Abstract
Stem cell-based therapy stands as a robust experimental treatment for ischemic stroke. Stem cells derived from fetal, embryonic, and adult tissues serve as potential sources for transplantable cells in the setting of ischemic stroke. However, the search continues for finding an optimal cell line for clinical use. Muse cells, a distinct subset of mesenchymal stem cells found sporadically in the connective tissue of nearly every organ, may be a suitable candidate due to its safety and accessibility. These cells have been investigated for therapeutic usage in chronic kidney disease, liver disease, acute myocardial infarction, and stroke. Muse cells display the ability to engraft and differentiate into the host neural network unlike many other cell lines which only display bystander immunomodulating effects. Taking advantage of this unique engraftment and differentiation mechanism behind Muse cells’ therapeutic effects on the central nervous system, as well as other organ systems, will undoubtedly advance the cells’ utility for cell-based regenerative medicine in stroke.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stroke is currently the fifth leading cause of death in the United States and can cause disabling neurological deficits including cognitive impairment, hemiparesis, sensory disturbance, and aphasia (Ovbiagele et al. 2013). Ischemic stroke comprises 87% of all stroke cases and is characterized by inadequate perfusion to vital organs like the brain, leading to oxygen and nutrient deprivation and eventually cell death (Benjamin et al. 2019; Sacco et al. 2013). The ischemic cascade following stroke is divided into three phases. The acute phase occurs within the first few hours after the ischemic event. Blood flow, ATP, and energy stores in the affected brain tissue plummet, causing ionic disruption and metabolic failure. The ensuing ionic imbalance and neurotransmitter release (glutamate excitotoxicity) promotes an excess influx of sodium and calcium into the cell. Increased intracellular calcium activates downstream phospholipases and proteases that degrade integral membrane components and proteins, while the surplus of sodium leads to cellular swelling (Lo et al. 2003). In addition, the production of oxygen free radicals and other reactive oxygen species during the acute phase causes further damage and cell death (Hao et al. 2014; Lakhan et al. 2009). The subacute phase occurs after the acute phase and lasts for the first few days after the ischemic event. During this phase, injured cerebral tissue releases cytokines, chemokines, cellular adhesion molecules (CAMs), and matrix metalloproteases (MMPs) (Lo et al. 2003; Stonesifer et al. 2017). The release of MMPs and immune cell modulators increases the permeability of the blood-brain barrier (BBB), allowing peripheral leukocytes to infiltrate and upregulate the inflammatory process (Hao et al. 2014; Stonesifer et al. 2017). However, neuroinflammation is a self-regulated process and eventually subsides to prepare for structural and functional reorganization (Iadecola and Anrather 2011). Thus, in the transition from the subacute to the chronic phase, inflammation resolves and tissue repair begins, but such endogenous regenerative process is not sufficient to confer functional recovery in stroke patients. Although the mechanism behind the reestablishment of homeostasis is still poorly understood, evidence suggests that it is orchestrated by mediators that suppress the inflammatory response. Major steps include the removal of dead cells and the introduction of exogenous treatments designed to deliver anti-inflammatory and pro-survival factors that promote tissue reconstruction and repair (Iadecola and Anrather 2011; Nathan and Ding 2010).
The complex regulation of the ischemic cascade changes the neural, vascular, and connective tissues in the affected areas of the brain (Krause et al. 2019). These changes and subsequent neurological deficits can persist long after the stroke itself and prevent patients from fully reintegrating into society. With the country’s aging population, the number of yearly cases is expected to increase. Projects indicate that 3.88% of the US population over the age of 18 will have a stroke by 2030 and the total annual stroke-related costs are expected to reach $240.67 billion (Ovbiagele et al. 2013). There are only two approved acute treatment options currently available – tissue plasminogen activator (tPA) and endovascular thrombectomy – despite the American Heath Association and American Stroke Associations’ emphasis on implementing effective acute and chronic stroke care. Unfortunately, their use is limited by short therapeutic time windows and risks of additional damage. Although rehabilitation is an option for chronic stroke care, functional recovery remains modest. With the central nervous system’s (CNS) limited capability to recover after injury, treatments to regenerate neural cells is an unmet need.
In 1988, Sharp et al. described the first successful cell transplant in animal models of ischemic brain injury using rat fetal neocortical cells (Mampalam et al. 1988). Studies that followed illustrated the ability of the grafted cells to integrate with the injured host brain and receive afferent fibers and vascularization (Grabowski et al. 1992a, b). Since these discoveries, the fields of stem-cell therapy and regenerative medicine have amounted impressive preclinical evidence of stem cell transplantation’s restorative effects on disorders of the CNS including ischemic stroke (Lindvall and Kokaia 2006; Song et al. 2018). However, evidence for the donor cells’ survival, differentiation, and functional integration in the host brain have repeatedly failed to translate in human clinical trials (Kondziolka et al. 2000, 2005; Savitz et al. 2005). As the search continues for cell source targeted for ischemic brain injury, it is important to keep in mind that the degree of repair depends primarily on the selection of appropriate cell types for transplantation. Embryonic stem cells (ES) and adult tissue-derived stem cells have unique characteristics that determine their specific responses to stroke. The following sections provide a concise overview of different stem cell types and their potential value in targeted stroke therapy.
2 Identifying the Optimal Cell Type for Stem Cell Transplantation
2.1 Embryonic Stem Cells
ES cells are derived from the embryonic inner cell mass (ICM) prior to the 5th day of development post-fertilization. These pluripotent cells can replicate indefinitely and differentiate into any cell type in the body. ES are isolated from the surrounding embryo by fine-needle aspiration, laser dissection, or by growing the ICM on the surface of feeder cells (Lee and Lee 2011). After purifying the cell-isolate, ES cells can be grown and maintained in vitro until they are ready for transplantation. In the context of targeted stroke therapy, ES develop into neuronal progenitor cells to assist in repairing damaged neurons and brain tissues. In addition, they promote angiogenesis, release neurotrophic factors such as erythropoietin, and upregulate neuroprotective factors such as BcL-2 (Liu et al. 2014). However, ethical concerns surrounding the destruction of embryos and high risk of tumorigenicity severely limit the use of ES cells in clinical applications (Stonesifer et al. 2017).
After implantation of the embryo on the 5th day of development, the ES cells of the ICM begin to permanently differentiate into more specialized cells and are no longer pluripotent. These new, more differentiated stem cells are multipotent and still have a strong capacity to self-renew but can only give rise to cells of one lineage. The ES cells eventually disappear completely, and the ‘adult’ multipotent stem cells are responsible for maintaining adult tissues. There are several key adult-tissue derived stem cells that may be beneficial in the post-stroke care of patients. Neural stem cells (NSCs) directly differentiate into the various neuronal cell types to expedite recovery (Zhao and Moore 2018). Induced pluripotent stem cells (iPSCs) are a recently discovered source of autologous ES-like cells. Extraembryonic, adipose, and dental-derived stem cells also improve stroke outcomes. Next, we will discuss bone-marrow derived stem cells, in particular mesenchymal stem cells (MSCs). Finally, we focus on Multilineage-differentiating stress enduring (Muse) cells which are primarily derived from bone marrow, but subsequently harvested in other tissues, such as adipose and umbilical cord.
2.2 Neural Stem Cells
NSCs form the entire central nervous system (CNS) by differentiating into neurons, astrocytes, and oligodendrocytes (Okano and Temple 2009). However, many NSCs terminally differentiate once neural development is complete, leaving only a small population in the subventricular zone (SVZ) and subgranular zone (SGZ) (Kempermann et al. 2015). The markedly reduced quantity of these stem cells limits the brain’s ability to renew itself after injury. Harvesting techniques using needle-aspiration or biopsy are dangerous, and while newer techniques such as magnetic isolation may be safer, they are still constrained by the scarcity of NSCs. Nevertheless, NSCs remain a prime candidate for stroke-therapy because hypoxia and injury stimulate these cells to migrate from the SVZ and SGZ to damaged tissue where they promote angiogenesis, neurogenesis, and secretion of various neuroprotective factors (Santilli et al. 2010; Zhang et al. 2014). These effects are most pronounced if NSC transplantation is autologous and administered within 72 h of insult (Chen et al. 2016). However, maintaining a premade store of autologous NSCs is impractical given the difficulty of obtaining these cells.
2.3 Induced Pluripotent Stem Cells
Unlike the other stem cell types, iPSCs are not normally present throughout development. Cellular differentiation is naturally a unidirectional process; however, scientific advancements have allowed researchers to reverse cell development such that stem cells can be artificially generated from terminally differentiated somatic cells like fibroblasts and blood cells. Exposing the cell to specialized genes and signals reprograms adult cells to become embryonic-like iPSCs capable of asymmetric division. The major advantage to using iPSCs is that adult cells can be easily harvested from any tissue source, converted into stem cells, then induced to become nearly any other type of cell, including T-regulatory cells, microglia, and other neural cell types. Like traditional stem cells, iPSCs reduce infarct size and modulate the immune system to create more suitable environments for recovery (Zents and Copray 2016). Despite the numerous benefits of iPSCs, their value is offset by two major flaws. First, although autologous in nature, they may still be rejected by the host (Zhao et al. 2011). Second, iPSCs have the highest tumorigenicity of any of the studied stem cell types (Liang et al. 2013).
2.4 Other Sources of Adult Stem Cells
Bone marrow, adipose and extraembryonic tissues (e.g., umbilical cord, placenta) are two sources of mesenchymal stem cells (MSCs). Adipose tissue is a type of loose connective tissue composed primarily of adipocytes. Adipose tissue-derived mesenchymal stem cells (AD-MSCs) are acquired by enzymatically digesting fat samples obtained from fat suctioning or excision. Transplantation with AD-MSCs improves neurological recovery, decreases the size of the infarct, and reduces inflammation (Gutiérrez-Fernández et al. 2013). Furthermore, treatment is very accessible due to the high prevalence of adipose tissue and the ability to administer treatment intravascularly with encouraging results (Gutiérrez-Fernández et al. 2013). However, treatment with AD-MSCs is diminished by its’ propensity to cause cancer cells to rapidly proliferate (Eterno et al. 2014).
While adipose tissue is plentiful, extraembryonic tissues like the umbilical cord and placenta are not. The umbilical cord arises from the placenta during gestation and together these organs connect the circulatory systems of the mother and fetus. As they are both shed after delivery, stem cells can be easily harvested from them (Gutiérrez-Fernández et al. 2013; Shinozuka et al. 2013). Tissue injury induces extraembryonic tissue-derived MSCs to inhibit immune cell migration, increase angiogenesis and neurogenesis, and potentially preserve neuroplasticity (Shinozuka et al. 2013). Despite the promising potential for neurological repair, extraembryonic stem cell use is constrained by the availability of placentas or umbilical cords to harvest cells from (Stonesifer et al. 2017).
Other sources of adult-tissue derived stem cells include breastmilk, menstrual blood, and dental tissue. Preliminary stroke models have shown that breast milk and menstrual blood-derived stem cells may also have beneficial effects, but few studies have investigated this thoroughly enough to warrant their consideration as a transplantation source (Stonesifer et al. 2017). Similarly, dental tissue-derived stem cells have been shown to preserve neurological function post-stroke, but their utility is minimized by the higher availability of other tissue sources with comparable outcomes (Stonesifer et al. 2017).
2.5 Bone Marrow-Derived Stem Cells
Because of its long track record of safety as graft source for hematologic diseases, the bone marrow has been extensively studied for stem cell therapy in stroke. Bone-marrow is a highly active spongy tissue that produces billions of new cells each day (Higgins 2015). There are four key multipotent cells that accomplish this extraordinary feat: hematopoietic stem cells (HSCs), endothelial stem cells (ESCs), very small embryonic-like stem cells (VSELs), and MSCs. This diversity in cell type makes bone-marrow an attractive target for stem cell harvesting. Typically, marrow is extracted from the iliac crest of anesthetized patients using needle-aspiration and is cryopreserved until it is ready for purification (Gorin 2019). These cells can then be isolated and transplanted into stroke patients, with each stem cell type having different effects. We will briefly consider HSCs, ESCs, and VSELs before discussing MSCs in detail.
HSCs develop into all the different types of blood cells in the body. In response to stroke and hypoxia, they preferentially differentiate through the myeloid lineage, which may be important in resolving the hypoxic environment (Felfly et al. 2010). The beneficial effects of HSC are limited by its tendency to promote inflammation, thereby delaying and possibly diminishing recovery (Kasahara et al. 2016).
EPCs are a potentially valuable transplant source due to their ability to repair the blood-brain barrier and brain vasculature, which are often compromised during or prior to the onset of stroke (Stonesifer et al. 2017). Damage to the blood-brain barrier allows inflammatory cells from the systemic circulation to migrate into the site of injury, leading to inflammation and even more damage. The strong angiogenic properties not only result in increased vessel density and reduced quantity of apoptotic cells, but also provide mild anti-inflammatory effects by limiting inappropriate immune cell access to the brain (Chen et al. 2008). Nevertheless, the usefulness of EPCs is limited by the difficulty in producing purified cell cultures.
VSELs are present in both the brain tissue and the blood in low quantities. They have excellent potential for stroke treatment due to their ability to differentiate into neurons, microglia, and oligodendrocytes (Hsiao et al. 2014). However, like EPCs, they are very difficult to harvest in clinically relevant numbers.
2.6 Mesenchymal Stem Cells
MSCs were originally isolated from bone marrow but have been harvested from multiple tissues including the umbilical cord, amniotic fluid, placenta, and adipose tissue (Friedenstein et al. 1966; McElreavey et al. 1991; Zuk et al. 2002). MSCs have been found to have a high potential for regeneration while maintaining multipotency. These cells exhibit plastic adherence, have the ability to self-renew, and exhibit a specific set of cell surface markers, such as cluster of differentiation (CD)73, CD90, and CD105, while lacking expression of CD14, CD34, CD45, and human leukocyte antigen-DR (HLA-DR) (Mushahary et al. 2018). MSCs have the ability to differentiate into mesodermal cells such as adipocytes, chondrocytes, myocytes, and osteocytes (Ullah et al. 2015). MSCs express various growth factors that are proven to facilitate tissue repair and maintain homeostasis within the immune system (Ma et al. 2014). MSCs therapeutic potential allows for the treatment of chronic diseases including Parkinson’s disease, Alzheimer disease, and Type 1 diabetes because of their ability to secrete anti-inflammatory molecules and immunoregulatory effects (Ullah et al. 2015). MSCs can interact with cells of the innate and adaptive immune system to control effector functions (Li and Hua 2017). The mechanism of MSCs involves the migration to injured tissues through specific target pathways where they inhibit the release of pro-inflammatory cytokines and help promote the survival and growth of the damaged cells.
MSCs have been used in vitro to expand the cells and differentiate into specific cell lineages. Cultured MSCs have been shown to modulate immune responses and reroute the progression of inflammatory diseases (Ma et al. 2014). As tissue injuries correspond with inflammation, MSCs can effectively mobilize to damaged tissue sites. Their mechanism of action involves modulating inflammatory processes and releasing growth factors to facilitate tissue repair (Ma et al. 2014). MSCs contain immunomodulatory features and secrete cytokines and immune receptors to maintain homeostasis and regulate the environment in the host tissue. Their multilineage potential and secretion of anti-inflammatory molecules make MSCs an effective treatment for chronic diseases (Ullah et al. 2015). When MSCs migrated to the site of damaged tissue, cytokines, toxins of infectious agents, and hypoxia allow for the release of growth factors that promote the development of fibroblasts, endothelial cells, and tissue progenitor cells which carry out tissue regeneration and repair (Ma et al. 2014). MSCs are useful for treatment of chronic diseases due to their functions in inflammatory niches but also immunomodulatory properties. The immunosuppressive functions of MSCs are triggered by the environment of the cells and allows for the release of inflammatory factors.
The therapeutic effects of MSCs allow for the cells to work in action with immune cells, stromal cells, and endothelial cells to promote tissue repair. In vitro, MSCs have the ability to differentiate into all the three lineages: ectoderm, mesoderm, and endoderm and act as a potential source for stem cell therapy for ischemic stroke (Ullah et al. 2015) (Kondziolka et al. 2005). The ability of MSCs to differentiate into several different types of tissues and expansive properties allows them to be used in stem cell-based therapies.(Mushahary et al. 2018)The use of MSCs to protect against ischemia/reperfusion, however, is influenced by the culture conditions that influence function and depends on how the MSCs are administered and expanded in vitro (Ma et al. 2014). In studies of ischemic stroke, MSCs are able to modulate an immune response and act neuroprotective, through stimulation of neurogenesis, oligodendrogenesis, astrogenesis, and angiogenesis (Dabrowska et al. 2019). MSCs derived from bone marrow are commonly used due to the secretion of neurotropic factors which help to stimulate cerebral repair processes. The use of MSCs demonstrates the ability to promote cell survival and modulate the immune response, however, in vivo studies indicate that MSCs do not functionally replace the injured cells and do not serve as a promising stem cell therapy to regenerate the injured neurons after an ischemic stroke.
2.7 Multilineage-Differentiating Stress Enduring (Muse) Cells
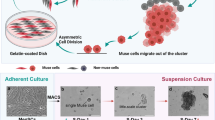
Reported in 2010 by Kuroda et al., Multilineage-differentiating stress enduring (Muse) cells are a subset of endogenous regenerative MSCs that reside in the peripheral blood and connective tissue of nearly all organs (Wakao et al. 2018). They are also found in mesenchymal tissues but are hypothesized to originate in the bone marrow where they make up ~0.03% of the mononucleated cell fraction (Tanaka et al. 2018). These cells possess the ability to self-renew, exhibit triploblastic differentiation, and regenerate a plethora of tissues when administered topically or intravenously. A small concentration is also present in peripheral blood, 0.01–0.2% of the mononucleated cell fraction, however this number may increase during injury or disease due to activation via stress (Tanaka et al. 2018; Wakao et al. 2014). Muse cells may be isolated and distinguished using the marker SSEA-3 (Wakao et al. 2011). Muse cells also reside in extraembryonic tissues such as the umbilical cord making them distinct from other somatic cells (Leng et al. 2019). Muse cells’ unique regenerative capacities could provide a feasible treatment for many diseases.
When compared to MSC’s, Muse cells have demonstrated the potential to fully engraft into the site of injury and replenish dead or ischemic tissue in vivo (Kuroda et al. 2018; Hu and Longaker 2017; Minatoguchi et al. 2018; Nishina et al. 2018; Uchida et al. 2018). In terms of ischemic stroke, MSC’s have shown the ability to regenerate damaged tissue in vitro, however in vivo models have not indicated a full incorporation into area of infarct (Ikegame et al. 2011). Although attenuation of post-stroke inflammation was visible in vivo, it is plausible that this is due to stimulation of MSC secretome inducing endogenous paracrine-mediated brain regeneration pathways (Dabrowska et al. 2019; Leong et al. 2012; Ishizaka et al. 2013; Doeppner et al. 2015). Muse cells have demonstrated paracrine characteristics alike those of MSC’s as well as the ability to travel and reside at injured sites (Tanaka et al. 2018). The regenerative capacities of Muse cells may provide a more beneficial cell-based therapy to treat ischemic stroke and other diseases when compared to MSC’s, however further investigation is necessary.
The pluripotency exhibited by Muse cells allows for Muse-cell based therapy to treat a wide range of diseases such as myocardial infarct (MI), stroke, chronic kidney disease, liver disease, chronic skin wounds, and soft tissue defects (Kuroda et al. 2018; Hu and Longaker 2017; Minatoguchi et al. 2018; Nishina et al. 2018; Uchida et al. 2018). These cells are non-tumorigenic and exhibit low telomerase activity making them a great candidate for cell-based therapy (Tanaka et al. 2018). Many of these diseases do not have a standard treatment besides end-stage transplantation, and a regenerative cell-based therapy may be a possible avenue to treat these diseases.
Endogenous Muse cells have shown to play an important role in the acute phase of MI. Acute Myocardial Infarct (AMI) patients with a higher concentration of endogenous Muse cells in the peripheral blood have shown greater progress in cardiac remodeling, cardiomyocyte regeneration, and cardiac function during chronic phase. Upon intravenous administration of exogenous allogenic Muse cells, AMI rabbit model exhibited a significant decrease in myocardial infarct size, a 6-month cardiac remodeling phase, and improved cardiac function over a long period of time without immunosuppressant treatment (Uchida et al. 2017). Muse-cell based therapy could also potentially be beneficial in treating patients suffering from an acute myocardial infarct (Minatoguchi et al. 2018). In a recent murine stroke model, cultured human bone marrow-derived Muse cells were administered to 2 weeks post-lacunar infarction. Transplantation during the subacute phase resulted in differentiation into neurons and oligodendrocytes, promoted neuronal reconstruction and improved overall brain function (Minatoguchi et al. 2018). Muse cells have demonstrated positive results both in vitro and in vivo to treat chronic liver disease (Ogura et al. 2014). Cell-based therapies using bone-marrow derived stem cells and peripheral blood-derived stem cells did not display efficacy in clinical trials when treating chronic liver disease (Ogura et al. 2014). Muse cells derived from human bone marrow were intravenously administered to immunodeficient mice with liver fibrosis. Spontaneous differentiation of the Muse cells into tissue compatible cells was exhibited as well as homing at the site of injury in the liver (Ogura et al. 2014). Chronic kidney disease may also utilize a Muse cell-based therapy (Uchida et al. 2018). There are many underlying causes of renal dysfunction and a cell-based therapy is needed as alternative to dialysis and transplantation. A rodent model of chronic kidney disease indicated differentiation at the site of injury of intravenously administered Muse cells into glomerular cells as well as improvement in renal function (Uchida et al. 2018). Chronic wounds and soft tissue defects have shown favorable improvement when treated with exogenous Muse cells. Research has shown that through differentiation of Muse cells into fibroblasts, keratinocytes and melanocytes, Muse cells may be an avenue of therapy for skin reconstruction (Hu and Longaker 2017). Muse cells exhibit regenerative characteristics that make them a potential candidate as a cell-based therapy for many diseases. The non-tumorigenic, pluripotent and regenerative abilities of these cells warrant their potential as therapy.
The ability of Muse cells to regenerate tissues have been exhibited in the brain, kidneys, liver, heart, and skin (Kuroda et al. 2018; Hu and Longaker 2017; Minatoguchi et al. 2018; Nishina et al. 2018; Uchida et al. 2018). Exogenous and endogenous Muse cells migrate to the injury through the peripheral blood, and home to the damaged host tissue site through the sphingosine-1-phosphate (S1P)-S1P receptor 2 (S1PR2) system (Yamada et al. 2018). Muse cells are able to survive the harsh environment at the target site due to their high stress tolerance (Alessio et al. 2018). Muse cells also possess immunomodulatory abilities allowing them to evade host immune cells at the injury site. Muse cells integrate into the site of injury and spontaneously differentiate into tissue compatible cells. In addition to differentiation at the site of injury, Muse cells were also found to exhibit paracrine characteristics through secretion of therapeutic factors such as hepatocyte growth factor, stem cell-derived factor 1, and epidermal growth factor that promoted functional recovery in tissue injuries (Tanaka et al. 2018). In the case of an ischemic stroke rodent model, 2–3 months after Muse cell transplantation the Muse cells had formed synapses with host neurons as well as integrated axons into the pyramidal tract (Uchida et al. 2016). Both of these findings resulted in improved motor function and somatosensory evoked potential. Findings indicate that Muse cells could potentially be used as a regenerative stem cell therapy for many diseases however further elucidation is necessary.
3 Preclinical Studies on Muse Cells
There have been several recent in vitro and in vivo studies exploring the efficacy of Muse cells in treating ischemic injury (Leng et al. 2019; Uchida et al. 2017; Yamada et al. 2018; Alessio et al. 2018). Preclinical studies showed that Muse cells migrate to the site of injury, incorporate into peri-infarct tissue, and differentiate spontaneously into cells that are congruous with injured tissue (Leng et al. 2019). In addition, Muse cells derived from adipose tissue have been shown to have anti-inflammatory activities, decreasing the secretion of cytokines, such as interferon-γ, which indicates their potential efficacy in ameliorating post-ischemic neuroinflammation (Uchida et al. 2016). When comparing Muse cells to non-Muse cells (cells other than Muse cells in MSCs), Muse cells differentiate into neurons and oligodendrocytes, remain integrated in the peri-infarct while non-Muse cells release therapeutic factors but do not replace ischemic cells (Dezawa et al. 2019). However, before the clinical application of Muse cells can be considered, the optimal timing, dosage, and means of delivery need to be further investigated.
In order to establish the optimal timing for Muse cell stroke therapy, preclinical trials investigating the differential effects of acute, subacute, and chronic delivery need to be examined. In a recent study, human fibroblast-derived Muse cells were transplanted stereotaxically into three regions near the ischemic cortex 2 days after the middle cerebral artery occlusion (MCAO). The Muse cells remained in the rat’s brain for 84 days. Substantial amelioration in neurological and motor performance was observed after more than 84 days (Alessio et al. 2018). Another study found that immunodeficient MCAO rat models showed recovery 35 days after acute transplantation of Muse cells (Dezawa et al. 2019). In an immunodeficient lacunar infarction mouse model, human bone marrow-derived Muse cells were transplanted into the site of the peri-lesion at the subacute stage of lacunar infarction (Uchida et al. 2017). At 56 days post transplantation, the Muse cells differentiated into NeuN, MAP 2 expressing neurons and GST-pi expressing oligodendrocytes, and the mice with the Muse cell transplantation showed substantial improvement in neurological function (Uchida et al. 2017). The cylinder test in a different study fetal porcine cells found that neurological and motor recovery were not significantly different between immunodeficient lacunar mice given either subacute treatment or chronic treatment (Abe et al. 2020). Moreover, the recovery time for ischemic animal models varies between acute and subacute treatment but may not differ between subacute and chronic delivery (Abe et al. 2020). While delivering Muse cells in the acute phase is ideal, delivery during subacute or chronic phases may still confer benefits.
Additionally, preclinical studies have investigated the efficacy of various does for Muse cell ischemic stroke treatment. Multilineage-differentiating stress-enduring cell-based product (CL2020) was injected through the cervical vein in an immunodeficient mouse lacunar model. CL2020 was administered in three different doses: high dose (5 × 104 cells/body), medium dose (1 × 104 cells/body) low dose (5 × 103 cells/body) at both the subacute phase and chronic phase. As seen in the cylinder test, the mice that were given the high dose demonstrated the greatest neurological and motor function improvement in both the subacute and chronic group when compared to the vehicle. For the mice which were given the high dose, their rehabilitation lasted up to 22 weeks (Abe et al. 2020). In another study, comparing the effects of Muse cells and non-Muse cells, a dosage 2.5 × 104 cells/body was used. The results indicated that the Muse cells improved neurological function of MCAO mice, as observed 35 days after transplantation (Dezawa et al. 2019). Furthermore, preclinical studies suggest that higher doses of Muse cell treatment are more effective in alleviating stroke-induced injury, bringing these cells closer to clinical application.
Before moving to clinical trials, the least invasive mode of delivery for Muse cell treatment must be established. Preclinical trials have examined intravenous injection as possible means of delivery for Muse cells (Dabrowska et al. 2019). The reparative properties of Muse cells through intravenous injection can be observed in a variety of tissues, such as the brain, liver, and skin (Leng et al. 2019). In the study that intravenously administered CL2020 to lacunar mice through the cervical vein, neurological and motor recovery was observed. When the human cells were depleted by the intraperitoneal injection of diphtheria toxin, the recovery was abolished, indicating that intravenous administration may be a viable, non-invasive method to deliver the cells (Abe et al. 2020).
Although the preclinical trials involving Muse cells are promising, there are some limitations. Allogeneic Muse cells can stay in the host brain as differentiated neuronal tissue for longer than 6 months (Dezawa et al. 2019). However, before moving to clinical trials, a long-term engraftment system, where donor cells continue to integrate themselves into the host’s nervous tissue must be engendered. Preclinical studies have demonstrated encouraging results regarding the efficacy of Muse cells in treating ischemic stroke models.
4 Clinical Studies on Muse Cells
Treatment of neuronal cells through stem cell transplantation evidently enhance the motor and cognitive recovery in rodent stroke models (Kondziolka et al. 2000). However, the same significant improvement was not seen in clinical trials (Kondziolka et al. 2000, 2005; Savitz et al. 2005). Savitz et al. (2005) conducted a clinical trial to observe the effects of fetal porcine neural cell transplantation. A burr hole was created during the surgical process to implant the fetal cells at the infarct site in the basal ganglia (Savitz et al. 2005). In preclinial studies, the specific cell transplantation was deemed safe in animal models for basal ganglia infarcts (Savitz et al. 2005). In the clinical trial, some patients did not display adverse effects while some improved in speech and motor functions over long periods of time (Savitz et al. 2005). Other patients, however, temporarily experienced motor deficits weeks after transplantation (Savitz et al. 2005). One patient specifically developed seizure a week after treatment, and the study was terminated by the FDA (Savitz et al. 2005). Some studies have suggested that stem cells are doing more harm than good. Amariglio et al. (2009) first reports a human brain tumor after the 13-year-old patient with Ataxia Telangiectasia (AT) underwent fetal neural stem cell therapy. Although the patient was healthy after the treatment, a small tumor was discovered adjacent to the site 4 years later (Amariglio et al. 2009). Through cytogenetic and molecular examinations, the tumor was concluded to have originated outside the body, suggesting that the tumor was derived from the transplantation (Amariglio et al. 2009). Incidents mentioned above raises concern for the safety of patients receiving cell therapy. With inconsistent results in clinical trials, a new method of approach should be developed for cell therapy to improve the safety and efficacy of the treatment.
Compared to other stem cells, MSC is the most suitable type of stem cell for neural cell therapy for ischemic stroke patients. One study examined the safety and efficiency of MSC treatment in nonacute ischemic strokes (Valeria Battistella et al. 2011). In the study, patients received the maximal amount of MSC (5 × 108 cells) during the clinical trial, but no adverse events were reported for 180 days after transplantation (Valeria Battistella et al. 2011). Even with large amounts of stem cells were introduced to stroke patients, the intervention did not cause detrimental effects to their health. This finding ensures the safety of nonacute stroke patients when undergoing this method of treatment. Additionally, long-term studies have demonstrated no significant side effects in patients treated with MSC (Jin Soo Lee et al. 2010). Patients treated with MSC did not develop malignant tumors and no significant structural change was observed after a year of treatment (Jin Soo Lee et al. 2010). Studies investigating cell therapy with MSC have consistently provided no evidence regarding health concerns or consequences. Due to its safety and accessibility, MSCs are a favorable source for cell therapy. However, some MSC studies are concerned about the lack of evidence of the stem cell’s efficiency.
Recent clinical trials have been conducted using Muse cells (CL2020) in Japan, further highlighting its safety and efficiency in ischemic strokes. Japanese studies consist of treatments for neonatal hypoxic ischemic encephalopathy, myocardial infarction, ischemic stroke, spinal cord injury, and epidermolysis bullosa (JapicCTI-183834, J.I. 2020; JapicCTI-184103, J.I. 2018; JapicCTI-184563, J.I. 2018; JapicCTI-194841, J.I. 2019; JapicCTI-195067, J.I. 2019). All clinical trials administered patients with allogenic CL2020 through intravenous infusion. Immunosuppression was not necessary during the clinical trials because of the HLA-G expression in human Muse cells. This molecule allows the stem cells to function in the target site without the living body and its immune system reacting (Shohei Wakao et al. 2014). Muse cell’s unique ability to develop into various types of cells and tissues allows it to target and repair damaged sites in vivo (Shohei Wakao et al. 2014). During clinical treatments for spinal cord, epidermolysis bullosa, and ischemic stroke, no significant evidence suggested that the intervention was detrimental or ineffective for patients with the mentioned conditions. Clinical studies for the three health conditions deemed CL2020 to be safe and efficient, further supporting the notion to utilize MSC and Muse cells for stem cell therapy (JapicCTI-184103, J.I. 2018; JapicCTI-184563, J.I. 2018; JapicCTI-194841, J.I. 2019). Muse cell therapy has also been explored for neonatal hypoxic ischemic encephalopathy patients (jRCT2043190112, J.I. 2020).
Muse cells can be directly administered to patients because of their unique anti-inflammatory and anti-immune mechanisms (Dezawa 2018; Young 2018). This mechanism prevents the body from rejecting the stem cell, avoiding the need to genetically manipulate the Muse cells for acceptance (Dezawa 2018). Additionally, unlike embryonic stem cells (ES) and induced pluripotent stem cells (iPS), Muse cells do not need to be administered to the target site directly (Dezawa 2018). Instead, Muse cells can be administered through intravenous injections, removing the need for surgical operations (Dezawa 2018). Due to these advantages, Muse cells bring new light to stem cell therapy. Further research and clinical studies should be conducted to investigate the efficiency and reliability of Muse cells in ischemic stroke.
5 Summary
Ischemic stroke, caused by areas of the brain being deprived of oxygen and nutrients, lead to neurological damages and cognitive impairments (Benjamin et al. 2019; Sacco et al. 2013). The affected areas acutely experience ionic disruptions and metabolic failures due to lack of sufficient blood flow, ATP, and energy. Chronically, the release of free radical oxygen species and inflammation cause additional damage and cell death within the affected regions of the brain (Hao et al. 2014; Lakhan et al. 2009). Unfortunately, tPA and endovascular thrombectomy are the only approved treatments for acute stroke and their use is limited by the narrow effective time window and risk for additional damage. Rehabilitation helps chronic management and stroke care but is does treat the loss of function. An intervention that could regenerate neural cells and restore lost function of the brain would be a valuable addition in our toolkit to treat stroke.
Stem cell therapy offers a potential solution, and preclinical studies have highlighted the regenerative abilities of donor stem cells to neural tissue (Lindvall and Kokaia 2006; Song et al. 2018; Kondziolka et al. 2000). However, ethical and logistical concerns limit the use of stem cells in regenerative medicine. For example, the extraction of ES involves the destruction of human embryos (Bernard and Parham 2009). iPSC, despite its impressive ability to become any cell type, is infamous for having the highest tumorigenicity, where cultured cells give rise to tumors over time (Liang et al. 2013). These issues bring controversy to stem cell research, hindering the advancement of stem cell therapy. Compared to previously mentioned stem cells, adult stem cells, such as MSCs, are more suitable for stem cell therapy. No ethical issues regarding their procurement is present because MSCs are harvested from the placenta, bone marrow, and umbilical cord (Shinozuka et al. 2013). Additionally, MSCs evidently possess the same proliferative ability as iPSC while also being safe to use (Valeria Battistella et al. 2011). However, MSCs possess their own limitations. Studies have shown the variability and heterogeneity of MSC when the stem cell is extracted from different donors and tissue source, making it difficult for different research groups to compare methods and results (Mohamed-Ahmed et al. 2018). To circumvent this and previous limitations, Muse cells, a distinct subset of MSCs, are favored in cell therapy for ischemic stroke. Preclinical studies have investigated the efficiency of Muse cells. Studies have highlighted the mobility and anti-inflammatory activities of Muse cells (Leng et al. 2019; Uchida et al. 2016). Unlike non-Muse MSCs, Muse cells are able to differentiate into neurons, replace ischemic cells, and remain integrated (Dezawa et al. 2019). Clinical trials have commenced to investigate the safety and efficiency of Muse cells. Muse cells were administered through intravenous injections, removing the need for surgical operations (Dezawa 2018). This was possible due to Muse cell’s ability to identify and repair damaged neural sites. Additionally, Muse cells were not genetically modified and no signs of rejection were observed in ischemic stroke patients because of the stem cells’ anti-inflammatory mechanism (JapicCTI-184103, J.I. 2018). The evidence generated from preclinical and clinical studies makes Muse cells the most suitable candidate for cell therapy in ischemic stroke due to its safety, accessibility, and efficiency. Further clinical studies should be conducted to determine the consistency of Muse cells.
References
Abe T, Aburakawa D, Niizuma K et al (2020) Intravenously transplanted human multilineage-differentiating stress-enduring cells afford brain repair in a mouse lacunar stroke model. Stroke 51(2):601–611
Alessio N, Squillaro T, Özcan S et al (2018) Stress and stem cells: adult muse cells tolerate extensive genotoxic stimuli better than mesenchymal stromal cells. Oncotarget 9(27):19328–19341
Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G (2009) Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6(2)
Benjamin EJ et al (2019) Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 139(10):e56–e528
Bernard L, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30(3):10
Chen ZZ et al (2008) Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci Lett 445(1)
Chen S et al (2016) Differentiation of isolated human umbilical cord mesenchymal stem cells into neural stem cells. Int J Ophthalmol 9(1):41–47
Dabrowska S et al (2019) Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation 16(1):178
Dezawa M (2018) Clinical trials of muse cells. Muse Cells 1103:3
Dezawa M, Niizuma K, Tominaga T (2019) Actualization of neural regenerative medicine by intravenous drip of donor-derived muse cells. Brain Nerve 71(8):895–900
Doeppner TR et al (2015) Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med 4(10):1131–1143
Eterno V et al (2014) Adipose-derived mesenchymal stem cells (ASCs) may favour breast cancer recurrence via HGF/c-met signaling. Oncotarget 5(3):613–633
Felfly H et al (2010) Hematopoietic stem cell transplantation protects mice from lethal stroke. Exp Neurol 225(2)
Friedenstein AJ, Piatetzky S II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16(3):381–390
Gorin NC (2019) Bone marrow harvesting for HSCT. In: The EBMT handbook. Springer
Grabowski M, Brundin P, Johansson BB (1992a) Fetal neocortical grafts implanted in adult hypertensive rats with cortical infarcts following a middle cerebral artery occlusion: ingrowth of afferent fibers from the host brain. Exp Neurol 116(2):105–121
Grabowski M et al (1992b) Vascularization of fetal neocortical grafts implanted in brain infarcts in spontaneously hypertensive rats. Neuroscience 51(3):673–682
Gutiérrez-Fernández M et al (2013) Adipose tissue-derived stem cells in stroke treatment: from bench to bedside. Discov Med 16(86):37–43
Hao L et al (2014) Stem cell-based therapies for ischemic stroke. Biomed Res Int 2014:468748
Higgins JM (2015) Red blood cell population dynamics. Clin Lab Med 35(1):43–57
Hsiao HH et al (2014) Acute cerebral infarct with elevated factor VIII level during the thrombocytopenic stage after hematopoietic stem cell transplant. Exp Clin Transplant 12(2):171–172
Hu MS, Longaker MT (2017) A MUSE for skin regeneration. J Invest Dermatol 137(12):2471–2472
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17(7):796–808
Ikegame Y et al (2011) Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13(6):675–685
Ishizaka S et al (2013) Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 44(3):720–726
JapicCTI-183834, J.I (2020) Exploratory study of CL2020 in patients with ST-elevation acute myocardial infarction
JapicCTI-184103, J.I (2018) A randomized, double-blind, placebo-controlled clinical study of CL2020 in ischemic stroke patient
JapicCTI-184563, J.I (2018) A clinical study of CL2020 in patients with epidermolysis bullosa
JapicCTI-194841, J.I (2019) A clinical study of CL2020 in patients with spinal cord injury
JapicCTI-195067, J.I (2019) A confirmatory study of CL2020 in patients with ST-elevation myocardial infarction
Jin Soo Lee JMH, Moon GJ, Lee PH, Ahn YH, Bang OY, STARTING Collaborators (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28(6):8
jRCT2043190112, J.I (2020) The clinical trial of CL2020 for neonatal hypoxic ischemic encephalopathy
Kasahara Y et al (2016) Transplantation of hematopoietic stem cells: intra-arterial versus intravenous administration impacts stroke outcomes in a murine model. Transl Res 176:69–80
Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 7(9):a018812
Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L (2000) Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55(4):5
Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell-Stephens T, Teraoka J (2005) Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg 103(1):8
Krause M et al (2019) Cell-based therapies for stroke: are we there yet? Front Neurol 10:656
Kuroda S et al (2018) Muse cell: a new paradigm for cell therapy and regenerative homeostasis in ischemic stroke. Adv Exp Med Biol 1103:187–198
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97
Lee JE, Lee DR (2011) Human embryonic stem cells: derivation, maintenance and cryopreservation. Int J Stem Cells 4(1):9–17
Leng Z, Sun D, Huang Z et al (2019) Quantitative analysis of SSEA3+ cells from human umbilical cord after magnetic sorting. Cell Transplant 28(7):907–923. 52
Leong WK et al (2012) Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med 1(3):177–187
Li N, Hua J (2017) Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci 74(13):2345–2360
Liang Y et al (2013) The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin J Cancer 32(4):205–212
Lindvall O, Kokaia Z (2006) Stem cells for the treatment of neurological disorders. Nature 441(7097):1094–1096
Liu S-J et al (2014) Co-grafting of neural stem cells with olfactory en sheathing cells promotes neuronal restoration in traumatic brain injury with an anti-inflammatory mechanism. J Neuroinflammation 11
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4(5):399–415
Ma S et al (2014) Immunobiology of mesenchymal stem cells. Cell Death Differ 21(2):216–225
Mampalam TJ et al (1988) Neuronal changes in fetal cortex transplanted to ischemic adult rat cortex. J Neurosurg 69(6):904–912
McElreavey KD et al (1991) Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton's jelly portion of human umbilical cord. Biochem Soc Trans 19(1):29S
Minatoguchi S et al (2018) Acute myocardial infarction, cardioprotection, and muse cells. Adv Exp Med Biol 1103:153–166
Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H et al (2018) Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther 9(1)
Mushahary D et al (2018) Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 93(1):19–31
Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140(6):871–882
Nishina T, Hoshikawa KT, Ueno Y (2018) Current cell-based therapies in the chronic liver diseases. Adv Exp Med Biol 1103:243–253
Ogura F et al (2014) Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev 23(7):717–728
Okano H, Temple S (2009) Cell types to order: temporal specification of CNS stem cells. Curr Opin Neurobiol 19(2):112–119
Ovbiagele B et al (2013) Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 44(8):2361–2375
Sacco RL et al (2013) An updated definition of stroke for the 21st century. Stroke 44(7):2064–2089
Santilli G et al (2010) Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One 5(1):e8575
Savitz SI et al (2005) Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovas Dis 20(2):7
Shinozuka K et al (2013) Stem cell transplantation for neuroprotection in stroke. Brain Sci 3(1):239–261
Shohei Wakao HA, Kushida Y, Dezawa M (2014) Muse cells, newly found non-tumorigenic pluripotent stem cells, reside in human mesenchymal tissues. Pathol Int 64(1):9
Song CG et al (2018) Stem cells: a promising candidate to treat neurological disorders. Neural Regen Res 13(7):1294–1304
Stonesifer C et al (2017) Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 158:94–131
Tanaka T, Nishigaki K, Minatoguchi S et al (2018) Mobilized Muse cells after acute myocardial infarction predict cardiac function and remodeling in the chronic phase. Circ J 82(2):561–571
Uchida H et al (2016) Transplantation of unique subpopulation of fibroblasts, Muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 34(1):160–173
Uchida H et al (2017) Human muse cells reconstruct neuronal circuitry in subacute lacunar stroke model. Stroke 48(2):428–435
Uchida N, Kumagai N, Kondo Y (2018) Application of muse cell therapy for kidney diseases. Adv Exp Med Biol 1103:199–218
Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells – current trends and future prospective. Biosci Rep 35(2)
Valeria Battistella GRdF, da Fonseca LMB, Mercante D, Gutfilen B, Goldenberg RCS, Dias JV, Kasai-Brunswick TH, Wajnberg E, Rosado-de-Castro PH, Alves-Leon SV, Mendez-Otero R, Andre C (2011) Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med 6(1):8
Wakao S et al (2011) Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci U S A 108(24):9875–9880
Wakao S et al (2014) Muse cells, newly found non-tumorigenic pluripotent stem cells, reside in human mesenchymal tissues. Pathol Int 64(1):1–9
Wakao S, Kushida Y, Dezawa M (2018) Basic characteristics of Muse cells. Adv Exp Med Biol 1103:13–41
Yamada Y et al (2018) S1P-S1PR2 Axis mediates homing of muse cells into damaged heart for long-lasting tissue repair and functional recovery after acute myocardial infarction. Circ Res 122(8):1069–1083
Young W (2018) Future of Muse cells. Muse Cells 1103:7
Zents K, Copray S (2016) The therapeutic potential of induced pluripotent stem cells after stroke: evidence from rodent models. Curr Stem Cell Res Ther 11(2):166–174
Zhang RL et al (2014) Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One 9(12):e113972
Zhao X, Moore D (2018) Neural stem cells: developmental mechanisms and disease modeling. Cell Tissue Res 371(1):1–6
Zhao T et al (2011) Immunogenicity of induced pluripotent stem cells. Nature 474(7350):212–215
Zuk PA et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Park, Y.J. et al. (2020). A Museum of Stem Cells Points to Muse Cells as Robust Transplantable Cells for Stroke: Review. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 11. Advances in Experimental Medicine and Biology(), vol 1312. Springer, Cham. https://doi.org/10.1007/5584_2020_596
Download citation
DOI: https://doi.org/10.1007/5584_2020_596
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71924-1
Online ISBN: 978-3-030-71925-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)