Abstract
We studied a potential impact of chronic psychosocial load on the allostatic biomarkers – cardiac vagal activity, inflammation, and oxidative stress in healthy undergraduate students. Continuous resting ECG signals were monitored in a group of 16 female healthy students (age: 23.2 ± 0.2 years, BMI: 20.9 ± 0.5 kg/m2) at two time periods: right after holiday (rest period) and a day before final exams (stress period). Vagal activity was quantified by spectral analysis of heart rate variability at high frequency band (HF-HRV). The immune response was assessed from the level of tumor necrosis factor-alpha (TNF-α) in plasma. In addition, mean RR intervals were evaluated. We found that HF-HRV was significantly reduced and the TNF-α was increased in the stress period compared with the rest period. No significant changes were found in the RR interval. In conclusion, allostatic load induced by stress and the accompanying greater immune response decreased cardiovagal regulation in healthy young subjects. These findings may help understand the pathway by which stress can influence health and disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The autonomic nervous system (ANS) plays a key role in health and disease. Normally, the activity of the sympathetic and parasympathetic branches is in dynamic balance indicating adaptability and flexibility of the organism under different challenges. The autonomic imbalance, in particular a decreased cardiac vagal function, may be associated with the development of risk factors for cardiovascular morbidity (Thayer and Lane 2007). Importantly, an increased inflammatory activity is now thought to play a major role in cardiovascular diseases. Thus, the function of the parasympathetic (vagal) tone associated with regulation of immune response has gained more attention (Thayer and Sternberg 2006).
Animal models suggest that low levels of parasympathetic activity can increase the magnitude of the inflammatory response, particularly tumor necrosis factor (TNF)-alpha (Borovikova et al. 2000). Therefore, the vagus nerve inhibitory function is critical in the regulation of the immune response via cholinergic anti-inflammatory pathway, in which efferent vagal activity tonically inhibits release of proinflammatory cytokines mediated by the interaction of acetylcholine with the alpha-7 nicotinic receptor on tissue macrophages (Huston and Tracey 2011). However, a major unanswered question in clinical application is whether it is possible to record neural activity in the vagus nerve as a surrogate marker of activity in the inflammatory reflex to determine the sensitivity of the immune response. From this aspect of view, the efferent vagal modulation can be quantified by the heart rate variability analysis.
Heart rate variability (HRV) – complex beat-to-beat oscillations in the heart rate around its mean value – is a noninvasive tool to measure autonomic regulatory inputs of the heart. The short-term HRV originates predominantly from the activity of the cardiac preganglionic vagal neurons of the nucleus ambiguus, which is crucial for cardio-respiratory coupling – respiratory sinus arrhythmia (RSA). This physiological phenomenon is characterized by a rhythmic increase in heart rate associated with inspiration when respiratory mechanisms in the brainstem attenuate the vagal efferent action on the heart and by a decrease in heart rate during expiratory phase when the vagal efferent influence on the heart is reinstated (Yasuma and Hayano 2004; Korpas and Jakus 2000). Thus, the RSA can be quantified by the HRV spectral analysis at respiratory-linked high frequency (HF-HRV) neuronal oscillations, providing important information about neurocardiac vagal function. It is important to note that recent studies provided human evidence that vagal activity indexed by HF-HRV is inversely related to inflammatory cytokines and, therefore, activation of efferent vagal pathway plays a role in the tonic inhibitory control of the release of proinflammatory mediators including TNF-α (Tonhajzerova et al. 2013; Jan et al. 2010; Marsland et al. 2007).
Furthermore, both autonomic and immune systems represent important allostatic systems regulating adaptive response of the organism to chronic load (McEwen 1998). In particular, TNF-α as a proinflammatory marker produced by macrophages and B lymphocytes has been shown to be associated with psychosocial stress. For example, higher TNF-α levels were found in burnout syndrome (von Känel et al. 2008), and a reduction of TNF-α after a 10-week relaxation program was reported in tinnitus sufferers; thus, the TNF-α has been postulated to be a stress-sensitive immunological marker (Weber et al. 2002). Importantly, the mechanistic role of autonomic dysregulation in the context of stress has been explored in a variety of animal or laboratory models. However, relatively few studies have addressed the association between autonomic dysfunction and chronic real-life stress in humans. Lucini et al. (2005) demonstrated that cardiac vagal control quantified by the HF-HRV is impaired in humans with symptoms of chronic psychosocial stress. In this regard, the vagally-mediated heart rate beat-to-beat oscillations could reflect the central-peripheral nervous system integration, in particular as a potential marker of stress (Porges 2009; Thayer and Lane 2007). Nonetheless, the vagal-immune interaction during chronic allostatic load on the body is still unclear.
In the present study we addressed the hypothesis that vagal-immune interaction is altered in response to the long-lasting mental load. The aim of the study was to evaluate the cardiac vagal function, indexed by HF-HRV, and immune activity, expressed by TNF-α, in response to mental stress evoked by day-to-day intensive study accompanying the exam period in healthy students.
2 Methods
This study was approved by the Ethics Committee of Jessenius Faculty of Medicine in Martin, Slovakia and was performed in accordance with the Declaration of Helsinki for Human Research. All subjects were carefully instructed about the study protocol and they gave their informed consent to participate prior to examination.
2.1 Subjects
We examined 16 young, female, healthy medical students (mean age 23.2 ± 0.5 years). The exclusion criteria were the following: history of respiratory, endocrinologic, cardiovascular, infectious, mental, or other diseases potentially influencing HRV (including obesity, underweight, overweight, alcohol, or drug abuse). Smokers also were excluded from this study. All subjects were instructed not to use substances which affect the cardiovascular system (caffeine, alcohol) for at least 12 h before the recording. Importantly, because hormonal changes during menstrual cycle can affect the cardiac autonomic regulation (Hirshoren et al. 2002), the females were included being in the proliferative menstrual phase. Anthropometric characteristics of the participants were examined using an InBody J10 (Biospace; Seoul, South Korea), with the technology of direct segmental multi-frequency bioimpedance analysis (DSM-BIA), and they are presented in Table 1.
2.2 Protocol
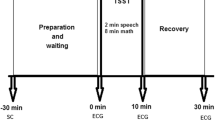
All subjects were examined under standard conditions: a quiet room in a light and temperature-controlled laboratory, in the morning between 9.00 a.m.–12.30 p.m., and after normal breakfast 2 h prior to the examination. The subjects were instructed to sit comfortably in a special armchair and not to speak or move unless necessary. A thoracic belt with ECG telemetric electrodes for R-R intervals recording with sampling frequency of 1,000 Hz (VarCorPF8, Olomouc, Czech Republic) was applied after initial 15 min of the rest period required for heart rate stabilization. Then, the subjects remained in the sitting position for a continuous ECG recording. All subjects were examined twice: at the winter term beginning after holidays (rest period) and at the exam period ending the day before the final exam (stress period).
2.3 Data Analysis
Spontaneous short-term heart rate variability: 300 R-R intervals segments were analyzed between the first and fifth minute of the recording. Slower oscillations and trends were eliminated using the detrending procedure of Tarvainen et al. (2002) and time series were resampled (resampling frequency of 2 Hz) to obtain equidistant time series using cubic spline interpolation. Subsequently, mean power spectrum of the analyzed segment was computed by fast Fourier transform (using window length of 256 samples) and spectral power in the high frequency band (HF: 0.15–0.4 Hz) was obtained by integration. We focused on the high frequency spectral power of the HRV (HF-HRV) reflecting mainly respiratory sinus arrhythmia indicating cardiovagal regulatory inputs. In addition, mean R-R interval was calculated.
2.4 Inflammatory Marker Assay
Blood samples were collected to EDTA tubes in the fasting state at least 3 h before the examination in both (rest and stress) periods. The blood was centrifuged immediately and kept frozen at −80 °C until analysis. The concentration of the proinflammatory marker – tumor necrosis factor-alpha (TNF-α) was assessed using commercially available human ultra-sensitive ELISA kits (Invitrogen; Camarillo, Canada).
2.5 Statistical Analysis
Data were expressed as means ± SE. The nongaussian/gaussian distribution was ascertained by the Lilliefors test. Because HF-HRV index had a skewed distribution, the values were logarithmically transformed to be able to use a t-paired test for normal distribution. P < 0.05 was considered as statistically significant. Statistical analysis was performed using a commercial software package SYSTAT ver. 10 for Windows (SSI, Richmond, CA).
3 Results
3.1 Heart Rate Variability
The high frequency oscillations of the heart rate variability (HF-HRV) were significantly reduced in the stress period compared with the rest period (p = 0.045). No significant changes were found in the mean RR intervals (Table 2).
3.2 Inflammatory Marker
The concentration of the inflammatory marker TNF-α was significantly higher in the stress period compared with the rest period (p = 0.025; Table 2).
4 Discussion
Chronic allostatic load, a burden of chronic stress and accompanying changes in personal behaviors, may lead to a disease in the long-run, mediated via autonomic, neuroendocrine, or immune system activity (McEwen 1998). Sympathetic predominance, vagal withdrawal, and baroreflex impairment represent the autonomic counterpart of the complex psychophysiological changes underlying the increase in cardiovascular risk associated with long-lasting stress (Rosengren et al. 2004). As previously noted, chronic psychological stress and the inflammatory response have been implicated in the etiology and pathogenesis of certain cardiovascular diseases, such as atherosclerosis, and of other diseases, e.g., obesity (Hamer et al. 2012). However, the response of vagal-immune pathway to allostatic load evoked by chronic stress is still unclear. In acute stress, Weber et al. (2010) demonstrated that healthy subjects with low HRV had delayed recovery of TNF-α up to an hour after the stressor had ended and suggest that vagal function is coordinated with the regulation of both acute as well as chronic inflammation in healthy humans. Our results support this hypothesis. Decreased cardiovagal regulation associated with a greater immune response as a result of allostatic load evoked by a long-lasting stress-related exam period could represent the pathomechanism by which dysregulated vagal-immune homeostasis increases the risk of inflammatory conditions associated with chronic real-life stress.
Several explanations are assumed for these findings. Firstly, the common central neural areas regulating both immune and cardiovagal functions were found in recent studies. For example, the neuroimaging research showed associations between vagally mediated HRV and activity in specific brain areas, such as prefrontal, anterior cingulate cortex, insula, or amygdala (Thayer et al. 2012). These brain areas are also associated with the regulation of immune responses (Rosenkranz et al. 2005). Thus, cortical structures are involved in immunomodulation at least partially via the neural concomitants of the cholinergic anti-inflammatory pathway (Thayer et al. 2011). Taken together, the altered vagal-immune communication can be explained by discrete stress-linked control abnormalities in the common neurobiological regulatory basis for both autonomic and immune systems. Furthermore, the neurovisceral theory emphasizes the tonic inhibitory control of the prefrontal cortex on the subcortical regions, including amygdala, linked to the regulation of the immune system via the vagus nerve (Thayer and Sternberg 2006). Consequently, lack of inhibitory input by the vagus nerve might lead to perturbations in the neuroimmune pathway involved with the allostatic overload as observed in our studied group.
Secondly, psychological processes linked to stress (e.g., chronic emotional state, exhaustion, anxiety/depressive symptoms) or life-style modifications (e.g., physical inactivity accompanying day-to-day intensive study) are associated with a decrease in vagal activity (Thayer et al. 2012; Tonhajzerova et al. 2010). Therefore, in agreement to the neurovisceral theory, disruption of vagal inhibitory function may contribute to emotion-related activation of proinflammatory pathways (Marsland et al. 2007). It seems that the question of whether the altered vagal-immune pathway associated with chronic real-life stress in healthy subjects is a pure result of disrupted neurobiological integrity linked to the cholinergic anti-inflammatory pathway or it is only a reflection of stress-related psychological expression and behavioral inflexibility remains unclear.
Our findings support the hypothesis that the interventions related to the regulation of both vagal control and inflammation may be of particular importance (Huston and Tracey 2011). In particular, psychological methods may be effective in parasympathetic activity increase. Initial findings show that hypnosis and meditation increase vagal nerve output and inhibit immune responses (Aubert et al. 2009). The possibility that these and other interventions might influence immunity through activation of the parasympathetic activity warrants further investigation and may have valuable clinical implications for the treatment of inflammatory and cardiovascular disorders (Marsland et al. 2007).
5 Conclusion
We conclude that long-lasting mental load is associated with an alteration in vagal-immune communication shaping the allostatic complex system: decreased vagal activity could lead to disruption of vagal inhibitory function on the inflammatory response resulting in an excess of proinflammatory cytokines. The illumination of the vagal-immune pathway in studies on chronic stress can help understand health and disease.
References
Aubert AE, Verheyden B, Beckers F, Tack J, Vandenberghe J (2009) Cardiac autonomic regulation under hypnosis assessed by heart rate variability: spectral analysis and fractal complexity. Neuropsychobiology 60:104–112
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462
Hamer M, Endrighi R, Poole L (2012) Physical activity, stress reduction, and mood: insight into immunological mechanisms. Methods Mol Biol 934:89–102
Hirshoren N, Tzoran I, Marienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G (2002) Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metabol 87:1569–1575
Huston JM, Tracey KJ (2011) The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 269:45–53
Jan BU, Coyle SM, Macor MA, Reddel M, Calvano SE, Lowry SF (2010) Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock 33:363–368
Korpas J, Jakus J (2000) The expiration reflex from vocal folds. Acta Physiol Hung 3:201–215
Lucini D, Di Fede G, Parati G, Pagani M (2005) Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension 46:1201–1206
Marsland AL, Gianaros PJ, Prather A, Jennings JR, Neumann SA, Manuck SB (2007) Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom Med 69:709–716
McEwen BS (1998) Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 840:33–44
Porges SW (2009) The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 76:86–90
Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S (2004) Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 case and 13648 controls from 52 countries (The INTERHEART study): case control study. Lancet 364:953–962
Rosenkranz MA, Busse WW, Johnston T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ (2005) Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci U S A 102:13319–13324
Tarvainen MP, Ranta-Aho PO, Karjalainen PA (2002) An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng 49:172–175
Thayer JF, Lane RD (2007) The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 74:224–242
Thayer JF, Sternberg E (2006) Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 1088:361–372
Thayer JF, Loerbroks A, Sternberg EM (2011) Inflammation and cardiorespiratory control: the role of the vagus nerve. Respirat Physiol Neurobiol 178:387–394
Thayer JF, Ahs F, Frederikson M, Sollers JJ III, Wager TD (2012) A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36:747–756
Tonhajzerova I, Ondrejka I, Javorka K, Turianikova Z, Farsky I, Javorka M (2010) Cardiac autonomic regulation is impaired in girls with major depression. Progr Neuro-Psychopharmacol Biol Psych 34:613–618
Tonhajzerova I, Mokra D, Visnovcova Z (2013) Vagal function indexed by respiratory sinus arrhythmia and cholinergic anti-inflammatory pathways. Resp Physiol Neurobiol 187:78–81
von Känel R, Bellingrath S, Kudielka BM (2008) Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J Psychosom Res 65:51–59
Weber C, Arck P, Mazurek B, Klapp BF (2002) Impact of a relaxation training on psychometric and immunologic parameters in tinnitus sufferers. J Psychosom Res 52:29–33
Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, Arck PC, Deter HC (2010) Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol 109:201–211
Yasuma F, Hayano J (2004) Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest 125:683–690
Acknowledgments
This work was supported by the European Center of Excellence Projects: Code 262201120016 and 262201120036, and by the National Research Grants: VEGA 1/0087/14, 1/0059/13, and UK/299/2013.
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Visnovcova, Z. et al. (2014). Alterations in Vagal-Immune Pathway in Long-Lasting Mental Stress. In: Pokorski, M. (eds) Oxidative Stress and Cardiorespiratory Function. Advances in Experimental Medicine and Biology(), vol 832. Springer, Cham. https://doi.org/10.1007/5584_2014_10
Download citation
DOI: https://doi.org/10.1007/5584_2014_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09721-3
Online ISBN: 978-3-319-09722-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)