs of MCS in CHC patients after a DAA treatment (Makara et al. 2015; Chak et al. 2015; Koga et al. 2017; Obata et al. 2017). Several cohort studies reported high percentages of patients who achieved a remarkable improvement of cryoglobulinemic vasculitis after HCV eradication with DAA therapy (Sise et al. 2016; Bonacci et al. 2017; Saadoun et al. 2017; Lauletta et al. 2017; Emery et al. 2017; Comarmond et al. 2017; Gragnani et al. 2018; Hassan et al. 2018; Miailhes et al. 2018; Pozzato et al. 2020) (Table 5); our meta-prop analysis on these percentages, shows an overall improvement in 78% of patients (95% CI: 0.69–0.86 p = < 0.001) (Fig. 1). A cohort study by Mahale et al. detected the incidence rate (IR) per 1,000 persons-year (Py) of CMS in HCV positive patients, either never treated (IR 1000 Py = 0.72; 95% CI = 0.66–0.78), or DAA-treated without SVR (IR 1000 Py =0.52; 95%CI = 0.41–0.67), or DAA-treated with SVR (IR 1000 Py =0.33; 95%CI: 0.21–0.5), showing that HCV RNA clearance is a protective factor in this setting; the adjusted hazard ratios (aHR) indicate no significant difference between treated patients without SVR vs. untreated, aHR 1.11, (95% CI = 0.85–1.45), whereas the differences between patients who reached SVR versus untreated and versus those treated without SVR ware both statistically significant, respectively 0.61 (95% CI = 0.39–0.94) and 0.55 (95% CI = 0.33–0.90) (Mahale et al. 2018). Cacoub et al. analyzed the effect of DAA-induced SVR obtained on HCV extrahepatic manifestations in a meta-analysis including 16 studies; the achievement of SVR was associated with a reduction in extrahepatic mortality (OR 0.44; 95% CI = 0.28–0.67), a higher complete remissions of clinical signs and symptoms of cryoglobulinemic vasculitis (OR 20.76; CI = 6.73–64.05) and with a greater efficacy in malignant B-cell lymphoproliferative diseases (OR 6.49; CI = 2.02–20.85) (Cacoub et al. 2018a).
Out of 12,985 HCV genotype 4 CHC patients successfully treated with second generation DAAs, Fayed et al. identified 50 patients with de novo detectable serum cryoglobulins and vascular renal affection 4.3 ± 1.3 months after treatment, the most common type of kidney affection observed in renal biopsies being membranoproliferative glomerulonephritis (52%); chronic kidney disease (CKD) developed in 46% of cases. The Authors concluded that de novo cryoglobulinemic glomerulonephritis and progression to CKD rarely complicate a successful DAA treatment (Fayed et al. 2018).
Concluding on this point, DAA treatment finds full application in CHC patients with MCS, since it has a good safety profile, induces HCV eradication in nearly 95% of patients and is associated with remissions of cryoglobulinemic vasculitis and with a reduction extrahepatic mortality. In addition, MCS infrequently occurs in CHC patients after HCV eradication.
9 DAAs and Lymphoma
Strongly characterized as a hepatotropic virus, HCV also infect and replicate within B and T cells (Sarhan et al. 2018) and is capable of driving clonal expansion of B lymphocytes (Kasama et al. 2011) within the complex HCV syndrome; therefore, HCV infection is associated and causally related to lymphomagenesis (Zignego et al. 1997). Worthy of notice, patients with HCV-driven type II mixed cryoglobulinemia are at increased risk for NHL (with a 35-fold higher risk than the general population) (Defrancesco et al. 2020). A vast study by the International Lymphoma Epidemiology Consortium and other epidemiologic studies have identified an association between chronic HCV infection and B-NHL subtypes, particularly with the diffuse large B-cell lymphoma (DLBCL), marginal zone lymphoma (MZL), and lymphoplasmacytic lymphoma) (Suarez et al. 2006; De Sanjose et al. 2008; Rattotti et al. 2019; Defrancesco et al. 2020). The double tropism and the double oncogenic potential of HCV is also underlined by some reports on HCV infected patients with both HCC and lymphoma (Shapira et al. 2001; Utsunomiya et al. 2009; Becker et al. 2010).
The first evidences for a link between HCV infection and lymphoma date back to the 1990s (Ferri et al. 1994; Pioltelli et al. 1996; Hanley et al. 1996; Galli et al. 1996; Brind et al. 1996; Zignego et al. 1997), either in association or, more rarely, in the absence of mixed cryoglobulinemia (De Vita et al. 1997, Luppi et al. 1996). Subsequently, the aetiological hypothesis was enriched by the detection of HCV RNA in in NHL lesions lymphoma samples (Ohsawa et al. 1998; Karavattathayyil et al. 2000) and by the demonstration of a positive correlation between viral replication and the risk to develop lymphoma (Amiel et al. 2000). This link was confirmed by a Meta-analysis published in 2006 (Dal Maso et al. 2006) and by the data from the Swiss Cohort Study on HIV/HCV coinfected patients (Franceschi et al. 2006). In 2003, an Italian multicentre case-control study confirmed that B-NHL may originate in CHC patients, suggesting a significant potential benefit of an antiviral treatment in limiting the burden of HCV-related haematological disease (Mele et al. 2003). The association of HCV infection with NHL was further confirmed in a case control study which, however, failed to find a significant correlation with Hodgkin Disease (Montella et al. 2001). Zhou et al., in 2016, proposed the HCV load as a prognostic factor in patients with HCV-positive diffuse large B cells lymphoma. (Zhou et al. 2016); in 2017, Shimono proposed HCV infection as an independent factor in the prognosis of follicular lymphoma (Shimono et al. 2017), In 2019, a meta-analysis by Zhu et al. reaffirmed the prominent role of HCV as a risk factor for NHL. (Zhu et al. 2019) and more recently, in 2020, a Turkish multicentre cohort study proposed HCV as a causative and prognostic factor for splenic marginal zone lymphoma (Okay et al. 2020).
It is worth noticing that some studies have highlighting geographic variations for the HCV-NHL association, suggesting a deeper evaluation of HCV genotypes and cofactors responsible of discrepancies. In detail, a meta-analysis found a strikingly positive association between HCV seropositivity and NHL only for Italian and Japanese patients (Matsuo et al. 2004); in the 2001 a prospective study on 1576 patients., concluded that HCV positivity was scarcely prevalent (1.83%) in patients with B-NHL in France (Hausfater et al. 2001).
It is worth reporting that a few other some studies denied the association between CHC and B-cell lymphoma (Collier et al. 1999; Avilés et al. 2003).
Considering the complex role of HCV in the related haematological disease the “Fondazione Italiana Linfomi” designed a specific “HCV prognostic score” to its management (Defrancesco et al. 2020).
Underlying mechanisms for HCV lymphomagenesis are far from being fully understood, but seem to revolve around chronic antigen-driven proliferation of B-cells, majorly mediated by viral proteins such as HCV core protein (Suarez et al. 2006; Alisi et al. 2007), and E2 envelope protein (Quinn et al. 2001; Douam et al. 2015), also observed in T cell lines (Zhao et al. 2006), with mechanisms promote a mutator phenotype of immunoglobulin and proto-oncogenes (e.g. Ig heavy chain, BCL-6, p53, and beta-catenin) (Machida et al. 2006a), increasing NF-κB expression and contrasting antiapoptotic functions (e.g. Bcl-2) (Defrancesco et al. 2020). Furthermore, HCV can upregulate B-cell receptor signalling (Dai et al. 2016) and trigger the enhancement of TLR4 expression along with IFN-beta and interleukin-6 production (Feldmann et al. 2006; Machida et al. 2006b). Additional mechanisms involve mitochondrial dysregulation and oxidative damage, with DNA damage, STAT3 activation (Machida et al. 2006a) and epigenetic alterations in microRNA (Peveling-Oberhag et al. 2012), also in DLBCL (Augello et al. 2014). These complex mechanisms are extensively discussed in some dedicated reviews (Landau et al. 2007; Visco and Finotto 2014). A role in HCV lymphomagenesis has been proposed also for genetic risk factors, like the fibronectin gene polymorphisms (Fabris et al. 2008) and MHC II (e.g HLA-DQ) (De Re et al. 2004, 2009).
Of notice, HCV-related lymphoproliferative diseases present peculiar molecular signature, with possible therapeutic implications (De Re et al. 2012; Peveling-Oberhag et al. 2013; Visco et al. 2017), including an increase in specific oncogene expression, such as Bcl-2, correlated with t(14;18) translocation which disappears following HCV eradication (Zignego et al. 2000). A long past use of IFN in HCV positive patients with lymphoma was linked to the well-known antiproliferative effect of this drug, although toxicity was not negligible and SVR was far from being satisfactory, since HCV eradication was achieved by a quarter of patients receiving Standard IFN-based therapy by approximately 50% of those treated with Peg-IFN + RBV. The Peg-IFN and first-generation DAA-based therapy has been demonstrated able to obtain the SVR in 65–75% of HCV-1 CHC patients. A meta-analysis published by Peveling-Oberhag et al. in 2016 confirmed the strong association between SVR and B-NHL regression, particularly in MZL, suggesting that antiviral treatment may function as a first-line therapeutic approach when I-CT is not immediately required (Peveling-Oberhag et al. 2016), leading to a better overall survival in case of SVR (Hosry et al. 2016, Masarone and Persico 2019). Of notice, Su et al. observed a reduced risk for lymphoma development in patients who received early successful therapy for HCV infection (Su et al. 2019).
Some case-reports published in In 2015 showed that the second generation DAAs exert a favourable clinical effect on HCV lymphoma after HCV clearance: Rossotti et al. described a case of splenic MZL obtaining a favourable rapid hemato-virologic response after a 16-week treatment with faldaprevir, deleobuvir and RBV (Rossotti et al. 2015); Sultanik et al. reported the case of a HCV-positive woman with disseminated extranodal MZL treated with 4 weeks sofosbuvir + RBV, followed by 12 weeks sofosbuvir + daclatasvir, obtaining HCV clearance and a concomitant regression of lymphoma (Sultanik et al. 2015); Lim et al. reported a case of MZL regression by sofosbuvir + RBV (Lim et al. 2015); Carrier et al. reported a satisfactory viro-hematologic response (Carrier et al. 2015). in 5 NHL patients treated with sofosbuvir plus simprevir or daclatasvir, combined with chemotherapy one patient with DLBCL.
The French “ANRS HC-13 Lympho-C” Study observed two prospective cohorts of HCV-B-NHL patients: the first of 61 patients receiving Peg-IFN + RBV (combined with the first generation DAAs telaprevir or boceprevir only in some of them), and the second of 10 patients treated with sofosbuvir plus ledipasvir or simeprevir or daclatasvir or RBV; SVR led to a reduced risk for lymphoma progression, but IFN based regimen was poorly tolerated in DLBCL patients, already weakened by previous chemotherapy (Alric et al. 2016). A beneficial clinical effect of DAA therapy with ombitasvir/paritaprevir/RBV and dasabuvir was described in a HCV patient with aggressive double-hit B-cell lymphoma. (Galati et al. 2016).
In 2016, Arcaini et al. analysed a cohort of 46 patients with HCV-related lymphoproliferative disorders (indolent B-NHL, majorly MZL, and chronic lymphatic leukaemia, CLL): 39 subjects received sofosbuvir plus simprevir or RBV or daclatasvir or ledipasvir, while 7 subjects received an alternative regimen (paritaprevir/ ritonavir/ ombitasvir ± dasabuvir ± RBV or faldaprevir/ deleobuvir/ RBV); 98% of patients achieved SVR, while a hematologic response was obtained in 67% of cases (complete in 12 patients, 26%), more prominent in MZL, while no CLL/SLL patient obtained hematologic regression (Arcaini et al. 2016). Frigeni et al. observed a cohort of 100 patients with indolent HCV-B-NHL (only one with decompensated cirrhosis who failed to obtain SVR, with presenting lymphoma progression); 66 patients were treated with a DAA regimen and the remaining with an IFN-based regimen; nodal involvement was apparently more severe and less responsive, cryoglobulinemia wasn’t a relevant outcome modifier, the strongest responsiveness was observed in MZL and, noticeably, SVR (either ± IFN) led to an augmented overall hematologic response (Frigeni et al. 2020).
In 2019, a meta-analysis reaffirmed a powerful association between HCV eradication by DAA and favorable hematologic outcomes for HCV-positive B-NHL (Masarone and Persico 2019). Of notice, other studies observed a reduced risk for lymphoma development in patients who received early successful therapy for HCV infection (Su et al. 2019; Iwane et al. 2019) and prevention of relapse of DLBCL and, more generally, of malignant lymphoma (Pellicelli et al. 2018). As a complementary outcome, DAA eradication in HCV-positive DLBCL may reduce the liver toxicity of immune-chemotherapy (I-CT), and it can be offered after or even during I-CT, with the advantage of a timely management (Occhipinti et al. 2018; Merli et al. 2019, 2020).
Although the favourable effect of HCV eradication on the course of lymphoproliferative diseases is evident, some limitations have been underlined. For example, Schiavinato et al. analysed the peripheral blood lymphocytes populations and Ig light chain κ/λ ratio variations as indicators for monoclonal B-cell response in 9 patients with CHC and lymphoproliferative disorders treated with Ombitasvir/Paritaprevir/Ritornavir/Dasabuvir plus RBV; although all patients reached SVR12 and a global reduction of B cells, they still presented monoclonal components (Schiavinato et al. 2017). Rodríguez de Santiago et al. warned the scientific community against an excessive optimism as 6 patients out of 9 HCV patients with a lymphoproliferative disease presented a persistence of monoclonal B lymphocytes in the bone marrow 1 year after SVR; in addition, two NHL patients required additional therapy (chemotherapy and/or immunotherapy) after SVR was achieved (Rodríguez de Santiago et al. 2018). Furthermore, in aggressive B-cells lymphoma, such as DLBCL, there is a limited evidence for the therapeutic aid by DAA (Visco amd Finotto 2014).
In conclusion, available evidence increasingly recognizes the beneficial role of HCV eradication in the treatment of HCV-related lymphoproliferative disorders, particularly obtained with the highly tolerable and effective IFN-free DAA-based regimens, evidence reinforcing the importance of HCV in lymphomagenesis. Antiviral therapy appears majorly important in patients with indolent NHL, but some recent information support the use of HCV eradication also in patients affected by the more aggressive HVC-positive DLBCL, even if further investigation is needed in this topic.
10 Conclusion
In the last decade, emphasis has been placed on the extrahepatic involvement of chronic HCV infection, now fully recognized as a systemic disease, having reversed the previous “liver-focused” holistic paradigm towards an HCV pleiotropic action. In addition to being hepatotropic, HCV is also a lymphotropic virus responsible for polyclonal B-lymphocyte expansion that leads to the development of extrahepatic manifestations, such as type II cryoglobulinemia and some types of B-cell non-Hodgkin lymphomas, such as lymphoplasmacytic lymphoma/immunocytoma and marginal-zone lymphomas. In addition, chronic HCV infection is considered a trigger for immune-mediated disorders through a crossover immune response to self-antigens due to sequence similarities between viral proteins and self-proteins (molecular mimicry theory) or through the activation of autoreactive T-cells due to viral-induced local inflammation (bystander activation theory). In fact, chronic HCV infection has been associated with autoimmune diseases such as psoriasis, lichen planus, Sjogren syndrome and autoimmune thyroiditis, with the presence of organ-specific circulating anti-thyroperoxidase and anti-thyroglobulin autoantibodies and with high titers of non-organ-specific antinuclear, anti-smooth muscle and anti-liver/kidney microsome autoantibodies.
Patients with HCV infection show an increased overall mortality compared to the normal population, probably related to a dysmetabolic syndrome and cytokine remodeling towards chronic systemic inflammation that triggers endothelial dysfunction in response to the HCV envelope protein.
Luckily, recent advances in anti-HCV therapy have led to more efficient well tolerated interferon-free DAA regimens, so most patients can achieve HCV eradication. Ninety-five per cent of CHC patients without cirrhosis treated with DAAs recover completely, but substantial clinical and sometimes even histological improvement is also observed in cirrhotic patients. The beneficial action of the eradication of HCV infection with DAAs is also exerted on the extra-hepatic manifestations of this infection, but some results are contradictory or difficult to explain.
In fact, the available data do not allow a conclusion on whether the eradication of HCV infection induces a persistent reduction in fasting glucose and HbA1c; nevertheless, most studies strongly indicate a good T2DM compensation in patients treated with DAAs. Further long-term prospective studies on the evolution of glucose metabolism in HCV patients who achieved SVR with DAA treatment, diabetic and non-diabetic, are needed to resolve the remaining disputes.
CHC patients frequently show low serum levels of Total and LDL cholesterol, which increase significantly after HCV eradication, in some cases beyond the pre-treatment levels, most likely because the interaction between interaction HCV / lipid metabolism ceases. Despite this negative effect, HCV eradication exerts an overall favorable action on the cardiovascular system, possibly eliminating numerous other harmful effects exerted by HCV on this system.
Mechanisms responsible for direct vascular and cardiac damage in HCV patients have been identified in the procoagulant imbalance and in the IR/T2DM ratio. Furthermore, HCV-core protein can induce an immune-mediated oxidative damage in myocardial tissue and is considered a direct cardiotropic virus, responsible for dilated, hypertrophic right ventricular arrhythmogenicity, cardiac fibrosis and myocarditis. An association has been observed between carotid atherosclerosis, carotid intima-media thickness, β stiffness and HCV core protein. Other studies have reported an increased risk of acute coronary syndrome (ACS) and acute myocardial infarction (AMI), with an association between the number of affected vessels and HCV viral load. The abolition of many negative effects due to DAA induced HCV eradication explains how the increase in IR, Total and LDL cholesterol induced by the same drugs are not very influential. In this regard, it should be also considered that the increase in IR is transitory and therefore has only a temporary negative influence.
Infecting kidney endothelium, tubular epithelial cells, renal infiltrating leukocytes and mesangial cells, HCV is responsible of several kidney lesions, like mixed cryoglobulinemic nephropathy, membranous-proliferative glomerulonephritis, and membranous nephropathy. This infection speeds CKD to an end-stage and has been identified as an independent predictor of death for dialysis patients. The DAAs-induced HCV eradication exerts a beneficial effect in CHC patients with CKD, even in those HIV coinfected, but some conflicting data persist on the effect of some DAA regimens on eGFR. Indeed, the favorable effect of DAA on eGFR is more evident in patients with mild or moderate CKD (stages CKD-3a/CKD-3b) than in those with a more severe illness (stages CKD-4/5).
HCV infection is associated with both mixed cryoglobulinemia and non-Hodgkin’s lymphoma, particularly B-cell NHL. MCS is currently considered as a B-cell benign lymphoproliferative disorder frequently induced by HCV infection, but it is also associated with autoimmune or lymphoproliferative disorders. HCV-induced MCS frequently shows a silent, indolent course, but in some cases, it may present a rapidly unfavorable, sometimes life-threatening outcome. Nearly 20% of HCV-related MCS patients show nephropathy at the time of first diagnosis, an index of unfavorable prognosis. DAA treatment finds full application in CHC patients with MCS, since it has a good safety profile, induces HCV eradication in nearly 95% of treated patients and is associated with remissions of cryoglobulinemic vasculitis and with a reduction extrahepatic mortality. In addition, MCS infrequently occurs in CHC patients after HCV eradication.
The role of HCV virus in the pathogenesis of lymphoproliferative diseases have been shown by several epidemiological studies and is now worldwide accepted. Available studies increasingly recognize the beneficial role of DAAs-induced HCV eradication in treating of HCV-related lymphoproliferative disorders. Antiviral therapy appears majorly important in patients with low-grade B-NHL, but some recent information support using DAAs to obtain HCV eradication also in patients affected by the more aggressive form NHL and even in HVC-positive DLBCL, in this case in combination with chemotherapy.
In conclusion, DAA-induced HCV eradication influences favorably all the extrahepatic manifestations of this infection, with the exception of lipid homeostasis, where the increase in TC and LDL cholesterol could favor, at least theoretically, the occurrence of cardiovascular events. This eventuality, however, is poorly perceived in most cases, possibly because overwhelmed by the effect of HCV eradication in abolishing numerous other harmful effects of HCV infection on cardiovascular system.
References
Abdel Alem S, Elsharkawy A, Fouad et al (2017) Improvement of glycemic state among responders to Sofosbuvir-based treatment regimens: single center experience. J Med Virol 89(12):2181–2187. https://doi.org/10.1002/jmv.24897
Adinolfi LE, Nevola R, Guerrera B et al (2018a) Hepatitis C virus clearance by direct-acting antiviral treatments and impact on insulin resistance in chronic hepatitis C patients. J Gastroenterol Hepatol 33(7):1379–1382. https://doi.org/10.1111/jgh.14067
Adinolfi LE, Rinaldi L, Nevola R (2018b) Chronic hepatitis C, atherosclerosis and cardiovascular disease: what impact of direct-acting antiviral treatments? World J Gastroenterol 24(41):4617–4621. https://doi.org/10.3748/wjg.v24.i41.4617
Adinolfi LE, Rinaldi L, Marrone A et al (2018c) The effect of sustained virological response by direct-acting antivirals on insulin resistance and diabetes mellitus in patients with chronic hepatitis C. Expert Rev Anti-Infect Ther 16(8):595–597. https://doi.org/10.1080/14787210.2018.1505500
Alessio L, Onorato L, Sangiovanni V et al (2020) DAA-based treatment for HIV-HCV-coinfected patients: analysis of factors of sustained virological response in a real-life study. Antivir Ther. https://doi.org/10.3851/IMP3353
Alisi A, Giannini C, Spaziani A et al (2007) Hepatitis C virus core protein enhances B lymphocyte proliferation. Dig Liver Dis 39(Suppl 1):S72–S75. https://doi.org/10.1016/s1590-8658(07)80015-6
Alric L, Besson C, Lapidus N et al (2016) Antiviral treatment of HCV-infected patients with B-cell non-Hodgkin lymphoma: ANRS HC-13 Lympho-C study. PLoS One 11(10):e0162965. https://doi.org/10.1371/journal.pone.0162965
Alter HJ, Seeff LB (2000) Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 20(1):17–35. https://doi.org/10.1055/s-2000-9505
Alter MJ, Margolis HS, Krawczynski K et al (1992) The natural history of community-acquired hepatitis C in the United States. The sentinel counties chronic non-A, non-B hepatitis study team. N Engl J Med 327(27):1899–1905. https://doi.org/10.1056/NEJM199212313272702
Álvarez-Ossorio MJ, Sarmento E, Castro R, Granados R et al (2018) Impact of interferon-free regimens on the glomerular filtration rate during treatment of chronic hepatitis C in a real-life cohort. J Viral Hepat 25(6):699–706. https://doi.org/10.1111/jvh.12867
Alyan O, Kacmaz F, Ozdemir O et al (2008) Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 72(12):1960–1965. https://doi.org/10.1253/circj.cj-08-0459
Amiel A, Kitay-Cohen Y, Fejgin MD et al (2000) Replication status as a marker for predisposition for lymphoma in patients with chronic hepatitis C with and without cryoglobulinemia. Exp Hematol 28(2):156–160. https://doi.org/10.1016/s0301-472x(99)00140-x
Arase Y, Suzuki F, Suzuki Y et al (2009) Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 49(3):739–744. https://doi.org/10.1002/hep.22703
Arase Y, Suzuki F, Kawamura Y et al (2011) Development rate of chronic kidney disease in hepatitis C virus patients with advanced fibrosis after interferon therapy. Hepatol Res 41(10):946–954. https://doi.org/10.1111/j.1872-034X.2011.00845.x
Arcaini L, Besson C, Frigeni M et al (2016) Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood 128(21):2527–2532. https://doi.org/10.1182/blood-2016-05-714667
Augello C, Gianelli U, Savi F et al (2014) MicroRNA as potential biomarker in HCV-associated diffuse large B-cell lymphoma. J Clin Pathol 67(8):697–701. https://doi.org/10.1136/jclinpath-2014-202352
Avilés A, Valdez L, Halabe J et al (2003) No association between lymphoma and hepatitis C virus. Med Oncol 20(2):165–168. https://doi.org/10.1385/MO:20:2:165
Bagaglio S, Uberti-Foppa C, Sagnelli C et al (2020) HIV-1 recombinant forms in immigrants regularly residing in Milan, northern Italy. https://doi.org/10.1007/s15010-020-01434-3
Becker DJ, Sevilla DW, O’Connor O (2010) Concurrent and apposed hepatocellular carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia in a patient with hepatitis C virus. Acta Haematol 123(2):77–80. https://doi.org/10.1159/000268853
Bedimo R, Abodunde O (2016) Metabolic and cardiovascular complications in HIV/HCV-co-infected patients. Curr HIV/AIDS Rep 13(6):328–339. https://doi.org/10.1007/s11904-016-0333-9
Beig J, Orr D, Harrison B, Gane E (2018) Hepatitis C virus eradication with new interferon-free treatment improves metabolic profile in hepatitis C virus-related liver transplant recipients. Liver Transpl 24(8):1031–1039. https://doi.org/10.1002/lt.25060
Bonacci M, Lens S, Londoño MC et al (2017) Virologic, clinical, and immune response outcomes of patients with hepatitis C virus-associated Cryoglobulinemia treated with direct-acting antivirals. Clin Gastroenterol Hepatol 15(4):575–583.e1. https://doi.org/10.1016/j.cgh.2016.09.158
Bose SK, Ray R (2014) Hepatitis C virus infection and insulin resistance. World J Diabetes 5(1):52–58. https://doi.org/10.4239/wjd.v5.i1.52
Brind AM, Watson JP, Burt A et al (1996) Non-Hodgkin’s lymphoma and hepatitis C virus infection. Leuk Lymphoma 21(1–2):127–130. https://doi.org/10.3109/10428199609067589
Brouet JC (1983) Les cryoglobulinémies [Cryoglobulinemias]. Presse Med 12(47):2991–2996. French
Butt AA, Yan P, Chew KW et al (2017) Risk of acute myocardial infarction among hepatitis C virus (HCV)-positive and HCV-negative men at various lipid levels: results from ERCHIVES. Clin Infect Dis 65(4):557–565. https://doi.org/10.1093/cid/cix359
Butt AA, Ren Y, Puenpatom A et al (2018) Effectiveness, treatment completion and safety of sofosbuvir/ledipasvir and paritaprevir/ritonavir/ombitasvir + dasabuvir in patients with chronic kidney disease: an ERCHIVES study. Aliment Pharmacol Ther 48(1):35–43. https://doi.org/10.1111/apt.14799
Butt AA, Yan P, Aslam S et al (2019) Hepatitis C virus treatment with directly acting agents reduces the risk of incident diabetes -results from ERCHIVES. Clin Infect Dis. https://doi.org/10.1093/cid/ciz304
Cacoub P, Saadoun D, Limal N et al (2005) Pegylated interferon alfa-2b and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum 52(3):911–915. https://doi.org/10.1002/art.20958
Cacoub P, Gragnani L, Comarmond C et al (2014) Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver dis 46(Suppl 5):S165–S173. https://doi.org/10.1016/j.dld.2014.10.005
Cacoub P, Desbois AC, Comarmond C et al (2018a) Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis. Gut 67(11):2025–2034. https://doi.org/10.1136/gutjnl-2018-316234
Cacoub P, Nahon P, Layese R et al (2018b) Prognostic value of viral eradication for major adverse cardiovascular events in hepatitis C cirrhotic patients. Am Heart J 198:4–17. https://doi.org/10.1016/j.ahj.2017.10.024
Calleja JL, Crespo J, Rincón D et al (2017) Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J Hepatol 66(6):1138–1148. https://doi.org/10.1016/j.jhep.2017.01.028
Calogero A, Sagnelli E, Creta M et al (2019) Eradication of HCV infection with the direct-acting antiviral therapy in renal allograft recipients. BioMed Res Int 4674560, 8 p. https://doi.org/10.1155/2019/4674560. Erratum to Eradication of HCV infection with the direct-acting antiviral therapy in renal allograft recipients. BioMed Res Int 2019: 8797329, 1 p, 2019. https://doi.org/10.1155/2019/8797329
Cammarota S, Citarella A, Guida A et al (2019) The inpatient hospital burden of comorbidities in HCV-infected patients: a population-based study in two Italian regions with high HCV endemicity (the BaCH study). PLoS One 14(7):e0219396. https://doi.org/10.1371/journal.pone.0219396
Carbonari M, Caprini E, Tedesco T et al (2005) Hepatitis C virus drives the unconstrained monoclonal expansion of VH1-69-expressing memory B cells in type II cryoglobulinemia: a model of infection-driven lymphomagenesis. J Immunol 174(10):6532–6539. https://doi.org/10.4049/jimmunol.174.10.6532
Carrier P, Jaccard A, Jacques J et al (2015) HCV-associated B-cell non-Hodgkin lymphomas and new direct antiviral agents. Liver Int 35(10):2222–2227. https://doi.org/10.1111/liv.12897
Carvalho JR, Velosa J, Serejo F (2018) Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication – comparison of the new direct-acting antiviral agents with the old regimens. Scand J Gastroenterol 53(7):857–863. https://doi.org/10.1080/00365521.2018.1473486
Chak E, Schulze C, Runyon BA (2015) Rapid resolution of hepatitis C virus-associated Cryoglobulin rash with use of direct-acting antivirals. Clin Gastroenterol Hepatol 13(11):e166–e167. https://doi.org/10.1016/j.cgh.2015.04.002
Charles ED, Green RM, Marukian S et al (2008) Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood 111(3):1344–1356. https://doi.org/10.1182/blood-2007-07-101717
Chaudhury CS, Sheehan J, Chairez C et al (2017) No improvement in hemoglobin A1c following hepatitis C viral clearance in patients with and without HIV. J Infect Dis 217(1):47–50. https://doi.org/10.1093/infdis/jix517
Ciancio A, Bosio R, Bo S et al (2018) Significant improvement of glycemic control in diabetic patients with HCV infection responding to direct-acting antiviral agents. J Med Virol 90(2):320–327. https://doi.org/10.1002/jmv.24954
Collier JD, Zanke B, Moore M et al (1999) No association between hepatitis C and B-cell lymphoma. Hepatology 29(4):1259–1261. https://doi.org/10.1002/hep.510290422
Comarmond C, Garrido M, Pol S, et al (2017) Direct-acting antiviral therapy restores immune tolerance to patients with hepatitis C virus-induced Cryoglobulinemia Vasculitis. Gastroenterology 152(8):2052–2062. e2. https://doi.org/10.1053/j.gastro.2017.02.037
Coppola N, Pisaturo M, Guastafierro S et al (2012) Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig Liver Dis 44(1).49–54
Coppola N, Zampino R, Bellini G et al (2014) Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clin Gastroenterol Hepatol 12(2):334–340. https://doi.org/10.1016/j.cgh.2013.05.008
Coppola N, Rosa Z, Cirillo G et al (2015) TM6SF2 E167K variant is associated with severe steatosis in chronic hepatitis C, regardless of PNPLA3 polymorphism. Liver Int 35(8):1959–1963. https://doi.org/10.1111/liv.12781
Coppola N, Portunato F, Buonomo AR et al (2019) Interferon-free regimens improve kidney function in patients with chronic hepatitis C infection. J Nephrol 32(5):763–773. https://doi.org/10.1007/s40620-019-00608-z
Corey KE, Ross AS, Wurcel et al (2006) Outcomes and treatment of acute hepatitis C virus infection in a United States population. Clin Gastroenterol Hepatol 4(10):1278–1282. https://doi.org/10.1016/j.cgh.2006.06.026
Cornella SL, Stine JG, Kelly V et al (2015) Persistence of mixed cryoglobulinemia despite cure of hepatitis C with new oral antiviral therapy including direct-acting antiviral sofosbuvir: a case series. Postgrad Med 127(4):413–417. https://doi.org/10.1080/00325481.2015.1021660
D’Ambrosio R, Pasulo L, Giorgini A et al (2020) Renal safety in 3264 HCV patients treated with DAA-based regimens: results from a large Italian real-life study. Dig Liver Dis 52(2):190–198. https://doi.org/10.1016/j.dld.2019.11.006
Dai CY, Chuang WL, Ho CK et al (2008) Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: a community-based study. J Hepatol 49(1):9–16. https://doi.org/10.1016/j.jhep.2008.03.016
Dai B, Chen AY, Corkum CP et al (2016) Hepatitis C virus upregulates B-cell receptor signaling: a novel mechanism for HCV-associated B-cell lymphoproliferative disorders. Oncogene 35(23):2979–2990. https://doi.org/10.1038/onc.2015.364
Dal Maso L, Franceschi S (2006) Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomark Prev 15(11):2078–2085. https://doi.org/10.1158/1055-9965.EPI-06-0308
Dammacco F, Sansonno D (2013) Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med 369(11):1035–1045. https://doi.org/10.1056/NEJMra1208642
Daniels D, Grytdal S, Wasley A et al (2009) Surveillance for acute viral hepatitis – United States, 2007. MMWR Surveill Summ 58(3):1–27
Dawood AA, Nooh MZ, Elgamal AA (2017) Factors associated with improved glycemic control by direct-acting antiviral agent treatment in Egyptian type 2 diabetes mellitus patients with chronic hepatitis C genotype 4. Diabetes Metab J 41(4):316–321. https://doi.org/10.4093/dmj.2017.41.4.316
De Re V, Caggiari L, Talamini R et al (2004) Hepatitis C virus-related hepatocellular carcinoma and B-cell lymphoma patients show a different profile of major histocompatibility complex class II alleles. Hum Immunol 65(11):1397–1404. https://doi.org/10.1016/j.humimm.2004.08.183
De Re V, Caggiari L, Monti G et al (2009) HLA DR-DQ combination associated with the increased risk of developing human HCV positive non-Hodgkin's lymphoma is related to the type II mixed cryoglobulinemia. Tissue Antigens 75(2):127–135. https://doi.org/10.1111/j.1399-0039.2009.01414.x
De Re V, Caggiari L, Garziera M et al (2012) Molecular signature in HCV-positive lymphomas. Clin Dev Immunol 2012:623465. https://doi.org/10.1155/2012/623465
de Sanjose S, Benavente Y, Vajdic CM et al (2008) Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the international lymphoma epidemiology consortium. Clin Gastroenterol Hepatol 6(4):451–458. https://doi.org/10.1016/j.cgh.2008.02.011
De Vita S, Sacco C, Sansonno D et al (1997) Characterization of overt B-cell lymphomas in patients with hepatitis C virus infection. Blood 90(2):776–782
Defrancesco I, Zerbi C, Rattotti S et al (2020) HCV infection and non-Hodgkin lymphomas: an evolving story. Clin Exp Med 20(3):321–328. https://doi.org/10.1007/s10238-020-00615-6
Desbois AC, Cacoub P (2017) Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J Gastroenterol 23(9):1697–1711. https://doi.org/10.3748/wjg.v23.i9.1697
Domont F, Cacoub P (2016) Chronic hepatitis C virus infection, a new cardiovascular risk factor? Liver Int 36(5):621–627. https://doi.org/10.1111/liv.13064
Douam F, Bobay LM, Maurin G et al (2015) Specialization of hepatitis C virus envelope glycoproteins for B lymphocytes in chronically infected patients. J Virol 90(2):992–1008. https://doi.org/10.1128/JVI.02516-15
Drazilova S, Gazda J, Janicko M et al (2018) Chronic hepatitis C association with diabetes mellitus and cardiovascular risk in the era of DAA therapy. Can J Gastroenterol Hepatol 2018:6150861. https://doi.org/10.1155/2018/6150861
El Sagheer G, Soliman E, Ahmad A et al (2018) Study of changes in lipid profile and insulin resistance in Egyptian patients with chronic hepatitis C genotype 4 in the era of DAAs. Libyan J Med 13(1):1435124. https://doi.org/10.1080/19932820.2018.1435124
El-Serag HB, Christie IC, Puenpatom A (2019) The effects of sustained virological response to direct-acting anti-viral therapy on the risk of extrahepatic manifestations of hepatitis C infection. Aliment Pharmacol Ther 49:1442–1447
Emery JS, Kuczynski M, La D et al (2017) Efficacy and safety of direct acting antivirals for the treatment of mixed Cryoglobulinemia. Am J Gastroenterol 112(8):1298–1308. https://doi.org/10.1038/ajg.2017.49
Esteban JI, Sauleda S, Quer J (2008) The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 48(1):148–162. https://doi.org/10.1016/j.jhep.2007.07.033
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, & European Association for the Study of the Liver (2018) EASL recommendations on treatment of hepatitis C 2018. J Hepatol 69(2):461–511. https://doi.org/10.1016/j.jhep.2018.03.026
Fabris M, Quartuccio L, Salvin S et al (2008) Fibronectin gene polymorphisms are associated with the development of B-cell lymphoma in type II mixed cryoglobulinemia. Ann Rheum Dis 67(1):80–83. https://doi.org/10.1136/ard.2006.067637
Fabrizi F, Donato FM, Messa P (2017) Hepatitis C and its metabolic complications in kidney disease. Ann Hepatol 16(6):851–861. https://doi.org/10.5604/01.3001.0010.5275
Fabrizi F, Cerutti R, Dixit V et al (2020) The impact of antiviral therapy for HCV on kidney disease: a systematic review and meta-analysis. Nefrologia 40(3):299–310. https://doi.org/10.1016/j.nefro.2019.07.007
Fabrizio C, Procopio A, Scudeller L et al (2017) HCV and diabetes: towards a ‘sustained’ glycaemic improvement after treatment with DAAs? Clin Microbiol Infect 23(5):342–343. https://doi.org/10.1016/j.cmi.2016.09.021
Fayed A, El Nokeety MM, Samy Abdelaziz T et al (2018) Incidence and characteristics of de novo renal Cryoglobulinemia after direct-acting antivirals treatment in an Egyptian hepatitis C cohort. Nephron 140(4):275–281. https://doi.org/10.1159/000493807
Feldmann G, Nischalke HD, Nattermann J et al (2006) Induction of interleukin-6 by hepatitis C virus core protein in hepatitis C-associated mixed cryoglobulinemia and B-cell non-Hodgkin's lymphoma. Clin Cancer Res 12(15):4491–4498. https://doi.org/10.1158/1078-0432.CCR-06-0154
Felmlee DJ, Hafirassou ML, Lefevre M et al (2013) Hepatitis C virus, cholesterol and lipoproteins--impact for the viral life cycle and pathogenesis of liver disease. Viruses 5(5):1292–1324. https://doi.org/10.3390/v5051292
Feng B, Eknoyan G, Guo ZS et al (2012) Effect of interferon-alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 27(2):640–646. https://doi.org/10.1093/ndt/gfr236
Ferri C, Caracciolo F, Zignego AL et al (1994) Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol 88(2):392–394. https://doi.org/10.1111/j.1365-2141.1994.tb05036.x
Fletcher NF, McKeating JA (2012) Hepatitis C virus and the brain. J Viral Hepat 19(5):301–306. https://doi.org/10.1111/j.1365-2893.2012.01591.x
Franceschi S, Polesel J, Rickenbach M et al (2006) Hepatitis C virus and non-Hodgkin’s lymphoma: findings from the Swiss HIV Cohort Study. Br J Cancer 95(11):1598–1602. https://doi.org/10.1038/sj.bjc.6603472
Frigeni M, Besson C, Visco C et al (2020) Interferon-free compared to interferon-based antiviral regimens as first-line therapy for B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Leukemia 34(5):1462–1466. https://doi.org/10.1038/s41375-019-0687-2
Frustaci A, Calabrese F, Chimenti C et al (2002) Lone hepatitis C virus myocarditis responsive to immunosuppressive therapy. Chest 122(4):1348–1356. https://doi.org/10.1378/chest.122.4.1348
Galati G, Rampa L, Vespasiani-Gentilucci U et al (2016) Hepatitis C and double-hit B cell lymphoma successfully treated by antiviral therapy. World J Hepatol 8(29):1244–1250. https://doi.org/10.4254/wjh.v8.i29.1244
Galli M, Pioltelli P, Zehender G et al (1996) HCV and lymphomagenesis. Lancet 348(9022):275. https://doi.org/10.1016/s0140-6736(05)65593-6
Gilad A, Fricker ZP, Hsieh A et al (2019) Sustained improvement in type 2 diabetes mellitus is common after treatment of hepatitis C virus with direct-acting antiviral therapy. J Clin Gastroenterol 53(8):616–620. https://doi.org/10.1097/MCG.0000000000001168
Giordanino C, Bugianesi E, Smedile A et al (2008) Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol 103(10):2481–2487. https://doi.org/10.1111/j.1572-0241.2008.02002.x
Gitto S, Cicero A, Loggi E et al (2018) Worsening of serum lipid profile after direct acting antiviral treatment. Ann Hepatol 17(1):64–75. https://doi.org/10.5604/01.3001.0010.7536
Global Hepatitis Report (2017) WHO The Polaris Observatory HCV Collaborators Lancet. Gastroenterol Hepatol 2: 161–176
González-Reimers E, Quintero-Platt G, Martín-González C et al (2016) Thrombin activation and liver inflammation in advanced hepatitis C virus infection. World J Gastroenterol 14(22(18)):4427–4437. https://doi.org/10.3748/wjg.v22.i18.4427
Goossens N, Negro F (2017) Cardiovascular manifestations of hepatitis C virus. Clin Liver Dis 21(3):465–473. https://doi.org/10.1016/j.cld.2017.03.003
Gragnani L, Fabbrizzi A, Triboli E et al (2014) Triple antiviral therapy in hepatitis C virus infection with or without mixed cryoglobulinaemia: a prospective, controlled pilot study. Dig Liver Dis 46(9):833–837. https://doi.org/10.1016/j.dld.2014.05.017
Gragnani L, Fognani E, Piluso A et al (2015) Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: a prospective, controlled, open-label, cohort study. Hepatology 61(4):1145–1153. https://doi.org/10.1002/hep.27623
Gragnani L, Cerretelli G, Lorini S et al (2018) Interferon-free therapy in hepatitis C virus mixed cryoglobulinaemia: a prospective, controlled, clinical and quality of life analysis. Aliment Pharmacol Ther 48(4):440–450
Hanley J, Jarvis L, Simmonds P et al (1996) HCV and non-Hodgkin lymphoma. Lancet 347(9011):1339. https://doi.org/10.1016/s0140-6736(96)90991-5
Hashimoto S, Yatsuhashi H, Abiru et al (2016) Rapid increase in serum low-density lipoprotein cholesterol concentration during hepatitis C interferon-free treatment. PLoS One 11(9):e0163644. https://doi.org/10.1371/journal.pone.0163644
Hassan AM, Osman HA, Mahmoud HS et al (2018) Sofosbuvir-daclatasvir improves hepatitis C virus-induced mixed cryoglobulinemia: Upper Egypt experience. Infect Drug Resist 11:895–901. https://doi.org/10.2147/IDR.S167093. Erratum in: Infect Drug Resist. 2019 May 29;12:1469
Hausfater P, Cacoub P, Sterkers Y et al (2001) Hepatitis C virus infection and lymphoproliferative diseases: prospective study on 1,576 patients in France. Am J Hematol 67(3):168–171. https://doi.org/10.1002/ajh.1101
Henson JB, Sise ME (2019) The association of hepatitis C infection with the onset of CKD and progression into ESRD. Semin Dial 32(2):108–118. https://doi.org/10.1111/sdi.12759
Hosry J, Mahale P, Turturro F et al (2016) Antiviral therapy improves overall survival in hepatitis C virus-infected patients who develop diffuse large B-cell lymphoma. Int J Cancer 139(11):2519–2528. https://doi.org/10.1002/ijc.30372
Hsu YH, Muo CH, Liu CY et al (2015a) Hepatitis C virus infection increases the risk of developing peripheral arterial disease: a 9-year population-based cohort study. J Hepatol 62(3):519–525. https://doi.org/10.1016/j.jhep.2014.09.022
Hsu YC, Ho HJ, Huang YT et al (2015b) Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut 64(3):495–503. https://doi.org/10.1136/gutjnl-2014-308163
Huang JF, Huang CF, Yeh ML et al (2017) The outcomes of glucose abnormalities in chronic hepatitis C patients receiving interferon-free direct antiviral agents. Kaohsiung J Med Sci 33:567–571
Hui JM, Sud A, Farrell et al (2003) Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology 125(6):1695–1704. https://doi.org/10.1053/j.gastro.2003.08.032
Hum J, Jou JH, Green et al (2017) Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care 40(9):1173–1180. https://doi.org/10.2337/dc17-0485
Humphries K, Darling JM, Barritt AS 4th (2014) Membranoproliferative glomerulonephritis, type II cryoglobulinemia and triple therapy for hepatitis C: a case series and review of the literature. Dig Dis Sci 59(8):2007–2012. https://doi.org/10.1007/s10620-014-3085-7
Ichikawa T, Miyaaki H, Miuma S et al (2019) Carotid intima-media thickness and small dense low-density lipoprotein cholesterol increase after one year of treatment with direct-acting antivirals in patients with hepatitis C virus infection. Intern Med 58(9):1209–1215. https://doi.org/10.2169/internalmedicine.1514-18
Ikeda A, Ikeda K, Takai et al (2017) Hepatitis C treatment with Sofosbuvir and Ledipasvir accompanied by immediate improvement in hemoglobin A1c. Digestion 96(4):228–230. https://doi.org/10.1159/000484237
Ishizaka Y, Ishizaka N, Takahashi E et al (2003) Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J 67(1):26–30. https://doi.org/10.1253/circj.67.26
Iwane K, Kayahara T, Takabatake H et al (2019) Recurrence of malignant lymphoma immediately after treatment for hepatitis C virus using direct-acting antivirals. Nihon Shokakibyo Gakkai Zasshi 116(2):177–183. Japanese. https://doi.org/10.11405/nisshoshi.116.177
Kanwal F, White DL, Tavakoli-Tabasi S et al (2014) Many patients with interleukin 28B genotypes associated with response to therapy are ineligible for treatment because of comorbidities. Clin Gastroenterol Hepatol 12(2): 327–333.e1. https://doi.org/10.1016/j.cgh.2013.08.034
Karavattathayyil SJ, Kalkeri G, Liu HJ et al (2000) Detection of hepatitis C virus RNA sequences in B-cell non-Hodgkin lymphoma. Am J Clin Pathol 113(3):391–398. https://doi.org/10.1309/REV9-FDTM-5NGC-HBWY
Kasama Y, Sekiguchi S, Saito M et al (2011) Persistent expression of the full genome of hepatitis C virus in B cells induces spontaneous development of B-cell lymphomas in vivo. Blood 116(23):4926–4933. https://doi.org/10.1182/blood-2010-05-283358
Katsi V, Felekos I, Skevofilax S et al (2015) Cardiovascular disease and hepatitis C virus infection: an irrelevant statement or a hot relationship? Cardiol Rev 23(1):11–17. https://doi.org/10.1097/CRD.0000000000000031
Kawagishi N, Suda G, Nakamura A et al (2018) Liver steatosis and dyslipidemia after HCV eradication by direct acting antiviral agents are synergistic risks of atherosclerosis. PLoS One 13(12):e0209615. https://doi.org/10.1371/journal.pone.0209615
Koga T, Kawashiri SY, Nakao K et al (2017) Successful ledipasvir + sofosbuvir treatment of active synovitis in a rheumatoid arthritis patient with hepatitis C virus-related mixed cryoglobulinemia. Mod Rheumatol 27(5):917–918. https://doi.org/10.1080/14397595.2016.1253814
Kuller LH, Tracy R, Belloso W et al (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5(10):e203. https://doi.org/10.1371/journal.pmed.0050203
Landau DA, Saadoun D, Calabrese LH et al (2007) The pathophysiology of HCV induced B-cell clonal disorders. Autoimmun Rev 6(8):581–587. https://doi.org/10.1016/j.autrev.2007.03.010
Lanini S, Bartolini B, Taibi C et al (2019) Early improvement of glycaemic control after virus clearance in patients with chronic hepatitis C and severe liver fibrosis: a cohort study. New Microbiol 42(3):139–144
Latt N, Alachkar N, Gurakar A (2012) Hepatitis C virus and its renal manifestations: a review and update. Gastroenterol Hepatol 8(7):434–445
Lauletta G, Russi S, Pavone F et al (2017) Direct-acting antiviral agents in the therapy of hepatitis C virus-related mixed cryoglobulinaemia: a single-centre experience. Arthritis Res Ther 19(1):74. https://doi.org/10.1186/s13075-017-1280-6
Lee MH, Yang HI, Lu SN et al (2012) Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis 206(4):469–477. https://doi.org/10.1093/infdis/jis385
Li T, Qu Y, Guo Y et al (2017) Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver Int 37(7):974–981. https://doi.org/10.1111/liv.13336
Li J, Gordon SC, Rupp LB et al (2019) Sustained virological response does not improve long-term glycaemic control in patients with type 2 diabetes and chronic hepatitis C. Liver Int 39(6):1027–1032. https://doi.org/10.1111/liv.14031
Lim LY, La D, Cserti-Gazdewich CM et al (2015) Lymphoma remission by interferon-free HCV eradication without chemotherapy. ACG Case Rep J 3(1):69–70. https://doi.org/10.14309/crj.2015.104
Lonardo A, Ballestri S, Guaraldi G et al (2016) Fatty liver is associated with an increased risk of diabetes and cardiovascular disease – evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol 22(44):9674–9693. https://doi.org/10.3748/wjg.v22.i44.9674
Loria P, Marchesini G, Nascimbeni F et al (2014) Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis 232(1):99–109. https://doi.org/10.1016/j.atherosclerosis.2013.10.030
Luppi M, Grazia Ferrari M, Bonaccorsi G et al (1996) Hepatitis C virus infection in subsets of neoplastic lymphoproliferations not associated with cryoglobulinemia. Leukemia 10(2):351–355
Machida K, Cheng KT, Lai CK et al (2006a) Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol 80(14):7199–7207. https://doi.org/10.1128/JVI.00321-06
Machida K, Cheng KT, Sung VM et al (2006b) Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol 80(2):866–874. https://doi.org/10.1128/JVI.80.2.866-874.2006
Maggi G, Bottelli R, Gola D et al (1996) Serum cholesterol and chronic hepatitis C. Ital J Gastroenterol 28(8):436–440
Mahale P, Engels EA, Li R et al (2018) The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut 67(3):553–561. https://doi.org/10.1136/gutjnl-2017-313983
Makara M, Sulyok M, Csacsovszki O et al (2015) Successful treatment of HCV-associated cryoglobulinemia with ombitasvir/paritaprevir/ritonavir, dasabuvir and ribavirin: a case report. J Clin Virol 72:66–68. https://doi.org/10.1016/j.jcv.2015.09.003
Maruyama S, Koda M, Oyake N et al (2013) Myocardial injury in patients with chronic hepatitis C infection. J Hepatol 58(1):11–15. https://doi.org/10.1016/j.jhep.2012.07.045
Marzouk D, Sass J, Bakr I et al (2007) Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut 56(8):1105–1110. https://doi.org/10.1136/gut.2006.091983
Masarone M, Persico M (2019) Hepatitis C virus infection and non-hepatocellular malignancies in the DAA era: a systematic review and meta-analysis. Liver Int 39(7):1292–1306. https://doi.org/10.1111/liv.14119
Matsumori A (2006) Role of hepatitis C virus in cardiomyopathies. Ernst Schering Res Found Workshop 55:99–120. https://doi.org/10.1007/3-540-30822-9_7
Matsumori A, Matoba Y, Nishio R et al (1996) Detection of hepatitis C virus RNA from the heart of patients with hypertrophic cardiomyopathy. Biochem Biophys Res Commun 222(3):678–682. https://doi.org/10.1006/bbrc.1996.0803
Matsumori A, Ohashi N, Hasegawa K et al (1998) Hepatitis C virus infection and heart diseases: a multicenter study in Japan. Jpn Circ J 62(5):389–391. https://doi.org/10.1253/jcj.62.389
Matsuo K, Kusano A, Sugumar A et al (2004) Effect of hepatitis C virus infection on the risk of non-Hodgkin's lymphoma: a meta-analysis of epidemiological studies. Cancer Sci 95(9):745–752. https://doi.org/10.1111/j.1349-7006.2004.tb03256.x
Mauss S, Berger F, Wehmeyer MH et al (2017) Effect of antiviral therapy for HCV on lipid levels. Antivir Ther 21(1):81–88. https://doi.org/10.3851/IMP3094
Mazzaro C, Franzin F, Tulissi P et al (1996) Regression of monoclonal B-cell expansion in patients affected by mixed cryoglobulinemia responsive to alpha-interferon therapy. Cancer 77(12):2604–2613
Mehta SH, Brancati FL, Sulkowski MS et al (2000) Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med 133(8):592–599. https://doi.org/10.7326/0003-4819-133-8-200010170-00009
Mehta DA, Cohen E, Charafeddine M et al (2017) Effect of hepatitis C treatment with Ombitasvir/Paritaprevir/R + Dasabuvir on renal, cardiovascular and metabolic Extrahepatic manifestations: a post-hoc analysis of phase 3 clinical trials. Infect Dis Ther 6(4):515–529. https://doi.org/10.1007/s40121-017-0171-0
Meissner EG, Lee YJ, Osinusi A et al (2015) Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology 61(3):790–801. https://doi.org/10.1002/hep.27424
Mele A, Pulsoni A, Bianco E et al (2003) Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood 102(3):996–999. https://doi.org/10.1182/blood-2002-10-3230
Merli M, Frigeni M, Alric L et al (2019) Direct-acting antivirals in hepatitis C virus-associated diffuse large B-cell lymphomas. Oncologist 24(8):e720–e729. https://doi.org/10.1634/theoncologist.2018-0331
Merli M, Defrancesco I, Visco C et al (2020) Direct-acting antivirals in relapsed or refractory hepatitis C virus-associated diffuse large B-cell lymphoma. Leuk Lymphoma 61(9):2122–2128. https://doi.org/10.1080/10428194.2020.1755859
Merz A, Long G, Hiet MS et al (2011) Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem 286(4):3018–3032. https://doi.org/10.1074/jbc.M110.175018
Miailhes P, Hartig-Lavie K, Virlogeux V, et al (2018) Benefit of direct-acting antiviral therapy for hepatitis C virus (HCV) in monoinfected and HIV-HCV-coinfected patients with mixed cryoglobulinaemia. Clin Microbiol Infect 24(11):1215.e1–1215.e4. https://doi.org/10.1016/j.cmi.2018.05.019
Minopetrou M, Hadziyannis E, Deutsch M et al (2013) Hepatitis C virus (HCV)-related cryoglobulinemia: cryoglobulin type and anti-HCV profile. Clin Vaccine Immunol 20(5):698–703. https://doi.org/10.1128/CVI.00720-12
Mohanty A, Salameh S, Butt AA (2019) Impact of direct acting antiviral agent therapy upon Extrahepatic manifestations of hepatitis C virus infection. Curr HIV/AIDS Rep 16(5):389–394. https://doi.org/10.1007/s11904-019-00466-1
Montella M, Crispo A, de Bellis G et al (2001) HCV and cancer: a case-control study in a high-endemic area. Liver 21(5):335–341. https://doi.org/10.1034/j.1600-0676.2001.210506.x
Montero N, Favà A, Rodriguez E et al (2018) Treatment for hepatitis C virus-associated mixed cryoglobulinaemia. Cochrane Database Syst Rev 5(5):CD011403. https://doi.org/10.1002/14651858.CD011403.pub2
Morales AL, Junga Z, Singla MB et al (2016) Hepatitis C eradication with sofosbuvir leads to significant metabolic changes. World J Hepatol 8(35):1557–1563. https://doi.org/10.4254/wjh.v8.i35.1557
Moucari R, Asselah T, Cazals-Hatem D et al (2008) Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 134(2):416–423. https://doi.org/10.1053/j.gastro.2007.11.010
Nahon P, Bourcier V, Layese R et al (2017) Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 152(1):142–156.e2. https://doi.org/10.1053/j.gastro.2016.09.009
Negro F (2014) Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol 61(1 Suppl):S69–S78. https://doi.org/10.1016/j.jhep.2014.08.003
Negro F, Forton D, Craxì A et al (2015) Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 149(6):1345–1360. https://doi.org/10.1053/j.gastro.2015.08.035
Nielsen SU, Bassendine MF, Burt AD et al (2006) Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol 80(5):2418–2428. https://doi.org/10.1128/JVI.80.5.2418-2428.2006
Novo G, Macaione F, Giannitrapani L et al (2018) Subclinical cardiovascular damage in patients with HCV cirrhosis before and after treatment with direct antiviral agents: a prospective study. Aliment Pharmacol Ther 48(7):740–749. https://doi.org/10.1111/apt.14934
Obata F, Murakami T, Miyagi J et al (2017) A case of rapid amelioration of hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis treated by interferon-free directly acting antivirals for HCV in the absence of immunosuppressant. CEN Case Rep 6(1):55–60. https://doi.org/10.1007/s13730-016-0244-z
Occhipinti V, Farina L, Viganò M et al (2018) Concomitant therapy with direct-acting antivirals and chemoimmunotherapy in HCV-associated diffuse large B-cell lymphoma. Dig Liver Dis 51(5):719–723. https://doi.org/10.1016/j.dld.2018.10.019
Ohsawa M, Tomita Y, Hashimoto M et al (1998) Hepatitis C viral genome in a subset of primary hepatic lymphomas. Mod Pathol 11(5):471–478
Okabe M, Fukuda K, Arakawa K et al (1997) Chronic variant of myocarditis associated with hepatitis C virus infection. Circulation 96(1):22–24. https://doi.org/10.1161/01.cir.96.1.22
Okay M, Aslan T, Özdemir E et al (2020) Splenic marginal zone lymphoma in Turkey: association with hepatitis B instead of hepatitis C virus as an etiologic and possible prognostic factor - A multicenter cohort study. Turk J Haematol 6(37(2)):84–90. https://doi.org/10.4274/tjh.galenos.2019.2019.0177
Oliveira CP, Kappel CR, Siqueira ER et al (2013) Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol 164(2):221–226. https://doi.org/10.1016/j.ijcard.2011.07.016
Osibogun O, Ogunmoroti O, Michos ED et al (2017) HIV/HCV coinfection and the risk of cardiovascular disease: a meta-analysis. J Viral Hepat 24(11):998–1004. https://doi.org/10.1111/jvh.12725
Pavone P, Tieghi T, d’Ettorre et al (2016) Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect 22(5):462.e1–462.e4623. https://doi.org/10.1016/j.cmi.2015.12.030
Pellicelli A, Giannelli V, Zoli V et al (2018) Antiviral therapy in hepatitis C-infected patients prevents relapse of diffuse large B cell lymphoma. Clin Exp Hepatol 4(3):197–200. https://doi.org/10.5114/ceh.2018.78124
Pepe A, Meloni A, Borsellino Z et al (2015) Myocardial fibrosis by late gadolinium enhancement cardiac magnetic resonance and hepatitis C virus infection in thalassemia major patients. J Cardiovasc Med 16(10):689–695. https://doi.org/10.2459/JCM.0000000000000278
Perazzo H, Castro R, Luz PM et al (2020) Effectiveness of generic direct-acting agents for the treatment of hepatitis C: systematic review and meta-analysis. Bull World Health Organ 98(3):188–197K. https://doi.org/10.2471/BLT.19.231522
Perticone M, Maio R, Tassone EJ et al (2015) Insulin-resistance HCV infection-related affects vascular stiffness in normotensives. Atherosclerosis 238(1):108–112. https://doi.org/10.1016/j.atherosclerosis.2014.11.025
Petta S, Torres D, Fazio G et al (2012) Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology 55(5):1317–1323. https://doi.org/10.1002/hep.25508
Petta S, Macaluso FS, Craxì A (2014) Cardiovascular diseases and HCV infection: a simple association or more? Gut 63(3):369–375. https://doi.org/10.1136/gutjnl-2013-30610
Petta S, Adinolfi LE, Fracanzani AL et al (2018) Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J Hepatol 69(1):18–24. https://doi.org/10.1016/j.jhep.2018.02.015
Peveling-Oberhag J, Crisman G, Schmidt A et al (2012) Dysregulation of global microRNA expression in splenic marginal zone lymphoma and influence of chronic hepatitis C virus infection. Leukemia 26(7):1654–1662. https://doi.org/10.1038/leu.2012.29
Peveling-Oberhag J, Arcaini L, Hansmann ML et al (2013) Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol 59(1):169–177. https://doi.org/10.1016/j.jhep.2013.03.018
Peveling-Oberhag J, Arcaini L, Bankov K et al (2016) The anti-lymphoma activity of antiviral therapy in HCV-associated B-cell non-Hodgkin lymphomas: a meta-analysis. J Viral Hepat 23(7):536–544. https://doi.org/10.1111/jvh.12518
Pioltelli P, Zehender G, Monti G et al (1996) HCV and non-Hodgkin lymphoma. Lancet 347(9001):624–625. https://doi.org/10.1016/s0140-6736(96)91328-8
Poynard T, Bedossa P, Opolon P (1997) Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet (London, England) 349(9055):825–832. https://doi.org/10.1016/s0140-6736(96)07642-8
Poynard T, Ratziu V, Benmanov Y et al (2000) Fibrosis in patients with chronic hepatitis C: detection and significance. Semin Liver Dis 20(1):47–55. https://doi.org/10.1055/s-2000-9258
Pozzato G, Mazzaro C, Artemova M et al (2020) Direct-acting antiviral agents for hepatitis C virus-mixed cryoglobulinaemia: dissociated virological and haematological responses. Br J Haematol. https://doi.org/10.1111/bjh.17036
Quinn ER, Chan CH, Hadlock KG et al (2001) The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood 98(13):3745–3749. https://doi.org/10.1182/blood.v98.13.3745
Rattotti S, Ferretti VV, Rusconi C et al (2019) Lymphomas associated with chronic hepatitis C virus infection: a prospective multicenter cohort study from the rete Ematologica Lombarda (REL) clinical network. Hematol Oncol 37(2):160–167. https://doi.org/10.1002/hon.2575
Revuelto Artigas T, Betriu Bars À, Zaragoza Velasco N et al (2019a) Antiviral treatment does not improve subclinical atheromatosis in patients with chronic hepatitis caused by hepatitis C virus. El tratamiento antiviral no mejora la ateromatosis subclínica en pacientes con hepatitis crónica por virus de la hepatitis C. Gastroenterol Hepatol 42(6):362–371. https://doi.org/10.1016/j.gastrohep.2019.02.002
Revuelto Artigas T, Zaragoza Velasco N, Gómez Arbones X et al (2019b) Chronic hepatitis C infection: An independent risk factor for subclinical atheromatosis. Infección crónica por el virus de la hepatitis C: un factor de riesgo independiente para la ateromatosis subclínica. Rev Clin Esp 219(6):293–302. https://doi.org/10.1016/j.rce.2018.12.007
Roccatello D, Saadoun D, Ramos-Casals M et al (2018) Cryoglobulinaemia. Nat Rev Dis Primers 2(4(1)):11. https://doi.org/10.1038/s41572-018-0009-4
Rodríguez de Santiago E, Velázquez Kennedy K, García González M et al (2018) HCV-positive lymphoma after sustained virological response with direct-acting antiviral agents: the game is not over after HCV eradication. J Viral Hepat 25(5):614–615. https://doi.org/10.1111/jvh.12843
Romano C, Cuomo G, Ferrara R et al (2018) Uncommon immune-mediated extrahepatic manifestations of HCV infection. Expert Rev Clin Immunol 14(12):1089–1099. https://doi.org/10.1080/1744666X.2018.1538790
Rossotti R, Travi G, Pazzi A et al (2015) Rapid clearance of HCV-related splenic marginal zone lymphoma under an interferon-free, NS3/NS4A inhibitor-based treatment. A case report. J Hepatol 62(1):234–237. https://doi.org/10.1016/j.jhep.2014.09.031
Saadoun D, Resche Rigon M, Thibault V et al (2014) Peg-IFNα/ribavirin/protease inhibitor combination in hepatitis C virus associated mixed cryoglobulinemia vasculitis: results at week 24. Ann Rheum Dis 73(5):831–837. https://doi.org/10.1136/annrheumdis-2012-202770
Saadoun D, Resche Rigon M, Pol S et al (2015) PegIFNα/ribavirin/protease inhibitor combination in severe hepatitis C virus-associated mixed cryoglobulinemia vasculitis. J Hepatol 62(1):24–30. https://doi.org/10.1016/j.jhep.2014.08.015
Saadoun D, Pol S, Ferfar Y, et al (2017) Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology 153(1):49–52.e5. https://doi.org/10.1053/j.gastro.2017.03.006
Sagnelli E, Pisaturo M, Stanzione M et al (2013) Clinical presentation, outcome, and response to therapy among patients with acute exacerbation of chronic hepatitis C. Clin Gastroenterol Hepatol 11(9):1174–1180.e11. https://doi.org/10.1016/j.cgh.2013.03.025
Sagnelli E, Macera M, Russo A et al (2019) Epidemiological and etiological variations in hepatocellular carcinoma. Infection. https://doi.org/10.1007/s15010-019-01345-y
Sagnelli C, Uberti-Foppa C, Cella E et al (2020) Molecular epidemiology of HIV-1 infection in immigrant population in norther Italy. Epidemiol Infect 148:e19. https://doi.org/10.1017/S0950268819002012
Sanchez MJ, Bergasa NV (2008) Hepatitis C associated cardiomyopathy: potential pathogenic mechanisms and clinical implications. Med Sci Monit 14(5):RA55–RA63
Santantonio T, Medda E, Ferrari C et al (2006) Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis 43(9):1154–1159. https://doi.org/10.1086/507640
Sarhan MA, Pham TN, Chen AY et al (2018) Hepatitis C virus infection of human T lymphocytes is mediated by CD5. J Virol 86(7):3723–3735. https://doi.org/10.1128/JVI.06956-11
Schiavinato A, Zanetto A, Pantano G et al (2017) Polyclonal and monoclonal B lymphocytes response in HCV-infected patients treated with direct-acting antiviral agents. J Viral Hepat 24(12):1168–1176. https://doi.org/10.1111/jvh.12746
Shapira MY, Muszkat M, Braunstein I et al (2001) Co-occurrence of hepatocellular carcinoma and lymphoma in patients with hepatitis C virus cirrhosis. J Clin Gastroenterol 32(4):368–369. https://doi.org/10.1097/00004836-200104000-00023
Shimono J, Miyoshi H, Kato T et al (2017) Hepatitis C virus infection is an independent prognostic factor in follicular lymphoma. Oncotarget 9(2):1717–1725. https://doi.org/10.18632/oncotarget.23138
Shin HP, Park JA, Burman B et al (2017) Efficacy and safety of sofosbuvir-based regimens for treatment in chronic hepatitis C genotype 1 patients with moderately impaired renal function. Clin Mol Hepatol 23(4):316–322. https://doi.org/10.3350/cmh.2016.0087
Sigon G, D’Ambrosio R, Clerici M et al (2019) Procoagulant imbalance influences cardiovascular and liver damage in chronic hepatitis C independently of steatosis. Liver Int. https://doi.org/10.1111/liv.14213
Simó R, Lecube A, Genescà J et al (2006) Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care 29(11):2462–2466. https://doi.org/10.2337/dc06-0456
Sise ME, Bloom AK, Wisocky J et al (2016) Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 63(2):408–417. https://doi.org/10.1002/hep.28297
Sise ME, Chute DF, Oppong Y et al (2020) Direct-acting antiviral therapy slows kidney function decline in patients with hepatitis C virus infection and chronic kidney disease. Kidney Int 97(1):193–201. https://doi.org/10.1016/j.kint.2019.04.030
Soeiro C, Gonçalves C, Marques et al (2018) Glomerular filtration rate change during chronic hepatitis C treatment with Sofosbuvir/Ledipasvir in HCV/HIV Coinfected patients treated with Tenofovir and a boosted protease inhibitor: an observational prospective study. BMC Infect Dis 18(1):364. https://doi.org/10.1186/s12879-018-3278-3
Soriano V, Barreiro P, de Mendoza C (2016) Hypoglycemia in a diabetic patient during hepatitis C therapy. Hepatology 63(6):2065–2066. https://doi.org/10.1002/hep.28137
Stine JG, Wynter JA, Niccum B et al (2017) Effect of treatment with direct acting antiviral on glycemic control in patients with diabetes mellitus and chronic hepatitis C. Ann Hepatol 16(2):215–220. https://doi.org/10.5604/16652681.1231581
Stroffolini T, Sagnelli E, Sagnelli C et al (2018) Geographical pattern of chronic liver diseases in Italy: results from two pooled national surveys Eur J Intern Med. pii: S0953–6205(18)30408–4. https://doi.org/10.1016/j.ejim.2018.10.015
Su TH, Liu CJ, Tseng TC et al (2019) Early antiviral therapy reduces the risk of lymphoma in patients with chronic hepatitis C infection. Aliment Pharmacol Ther 49(3):331–339. https://doi.org/10.1111/apt.15101
Suarez F, Lortholary O, Hermine O et al (2006) Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood 107(8):3034–3044. https://doi.org/10.1182/blood-2005-09-3679
Sultanik P, Klotz C, Brault P et al (2015) Regression of an HCV-associated disseminated marginal zone lymphoma under IFN-free antiviral treatment. Blood 125(15):2446–2447. https://doi.org/10.1182/blood-2014-12-618652
Tada S, Saito H, Ebinuma H et al (2009) Treatment of hepatitis C virus with peg-interferon and ribavirin combination therapy significantly affects lipid metabolism. Hepatol Res 39(2):195–199. https://doi.org/10.1111/j.1872-034X.2008.00439.x
Taramasso L, Di Biagio A, Bovis F et al (2018) Trend of estimated glomerular filtration rate during ombistasvir/paritaprevir/ritonavir plus dasabuvir ± ribavirin in HIV/HCV co-infected patients. PLoS One 13(2):e0192627. https://doi.org/10.1371/journal.pone.0192627
Targher G, Bertolini L, Padovani R et al (2007) Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol 46(6):1126–1132. https://doi.org/10.1016/j.jhep.2007.01.021
Teegen EM, Dürr M, Maurer MM et al (2019) Evaluation of histological dynamics, kidney function and diabetes in liver transplant patients after antiviral treatment with direct-acting antivirals: therapy of HCV-recurrence. Transpl Infect Dis 21
Terrier B, Karras A, Cluzel P et al (2013) Presentation and prognosis of cardiac involvement in hepatitis C virus-related vasculitis. Am J Cardiol 111(2):265–272. https://doi.org/10.1016/j.amjcard.2012.09.028
Tomiyama H, Arai T, Hirose K et al (2003) Hepatitis C virus seropositivity, but not hepatitis B virus carrier or seropositivity, associated with increased pulse wave velocity. Atherosclerosis 166(2):401–403. https://doi.org/10.1016/s0021-9150(02)00388-x
Tran TT, Mehta D, Goldstein A et al (2017) Potential effect of hepatitis C treatment on renal, cardiovascular and metabolic extrahepatic manifestations: results from clinical trials of ombitasvir/paritaprevir/ritonavir and dasabuvir ± ribavirin. J Hepatol 66(1):S302. https://doi.org/10.1016/s0168-8278(17)30921-2
Tran TT, Mehta D, Mensa F et al (2018) Pan-genotypic hepatitis C treatment with Glecaprevir and Pibrentasvir for 8 weeks resulted in improved cardiovascular and metabolic outcomes and stable renal function: a post-hoc analysis of phase 3 clinical trials. Infect Dis Ther 7(4):473–484. https://doi.org/10.1007/s40121-018-0218-x
Tsai MS, Hsu YC, Yu PC et al (2015) Long-term risk of acute coronary syndrome in hepatitis C virus infected patients without antiviral treatment: a cohort study from an endemic area. Int J Cardiol 181:27–29. https://doi.org/10.1016/j.ijcard.2014.11.200
Tsui JI, Whooley MA, Monto A et al (2009) Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the heart and soul study. J Card Fail 15(5):451–456. https://doi.org/10.1016/j.cardfail.2008.12.003
Urbaczek AC, Ribeiro LCDEA, Ximenes VF et al (2014) Inflammatory response of endothelial cells to hepatitis C virus recombinant envelope glycoprotein 2 protein exposure. Mem Inst Oswaldo Cruz 109(6):748–756. https://doi.org/10.1590/0074-0276140090
Utsunomiya T, Okamoto M, Tsujita E et al (2009) Hepatocellular carcinoma infiltrated with non-Hodgkin’s lymphoma: report of a case. Surg Today 39(11):1010–1012. https://doi.org/10.1007/s00595-009-3966-0
Vanni E, Bugianesi E, Saracco G (2016) Treatment of type 2 diabetes mellitus by viral eradication in chronic hepatitis C: myth or reality? Dig Liver Dis 48(2):105–111. https://doi.org/10.1016/j.dld.2015.10.016
Vassalle C, Masini S, Bianchi F et al (2004) Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 90(5):565–566. https://doi.org/10.1136/hrt.2003.018937
Vassalle C, Petta S, Pepe A et al (2018) Expert opinion on managing chronic HCV in patients with cardiovascular disease. Antivir Ther 23(Suppl 2):35–46. https://doi.org/10.3851/IMP3248
Visco C, Finotto S (2014) Hepatitis C virus and diffuse large B-cell lymphoma: pathogenesis, behavior and treatment. World J Gastroenterol 20(32):11054–11061. https://doi.org/10.3748/wjg.v20.i32.11054
Visco C, Wang J, Tisi MC et al (2017) Hepatitis C virus positive diffuse large B-cell lymphomas have distinct molecular features and lack BCL2 translocations. Br J Cancer 117(11):1685–1688. https://doi.org/10.1038/bjc.2017.345
Wang CS, Wang ST, Yao WJ et al (2007) Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol 166(2):196–203. https://doi.org/10.1093/aje/kwm061
Waris G, Felmlee DJ, Negro F et al (2007) Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J Virol 81(15):8122–8130. https://doi.org/10.1128/JVI.00125-07
Webster DP, Klenerman P, Dusheiko GM (2015) Hepatitis C. Lancet 385(9973):1124–1135. https://doi.org/10.1016/S0140-6736(14)62401-6
Weidner P, Boettche D, Zimmerer T et al (2018) Impact of direct acting antiviral (DAA) treatment on glucose metabolism and reduction of pre-diabetes in patients with chronic hepatitis C. J Gastrointestin Liver Dis 27(3):281–289. https://doi.org/10.15403/jgld.2014.1121.273.daa
Wong AH, Sie J, Chen A et al (2020) Glycemic control after initiating direct-acting antiviral agents in patients with hepatitis C virus and type 2 diabetes mellitus using the United States integrated healthcare system. J Res Pharm Pract 9(1):16–23. https://doi.org/10.4103/jrpp.JRPP_19_110
Younossi ZM, Stepanova M, Nader F et al (2013) Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther 37(6):647–652. https://doi.org/10.1111/apt.12234
Younossi ZM, Elsheikh E, Stepanova M et al (2015) Ledipasvir/sofosbuvir treatment of hepatitis C virus is associated with reduction in serum apolipoprotein levels. J Viral Hepat 22(12):977–982. https://doi.org/10.1111/jvh.12448
Younossi ZM, Stepanova M, Estep M et al (2016) Dysregulation of distal cholesterol biosynthesis in association with relapse and advanced disease in CHC genotype 2 and 3 treated with sofosbuvir and ribavirin. J Hepatol 64(1):29–36. https://doi.org/10.1016/j.jhep.2015.08.027
Zhao LJ, Zhang XL, Zhao P et al (2006) Up-regulation of ERK and p38 MAPK signaling pathways by hepatitis C virus E2 envelope protein in human T lymphoma cell line. J Leukoc Biol 80(2):424–432. https://doi.org/10.1189/jlb.0106014
Zhu X, Jing L, Li X (2019) Hepatitis C virus infection is a risk factor for non-Hodgkin lymphoma: a MOOSE-compliant meta-analysis. Medicine (Baltimore) 98(11):e14755. https://doi.org/10.1097/MD.0000000000014755
Zignego AL, Ferri C, Giannini C et al (1997) Hepatitis C virus infection in mixed cryoglobulinemia and B-cell non-Hodgkin’s lymphoma: evidence for a pathogenetic role. Arch Virol 142(3):545–555. https://doi.org/10.1007/s007050050100
Zignego AL, Giannelli F, Marrocchi ME et al (2000) T(14;18) translocation in chronic hepatitis C virus infection. Hepatology 31(2):474–479. https://doi.org/10.1002/hep.510310230
Zignego AL, Giannini C, Monti M et al (2007) Hepatitis C virus lymphotropism: lessons from a decade of studies. Dig Liver Dis 39(Suppl 1):S38–S45. https://doi.org/10.1016/s1590-8658(07)80009-0
Conflict-of-Interest Statement
All the authors of the manuscript declare they have no conflict of interest in connection with this paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sagnelli, E. et al. (2020). Impact of DAA-Based Regimens on HCV-Related Extra-Hepatic Damage: A Narrative Review. In: Donelli, G. (eds) Advances in Microbiology, Infectious Diseases and Public Health. Advances in Experimental Medicine and Biology(), vol 1323. Springer, Cham. https://doi.org/10.1007/5584_2020_604
Download citation
DOI: https://doi.org/10.1007/5584_2020_604
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71201-3
Online ISBN: 978-3-030-71202-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)