Abstract
Liver diseases account for one of the leading causes of deaths in global health care. Furthermore, chronic liver failure such as liver cirrhosis is, namely, responsible for these fatal conditions. However, only liver transplantation is an established treatment for this end-stage condition, although the availability of this salvage treatment option is quite limited. Thus, the novel therapy such as artificial liver devices or cellular administration has been regarded as feasible. Especially cellular therapies have been proposed in decades. The technical advancement and progress of understanding of cellular differentiation have contributed to the development of basis of cellular therapy. This attractive therapeutic option has been advanced from original embryonic stem cells to more effective cellular fractions such as Muse cells. Indeed several cellular therapies including bone marrow-derived stem cells or peripheral blood-derived stem cells were initiated; the recent most organized clinical trials could not demonstrate its efficacy. Thus, truly innovative cellular therapy is needed to meet the scientific demands, and Muse cell administration is the remaining approach to this. In this article, we will discuss the current development and status of cellular therapy toward chronic liver failure.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
It is well known that chronic liver diseases including viral infection (hepatitis B virus, hepatitis C virus), autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), nonalcoholic steatohepatitis (NASH), alcoholic hepatitis (AH), and some others may lead to the development of liver cirrhosis and ultimately end-stage liver failure [1]. Although the causes of liver cirrhosis differ according to area or county, no life-saving treatment for this condition other than liver transplantation currently exists. A recent etiological survey has confirmed that HBV infection, HCV infection, alcoholic liver disease, and NASH are the leading causes of liver failure [1]. However, the development of effective antiviral treatments has changed the clinical situation. It is estimated that the incidence of HCV-related cirrhosis will decrease dramatically within the next few decades [2]. Also, the introduction of effective nucleos(t)ide analogues for HBV infection has successfully suppressed the replication of HBV, resulting in clinically significant and durable suppression of hepatic fibrosis and inflammation in the long term. Thus, the current major causes of liver cirrhosis are remaining liver diseases such as NASH, for which no fundamental treatments have been established. Moreover, there are still many patients with established liver failure who continuously suffer from complications such as ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, and ultimately hepatocellular carcinoma (HCC) [1]. Currently, liver transplantation, either cadaveric or from living donors, is recognized as the only option for end-stage liver disease. However, its use is limited by a shortage of donors, a high incidence of surgical complications, and high medical costs. In this situation, the development of medical therapies other than liver transplantation would be desirable [3]. The present strategy of medical therapy for liver cirrhosis is (1) resolution of hepatic fibrosis, (2) recovery of hepatic function (both synthetic and metabolic), and (3) reducing the incidence of complications [4]. Recently, various anti-fibrotic drugs have been investigated in clinical trials, including the apoptosis signal-regulating kinase 1 (ASK1) inhibitor, selonsertib [5]. To reverse the decline in the synthetic function of the liver, several nutritional therapies such as branched-chain amino acid (BCAA) supplementation have been applied, although their effects have been proven only for patients with comparative reversal of decompensated cirrhosis. Furthermore, artificial liver support including extracorporeal xenogeneic hepatocyte-based approaches has demonstrated limited effects in patients with chronic liver failure. As a consequence, these forms of artificial liver support are merely regarded as temporary bridging therapies to liver transplantation. Moreover, no rational approach has been established for prevention of liver cirrhosis in patients with end-stage liver diseases. Against this background, the development and introduction of novel therapies for end-stage liver diseases would seem to be desirable. Among them, cell-based therapy has been regarded as very promising. The purpose of cell therapy is for grafted cells to migrate to damaged organs and participate in tissue recovery. For this purpose, cell therapy would seem to be a more effective approach than the use of artificial extracorporeal devices for hepatic disease. The specific characteristics of the liver, such as its ample blood supply, a marked capacity for regeneration, and comparatively easy access from the body surface, are all amenable to the development of novel forms of cellular therapy for intractable end-stage liver disease. For example, as access routes for cellular infusion, transplanted cells can be injected peripherally, intra-arterially (via the hepatic artery), or via the portal vein. However, when considering the possible complications of cell therapy, administration of cells via a peripheral vein may decrease the risk of such complications. In this review, we discuss the current status of cell-based therapy for end-stage liver diseases.
13.2 Stem Cells
Stem cells are known to have various specific abilities such as self-renewal and differentiation. There are several types of stem cells in mammals, and embryonic stem (ES) cells are the most prototypic. However, due to their limited accessibility and ethical issues, the clinical application of ES cells has a number of specific hurdles. Another type of stem cell is the mesenchymal stem cell (MSC). The application of MSCs as a source of cell therapy has been investigated worldwide for numerous conditions. MSCs have the advantage of easy accessibility; they can be obtained even from medical waste tissues such as adipose tissue, umbilical tissue, and dental pulp. Another type of stem cell is hematopoietic stem cells, which are reported to differentiate into hepatocyte-like cells under certain conditions [6]. A number of studies using animal models and some human investigational trials have described their application for hepatic regenerative therapy [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Unfortunately, however, most of those studies were hampered by significant bias [29]. Table 13.1 summarizes the major clinical trials of cell therapy for liver cirrhosis. The majority of cellular sources have been autologous bone marrow or allogenic umbilical cord. Clinical trials of this form of cell therapy have obtained data based on laboratory tests (albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), etc.), residual hepatic function (Child-Pugh score), MELD (model for end-stage liver disease) score, clinical symptoms (hepatic encephalopathy, anemia, edema, ascites fluid), and occasionally histological evaluation. In these clinical trials, umbilical cord MSCs were administered via a peripheral vein, and the number of cells infused was usually around 5.0 × 106 cells per kg body weight, being given three times at 4-week intervals. Some of the studies reported an improvement in the serum levels of albumin and total bilirubin, a decrease of the MELD score, or an improvement of clinical symptoms such as ascites at the end of the observation period [25, 29]. Clinical trials using bone marrow-derived MSCs as the cell source have made use of autologous bone marrow and administration via various routes such as the hepatic artery, portal vein, peripheral veins, or intrahepatic vessels. The number of transplanted cells in those studies ranged between 3.4 × 108 and 0.75 × 106/patient [29]. Although some studies reported an improvement of surrogate markers, the results were not consistent [20, 29, 30]. A few explorative clinical studies of MSC administration resulted in partial improvement of hepatic reserve in patients with alcoholic cirrhosis [22, 31]. However, a recent well-conducted randomized trial concluded that there was no beneficial effect of MSC administration combined with administration of granulocyte colony-stimulating factor [32]. This disappointing result further emphasizes the need for novel cell-based therapies for chronic liver failure [33]. At least, we need to summarize the reasons for this trial failure in scientific views. This includes the fundamental questions such as the candidacy of MSC as the cellular source toward the organ like the liver, which is one of the largest organs consisting mammalian body.
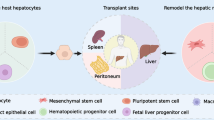
13.3 Future Novel Cellular Therapies Including Muse Cell Administration Toward Chronic Liver Failures (Fig. 13.1)
Current forms of cellular therapy require harvesting of MSCs from bone marrow and a certain period of time to prepare a sufficient number of pure cells, which limits the clinical application of this approach, especially in emergency cases such as acute liver failure. ES cells were initially reported as the potential cell source for administration. However, tumorigenicity and ethical issues for using fertilized egg are major barriers for feasibility of ES cells, and there are numerous issues to be overcome before reaching clinical trials with this cell source. Inducible pluripotent stem (iPS) cells have been engineered to overcome these difficulties and can theoretically differentiate into various types of cells, tissues, and organs [18, 34,35,36]. Although this approach is reported safe so far, there are still significant concerns about the artificial introduction of exogenous genes such as retroviral vectors [37]. Since the long-term efficacy and safety of iPS cell administration have not been proved, we need to be very careful about its clinical application as a standard form of care.
Current concept of cell-based therapy for chronic liver failure
The major possible cellular source was either mesenchymal stem cells (MSCs), Muse cells, or iPS cells. The former two cells were essentially isolated from bone marrow, whereas the latter could be transformed by genetic modification. Muse cells could be subspecialized population of MSCs (see text). Although these concepts have been proposed, none of these have proven their efficacy in phase II clinical trials in chronic liver failure
Multilineage-differentiating stress-enduring (Muse) cells are a form of mesenchymal stem cell with several of the novel characteristics of non-tumorigenic pluripotent characters [38, 39]. The previous reports demonstrated the capabilities of pluripotent differential abilities of Muse cells into liver-constituting cells such as hepatocytes [6, 40,41,42]. As Muse cells are able to recognize the sites of tissue damage/injury, thus contributing to tissue repair and promoting the improvement of organ function, their application to cellular therapy has naturally attracted attention. Besides differentiation capability, Muse cells also have other technical advantages over traditional MSCs. Muse cells were reported to home specifically into damaged tissue after intravenous injection and keep engrafted as tissue-specific cells for a longer period over several months, while majority of MSCs other than Muse cells, namely, non-Muse MSCs, basically do not home into damaged tissue nor they engraft as differentiated cells in the tissue [43]. The most recent study revealed that the sphingosine-1-phosphate is the major migratory factor of Muse cells, which will in turn be utilized for more efficient future isolation methods [44]. Interestingly, this humoral factor has been reported to be important by independent researchers [13]. Moreover, there is no need for Muse cells to introduce exogenous genes for acquiring pluripotency, which is essential for iPS cells, because Muse cells are already pluripotent. Besides safety profiles, Muse cells have superiority over MSC, ES cells, or iPS cells; without any prior gene introductions, Muse cells can selectively home into damaged tissue and efficiently replenish tissue-specific cells by intravenous injection. Exploiting this property, Muse cell administration has been reportedly effective in animal models with cerebral infarction, nephropathy, myocardial infarction, and liver resection [45,46,47,48,49]. The recent report by Iseki et al. used the Muse cell administration in mice model of liver cirrhosis [46]. In this report, the authors demonstrated that intravenously injected Muse cells have been recruited selectively to the liver and not to other organs. Moreover, Muse cells spontaneously differentiated in the damaged liver tissue into hepatocyte marker-positive cells without fusing with host hepatocytes [46]. These differentiated cells express major hepatocyte markers such as HepPar-1, albumin, and ant1-trypsin. They also expressed cytochrome (CPY) 1A2, an enzyme for detoxication, and glucose-6-phosphatase, an enzyme for glyocolysis, as representative markers of hepatocytes. As a consequence, the elevation of serum albumin levels and the decrease of total bilirubin levels were delivered by intravenous administration of Muse cells [46]. Surprisingly, even hepatic fibrosis has been improved in this animal model of Muse cell administration [46]. One of the explanations for the fibrolytic activities of Muse cells is the production of matrix metalloproteinases (MMPs) [40]. Since the liver is an organ playing a significant immunological role, it is capable of inducing transplantation-related immunological tolerance. Therefore, the liver could be a better target for Muse cell administration than other solid organs.
For organ repair after specific forms of injury, stem cells need to contribute to the replenishment of tissue-specific cells that are actually functional in situ. Muse cell administration in a mouse model of partial hepatectomy has shown that Muse cells differentiate spontaneously into major liver components, including hepatocytes, cholangiocytes, sinusoidal endothelial cells, and Kupffer cells [47]. Not only do Muse cells have the ability to home to and accumulate in damaged organs, they can also contribute to the resolution of inflammation and fibrosis [47]. Thus, based on the results obtained from small-animal models, Muse cell treatment appears to have promising as a novel regenerative treatment for liver cirrhosis. The safety of MSC administration therapy has been reported by several clinical trials [30]. Muse cells are a subpopulation of MSC and thus are expected to be safe. As for the efficacy, since the liver is the largest human organ, high efficacy for homing into the damaged liver and for engraftment as functional hepatocytes is key point for outcomes, which should be estimated in large-animal models (i.e., swine, etc.). Establishment of suitable models involving large animals with chronic liver failure and fibrosis will help to clarify a life-size efficacy and safety of Muse cell administration, leading in turn to human clinical trials. In this viewpoint, the most recent press release announcing the launch of clinical trials of Muse cell administration to evaluate its efficacy and safety in acute myocardial infarction has given a definite conviction of the application of this fascinating cell administration toward liver diseases in the near future [50].
References
Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I et al (2016) Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol 51(7):629–650
Naggie S, Muir AJ (2017) Oral combination therapies for hepatitis C virus infection: successes, challenges, and unmet needs. Annu Rev Med 68:345–358
Forbes SJ, Newsome PN (2012) New horizons for stem cell therapy in liver disease. J Hepatol 56(2):496–499
Terai S, Tsuchiya A (2017) Status of and candidates for cell therapy in liver cirrhosis: overcoming the “point of no return” in advanced liver cirrhosis. J Gastroenterol 52(2):129–140
Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H et al (2018) The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 67(2):549–559
Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J et al (2000) Hepatocytes from non-hepatic adult stem cells. Nature 406(6793):257
Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N et al (2011) Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol 23(10):936–941
Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS et al (2013) Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transpl 27(4):607–612
Andreone P, Catani L, Margini C, Brodosi L, Lorenzini S, Sollazzo D et al (2015) Reinfusion of highly purified CD133+ bone marrow-derived stem/progenitor cells in patients with end-stage liver disease: a phase I clinical trial. Dig Liver Dis 47(12):1059–1066
Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F et al (2015) Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut 64(12):1949–1960
El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S et al (2012) Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev 8(3):972–981
Kharaziha P, Hellstrom PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F et al (2009) Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 21(10):1199–1205
King A, Houlihan DD, Kavanagh D, Haldar D, Luu N, Owen A et al (2017) Sphingosine-1-phosphate prevents egress of hematopoietic stem cells from liver to reduce fibrosis. Gastroenterology 153(1):233–248 e16
Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N et al (2007) Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol 13(24):3359–3363
Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C et al (2011) Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 54(3):820–828
Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H et al (2004) Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 40(6):1304–1311
Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA et al (2014) Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther 5(3):70
Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C et al (2010) Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51(1):297–305
Sokal EM, Lombard CA, Roelants V, Najimi M, Varma S, Sargiacomo C et al (2017) Biodistribution of liver-derived mesenchymal stem cells after peripheral injection in a hemophilia a patient. Transplantation 101(8):1845–1851
Spahr L, Chalandon Y, Terraz S, Kindler V, Rubbia-Brandt L, Frossard JL et al (2013) Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One 8(1):e53719
Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H et al (2016) Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology 64(6):2185–2197
Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y et al (2006) Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 24(10):2292–2298
Wang L, Li J, Liu H, Li Y, Fu J, Sun Y et al (2013) Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol 28(Suppl 1):85–92
Xu L, Gong Y, Wang B, Shi K, Hou Y, Wang L et al (2014) Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol 29(8):1620–1628
Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J et al (2012) Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 27(Suppl 2):112–120
Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z et al (2012) Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med 1(10):725–731
Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY et al (2014) Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 34(1):33–41
Lanthier N, Lin-Marq N, Rubbia-Brandt L, Clement S, Goossens N, Spahr L (2017) Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Res Ther 8(1):88
Moore JK, Stutchfield BM, Forbes SJ (2014) Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther 39(7):673–685
Trounson A, McDonald C (2015) Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17(1):11–22
Saito T, Okumoto K, Haga H, Nishise Y, Ishii R, Sato C et al (2011) Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev 20(9):1503–1510
Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J et al (2018) Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol 3(1):25–36
Lanthier N (2018) Haemopoietic stem cell therapy in cirrhosis: the end of the story? Lancet Gastroenterol Hepatol. 3(1):3–5
Takayama K, Morisaki Y, Kuno S, Nagamoto Y, Harada K, Furukawa N et al (2014) Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc Natl Acad Sci USA 111(47):16772–16777
Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G et al (2010) Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest 120(9):3127–3136
Iwamuro M, Komaki T, Kubota Y, Seita M, Kawamoto H, Yuasa T et al (2010) Hepatic differentiation of mouse iPS cells in vitro. Cell Transplant 19(6):841–847
Normile D (2017) iPS cell therapy reported safe. Science 355(6330):1109–1110
Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H et al (2010) Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA 107(19):8639–8643
Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M (2013) Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 8(7):1391–1415
Iseki M, Kushida Y, Wakao S, Akimoto T, Mizuma M, Motoi F et al (2017) Muse cells, nontumorigenic pluripotent-like stem cells, have liver regeneration capacity through specific homing and cell replacement in a mouse model of liver fibrosis. Cell Transplant 26(5):821–840
Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S et al (2014) Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev 23(7):717–728
Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H et al (2011) Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA 108(24):9875–9880
Dezawa M (2016) Muse cells provide the pluripotency of mesenchymal stem cells: direct contribution of Muse cells to tissue regeneration. Cell Transplant 25(5):849–861
Tanaka T, Nishigaki K, Minatoguchi S, Nawa T, Yamada Y, Kanamori H et al (2018) Mobilized Muse cells after acute myocardial infarction predict cardiac function and remodeling in the chronic phase. Circ J 82(2):561–571
Uchida N, Kushida Y, Kitada M, Wakao S, Kumagai N, Kuroda Y et al (2017) Beneficial effects of systemically administered human Muse cells in adriamycin nephropathy. J Am Soc Nephrol 28(10):2946–2960
Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S et al (2016) Transplantation of unique subpopulation of fibroblasts, Muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 34(1):160–173
Katagiri H, Kushida Y, Nojima M, Kuroda Y, Wakao S, Ishida K et al (2016) A distinct subpopulation of bone marrow mesenchymal stem cells, Muse cells, directly commit to the replacement of liver components. Am J Transplant 16(2):468–483
Yamauchi T, Kuroda Y, Morita T, Shichinohe H, Houkin K, Dezawa M et al (2015) Therapeutic effects of human multilineage-differentiating stress enduring (MUSE) cell transplantation into infarct brain of mice. PLoS One 10(3):e0116009
Kinoshita K, Kuno S, Ishimine H, Aoi N, Mineda K, Kato H et al (2015) Therapeutic potential of adipose-derived SSEA-3-positive Muse cells for treating diabetic skin ulcers. Stem Cells Transl Med 4(2):146–155
Life Science Institute, Inc., Press release http://www.lsii.co.jp/pdf/20180115-1.pdf (Japanese)
Acknowledgment
This work was supported in part by the Grant-in-Aid for Scientific Research (B) (Grant #JP16H05283) from JSPS and CREST (grant #17gm0610001h0006) and Research Program on Hepatitis from AMED.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK, part of Springer Nature
About this chapter
Cite this chapter
Nishina, T., Hoshikawa, K.T., Ueno, Y. (2018). Current Cell-Based Therapies in the Chronic Liver Diseases. In: Dezawa, M. (eds) Muse Cells. Advances in Experimental Medicine and Biology, vol 1103. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56847-6_13
Download citation
DOI: https://doi.org/10.1007/978-4-431-56847-6_13
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56845-2
Online ISBN: 978-4-431-56847-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)