Abstract
The carotid body is a highly specialized chemoreceptive organ of neural crest origin whose role is to detect changes in arterial oxygen content. The sensory units are the chemoreceptor cells, which are neuronal-like cells, surrounded by sustentacular or glial-like cells. It is suggested that the carotid body contains self-renewing multipotent stem cells, which are putatively represented by glial-like sustentacular cells. The mechanisms of renewal of neuronal-like cells are unclear. Recently, we have demonstrated the expression of galanin, a peptide promoting neurogenesis, in chemoreceptor cells in the human CB. Thus, in the present study we seek to determine whether galanin expression in chemoreceptor cells could be matched with that of nestin, a peptide that is a marker of multipotent neural stem cells, or rather with the glial fibrillary acidic protein (GFAP), a marker for glial cells. The latter would underscore the pluasibly essential role of sustentacular cells in the self-renewal capability of chemorecetors. We found that galanin expression is matched with nestin in chemoreceptor cells of the human carotid body, but not with that of GFAP. Thus, galanin expression in chemoreceptor cells could provide a signal for neurogenesis and chemoreceptor cell differentiation in the carotid body.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The carotid body (CB) is a paired arterial chemosensitive organ devoted to acute oxygen-sensing and oxygen homeostasis. The organ generates the stimulatory hypoxic ventilatory response to hypoxia. The CB originates from the neural crest, has a lobular organization, with the lobes separated by thin connective septa. The CB chemosensing core constitutes an intricate network of blood vessels that originate from a branch of the external carotid artery, and which spread into the parenchyma of the CB. The chemosensitive units consist of glomus or type I or chemoreceptor cells, which are neuronal-like cells, surrounded by Type II or sustentacular cells (López-Barneo et al. 2008; Prabhakar 2000; 2006; Verna 1979). The sustentacular cells are glial-like cells that have a supportive role and express the glial fibrillary acidic protein (GFAP) (Pallot 1987). Chemoreceptor cells release a number of neurotransmitters and neuromodulators in response to stimulating conditions (Porzionato et al. 2008; Shirahata et al. 2007; Nurse 2005; Iturriaga and Alcayaga 2004). This, in turn, elicits afferent discharge in the sinus nerve, which is conveyed through the glossopharyngeal nerve to the petrosal ganglion (Iturriaga et al. 2007).
CB tissue undergoes modifications with age; the connective tissue compartment increases, which results in a reduction of sensory elements. Analogous changes have been reported in young drug-addicted subjects (Zara et al. 2013; Porzionato et al. 2005; Di Giulio et al. 2003). But the opposite process, enlargement of sensory elements, is possible. The organ enlarges to several-fold its normal size in chronic hypoxia, which also is seen in patients with cardiopulmonary disease (Wang and Bisgard 2002; McGregor et al. 1984; Heath et al. 1982). This occurs through the production of new neuronal-like cells by activation of a resident population of neural crest-derived progenitor cells. Further, on returning to normoxic condition, the original size of the CB is restored, with about half of chemoreceptor cell mass replaced by the newly formed cells, which indicates a striking regenerative power. The newly formed cells have the same neurochemical composure and electrophysiological properties as the indigenous glomus cells. Consequently, the CB has been considered a neurogenic center with a distinct physiological function in adult life. It is suggested that the organ contains self-renewing multipotent stem cells, which are putatively represented by glial cells (Pardal et al. 2007). However, the exact mechanisms of renewal of neuronal-like cells remain unclear. Recently, we have demonstrated the expression of galanin in neuronal-like, chemoreceptor cells in the human CB (Mazzatenta et al. 2014). Galanin is a neurotrophic/neuroprotective peptide involved in neural plasticity. This peptide upregulates genes involved in neuronal signaling pathways, and neuronal differentiation. It also promotes neurogenesis (Cordeo-Llana et al. 2014; Ma et al. 2008). Therefore, in the present study we set out to examine whether galanin expression could be matched with that of nestin, a peptide that is conducive to the proper self-renewal of neural stem cells (Park et al. 2010) or rather with the GFAP peptide, which would underscore the suggested essential role of glial-like cells, above outlined, in the self-renewal of cells in the CB.
2 Methods
The study was approved by a local Ethics Committee and was performed according to the Italian law on the use of human autopsy tissue. Human carotid bodies (n = 12) were collected from 12 to 72 h post-mortem (the age range of deceased subjects was 27–76 years). The exclusion criteria were signs of past cardiac hypertrophy, myocardial infarction found during autopsy, history of other chronic pulmonary or cardiovascular disease, and degenerative tissue alterations detected in histologically stained tissue specimens. Further, the possible influence of the death-to-autopsy interval on the state of tissue specimens was examined by way of a statistical method (for details see Porzionato et al. 2005).
The specimens were fixed in 10 % formalin, embedded in paraffin, sectioned (3 μ thick), subjected to the Mallory trichrome histological staining (Bio Optica; Milan, Italy). The immunohistochemistry consisted of the following: H-11 mouse monoclonal anti-galanin antibody (sc166431; Biotechnology; Santa Cruz, CA), H1α 67 anti-hypoxia inducible factor (HIF-1 α) antibody (sc-53546; Biotechnology; Santa Cruz, CA), and the developing kits (HRP Polymer/DAB Plus Chromogen and Lab Vision UltraVision LP Detection System; Thermo Fisher Scientific, Carlsbad, CA). Serial sections were examined under light microscopy (Leica DM 4000 microscope), equipped with Leica DFC 320 digital acquisition system. The QWin Plus 3.5 software (Leica, Cambridge, UK) was used to digitalize images and to compute areas positive for antibodies. Statistical analysis employed one-way ANOVA, with α set at 0.001, unless otherwise specified. Commercial SPSS and Origin packets were used for statistical analysis.
3 Results
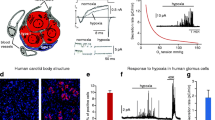
Figure 1, panels A and B, demonstrates the anatomical organization of the human CB. There are clusters of chemoreceptor cells, surrounded peripherally by connective tissue. The clusters are cut through by a network of capillaries. The anatomical structure highlighted in the histological staining revealed fairly well preserved CB parenchyma despite dealing with autopsized tissue. Panels C and D of the figure demonstrate immunohistochemical staining for HIF-1 alpha in chemoreceptor cells, which was used for the verification of visualization of these cells.
Panels A and B – anatomical structure of a human autopsized carotid body, stained with the Mallory trichrome technique; panels C and D – immunohistochemistry of HIF-1 alpha expression in chemoreceptor cells, taken as their marker. Panels B and D show doubly magnified parts of the clusters of chemoreceptor cells surrounded by connective septa as outlined by rectangles in the corresponding upper panels
The immunohistochemical investigation of the human carotid body parenchyma yielded the following findings. The expression of galanin and nestin was found in chemoreceptor cells, whereas that of GFAP in sustentacular cells surrounding the clusters of the former cells (Fig. 2A, B, and C). Densitometry, a quantitative measurement of optical density of immunostaining expressed as the percentage of positively stained area, yielded confirmatory evidence for the coexpression of galanin and nestin in chemoreceptor cells (one-way ANOVA pointed to insignificant differences between the two neuropeptide expression; F(1.10) = 1.03; p = 0.33), while there was significantly different expression between galanin and GFAP (F(1.10) = 5.3; p < 0.05).
Panel A – expression of galanin in carotid chemoreceptor cells; panel B – expression of nestin in carotid chemoreceptor cells, and panel C – expression of glial fibrillary acidic protein (GFAP) in sustentacular cells of the human carotid body. Insets in all three panel show zoomed in details. Panel D – comparison of densitometry assessment of immunostaining of the three neuropeptides in the human carotid body
4 Discussion
As shown by Pardal et al. (2007), nestin and GFAP are markers of differentiating new neuronal-like and glial-like cells, respectively. In the present study we investigated the hypothesis that galanin could be involved in the mechanism of neuronal-like differentiation in the CB. We addressed the issue in an immunohistochemical examination performed in serial sections of CB parenchyma, in which we sought to determine whether galanin would colocalize with nestin or rather with GFAP. We found that the expression of galanin colocalizes with that of nestin in chemoreceptor cells, but not with GFAP. The immunohistochemical findings were strengthened by quantitative densitometry, which pointed to the lack of difference between the expressions of galanin and nestin, and to an appreciable difference between galanin and GFAP.
Galanin expression has been shown throughout both central and peripheral nervous systems, including the CB of rat, monkey, guinea pig, chicken, and man (Mazzatenta et al. 2014; Finley et al. 1995; Ichikawa and Helke 1993; Kameda 1989; Heym and Kummer 1989). This neuropeptide in animals consists of 29 amino acids, while it has 30 amino acids in man. It is a highly inducible neuropeptide that is upregulated in the nervous system after pathological disturbance, where its N-terminal region is crucial for biological activity (Branchek et al. 2000). Biological effects of galanin are mediated through three different G-protein coupled receptors: GalR1, GalR2, and GalR3 (Branchek et al. 2000; Florén et al. 2000). The GalR3 has not been detected in carotid chemoreceptor cells in the rat (Porzionato et al. 2010), although it is present in the rat brain (Smith et al. 1998). The GalR1 sustains a transduction cascade through inhibition of adenylyl cyclase, while the GalR2 acts through the activation of phospholipase C and protein kinase C. Therefore, galanin can regulate differentiating neural cells and it participates in the development and regulation of plasticity of the nervous system (Wittau et al. 2000; Wang et al. 1998).
The CB undergoes size adaptation in chronic hypoxia, which leads to an increase in the organ’s volume, due to the formation of new neuronal-like, chemoreceptor cells. Conversely, when returned to normoxia, there is a corresponding decrease in CB size. The sensory cells can be renewed by the activation of a resident population of neural-crest-derived progenitors (Wang and Bisgard 2002; McGregor et al. 1984; Heath et al. 1982). These adaptive phenomena point to the probable presence of self-renewing, multipotent stem cells in the CB (Pardal et al. 2007). The ability for self-renewing seems crucial for the physiological role of the CB, since in adult life it enables to maintain a population of cells that can differentiate into neuronal-like cells on demand (Mazzatenta et al. 2014).
In conclusion, we believe we have shown that both galanin and nestin are coexpressed in a population of neuronal-like, chemoreceptor cells in the human CB. The finding reinforces our hypothesis that galanin could be involved in the mechanism of chemoreceptor cell differentiation, rather than glial fibrillary acidic protein present in sustentacular glial-like cells.
References
Branchek TA, Smith KE, Gerald C, Walker MW (2000) Galanin receptor subtypes. Trends Pharmacol Sci 21:109–117
Cordeo-Llana O, Rinaldi F, Brennan PA, Wynick D, Caldwell MA (2014) Galanin promotes neuronal differentiation from neuronal progenitor cells in vitro and contributes to the generation of new olfactory neurons in the adult mouse brain. Exp Neurol 256:93–104
Di Giulio C, Cacchio M, Bianchi G, Rapino C, Di Ilio C (2003) Selected contribution: carotid body as a model for aging studies: is there a link between oxygen and aging? J Appl Physiol 95:1755–1758
Finley JC, Erickson JT, Katz DM (1995) Galanin expression in carotid body afferent neurons. Neuroscience 68:937–942
Florén A, Land T, Langel Ü (2000) Galanin receptor subtypes and ligand binding. Neuropeptides 34:331–337
Heath D, Smith P, Jago R (1982) Hyperplasia of the carotid body. J Pathol 138:115–127
Heym C, Kummer W (1989) Immunohistochemical distribution and colocalization of regulatory peptides in the carotid body. J Electron Microsc Tech 12:331–342
Ichikawa H, Helke CJ (1993) Distribution, origin and plasticity of galanin-immunoreactivity in the rat carotid body. Neuroscience 52:757–767
Iturriaga R, Alcayaga J (2004) Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev 47:46–53
Iturriaga R, Varas R, Alcayaga J (2007) Electrical and pharmacological properties of petrosal ganglion neurons that innervate the carotid body. Respir Physiol Neurobiol 157:130–139
Kameda Y (1989) Distribution of CGRP-, somatostatin-, galanin-, VIP- and substance P-immunoreactive nerve fibers in the chicken carotid body. Cell Tissue Res 257:623–629
López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI (2008) Carotid body oxygen sensing. Eur Respir J 32:1386–1398
Ma J, Shan L, Yu LL, Yuan CG (2008) Expression of galanin and galanin receptors in neurogenesis regions of adult mouse brain and effect of galanin on the neural stem cell's differentiation. Fen Zi Xi Bao Sheng Wu Xue Bao 41:359–366
Mazzatenta A, Marconi GD, Zara S, Cataldi A, Porzionato A, Di Giulio C (2014) In the carotid body, galanin is a signal for neurogenesis in young, and for neurodegeneration in the old and in drug-addicted subjects. Front Physiol 5:427. doi:10.3389/fphys.2014.00427
McGregor KH, Gil J, Lahiri S (1984) A morphometric study of the carotid body in chronically hypoxic rats. J Appl Physiol 57:1430–1438
Nurse CA (2005) Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120:1–9
Pallot DJ (1987) The mammalian carotid body. Adv Anat Embryol Cell Biol 102:1–91
Pardal R, Ortega-Sáenz P, Duran R, López-Barneo J (2007) Glia-like stem cells sustain shysiologic neurogenesis in the adult mammalian carotid body. Cell 131:364–377
Park D, Xiang AP, Mao FF, Zhang L, Di CG, Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, Walton N, Lahn BT (2010) Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 28:2162–2171
Porzionato A, Macchi V, Guidolin D, Parenti A, Ferrara SD, De Caro R (2005) Histopathology of the carotid body in heroin addiction. Possible chemosensitive impairment. Histopathology 46:296–306
Porzionato A, Macchi V, Parenti A, De Caro R (2008) Trophic factors in the carotid body. Int Rev Cell Mol Biol 269:1–58
Porzionato A, Macchi V, Barzon L, Masi G, Belloni A, Parenti A, Palù G, De Caro R (2010) Expression and distribution of galanin receptor subtypes in the rat carotid body. Mol Med Rep 3:37–41
Prabhakar NR (2000) Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol 88:2287–2295
Prabhakar NR (2006) O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol 91:17–23
Shirahata M, Balbir A, Otsubo T, Fitzgerald RS (2007) Role of acetylcholine in neurotransmission of the carotid body. Respir Physiol Neurobiol 157:93–105
Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA (1998) Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem 273:23321–2336
Verna A (1979) Ulstrastructure of the carotid body in the mammals. Int Rev Cytol 60:271–330
Wang ZY, Bisgard GE (2002) Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech 59:168–177
Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE (1998) Differential intracellular signalling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry 37:6711–6717
Wittau N, Grosse R, Kalkbrenner F, Gohla A, Schultz G, Gudermann T (2000) The galanin receptor type 2 initiates multiple signalling pathways in small cell lung cancer cells by coupling to G(q), G(i) and G(12) proteins. Oncogene 19:4199–4209
Zara S, Porzionato A, De Colli M, Macchi V, Cataldi A, De Caro R, Di Giulio C (2013) Human carotid body neuroglobin, vascular endothelial growth factor and inducible nitric oxide synthase expression in heroin addiction. Histol Histopathol 28:903–911
Conflict of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mazzatenta, A. et al. (2015). Coexpression of Galanin and Nestin in the Chemoreceptor Cells of the Human Carotid Body. In: Pokorski, M. (eds) Respirology. Advances in Experimental Medicine and Biology(), vol 885. Springer, Cham. https://doi.org/10.1007/5584_2015_189
Download citation
DOI: https://doi.org/10.1007/5584_2015_189
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25851-5
Online ISBN: 978-3-319-25853-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)