Abstract
The incidence of chronic inflammatory diseases (CIDs) is increasing worldwide. Their dramatic rise associated with limited effective strategies to slow down these epidemics calls for a better understanding of their pathophysiology in order to decrease the burdens on childhood. Several cross-sectional studies have demonstrated the association between intestinal dysbiosis and active diseases. Although informative, these studies do not mechanistically link alterations of the microflora with disease pathogenesis and, therefore, with potential therapeutic targets. More prospective studies are needed to determine whether intestinal dysbiosis plays a causative role in the onset and development of CIDs. Furthermore, given the complexity of the microflora interaction with the host, it is necessary to design a systems-level model of interactions between the host and the development of disease by integrating microbiome, metagenomics, metatranscriptomics, and metabolomics with either clinical either environmental data.
In this chapter we will discuss the current knowledge regarding the microbiome’s contribution to celiac disease and inflammatory bowel disease with a particular focus on how probiotics may be used as potential preventive therapy for CIDs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 The Potential Role of the Human Microbiome in CIDs
In the last three to four decades, the incidence of allergies and autoimmune diseases has been dramatically rising in the United States (USA) with a significant impact on the pediatric population (Bach 2002). The “hygiene hypothesis” suggests that this rise is mainly due to the reduced number of infections occurring at early age (Greenwood 1968, Strachan 1989); however, its accuracy is continuously revised (Versini et al. 2015). Since the inception of the Human Microbiome Project, our knowledge about the composition and function of the human microbiome has been rapidly expanding.
It is now well established that the microbiome can affect many key functions of the symbiotic host, including gut mucosal barrier maturation and to the development and function of the gut-associated lymphoid tissue (GALT).
Recent studies have shown that a specific microflora is present in both the human placenta (Aagaard et al. 2014) and meconium (Vicario et al. 2010), therefore suggesting that the establishment of the microbiome may occur already during the prenatal period. This is confirmed by in vivo animal studies showing how the maternal commensal flora presented to the fetus during the last trimester of pregnancy contributes to its immune system maturation and oral tolerance establishment (Rescigno et al. 2001). Furthermore, research on the effect of delivery mode, maternal/infant diet, antibiotic exposure, and surrounding environment has suggested that all these factors may significantly affect the early development of the infant intestinal microbiome, in particular within the first 3 years of life (Dominguez-Bello et al. 2010, Azad et al. 2013). Hence, it is plausible to infer that changes in the development of a healthy microbiome ecosystem occurring early in life may have lasting effects (Yatsunenko et al. 2012). Several studies have demonstrated the protective role that exposure to healthy and diverse commensal species early in life have against CIDs (Fig. 1). During the last decades, the need for knowledge about human microflora composition and function including the study of microbiome, metagenome, metatranscriptome, and metabolome has been made possible by the vast array of new technologies that have become available. With the field growing, a multi-omic systems biology research approach in pediatrics could amplify our understanding of many common pediatric diseases in a revolutionary way. This would help to identify possible targets for disease treatment, as well as for its prevention.
Stages of immune system maturation and enhancement of barrier function during the first years of life. The initial seeding of the microbiota starts during the prenatal stage in utero where the commensal flora present in the maternal placenta initiates signaling with the fetal mucosa (1). At birth the newborn is directly exposed to the mother (via delivery and breast milk) and environment-derived bacterial antigens. This interaction induces epithelial cells differentiation, tight junction enhancement, and immune cells proliferation (2). Secretory IgA (SIgA) favor diversity in the bacteria community (3)
2 Pathogenesis of Celiac Disease and Inflammatory Bowel Diseases
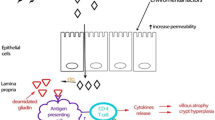
Celiac disease (CeD) and inflammatory bowel diseases (IBD) are chronic inflammatory intestinal conditions that overall affect between 0.5% and 1% of the worldwide population (Reilly et al. 2012). While gluten has been recognized to be the external trigger responsible for the onset of CeD, the precise pathogenesis mechanisms responsible for IBD are still unknown (Kaplan 2015). For both conditions environmental and genetic risk factors have been described to play a role. Development of CeD has been strongly associated with HLA DQ2 and/or DQ8. These specific haplotypes of the human leukocyte antigen system contribute to 40% of the genetic variance with 95% of CeD patients carrying an HLA DQ2 and 5% a HLA DQ8 (Leonard et al. 2015b). Additionally to the HLA genotype, genome-wide association studies (GWAS) have identified more genes associated with CeD. Among these genes, interleukin-18 (IL18), interleukin-21 (IL21), and chemokine receptors CCR1, CCR2, and CCR5 are involved in immune function, while others, such as MYO9B, PARD3, and MAG12, are associated with defects in intestinal permeability (Leonard et al. 2015b). Several studies in monozygotic and dizygotic twins have provided powerful evidence of the genetic susceptibility that contributes to the development of IBD (Thompson et al. 1996, Orholm et al. 2000). Nine IBD susceptibility loci have been described, some specific for Crohn’s disease and others related to IBD as a whole (Lees and Satsangi 2009). Furthermore GWAS studies have highlighted 50 loci specifically associated with IBD including genes involved in bacterial recognition (NOD2), autophagy (ATG16L1 and IRGM), immune response (IL23R, IL12B, STTA3, JAK2), as well as specific HLA loci (Lees and Satsangi 2009). Both CeD and IBD pathogeneses are characterized by increased intestinal permeability that contributes to the overall chronic intestinal inflammation. In CeD patients the interaction between gliadin peptides and G protein-coupled receptor CXCR3 on enterocytes (Lammers et al. 2008) triggers the MyD88-dependent release of zonulin, a potent intestinal barrier function (Tripathi et al. 2009, Drago et al. 2006). The consequent translocation of gliadin peptides into the lamina propria activates the immune response machinery. Interleukin-15 (IL15) production from enterocytes has an apoptotic and pro-inflammatory effect on the epithelium (Escudero-Hernandez et al. 2017, Maiuri et al. 2000, Malamut et al. 2010); release of intereleukin-8 (IL8) leads to neutrophils recruitment (Lammers et al. 2011, Lammers et al. 2015), followed by macrophages activation and proliferation of intraepithelial cytotoxic lymphocytes. After transglutaminase-induced deamidation, gliadin peptides are recognized in the lamina propria by Th1 and Th17 cells (Garrote et al. 2008). This leads to production of pro-inflammatory cytokines such as interferon gamma (IFNγ), interleukin-17 (IL17) and tumor necrosis factor-alpha (TNFα), and activation of humoral immune response with IgA and IgG anti-Ttg antibodies production (Fasano 2011). Finally the inflammatory response is exacerbated by the defective function of T regulatory cells (Serena et al. 2017, Granzotto et al. 2009).
Contrary to CeD, the external trigger for IBD is not known, and its pathogenesis is still unclear (Kaplan 2015). Increasing evidence suggests the contribution of impaired barrier function to the disease (Chassaing and Darfeuille-Michaud 2011). IBD patients are characterized by increased paracellular permeability and abnormalities in tight junctions (TJs) proteins expression and distribution. Furthermore the abundant production of pro-inflammatory TNFα has been associated with TJs transcription regulation, and patients with ulcerative colitis have been shown to have defective mucosal barrier structure (Rosenstiel et al. 2007). The increased intestinal permeability characterizing IBD patients promotes bacterial translocation through the intestinal mucosa (Chassaing and Darfeuille-Michaud 2011). The interaction between commensal microbes and specific epithelial receptors triggers in susceptible individuals a chronically active inflammation that causes the perpetuation of the disease (Fiorucci et al. 2002). Dendritic cells and macrophages recognize microbes through pattern recognition receptors. Their activation stimulates pro-inflammatory signaling cascades that lead to production of a wide range of cytokines (IL6, IL12, TGFβ) and initiation of adaptive immune response with proliferation of Th1/Th17 cells and production of IFNγ, TNFα, and interleukin-2 (IL2) (Lees and Satsangi 2009). Patients with IBD have been shown to exhibit a reduced number of peripheral T regulatory cells with decreased suppressive function in the mucosa (Eastaff-Leung et al. 2010).
Overall both CeD and IBD are multifactorial and complex conditions in which environment and genetic susceptibility equally contribute to their onset and development.
3 Role of Microbiome in CeD and IBD
3.1 Celiac Disease
CeD represents a unique model of autoimmunity in that the genetic predisposition with HLA DQ2 and/or DQ8 (Schuppan 2000) and the environmental trigger (gluten) are known. Furthermore disease-specific autoantibodies have been identified and can be routinely measured. The precise moment in which the loss of tolerance to gluten occurs can be precisely determined by knowing when the environmental trigger (gluten) has been introduced in the diet and, most importantly, by frequently screening genetically predisposed individuals for the appearance of tissue transglutaminase (tTG) autoantibodies (Schuppan 2000).
Dysbiosis has been associated with the development of CeD by several groups. In vitro studies have shown that commensal bacteria can affect the digestion of gliadin, as well as cytokines production and intestinal barrier function upon stimulation with gliadin (Laparra and Sanz 2010, Lindfors et al. 2008). Numerous studies have described the microbial composition and phylogenetic diversity of fecal and small intestinal samples in CeD patients compared to healthy controls and how these can be related to age, disease status, and symptoms (Wacklin et al. 2013). Additionally to alterations in the microbiome compositions, also changes in production of specific metabolites such as short chain fatty acids (SCFA) in the stools have been described in active CeD patients and associated with the described dysbiosis (Di Cagno et al. 2011). However, the lack of standardization in specimen collection, analysis pipelines, and patients’ information make it difficult to compare these studies.
Given the high HLA DQ2/DQ8 penetrance that characterizes CeD patients, evaluating the association between differences in the colonization of the gastrointestinal tract and the genetic makeup may contribute to determine the infant’s future risk of developing CeD (Tjellstrom et al. 2013, Olivares et al. 2015). Previous studies have shown that genetically predisposed infants carrying an HLA DQ2 are characterized by an increased abundance of Firmicutes and Proteobacteria compared to control infants (Sellitto et al. 2012). Similarly, at-risk infants (first-degree relatives of CeD patients) genetically compatible with CeD showed a reduction in Bacteroidetes, a higher abundance of Firmicutes, and slower microbiota during the first 2 years after birth (Sellitto et al. 2012).
Lionetti et al. have shown that a later introduction of gluten in the infant’s diet only transiently delays the development of CeD but do not prevent its onset (Lionetti et al. 2014). Another study has demonstrated that introducing small amounts of gluten between 16 and 24 weeks does not influence the overall incidence of CeD (Vriezinga et al. 2014). While the timing of gluten introduction does not seem to have pathological and preventive relevance, the effect that gluten has on altering the host microbiome in genetically predisposed individuals is well established. Firmicutes and Proteobacteria phyla have been shown to change in abundance upon introduction of gluten in the diet (Sellitto et al. 2012). Furthermore, this same study showed how kids that developed some kind of autoimmunity were characterized by high levels of lactate and high abundance of Lactobacilli species in the stools preceding the first detection of positive CD antibodies, therefore suggesting the possibility of identifying biomarker predictors in the microbiota (Sellitto et al. 2012).
3.2 Inflammatory Bowel Disease
Although the involvement of microorganisms in the pathogenesis of IBD has been previously suggested, data confirming the causative role of specific pathogens in triggering the chronic inflammatory cascade that characterizes IBD are still missing. Although specific mutations in pattern recognition receptors (PRR) have been linked to IBD (Corridoni et al. 2014), these genetic mutations appeared to be present only in a subgroup of IBD patients. This finding suggests that genetic makeup can only partially explain IBD. Hence the hypothesis that additionally to the genetic predisposition, other factors may contribute to the immune dysregulation that characterizes IBD (Barclay et al. 2008). Recent studies have shown that an altered immune response to commensal microflora is able to trigger IBD pathogenesis and that this exacerbated immune response that follows is driven, in turn, by specific genetic makeup and dysbiosis (Khor et al. 2011, Manichanh et al. 2012, Gevers et al. 2014, Morgan et al. 2012). Although the lack of consistency in studies design and data analysis makes the comparison among these studies very difficult, reduction in biodiversity and altered abundance of different taxa (Morgan et al. 2012, Manichanh et al. 2006) are findings that have been confirmed by the majority of research groups. A study from Manichanh et al. performed in Crohn’s (CH) patients reported an increased abundance in Bacteroidetes and Firmicutes as compared to control individuals. Interestingly the number of Firmicutes’ rybotypes is reduced in CH patients (Manichanh et al. 2006). A study looking at the microbiome composition in CH patients and how this relates to disease activity shows that the acute phase of the disease is characterized by decreased diversity of Bacteroidetes as compared to the remission patients (Seksik et al. 2003). In addition to the disease activity, also the site of the disease inflammation appears to contribute to the microbiota composition with higher Firmicutes diversity in subjects with colonic disease and reduced diversity in patients with affected ileum (Willing et al. 2010). As expected, the site of sampling has been shown to influence microbiome analysis data in IBD studies. Mucosal samples from CH patients show increased abundance of Enterobacteriaceae and reduced diversity of Feacalibacteria, while analysis from fecal samples gave opposite results (Swidsinski et al. 2002, Chassaing and Darfeuille-Michaud 2011).
Data on intestinal dysbiosis in pediatric IBD patients are scarcer. Two unreported strains of adhesive-invasive E. coli (AIEC) were in pediatric IBD patients and were associated with upregulation of CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6), tumor necrosis factor-α, and IL8 gene/protein expression (Negroni et al. 2012). Furthermore children with ulcerative colitis that were not responsive to steroids appeared to have decreased fecal microbiome diversity with abundant Firmicutes, Verrucomicrobia, and Lentisphaerae (Michail et al. 2012).
While the above studies are mainly descriptive, Morgan et al. described the association between microbial dysfunction metabolism and bacterial signaling pathways suggesting a mechanistic link between intestinal dysbiosis and IBD onset (Morgan et al. 2012).
4 Possible Probiotics Interventions to Manipulate Microbiome and Challenges to Their Implementation
For long time, probiotics have been considered promising candidates for treatment and prevention of CIDs such as CeD and IBD. If the causative role of dysbiosis on CIDs was confirmed, the manipulation of microbiome composition via probiotics in order to resupply/rebalance the gut ecosystem should improve the overall health of the microflora and therefore contribute to the disease prevention. While the theory of probiotics use is exciting, its efficacy is still under debate, mainly due by the still uncertain idea of what a healthy microbiota consists of. Currently, diversity is considered the main characteristic for a healthy microbiome. Several studies have shown the association between reduced microbial diversity and many diseases (Falony et al. 2016). The use of probiotics can be challenging also for technical reasons: while most studies describe dysbiosis at phylum level, the most commonly available probiotics contain specific species, therefore creating a gap between our knowledge on microbiome and potential therapies. Mechanistic studies looking at how specific probiotics may positively impact intestinal health failed in taking in considerations the complex biological networks that play a part in disease development. Therefore, it is desirable for the future research to focus on how specific microbiota signatures may contribute to the disease development before exploring therapeutic treatments. An example relative to what stated above is presented by a recent phase 3 clinical trial about NEC prevention in which data showed the lack of efficacy in administering the probiotic Bifidobacterium breve to premature infants (Costeloe et al. 2016). While this probiotic was chosen among others because it is commercially available and because of its reported positive nutritional outcomes, no data confirmed its benefits in preventing NEC. A prospective cross-sectional observational birth cohort study describing the microbiome profile of premature infants later affected by NEC showed no evidence that reduction of Bifidobacteria could be associated with NEC development, therefore predicting the failure of the preventive intervention with Bifidobacterium breve.
This work highlights the importance of identifying the microbiome signature associated to a specific condition before designing an interventional study. Knowledge about the microbiome composition paired with mechanistic understanding of how specific bacteria contribute to the disease development would greatly help in using probiotics in a more effective manner.
5 Use of Probiotics for CeD and IBD Prevention and/or Treatment
5.1 Celiac Disease
Probiotics have been used in several clinical trials as an additional treatment to the gluten free diet (Table 1). Probiotics, in fact, are not only potent modulator of immune response and barrier function, but they also represent an important source of endopeptidases to detoxify nondigestible gluten epitopes (Francavilla et al. 2017, Madsen et al. 2001). Specific Lactobacilli species have been shown to lyse the proline−/glutamine-rich gluten peptides reducing their immunotoxicity and to decrease gluten concentration to less than 10 ppm (Rizzello et al. 2007). The efficacy of Lactobacilli in digesting gluten has been proved in vivo by challenging CD patients in remission with probiotic predigested sweet gluten containing baked goods. The lack of worsening in hematological, serological parameters, and intestinal permeability during 60 days of challenge in the participants suggested that Lactobacilli-derived endopeptidases were able to completely degrade gluten and annul its toxicity for CeD patients (Francavilla et al. 2017). De Simone Formulation, a probiotic mixture of eight lactic acid and Bifidobacteria, has been shown to hydrolyze completely several α2-gliadin-derived epitopes and regulate the epithelial barrier function by stabilizing tight junctions (De Angelis et al. 2006, Madsen et al. 2001). Orlando et al. have shown that Lactobacillus rhamnosus is able to reduce the gliadin-mediated production of polyamines, therefore restoring the intestinal barrier function and reducing zonulin release (Orlando et al. 2014). In a similar study in which CaCo2 cells were stimulated with gliadin peptides, the authors have demonstrated that fermentation of gliadin synthetic peptides with Lactobacillus paracasei was interfering with their entrance in the epithelial compartment (Sarno et al. 2014).
Additionally to the endopeptidases activities, probiotics have been also demonstrated to reduce the gluten-induced inflammation by modulating the immune response. Bifidobacterium breve has been shown to prevent intestinal inflammation in CeD pediatric patients by inducing production of anti-inflammatory cytokine interleukin-10 (IL10) by Tr1 cells and reducing TNFα serum level (Zheng et al. 2014, Jeon et al. 2012). Other two studies conducted in children with CeD have demonstrated that consumption of different strains of B. breve for 3 months reconstituted the microbiome composition by reducing the abundance of Verrucomicrobia and production of short chain fatty acids (Primec et al. 2017), re-establishing the physiological Firmicutes/Bacteroides ratio and increasing the Actinobacteria abundance (Primec et al. 2017). Similarly, Olivares et al. have shown that administration of Bifidobacterium longum in children with CeD triggered a reduction of peripheral CD3+ T cells as well as decreased levels of TNFα and fecal IgA (Olivares et al. 2018).
While the number of studies involving probiotic preparations as a treatment for CeD is substantial, the literature about the use of probiotic as preventive measure in genetically predisposed individuals is rather modest. Given the important role that the microbiome plays in the development of the immune system, a deeper understanding on how shaping the microbiome composition may influence the final outcome of the disease is fundamental. Additionally the clinical trials developed until these days do not follow the enrolled subjects for extensive time. Further studies are deemed to better understand the long-term effect of the use of probiotics in the contest of CeD to better understand how to use them in a safe and proper manner.
5.2 Inflammatory Bowel Disease
Probiotics have been considered possible therapeutic tools for IBD for long time (Table 1). They can influence the microbiome composition and function, produce antibacterial substances, enhance barrier function, reduce intestinal permeability, and modulate innate and adaptive immune response (Orel and Kamhi Trop 2014).
Several groups have explored the potential of De Simone Formulation as possible treatment for IBD. The probiotic mixture has been shown to significantly enhance the remission stage of patients with ulcerative colitis (UC) when administered in combination with immunosuppressants and/or amynosalicylates (Bibiloni et al. 2005, Tursi et al. 2010). Furthermore De Simone Formulation has been also shown to be useful in maintaining the remission stage in UC patients both in adult and pediatric populations (Miele et al. 2009). Kato et al. tested Bifidobacteria-fermented milk (BFM) supplementation as a dietary adjunct to treat UC and concluded that the supplementation was more effective than conventional treatment (Kato et al. 2004). Similarly, Ishikawa et al. found that BFM supplementation triggered a reduction in the relative proportion of Bacteroidaceae and butyrate production in UC patients (Ishikawa et al. 2003). A prospective randomized placebo-controlled study showed that Lactobacillus reuteri improved the inflammation in UC patients by increasing IL10 production and reducing the levels of pro-inflammatory interleukin-1β (IL1β), TNFα, and IL8 (Oliva et al. 2012). Similarly, also Bifidobacterium longum and Bifidobacterium breve have been shown to reduce inflammation in UC patients as well as increase regeneration of epithelial cells (Furrie et al. 2005). Treatment with Lactobacillus rhamnosus GG significantly improved the clinical activity of CH pediatric patients by enhancing barrier function (Butterworth et al. 2008) as well as maintaining the disease remission (Schultz et al. 2004). Other studies however did not find the same promising results in their study (Gupta et al. 2000). Fujimori et al. conducted an open-label study in which patients with CH were treated with two probiotic preparations containing Bifidobacterium breve, Lactobacillus casei, and Bifidobacterium longum. The study showed an amelioration of clinical symptoms in the cohort and concluded that high doses of probiotics could be safely used as co-therapy for the treatment of CH (Fujimori et al. 2007). Finally different studies utilizing Bifidobacteria strains have shown promising results in CH patients with improvement of the mean histological score and reduction of pro-inflammatory TNFα (Steed et al. 2010).
While probiotics have great potential for future therapeutic approaches in IBD, further studies are necessary to ameliorate the specific conditions of the treatment.
6 Prospective Longitudinal Studies as Ideal Approach to Identify Targets for Disease Prevention Through Probiotics: The CDGEMM Proof of Concept Project
Different groups have reported that genetic risk for developing autoimmune diseases is associated with alterations in the microbiota composition (Sellitto et al. 2012, Olivares et al. 2015). For example, reduced abundance of Bacteroidetes and increased Firmicutes were described in infants at risk for CeD (Sellitto et al. 2012). The microbiota of these infants also showed a delay in maturation at 2 years of age (Sellitto et al. 2012) as compared to not at-risk control group, while the maturation was complete in not at-risk infants at 1 year (Palmer et al. 2007). Additionally, this same study showed that preceding the detection of positive antibodies, the microbiome of infants that developed an autoimmunity was characterized by reduction in Lactobacillus species (Sellitto et al. 2012). Similar data were confirmed in a later study in which differences between the microbiota compositions at 1 month of age were observed, with infants genetically predisposed showing increased Firmicutes and Proteobacteria compared to infants without a genetic predisposition (Olivares et al. 2015).
Although extremely informative, these studies have the limitation of having a cross-sectional design, therefore assuming that alterations in the microbiome are causative of the disease. However, changes in the microbiome may also represent a consequence of the disease rather than the cause. To correct this limitation, longitudinal prospective studies are needed to link the microbiome composition and function with the pathogenesis of CIDs.
Two longitudinal studies prospectively screened infants, with a first-degree family member with CeD, from birth and found that CeD can develop very early in life. The first study found that 16% of infants with a first-degree relative with CeD and carrying HLA DQ2 and/or DQ8 will develop CeD by age 5 (Lionetti et al. 2014). The second study demonstrated that 38% of infants with first-degree relatives of CeD patients and carrying two copies of DQ2 will develop CeD by age 5 (Vriezinga et al. 2014). These findings support the hypothesis that early environmental factors may strongly contribute to the development of CeD.
Unfortunately, the abovementioned studies did not look at microbiome composition or function. To date no large-scale, longitudinal studies have defined the mechanistic contribution of the gut microbiota in the pathogenesis of CeD. The Celiac Disease Genomic, Environmental, Microbiome, and Metabolomic (CDGEMM) has been designed to answer this question. Its aim is to understand the role that the gut microbiome plays in early steps involved in the pathogenesis of autoimmunity (Leonard et al. 2015a). This study is conceptualized based on the hypothesis that the combination of gluten and specific microbiota in infants genetically at risk for CeD triggers specific metabolic pathways that can epigenetically change the function of the immune system leading to the loss of gluten tolerance and subsequent onset of the autoimmune process. The ultimate goal of the CDGEMM study is the identification and validation of specific microbiome and metabolomic profiles able to predict loss of tolerance in children genetically at risk of autoimmunity.
7 Concluding Remarks and Future Directions
The incidence of CIDs is alarmingly rising. While research about the function of intestinal microbiome is expanding, therapeutic and preventive applicability are still poor. Recent studies have evaluated the microbiota focusing on the phylogenetic differences, different time points, different samples types, and different analysis tools. The focus of these studies has been mostly on the bacterial component of the microbiome excluding analysis on virome, fungi, and parasites. Additional studies looking at the mechanisms through which the microflora interacts with the host are needed. This is the only way we will be able to understand how to design interventions for treatment and prevention.
The transition from descriptive to mechanistic microbiome studies is fundamental to succeed in translational medicine. Researchers working with pediatric populations are the only ones that can start this transition given the hypothesis that the development of the microbiome during the first 1000 days of life has a lasting effect on an individual’s future health and risk of disease.
Large-scale, multicenter, prospective, longitudinal, multi-omic studies are required to design therapeutic strategies and targeted prevention therapies using probiotics as tool to modulate the microbiome composition and function. Ideally, data collection should start before the development of the disease in order to understand the role that the microbiome plays in the development of disease.
References
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J (2014) The placenta harbors a unique microbiome. Sci Transl Med 6:237ra65
Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL, Investigators CS (2013) Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185:385–394
Bach JF (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347:911–920
Barclay AR, Morrison DJ, Weaver LT (2008) What is the role of the metabolic activity of the gut microbiota in inflammatory bowel disease? Probing for answers with stable isotopes. J Pediatr Gastroenterol Nutr 46:486–495
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB (2005) VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100:1539–1546
Butterworth AD, Thomas AG, Akobeng AK (2008) Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 3:CD006634
Chassaing B, Darfeuille-Michaud A (2011) The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1720–1728
Corridoni D, Arseneau KO, Cifone MG, Cominelli F (2014) The dual role of nod-like receptors in mucosal innate immunity and chronic intestinal inflammation. Front Immunol 5:317
Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR, Probiotics in Preterm Infants Study Collaborative, G (2016) Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 387:649–660
De Angelis M, Rizzello CG, Fasano A, Clemente MG, De Simone C, Silano M, De Vincenzi M, Losito I, Gobbetti M (2006) VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim Biophys Acta 1762:80–93
Di Cagno R, De Angelis M, De Pasquale I, Ndagijimana M, Vernocchi P, Ricciuti P, Gagliardi F, Laghi L, Crecchio C, Guerzoni ME, Gobbetti M, Francavilla R (2011) Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol 11:219
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 107:11971–11975
Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D’agate C, Not T, Zampini L, Catassi C, Fasano A (2006) Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 41:408–419
Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S (2010) Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol 30:80–89
Escudero-Hernandez C, Plaza-Izurieta L, Garrote JA, Bilbao JR, CEGEC, Arranz E (2017) Association of the IL-15 and IL-15Ralpha genes with celiac disease. Cytokine 99:73–79
Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J (2016) Population-level analysis of gut microbiome variation. Science 352:560–564
Fasano A (2011) Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 91:151–175
Fiorucci S, Distrutti E, Mencarelli A, Barbanti M, Palazzini E, Morelli A (2002) Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion 66:246–256
Francavilla R, De Angelis M, Rizzello CG, Cavallo N, Dal Bello F, Gobbetti M (2017) Selected probiotic lactobacilli have the capacity to hydrolyze gluten peptides during simulated gastrointestinal digestion. Appl Environ Microbiol 83(14):e00376–e00317
Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A, Kobayashi T, Sekita Y, Seo T, Sakamoto C (2007) High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J Gastroenterol Hepatol 22:1199–1204
Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’Neil DA, Macfarlane GT (2005) Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54:242–249
Garrote JA, Gomez-Gonzalez E, Bernardo D, Arranz E, Chirdo F (2008) Celiac disease pathogenesis: the proinflammatory cytokine network. J Pediatr Gastroenterol Nutr 47(Suppl 1):S27–S32
Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, Gonzalez A, Mcdonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ (2014) The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15:382–392
Granzotto M, Dal Bo S, Quaglia S, Tommasini A, Piscianz E, Valencic E, Ferrara F, Martelossi S, Ventura A, Not T (2009) Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci 54:1513–1519
Greenwood BM (1968) Autoimmune disease and parasitic infections in Nigerians. Lancet 2:380–382
Gupta P, Andrew H, Kirschner BS, Guandalini S (2000) Is lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 31:453–457
Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T (2003) Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr 22:56–63
Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K (2012) Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog 8:e1002714
Kaplan GG (2015) The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 12:720–727
Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, Arakawa Y (2004) Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther 20:1133–1141
Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474:307–317
Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A (2008) Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135:194–204 e3
Lammers KM, Khandelwal S, Chaudhry F, Kryszak D, Puppa EL, Casolaro V, Fasano A (2011) Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology 132:432–440
Lammers KM, Chieppa M, Liu L, Liu S, Omatsu T, Janka-Junttila M, Casolaro V, Reinecker HC, Parent CA, Fasano A (2015) Gliadin induces neutrophil migration via engagement of the formyl peptide receptor, FPR1. PLoS One 10:e0138338
Laparra JM, Sanz Y (2010) Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J Cell Biochem 109:801–807
Lees CW, Satsangi J (2009) Genetics of inflammatory bowel disease: implications for disease pathogenesis and natural history. Expert Rev Gastroenterol Hepatol 3:513–534
Leonard MM, Camhi S, Huedo-Medina TB, Fasano A (2015a) Celiac disease genomic, environmental, microbiome, and metabolomic (CDGEMM) study design: approach to the future of personalized prevention of celiac disease. Nutrients 7:9325–9336
Leonard MM, Serena G, Sturgeon C, Fasano A (2015b) Genetics and celiac disease: the importance of screening. Expert Rev Gastroenterol Hepatol 9:209–215
Lindfors K, Blomqvist T, Juuti-Uusitalo K, Stenman S, Venalainen J, Maki M, Kaukinen K (2008) Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin Exp Immunol 152:552–558
Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, Amarri S, Barbato M, Barbera C, Barera G, Bellantoni A, Castellano E, Guariso G, Limongelli MG, Pellegrino S, Polloni C, Ughi C, Zuin G, Fasano A, Catassi C, Weaning SWGO, Risk CD (2014) Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 371:1295–1303
Madsen K, Cornish A, Soper P, Mckaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C (2001) Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580–591
Maiuri L, Ciacci C, Auricchio S, Brown V, Quaratino S, Londei M (2000) Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119:996–1006
Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, Mention JJ, Rahmi G, Kiyono H, Butz EA, Brousse N, Cellier C, Cerf-Bensussan N, Meresse B (2010) IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest 120:2131–2143
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J (2006) Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55:205–211
Manichanh C, Borruel N, Casellas F, Guarner F (2012) The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 9:599–608
Michail S, Durbin M, Turner D, Griffiths AM, Mack DR, Hyams J, Leleiko N, Kenche H, Stolfi A, Wine E (2012) Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis 18:1799–1808
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A (2009) Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 104:437–443
Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, Leleiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13:R79
Negroni A, Costanzo M, Vitali R, Superti F, Bertuccini L, Tinari A, Minelli F, Di Nardo G, Nuti F, Pierdomenico M, Cucchiara S, Stronati L (2012) Characterization of adherent-invasive Escherichia coli isolated from pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 18:913–924
Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, Cucchiara S, Stronati L (2012) Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther 35:327–334
Olivares M, Neef A, Castillejo G, Palma GD, Varea V, Capilla A, Palau F, Nova E, Marcos A, Polanco I, Ribes-Koninckx C, Ortigosa L, Izquierdo L, Sanz Y (2015) The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 64:406–417
Olivares M, Benitez-Paez A, De Palma G, Capilla A, Nova E, Castillejo G, Varea V, Marcos A, Garrote JA, Polanco I, Donat E, Ribes-Koninckx C, Calvo C, Ortigosa L, Palau F, Sanz Y (2018) Increased prevalence of pathogenic bacteria in the gut microbiota of infants at risk of developing celiac disease: the PROFICEL study. Gut Microbes 9(6):551–558
Orel R, Kamhi Trop T (2014) Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J Gastroenterol 20:11505–11524
Orholm M, Binder V, Sorensen TI, Rasmussen LP, Kyvik KO (2000) Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand J Gastroenterol 35:1075–1081
Orlando A, Linsalata M, Notarnicola M, Tutino V, Russo F (2014) Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: the role of cellular polyamines. BMC Microbiol 14:19
Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO (2007) Development of the human infant intestinal microbiota. PLoS Biol 5:e177
Primec M, Micetic-Turk D, Langerholc T (2017) Analysis of short-chain fatty acids in human feces: a scoping review. Anal Biochem 526:9–21
Reilly NR, Fasano A, Green PH (2012) Presentation of celiac disease. Gastrointest Endosc Clin N Am 22:613–621
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367
Rizzello CG, De Angelis M, Di Cagno R, Camarca A, Silano M, Losito I, De Vincenzi M, De Bari MD, Palmisano F, Maurano F, Gianfrani C, Gobbetti M (2007) Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol 73:4499–4507
Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, Hellmig S, Nikolaus S, Folsch UR, Helmke B, Autschbach F, Schirmacher P, Kioschis P, Hafner M, Poustka A, Mollenhauer J, Schreiber S (2007) Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol 178:8203–8211
Sarno M, Lania G, Cuomo M, Nigro F, Passannanti F, Budelli A, Fasano F, Troncone R, Auricchio S, Barone MV, Nigro R, Nanayakkara M (2014) Lactobacillus paracasei CBA L74 interferes with gliadin peptides entrance in Caco-2 cells. Int J Food Sci Nutr 65:953–959
Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC (2004) Lactobacillus GG in inducing and maintaining remission of Crohn’s disease. BMC Gastroenterol 4:5
Schuppan D (2000) Current concepts of celiac disease pathogenesis. Gastroenterology 119:234–242
Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J (2003) Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 52:237–242
Sellitto M, Bai G, Serena G, Fricke WF, Sturgeon C, Gajer P, White JR, Koenig SS, Sakamoto J, Boothe D, Gicquelais R, Kryszak D, Puppa E, Catassi C, Ravel J, Fasano A (2012) Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS One 7:e33387
Serena G, Yan S, Camhi S, Patel S, Lima RS, Sapone A, Leonard MM, Mukherjee R, Nath BJ, Lammers KM, Fasano A (2017) Proinflammatory cytokine interferon-gamma and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin Exp Immunol 187:490–506
Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, Cummings JH, Macfarlane S (2010) Clinical trial: the microbiological and immunological effects of synbiotic consumption – a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther 32:872–883
Strachan DP (1989) Hay fever, hygiene, and household size. BMJ 299:1259–1260
Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H (2002) Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54
Thompson NP, Driscoll R, Pounder RE, Wakefield AJ (1996) Genetics versus environment in inflammatory bowel disease: results of a British twin study. BMJ 312:95–96
Tjellstrom B, Hogberg L, Stenhammar L, Falth-Magnusson K, Magnusson KE, Norin E, Sundqvist T, Midtvedt T (2013) Faecal short-chain fatty acid pattern in childhood coeliac disease is normalised after more than one year’s gluten-free diet. Microb Ecol Health Dis 24:1–5
Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A (2009) Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 106:16799–16804
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, Modeo ME, Rodino S, D’amico T, Sebkova L, Sacca N, Di Giulio E, Luzza F, Imeneo M, Larussa T, Di Rosa S, Annese V, Danese S, Gasbarrini A (2010) Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 105:2218–2227
Versini M, Jeandel PY, Bashi T, Bizzaro G, Blank M, Shoenfeld Y (2015) Unraveling the hygiene hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med 13:81
Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, Putnam PE, Abonia JP, Santos J, Rothenberg ME (2010) Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut 59:12–20
Vriezinga SL, Auricchio R, Bravi E, Castillejo G, Chmielewska A, Crespo Escobar P, Kolacek S, Koletzko S, Korponay-Szabo IR, Mummert E, Polanco I, Putter H, Ribes-Koninckx C, Shamir R, Szajewska H, Werkstetter K, Greco L, Gyimesi J, Hartman C, Hogen Esch C, Hopman E, Ivarsson A, Koltai T, Koning F, Martinez-Ojinaga E, Te Marvelde C, Pavic A, Romanos J, Stoopman E, Villanacci V, Wijmenga C, Troncone R, Mearin ML (2014) Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 371:1304–1315
Wacklin P, Kaukinen K, Tuovinen E, Collin P, Lindfors K, Partanen J, Maki M, Matto J (2013) The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm Bowel Dis 19:934–941
Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Jarnerot G, Tysk C, Jansson JK, Engstrand L (2010) A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139(1844–1854):e1
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227
Zheng B, Van Bergenhenegouwen J, Overbeek S, Van De Kant HJ, Garssen J, Folkerts G, Vos P, Morgan ME, Kraneveld AD (2014) Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. PLoS One 9:e95441
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Serena, G., Fasano, A. (2018). Use of Probiotics to Prevent Celiac Disease and IBD in Pediatrics. In: Guandalini, S., Indrio, F. (eds) Probiotics and Child Gastrointestinal Health. Advances in Experimental Medicine and Biology(), vol 1125. Springer, Cham. https://doi.org/10.1007/5584_2018_317
Download citation
DOI: https://doi.org/10.1007/5584_2018_317
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14635-1
Online ISBN: 978-3-030-14636-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)