Abstract
Human eye is one of the important organs in human body, with iris, pupil, sclera, cornea, lens, retina and optic nerve. Many important eye diseases as well as systemic diseases manifest themselves in the retina. The most widespread causes of blindness in the industrialized world are glaucoma, Age Related Macular Degeneration (ARMD), Diabetic Retinopathy (DR) and Diabetic Macula Edema (DME). The development of a retinal image analysis system is a demanding research topic for early detection, progression analysis and diagnosis of eye diseases. Early diagnosis and treatment of retinal diseases are essential to prevent vision loss. The huge and growing number of retinal disease affected patients, cost of current hospital-based detection methods (by eye care specialists) and scarcity in the number of ophthalmologists are the barriers to achieve the recommended screening compliance in the patient who is at the risk of retinal diseases. Developing an automated system which uses pattern recognition, computer vision and machine learning to diagnose retinal diseases is a potential solution to this problem. Damage to the tiny blood vessels in the retina in the posterior part of the eye due to diabetes is named as DR. Diabetes is a disease which occurs when the pancreas does not secrete enough insulin or the body does not utilize it properly. This disease slowly affects the circulatory system including that of the retina. As diabetes intensifies, the vision of a patient may start deteriorating and leading to DR. The retinal landmarks like OD and blood vessels, white lesions and red lesions are segmented to develop automated screening system for DR. DME is an advanced symptom of DR that can lead to irreversible vision loss. DME is a general term defined as retinal thickening or exudates present within 2 disk diameter of the fovea center; it can either focal or diffuse DME in distribution. In this paper, review the algorithms used in diagnosis of DR and DME.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic edema
- Macula
- Blood vessels
- Classification
- Diabetic retinopathy
- Exudates
- Hemorrhages
- Macula
- Microanaurysms
- Optic disc
- Segmentation
1 Introduction
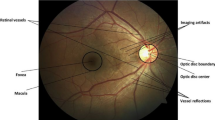
The human eye is a sensory organ for vision. It is well designed to collect the significant information about the environment around. The retina is the light sensitive tissue that lies in the posterior segment of the eye. It collects light focused from the lens, converts light signal into neural signal and then sends these signals to optic nerve. The optic nerve carries the signal to the brain; it helps to process the image. The color fundus retinal photography records interior surface of the eye that includes retina, retinal vasculature, Optic Disc (OD), macula and posterior pole. It helps to interpret retinal landmarks and is used for the screening of patients suffering from sight-threatening retinal diseases. The fact from World Health Organization (WHO) divulges that almost 285 million people are estimated to be visually impaired, among them 39 million people are blind and 246 million people have low vision. Approximately, 12.3%, 8.7% and 4.8% of visual impairment are caused by eye diseases like Glaucoma, Age-Related Macular Degeneration (ARMD) and Diabetic Retinopathy (DR).
Diabetes or Diabetes mellitus is a disease which occurs when the pancreas does not secrete enough insulin or the body is unable to utilize it properly. The disease slowly affects the circulatory system of the human body and retina. As diabetes intensifies, the vision of a patient may start to deteriorate and lead to DR. DR is an adverse change in retinal Blood Vessels (BVs) leading to vision loss without any symptoms. Diabetic Macula Edema (DME) is the advanced symptom of DR, and leads to irreversible vision loss. The swelling happens in the macula region due to leakage of fluid from BVs within the macula that appears as the presence of Exudates (EXs) in macula region. Early diagnosis and treatment of retinal diseases are essential to prevent vision loss, and it would significantly reduce the workload for the ophthalmologist.
2 Fundus Photography
The drawing of retinal vasculature including OD and fovea was published by Purkyne in 1823. Figure 1 shows the first diagrammatic representation of retina.
The first image of the retina was published by Van Trigt, a Dutch ophthalmologist in 1853 for analysis of retina. Figure 2 shows the first retinal image captured by Van Trigt.
After few years, the first photographic image of the retina was captured by Gerloff, a German ophthalmologist in 1891 that shows the BVs clearly (Abràmoff et al. 2010). The fundus camera is a complex optical system used for illuminating and imaging the retina of the eye. These devices generally consist of a microscope attached with a digital camera. It provides an upright, magnified view of the interior surface of the eye called fundus. The fundus images are two-dimensional representations of three-dimensional retinal tissue projected on to the imaging plane obtained using reflected light. The widely used retinal color fundus photographs have represented the amount of the reflected Red, Green and Blue wavebands as determined by the spectral sensitivity of the sensor.
In general to avoid misinterpretations, at least four images of the fundus are taken during eye examination for both left and right eye. For each eye, the images will be obtained with centers as Macula and OD separately. The names of the different types of images are given below.
-
Macula Centered Left Eye (MCLE)
-
Macula Centered Right Eye (MCRE)
-
Optic Disc Centered Left Eye (ODCLE)
-
Optic Disc Centered Right Eye (ODCRE)
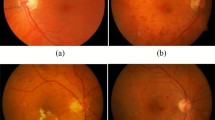
Eye disease like glaucoma is diagnosed using ODCLE and ODCRE, and retinal diseases like ARMD, DR and DME are identified using MCLE and MCRE. A healthy retinal image in MCLE and MCRE may contain clear anatomical structures like macula, OD and BVs, whereas an abnormal image shows the pathological features along with distorted anatomical features. The location of the OD differentiates the left and the right eye images. In the retinal image of a left eye, the macula is located on the right of the OD and in a right eye, macula is located on the left of the OD as shown in Fig. 3a–d.
3 Retinal Image Analysis System
Human retinal images have gained an important role in the detection and diagnosis of many eye diseases. Irrespective of the techniques used, the retinal images are mandatory for this application because the abnormalities are clearly observed in the retina than in any other part of the human eye. Retinal fundus images are easily storable and can be transmitted to anywhere at any time. It can be processed and analyzed to improve image quality and perform objective quantitative analysis. Retinal image analysis system is used for diagnosing and treatment of several diseases related to eye. Examples of such diseases are glaucoma, ARMD, DR, DME and other chronic diseases that may introduce complications in the retina such as cardiovascular and kidney diseases. Early diagnosis and timely treatment of eye diseases are essential to prevent vision loss. It would significantly reduce the workload of the ophthalmologists. The huge and growing number of retinal disease affected patients, cost of current hospital-based detection methods (by eye care specialists) and scarcity in the number of ophthalmologists are the barriers to achieve the recommended screening compliance in the patient who is at the risk of retinal diseases. Developing a retinal image analysis system using image processing, pattern recognition, machine vision and machine learning algorithms to diagnose retinal diseases is a potential solution to this problem. Numerous research efforts have been made in the area of retinal image analysis. Broadly categorized six components in retinal image analysis systems (RIAS) are explained below.
-
A.
Image acquisition: The first stage of RIAS is image acquisition. The color retinal fundus images for processing, implementation and testing of the developed RIAS can be taken from the publicly available retinal image database and in addition to that images are collected from Bejan Singh Eye Hospital, Nagerkoil.
-
B.
Image preprocessing: The retinal images, consisting of different sizes and taken from different cameras are used as input data for preprocessing. In retinal image acquisition, the retinal images’ quality has been affected by factors such as medical opacities, defocus or presence of artifact. The image quality is improved by image enhancement and restoration techniques. Image enhancement is used to improve or develop the retinal image for further processing and analysis. Techniques like histogram equalization, adaptive histogram equalization and contrast-limited adaptive histogram equalization are used. Image restoration removes salt-and-pepper noise, Gaussian noise and periodic noise by transforming the image to a different structure called Fourier transform, then applying noise filters to transform back to the original image.
-
C.
Image segmentation: Segmentation subdivides an image into its constituent parts or objects. Segmentation based on discontinuity attempt is to partition the image by detecting abrupt changes in gray level such as point, line, and edge. Segmentation based on similarity attempt is to create uniform regions by grouping together connected pixels that satisfy predefined similarity criteria (Gonzalez and Woods 2002). Therefore, the results of segmentation depend critically on these criteria and on the definition of connectivity. The algorithms are implemented by combining the process like thresholding, edge detection, filters and morphological processing. It has been used for creating mechanism of detecting edges, background removal as well as for finding the specific shape of retinal objects. Segmentation process in retinal images involves the identification of common retinal landmarks like OD, OC, BVs, macula and fovea or the identification of retinal lesions such as EXs, Cotton Wool Spots (CWSs), Microaneurysms (MAs), Hemorrhages (HAs) and Choroidal NeoVascularizations (CNVs). Many subsequent tasks such as retinal image classification rely on the quality of this segmentation process. Figure 4 shows the retinal image analysis system.
-
D.
Feature extraction and statistical analysis: The goal of RIAS is to extract useful retinal landmarks and lesions. The segmentation is used to reduce the amount of image data. Feature extraction technique has been used to extract image-based features which are application-dependent like texture, shape or statistical features. The resultant segmented images are labeled with clearly defined objects which are represented and processed independently. It treats each object (retinal landmark or lesion) as a binary image where the labeled object has the value of ‘1’ and everything else is ‘0’. The features like area, perimeter, mean, variance, and compactness ratio have been extracted, and most suitable features have been selected by performing statistical analysis for image classification.
-
E.
Image classification: An ultimate alternative to the conventional disease identification technique is automated disease classification technique. The algorithm is used to analyze retinal images and classify with confirmed diagnosis of the state of (or absence) the disease. The computational techniques employed for medical image analysis can be broadly classified into Artificial Intelligence (AI)-based techniques and conventional-based techniques. Retinal image classification is performed to assign a class for a retinal image such as a normal retina or a retina with lesion, i.e. a retina affected with glaucoma, ARMD, DR or DME.
4 Retinal Manifestation of Eye Diseases
4.1 Diabetic Retinopathy
The diabetes is a chronic condition associated with high blood sugar levels. All patients who have been diagnosed with type-I or type-II diabetes are at risk of developing DR. (Zaki et al. 2016). The consequence of untreated diabetes may lead to progression of DR. DR is an ocular manifestation of diabetes, and diabetics are at risk of vision loss due to DR. (Olson et al. 2015). Figure 5 shows the vision of normal and DR affected patients DR is a progressive disease of the retina that involves pathological changes of BVs and abnormal lesions such as MAs, HAs, EXs and CWSs that appear in fundus images
The effect of DR in human vision. (a) Normal vision (b) Vision of patient with DR. (Source: http://www.professionaleye.com/faqs/diabetic-eyecare-faq.aspx)
4.1.1 Signs and Symptoms of DR
The diabetic patients affected by DR have no early symptoms and signs that may cause damage to the retina eventually leading to blindness. The most important abnormal lesions present in fundus images are discussed below.
-
A.
Micro-aneurysms: The presence of MAs in retina is the first major symptom of the disease and caused by focal dilations of small BVs. MAs appear as small, round-shaped and red dots. The diameter of the MAs is less than 125 μm and less than the diameter of major optic veins, when crossing the thin BVs leads to smaller circular dots that are similar to MAs (Lazar and Hajdu 2012).
-
B.
Hemorrhages: The next major symptom of DR is HAs which are further divided into dot or blot HAs. The wall of thin BVs or MAs is weakened, ruptured, and this gives rise to HAs (Akram et al. 2013). Dot HAs appear as bright small red dots, and blot HAs appear as dark large red lesions. Retinal HAs are caused by pre-proliferative, proliferative DR, retinal ischemia, abnormal BVs in hypertension and malaria. HAs occur in the deeper layers of the retina when blood leaks from BVs (Tang et al. 2012).
-
C.
Exudates: The fragile and abnormal BVs leak watery fluid like proteins and lipids that cause EXs to appear. Exudates are yellow-white areas with sharp margins. EXs appear as individual dots, patches or in partial or complete rings surrounding MAs or zones of retinal edema (Mahendran and Dhanasekaran 2015).
-
D.
Cotton wool spots: The soft exudates are also known as CWSs. They are round or oval in shape with soft and feathery edges and white or pale yellow in color. CWs occur because of swelling of the surface layer of the retina. The absence of normal blood flow will affect the oxygen supply to retina and cause swelling. Figure 6 shows the physical signs of DR.
The Fundus image (a) Normal (b) Microaneurysms (c) Hemorrhages (d) Exudates (e) Cotton wool spots. (Source: http://cecas.clemson.edu/~ahoover/stare/)
4.1.2 Classification of DR
DR is broadly classified into two stages, and they are non-proliferative DR (NPDR) and proliferative DR (PDR). DR is commonly classified into the following stages.
-
A.
Mild non-proliferative diabetic retinopathy: At this earliest stage, MAs may occur. MAs are small balloon like swellings in the retina’s tiny BVs.
-
B.
Moderate non-proliferative diabetic retinopathy: As the disease progresses, some BVs that nourish the retina are blocked.
-
C.
Severe non-proliferative diabetic retinopathy: Many more small BVs are blocked, depriving several areas of the retina with their blood supply. These areas of the retina send signals to the body to grow new BVs for nourishment (Yun et al. 2008).
-
D.
Very Severe non-proliferative diabetic retinopathy: BVs broken completely in this stage
-
E.
Proliferative diabetic retinopathy: At this advanced stage, the signals sent by the retina for nourishment trigger the growth of new BVs. Figure 7 shows the stages of DR.
4.2 Diabetic Macula Edema
Prolonged diabetes can damage the tiny BVs in the retina leading to DR. These damaged BVs leak fluid that gets deposited near macula region leading to distortion in central vision known as DME. The DME may affect up to 10% of people with diabetes. DME can occur at any stage of DR although it is more likely to occur as the DR progresses (Akram et al. 2013).
4.2.1 Types of DME
There are two major types of DME.
-
A.
Clinically significant macula edema (CSME): It is the mild form of edema in which EXs are located at a distance from the center of macula region, and the vision is not affected.
-
B.
Non-Clinically significant macula edema (NCSME): It is the severe form of edema in which EXs are deposited in and around macula region or on the center of macula (called fovea), and the central vision is affected. Figure 8 shows the different stages of edema in digital retinal images.
Retinal fundus image (a) Healthy retinal image (b) non-CSME retinal image (c) CSME retinal image. (Source: Akram et al. 2014)
4.2.2 Signs and Symptoms of DME
The diabetic patients, who are affected with DR, are at risk of developing DME. The major symptoms of DME are blurred vision, double vision and sudden increase in eye floaters. There are three different signs for the presence of CSME, as defined by Early Treatment of Diabetic Retinopathy Study (ETDRS):
-
1.
The increase in retinal thickness ≤500 μm of the center of the fovea.
-
2.
EXs present in ≤500 μm of the center of the fovea with increased retinal thickness.
-
3.
The increase in retinal thickness ≥1 disc diameter with at least within 1 disc diameter at the center of the fovea (Ciulla et al. 2003). Figure 9 shows the diagrammatic representation of CSME
Diagrammatic illustration of clinically significant macula edema. (Source: https://theophthalmologist.com/issues/0815/diabetic-macular-edema-the-crest-of-the-diabetic-tsunami/)
4.2.3 Diagnosis of DME
The following examination is performed to provide information for treatment and follow up.
-
A.
Biomicroscopy: It is a routine clinical examination tool used to view all ocular structures of the eye. The slit lamps are used in illumination system that delivers bright, sharp and thin light to the focal plane to view ocular structures.
-
B.
Fluorescein Angiography: It is performed by injecting fluorescein into the circulatory system. The fundus camera is used to capture image where image intensity represents amount of emitted photons.
-
C.
OCT: It captures reflected light from ocular structures to create a cross-sectional image of the retina. The OCT is used to capture three-dimensional structural changes of retina from DME and retinal detachment.
-
D.
Color fundus photography: It is used to capture three-dimensional ocular structures into two-dimensional fundus images using fundus camera. The low power microscope provides vertical and magnified view of inner surface of the eye attached on the top of the camera (Mookiah et al. 2015).
4.2.4 Management of DME
The key part of treating and preventing DME is done by controlling blood glucose level and blood pressure. The main goal of laser surgery used in the treatment of DME is to reduce 50% of the risk of moderate vision loss. The ophthalmologist focuses the tiny laser pulses on areas near the macula where EXs are leaking and seals the leakage of BVs that causes swelling in macula region. The use of anti-VEGF has proven to reduce DME by shutting down the leakage in BVs and stop the growth of abnormal BVs. It automatically reduces the amount of leakage in macula and prevent vision loss (González Cortés 2015).
5 Retinal Image Database
There are several publicly available databases for research purpose which have been provided by research organizations and educational institutes all over the world as described below. DRIVE, STARE, DIARETDB, MESSIDOR, DRISHTI-DS and Indian eye image database have been used for testing and evaluating the developed algorithms.
-
A.
DRIVE database
The DRIVE (Digital Retinal Images for Vessel Extraction) consists of 40 color fundus photographs. It is available for public from their website (http://www.isi.uu.nl/Research/Databases/DRIVE/). The photographs were obtained from a DR screening program in Netherlands. The screening population consisted of 453 subjects between 31 and 86 years of age. The images available in the database are acquired using a Canon CR5 non-mydriatic 3CCD camera with a 45° Field of View (FOV). Each image in the database is captured using 8 bits per plane at 768 × 584 pixel resolution. The FOV of each image is circular with a diameter of approximately 540 pixels. A set of 40 images has been divided into training and testing sets containing 20 images in each set. A single and two manual segmentation of the vasculature are available for training and testing sets. Among the two manual segmentations, one is used as gold standard and the other one is used to compare the computer-generated segmentation.
-
B.
STARE database
The STARE (STructured Analysis of the REtina) consists of a retinal color fundus photograph (Hoover et al. 2000). It is available for educational and research purposes from the website (http://cecas.clemson.edu/~ahoover/stare/). The STARE Project was conceived and initiated in 1975 by Michael Goldbaum, at the University of California, San Diego, and had been funded by the National Institutes of Health, the USA since 1986. During this time, over thirty people have contributed to the project, with backgrounds ranging from medicine to science to engineering. Images and clinical data have been provided by the Shiley Eye Center at the University of California, San Diego, and by the Veterans Administration Medical Center in San Diego. The images available in the database were captured using a TopCon TRV-50 fundus camera at 35° FOV, and subsequently digitized at 605 × 700, 24-bit pixel. The dataset contains 31 normal retina images and 50 diseased retina images with expert annotation of manifestation visible in the retinal images.
-
C.
DIARETDB database
This database consists of two sets, namely DIARETDB0 and DIARETDB1 for DR detection from retinal images (Kauppi et al. 2006, 2007). The DIARETDB0 and DIARETDB1 are available for public in the website (http://www.it.lut.fi/project/imageret/diaretdb0/) and (http://www.it.lut.fi/project/imageret/diaretdb1/). The DIARETDB0 database consists of 130 color retinal images of which 20 are normal and 100 contains signs of DR like EXs, CWSs, MAs, HAs and neovascularization with unknown camera settings. The images in the database were captured using 1500 × 1152 pixels resolution with 50°FOV. The DIARETDB1 database consists of 89 color retinal images of which 5 are normal and 84 with early signs of DR like MAs with varying camera settings. The images in the database were captured using 1500 × 1152 pixels resolutions with 50°FOV.
-
D.
MESSIDOR database
The MESSIDOR (Methods to evaluate segmentation and indexing techniques in the field of retinal ophthalmology) consists of 1200 retinal images (Decencière et al. 2014). The database is available for direct download for research purpose from (http://messidor.crihan.fr.). The images in the database were acquired using 3 ophthalmologic departments using a color video 3CCD camera on a Topcon TRC NW6 non-mydriatic retinograph with a 45°FOV. The images were captured using 8 bits per plane at 1440 × 960, 2240 × 1488 or 2304 × 1536 pixels. The 1200 images are packaged in 3 sets, as per ophthalmologic department. Each set is divided into four zipped subsets containing each 100 images in Tagged Image File Format (TIFF) and an excel file with medical diagnoses of each image.
-
E.
DRISHTI-GS database
Drishti-GS data set consists of 101 retinal images (Sivaswamy et al. 2015). The images in the database are available for public in (http://cvit.iiit.ac.in/projects/mip/drishti-gs/mip-dataset2/Home.php). The images available in the database were collected from Aravind Eye Hospital, Madurai. The patients were between 40 and 80 years of age and OD centered images with dimensions of 2896 × 1944 pixels. Expert annotated images were collected from ophthalmologists with clinical experience of 3, 5, 9 and 20 years.
6 Detection of Diabetic Retinopathy
The growing number of diabetic patients has largely motivated the researchers in developing automated tools to facilitate the screening and evaluation procedures for DR. The literatures related to segmentation of BVs, segmentation of EXs, detection of MAs, identification of HAs and classification of DR are discussed in the following subsections.
6.1 Segmentation of Blood Vessel
BV segmentation is the initial step in the analysis of retina. It is carried out to locate anatomical features like OD and macula. Many researchers have applied various algorithms to segment the BVs successfully. The algorithms and the methodology used for the vessel segmentation are categorized as follows.
6.1.1 Supervised Methods
Aslani and Sarnel (2016) have proposed a pixel classification-based supervised learning for segmentation of blood vessels. The features like contrast enhanced intensity, vesselness measure, intensity of morphological transformed image, multi-scale response of Gabor wavelet and bar-selective combination of shifted filter responses are extracted, and classification of vessel or non-vessel pixels are performed by RF. This algorithm has achieved an SE of 0.7556, SPE of 0.9837, ACC of 0.9605 and area under curve of 0.9789 (Aslani and Sarnel 2016).
Roychowdhury et al. (2014) have developed a three-stage approach to segment BVs. First, the high pass filtering and top hat reconstruction are applied in green plane image to obtain binary image. Second, the major BVs are segmented from regions common to binary image and remaining pixels to construct vessel sub image. The vessel sub images are subjected to two-class classification by Gaussian mixture model. Third, the major vessels are combined with classified pixels in the sub image to get final segmentation output. This methodology has yielded an ACC of 95.2%, 95.15% and 95.3% for DRIVE, STARE and CHASE-DB1 databases with computation time of 3.1 s, 6.7 s and 11.7 s respectively (Roychowdhury et al. 2014).
Wang et al. (2015) have performed hierarchical retinal BV segmentation based on feature and ensemble learning. Histogram equalization and Gaussian filtering are employed as preprocessing steps to remove uneven illumination and improve contrast. The convolutional neural networks are used to extract the required features, and RF is employed to classify based on ensemble method. This methodology has attained an SE of 0.8173, 0.8104, SPE of 0.9733, 09791, ACC of 0.9767, 0.9813 and area under curve of 0.9475, 0.9751 in DRIVE and STARE databases respectively (Wang et al. 2015).
6.1.2 Matched Filters
Kar and Maity (2016a) have employed curvelet transform, matched filter and kernal fuzzy c means to enhance retinal BVs. The enhancement of retinal BVs is done by curvelet transform, and extraction of vessel silhouette is performed by kernel fuzzy c means on matched filter. The step and ramp like signals from vessel structure are distinguished by matched filter and Laplacian of Gaussian (LOG) filter. The proposed method has yielded an SE of 0.7577, 0.7549, false positive rate of 0.0212, 0.0301, SPE of 0.9788, 0.9699 and ACC of 0.9730, 0.9741 respectively in normal/abnormal cases in STARE database (Kar and Maity 2016a).
Kar and Maity (2016b) have employed curvelet transform, matched filter and fuzzy conditional entropy to segment BVs. The removal of noise and improvement of contrast are done by curvelet transform and sequential band pass filter. The different types of vessel silhouettes are extracted by fuzzy conditional entropy on matched filter response. The thin, medium and thick vessels are segmented by multiple thresholding. The proposed method has yielded a true positive rate of 76.32%, 72.82%, false positive rate of 1.99%, 2.6% and an ACC of 96.28%, 96.16% respectively for the DRIVE and STARE databases (Kar and Maity 2016b).
Singh and Srivastava (2016) have proposed Gumble Probability Distribution Function (GPDF)-based matched filter to segment retinal BVs. The PCA-based gray scale conversion and CLAHE are applied as preprocessing steps to enhance retinal BVs. The matched filter response is generated by GPDF-based matched filter. The vessel segmentation is done by entropy-based optimal thresholding, length filtering and removing outer artifacts. The methodology has attained a true positive rate of 0.7594, 0.7939, false positive rate of 0.0292, 0.0624 and an ACC of 0.9522, 0.9270 respectively for DRIVE and STARE databases (Singh and Srivastava 2016).
6.1.3 Thresholding-Based Approach
Christodoulidis et al. (2016) have developed multi-scale tensor voting to segment retinal BVs. The multi-scale line detection is employed to segment small retinal BVs. Adaptive thresholding is used to segment medium and large sized BVs, and multi-scale tensor voting is employed for reconnection of fragmented vessels at variable distances. The proposed method has yielded an improved vessel detection rate of 7.8% against the original multi-scale line detection method (Christodoulidis et al. 2016).
Imani et al. (2015) have employed the Morphological Component Analysis (MCA) to enhance BVs. The lesions and BVs are separated by MCA, and retinal vessels are enhanced by Morlet wavelet transform. Adaptive thresholding is employed to segment final vessel map. The proposed method has yielded an SE of 75.24%, 75.02%, SPE of 97.53%, 97.45% and ACC of 95.23%, 95.90% respectively (Imani et al. 2015).
6.1.4 Other Methods
GeethaRamani and Balasubramanian (2016) have employed image processing and data mining techniques to segment BVs. The image pre-processing, supervised and unsupervised method and image post-processing are the three major steps in the segmentation of BVs. The preprocessing steps used in this algorithm include image cropping, color transformation, color channel extraction, contrast enhancement, Gabor filtering and half wave rectification. Feature extraction is performed by PCA, and classification of vessel or non-vessel cluster is done by k-means clustering. The segmentation of BVs is done by k-means clustering and ensemble classifier. Mathematical morphology and connected component analysis are used as post-processing step. This methodology has yielded an SE of 70.79%, SPE of 97.78%, ACC of 95.36% and Positive Predictive Value (PPV) of 75.76% respectively in DRIVE database (GeethaRamani and Balasubramanian 2016).
Kovács and Hajdu (2016) have performed a self-calibrating approach for segmentation of BVs. The ROI is selected, and binary segmentation is performed by template matching. The edge reconstruction, thin and low contrast vessels are extracted by the trained database. This methodology has yielded an average ACC of 94.94% and 96.10% respectively in DRIVE and STARE databases (Kovács and Hajdu 2016).
Jebaseeli et al. (2019) has used Tandem PCNN model to segment the BVs and deep learning SVM for classification of BVs. The proposed method achieved the SE of 80.61%, SPE of 99.54% and ACC of 99.49% (Jebaseeli et al. 2019). Dash and Senapati (2020) had developed the algorithm to segment BVs by combining discrete wavelet transform (DWT) with Tyler Coye algorithm and achieved the SE of 74.03%, 99.05% and 96.61% (Dash and Senapati 2020). Jiang et al. (2018) used convolution network and transfer learning to segment BVs. The success rate of this algorithm is increased to 1–2% than the existing method (Jiang et al. 2018). Table 1 shows the comparison of performance measures of Blood vessel detection algorithm.
7 Detection of Diabetic Macula Edema
In the literature, numerous examples of the application of digital imaging techniques used in identification of DME can be found. There have been a few research investigations to identify EXs in macula region in the literature.
Akram et al. (2014) have presented a novel method for EX and macula detection by binary map of candidate using filter bank and Gaussian mixture model. A hybrid classifier is an ensemble of Gaussian mixture model and SVM used for classification of images into normal, CSME and non-CSME. This method has yielded an SE, SPE and ACC of 97.3%, 95.9% and 96.8% respectively on HEI-MED and MESSIDOR databases (Akram et al. 2014).
Giancardo et al. (2012) have proposed color, wavelet decomposition and automatic lesion segmentation for diagnosis of DME. The correlation between color/wavelet analysis and EXs probability map is captured by five measures like mean, median, SD, minimum and maximum. The probabilistic, geometric and tree-based techniques are used for classification of DME. The effectiveness of the algorithm is tested with the performance measure of AUC between 0.88 and 0.94 on MESSIDOR database (Giancardo et al. 2012).
Deepak and Sivaswamy (2011) have proposed a supervised learning for DME detection. The exudates are detected by motion pattern generation, and severity of abnormality is calculated by rotational asymmetry of the macular region. This algorithm is evaluated by an ACC of 81% for the moderate case and 100% for the severe case (Deepak and Sivaswamy 2011).
Srinivas et al. (2019) has analyzed the effect of Intravitreal ranibizumab on hard exudates in DME. This work can be extended with large sample size and duration more than 1 year (Srinivas et al. 2019).
8 Conclusion
A survey of techniques for the automatic detection of DR and DME has been presented in this research article. The proposed method involves two phases
-
(a)
Diagnosis of DR
-
(b)
Detection of DME.
The intention in this research work is to identify abnormalities from retinal images, locate and segment important structural indicators of eye diseases to extract various features from retinal structures and to classify them based on their severity. Considering the current circumstances in recent developments, the study focuses on, Differentiating bright and dark lesions of varying shapes, size and appearance as appropriate lesion to classify stages of DR and detecting EXs in macula region for diagnosis of DME
References
Abràmoff MD, Garvin MK, Sonka M (2010) Retinal imaging and image analysis. IEEE Rev Biomed Eng 3:169–208
Akram MU, Khalid S, Khan SA (2013) Identification and classification of microaneurysms for early detection of diabetic retinopathy. Pattern Recogn 46(1):107–116
Akram MU, Tariq A, Khan SA, Javed MY (2014) Automated detection of exudates and macula for grading of diabetic macular edema. Comput Methods Prog Biomed 114(2):141–152
Aslani S, Sarnel H (2016) A new supervised retinal vessel segmentation method based on robust hybrid features. Biomed Signal Process Control 30:1–2
Christodoulidis A, Hurtut T, Tahar HB, Cheriet F (2016) A multi-scale tensor voting approach for small retinal vessel segmentation in high resolution fundus images. Comput Med Imaging Graph 52:28–43
Ciulla TA, Amador AG, Zinman B (2003 Sep 1) Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 26(9):2653–2664
Dash S, Senapati MR (2020) Enhancing detection of retinal blood vessels by combined approach of DWT, Tyler Coye and Gamma correction. Biomed Signal Process Control 57:101740
Decencière E, Zhang X, Cazuguel G, Lay B, Cochener B, Trone C, Gain P, Ordonez R, Massin P, Erginay A, Charton B (2014) Feedback on a publicly distributed image database: the Messidor database. Image Anal Stereol 33(3):231–234
Deepak KS, Sivaswamy J (2011) Automatic assessment of macular edema from color retinal images. IEEE Trans Med Imaging 31(3):766–776
GeethaRamani R, Balasubramanian L (2016) Retinal blood vessel segmentation employing image processing and data mining techniques for computerized retinal image analysis. Biocybern Biomed Eng 36(1):102–118
Giancardo L, Meriaudeau F, Karnowski TP, Li Y, Garg S, Tobin KW Jr, Chaum E (2012) Exudate-based diabetic macular edema detection in fundus images using publicly available datasets. Med Image Anal 16(1):216–226
González Cortés JH (2015) Treatment of diabetic macular edema (DME): shifting paradigms. Med Univ 17(69):243–247
Gonzalez RC, Woods RE (eds) (2002) Digital image processing. Prentice Hall Press, Upper Saddle River, p 8. ISBN 0-201-18075
Hoover AD, Kouznetsova V, Goldbaum M (2000) Locating blood vessels in retinal images by piecewise threshold probing of a matched filter response. IEEE Trans Med Imaging 19(3):203–210
Imani E, Javidi M, Pourreza HR (2015) Improvement of retinal blood vessel detection using morphological component analysis. Comput Methods Prog Biomed 118(3):263–279
Jebaseeli TJ, Durai CA, Peter JD (2019) Retinal blood vessel segmentation from diabetic retinopathy images using tandem PCNN model and deep learning based SVM. Optik 199:163328
Jiang Z, Zhang H, Wang Y, Ko SB (2018) Retinal blood vessel segmentation using fully convolutional network with transfer learning. Comput Med Imaging Graph 68:1–5
Kar SS, Maity SP (2016a) Blood vessel extraction and optic disc removal using curvelet transform and kernel fuzzy c-means. Comput Biol Med 70:174–189
Kar SS, Maity SP (2016b) Retinal blood vessel extraction using tunable bandpass filter and fuzzy conditional entropy. Comput Methods Prog Biomed 133:111–132
Kauppi T, Kalesnykiene V, Kamarainen JK, Lensu L, Sorri I, Uusitalo H, Kälviäinen H, Pietilä J (2006) DIARETDB0: Evaluation database and methodology for diabetic retinopathy algorithms. Lappeenranta University of Technology, Finland, Machine Vision and Pattern Recognition Research Group, p 134
Kauppi, T, Kalesnykiene, V, Kamarainen, J.-K, Lensu, L, Sorri, I, Raninen A, Voutilainen R, Uusitalo, H, Kälviäinen, H & Pietilä, J (2007) DIARETDB1 diabetic retinopathy database and evaluation protocol, in proceedings of the eleventh conference on Medical Image Understanding and Analysis, pp 1–10
Kovács G, Hajdu A (2016) A self-calibrating approach for the segmentation of retinal vessels by template matching and contour reconstruction. Med Image Anal 29:24–46
Lazar I, Hajdu A (2012) Retinal microaneurysm detection through local rotating cross-section profile analysis. IEEE Trans Med Imaging 32(2):400–407
Mahendran G, Dhanasekaran R (2015) Investigation of the severity level of diabetic retinopathy using supervised classifier algorithms. Comput Electr Eng 45:312–323
Mookiah MR, Acharya UR, Fujita H, Koh JE, Tan JH, Chua CK, Bhandary SV, Noronha K, Laude A, Tong L (2015) Automated detection of age-related macular degeneration using empirical mode decomposition. Knowl-Based Syst 89:654–668
Olson JL, Asadi-Zeydabadi M, Tagg R (2015) Theoretical estimation of retinal oxygenation in chronic diabetic retinopathy. Comput Biol Med 58:154–162
Roychowdhury S, Koozekanani DD, Parhi KK (2014) Blood vessel segmentation of fundus images by major vessel extraction and subimage classification. IEEE J Biomed Health Inform 19(3):1118–1128
Singh NP, Srivastava R (2016) Retinal blood vessels segmentation by using Gumbel probability distribution function based matched filter. Comput Methods Prog Biomed 129:40–50
Sivaswamy J, Krishnadas S, Chakravarty A, Joshi G, Tabish AS (2015) A comprehensive retinal image dataset for the assessment of glaucoma from the optic nerve head analysis. JSM Biomed Imaging Data Pap 2(1):1004
Srinivas S, Verma A, Nittala MG, Alagorie AR, Nassisi M, Gasperini J, Sadda SR (2019) Effect of intravitreal ranibizumab on intraretinal hard exudates in eyes with diabetic macular edema. Am J Ophthalmol
Tang L, Niemeijer M, Reinhardt JM, Garvin MK, Abramoff MD (2012) Splat feature classification with application to retinal hemorrhage detection in fundus images. IEEE Trans Med Imaging 32(2):364–375
Wang S, Yin Y, Cao G, Wei B, Zheng Y, Yang G (2015) Hierarchical retinal blood vessel segmentation based on feature and ensemble learning. Neurocomputing 149:708–717
Yun WL, Acharya UR, Venkatesh YV, Chee C, Min LC, Ng EY (2008) Identification of different stages of diabetic retinopathy using retinal optical images. Inf Sci 178(1):106–121
Zaki WM, Zulkifley MA, Hussain A, Halim WH, Mustafa NB, Ting LS (2016) Diabetic retinopathy assessment: towards an automated system. Biomed Signal Process Control 24:72–82
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Suriyasekeran, K., Santhanamahalingam, S., Duraisamy, M. (2020). Algorithms for Diagnosis of Diabetic Retinopathy and Diabetic Macula Edema- A Review. In: Islam, M.S. (eds) Diabetes: from Research to Clinical Practice. Advances in Experimental Medicine and Biology(), vol 1307. Springer, Cham. https://doi.org/10.1007/5584_2020_499
Download citation
DOI: https://doi.org/10.1007/5584_2020_499
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51088-6
Online ISBN: 978-3-030-51089-3
eBook Packages: MedicineMedicine (R0)