Abstract
Reduced heart rate variability (HRV) constitutes a widely used marker of cardiac autonomic inflexibility which has been linked to disorders such as panic disorder (PD). To date, the pathophysiological mechanisms whereby panic leads to attenuated HRV are not fully elucidated. We aimed to investigate the hypothesis that PD patients show pathological reactivity both in response to interoceptive and psychosocial stress in comparison to healthy individuals. We performed a controlled study on 38 patients diagnosed with PD [20 males and 18 females aged 35.55 ± 10.12 years, mean ± standard deviation] and 23 age and gender matched healthy control participants. Distress was induced using the Trier Social Stress Test (TSST) and the dexamethasone–corticotropin-releasing-hormone (DEX–CRH) test. We assessed HRV prior to, during, and post-stress induction using the root mean square successive differences (RMSSD) as well as spectral analysis (high frequency; HF and low frequency; LF). Statistical analyses revealed significant main effects of time for mean heart rate (HR), HF, LF (solely DEX–CRH), LFHF-ratio (solely TSST) and the RMSSD. Significant interaction effects were observed with more pronounced increases in mean HR (TSST) and LFHF-ratio (DEX–CRH) in the healthy control participants. No significant main effects of group were observed. Overall, our results indicate “normal” HRV parameters in patients with PD. The HRV of PD patients is no worse than that of healthy control participants since the HRV profiles were similar between the study groups. The current study is one of rather rarely published studies which was unable to show an influence of PD on HRV. Implications for future studies are under discussion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panic disorder (PD) is a clinical and socioeconomic problem with a lifetime prevalence of up to 4.7% (Goodwin et al. 2005) and annual overall costs of up to 23.5 million US$ per million inhabitants (Smit et al. 2006). Furthermore, PD has been linked to an increase in cardiovascular disease and mortality (Albert et al. 2005; Fleet et al. 2000; Katerndahl 2008; Smoller et al. 2007). Patients experience panic attacks characterized by symptoms of autonomic arousal such as palpitations, hyperventilation, dizziness, tremor, chest discomfort, sweating, and hot and cold flushes (Woodward et al. 2009). Alarming somatic symptoms and cardiovascular symptoms, in particular, are among the hallmarks of panic attacks, along with an intense fear of dying and loss of control (Katerndahl 2008). The autonomic nervous system appears to be intimately involved in the initiation and manifestation of panic attacks (Friedman and Thayer 1998). The increased risk of cardiac events associated with PD has been linked to the abnormal or deregulated autonomic system, in particular to alterations in heart rate (HR), and heart rate variability [HRV; (Friedman 2007].
The HRV is a measurement of the beat-to-beat difference in HR. It is mediated by the parasympathetic and sympathetic cardiac nerves and reflects the capacity for the parasympathetic inhibition of autonomic arousal. A high HRV reflects a healthy autonomic nervous system that is able to respond to changing environmental circumstances (Porges 2011; Thayer and Lane 2009). By contrast, a decreased HRV is a marker of the autonomic inflexibility of various clinical disorders (Bloomfield et al. 1997; Ponikowski et al. 1997; Thayer and Lane 2000) and ill health (Dekker et al. 2000) which may precede more systemic problems such as inflammatory-mediated atherosclerosis and ventricular fibrillation (Thayer et al. 2010), especially in younger samples. The power spectrum analysis of the HRV assesses the quantitative contribution of high frequent (HF) and low frequent (LF) components to the total variance (power) of HR. Previous studies suggested that HF is a marker of the parasympathetic vagal tone that depends on respiration (Stein and Kleiger 1999; Task Force 1996). LF power has usually been proposed to be a marker of both, sympathetic and parasympathetic activity (Cohen et al. 2000; Winchell and Hoyt 1996). The ratio of LF power to HF power (LF/HF ratio) has often been interpreted as an index of cardiac sympathovagal balance (Alvarenga et al. 2006). This interpretation has recently been challenged by a growing body of evidence suggesting that the LF component of HRV is not a specific marker for cardiac sympathetic outflow (Billman 2007; Eckberg 1997; Goldstein et al. 2011; Pellegrino and Schiller 2015) but a marker of baroreflex function (Goldstein et al. 2011).
In clinical psychological research, different kinds of stressors were used to evaluate HRV in patients diagnosed with PD: (1) interoceptive stressors including orthostatic challenges, hyperventilation, and sympathomimetic drug intake (e.g. yohimbine, lactate) as well as (2) psychosocial stressors, including the Trier Social Stress Test (TSST; Kirschbaum et al. (1993)) and stress recall paradigms. Available studies reported mixed findings. (1) Some evidence exists for lower vagal tone in patients with PD in response to orthostatic maneuvers (Yeragani et al. 1993, 1997), to lactate infusion (Yeragani et al. 1994) and isoproterenol (Yeragani et al. 1995b). These results combine well with studies on resting HRV, also suggesting decreased variability at supine rest in PD patients (Klein et al. 1995; Middleton et al. 1994; Yeragani et al. 1990). However, some studies resulted in increased HRV parameters in response to orthostatic challenge (Yeragani et al. 1995a) and sympathomimetic drug intake (isoproterenol: Pohl and Yeragani (2001); yohimbine: (Albus et al. 1992; Yeragani et al. 1992). Beyond these findings of an altered HRV, some studies reported no differences in HR or vagal tone on orthostasis between patients with PD and healthy control study participants, suggesting “normal” cardiac autonomic reactivity in PD (orthostatic change: (Stein and Asmundson 1994; Yeragani et al. 1990); hyperventilation: Asmundson and Stein (1994)). These findings conflict with results by Phebe et al. (1997) who did not show an increase in sympathetic activity upon orthostatic challenge in unmedicated PD patients. They concluded that PD patients lack the normal baroreflex response (parasympathetic decrease) found in healthy control participants. (2) Effects of psychosocial stress induction on HRV parameters combine well with increased HRV parameters in response to sympathomimetics (Albus et al. 1992; Pohl and Yeragani 2001; Yeragani et al. 1992). Specifically, in a stress recall paradigm Cohen et al. (2000) described an analysis of HRV during the recall of a panic attack. In patients with PD, a significant increase in HR, LF, HF, and the LF/HF-ratio have been observed compared to the healthy control participants. Therefore, during recall, the PD patients seemed to have elevations in sympathetic (HR) and in parasympathetic activity (LF, HF, LF/HF-ratio). Psychosocial stress induction, using the TSST, also led to an increased HR and LF/HF-ratio, but to a decreased RMSSD, a time-domain measure of HRV estimating high-frequency variations in HR due to the parasympathetic control of the HRV (Petrowski et al. 2010).
The current study aimed at investigating alterations in HRV parameters following psychosocial stress induction using the TSST and interoceptive stress induction using the dexamethasone–corticotropin-releasing-hormone-test (DEX–CRH test). We investigated whether our previous findings of increased frequency domain parameters and decreased RMSSD in PD patients in response to the TSST were replicated in the present independently collected HRV data and whether this became evident in response to the DEX–CRH test as well. The DEX–CRH test induces hormonal stress, which is uncomfortable for the participants and leads to interoceptive stress. However, it is unclear whether the (cardiac) autonomic control after the CRH challenge becomes altered. Since the cortisol response increases due to CRH-injection (Erhardt et al. 2006; Petrowski et al. 2012), HR should increase and HRV should decrease. We hypothesized that patients with PD would show decreased HRV both after psychosocial stress induction and the DEX injection compared to healthy control participants.
Methods
Study Participants and Procedures
This study was conducted as part of a trial on the dissociation between ACTH and cortisol response in the DEX–CRH test previously published (Petrowski et al. 2012). The patients for the study were recruited at the University Hospital of the Technical University Dresden, Germany from 2008 to 2011. The Structured Clinical Interview (SCID; (Spitzer et al. 1992; Wittchen 1993) for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) was used to ascertain a diagnosis of PD with or without agoraphobia (American Psychiatric Association 2000). The general diagnostic exclusion criterion for all study participants was any acute and/or chronic medical illness as assessed by a physical examination and a routine laboratory test. Further, the study protocol only allowed participants aged 18–65 who smoked no more than ten cigarettes/day and whose body mass index (BMI) did not exceed 27 kg/m2. The healthy control participants were recruited through newspaper advertisements and matched to the patient samples by age and gender. Inclusion in the healthy control group was ensured if study participants did not report a lifetime history of any mental disorder according to the SCID.
The first patient sample consisted of 48 patients with a current diagnosis of PD and was recruited prior to treatment between May 2008 and September 2011. The patients were sent to our outpatient clinic by their general practitioner. Five patients had to be excluded due to thyroid hormone therapy. Three patients had to be excluded due to a panic attack during DEX–CRH testing, and one patient had to be excluded due to benzodiazepine drug treatment. For the HRV-analyses during the DEX–CRH test, one PD patient and nine healthy control participants were excluded due to the substandard quality of the HR recording, resulting in a final sample of n = 38 patients with PD, all recruited and tested in our anxiety outpatient clinic, and n = 32 healthy control participants. Ten of the patients with PD met the criteria for PD without agoraphobia and 29 for PD with agoraphobia. The characteristics of both study groups are summarized in Table 1. All the study participants provided written informed consent. The study protocol was approved by the local Ethics Committee of the Medical Faculty of the Technical University of Dresden, Germany (No#EK460230008).
Interventions
The TSST and DEX–CRH test were performed in random order on different days. The female participants were tested in the luteal phase in order to standardize the influence of the menstrual cycle.
TSST
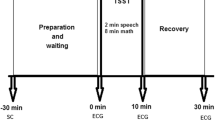
All participants performed the standardized Trier Social Stress Test (TSST). The TSST was developed for moderate psychosocial stress induction under laboratory conditions. The test protocol and effects of this procedure were described previously (Kirschbaum et al. 1993; Petrowski et al. 2010). In brief, participants were asked to assume the role of a job applicant who was invited to a personal interview with the company’s staff managers (selection committee). The participant was told that after a preparation period of 5 min duration they should introduce themselves in a free speech of 5 min duration and convince the committee that they were the perfect applicant for the vacant position. Then, a mental arithmetic task with the participants counting backwards from 2083 in increments of 13 was conducted. After the mock job interview and the task of mental arithmetic were completed, a five-minute-resting period followed. Blood samples for cortisol analysis were taken 75 and 1 min before the TSST as well as 1, 10, 20, 30, 45 and 60 min post-TSST. To account for cortisol circadian rhythm and DEX–CRH schedule, TSST was performed in the afternoon not earlier than 1400 h.
DEX–CRH Test
Further, all of the participants were scheduled for the DEX–CRH test according to the procedure published by Schreiber et al. (1996) and Heuser et al. (1994). An oral dosage of 1.5 mg Dexamethasone was administered at 2300 h. The study participants reported to the clinic at 1400 h the following day. They were asked to refrain from eating, drinking, and smoking at least 2 h before testing and during the three -and a-half hours of testing. They rested in a comfortable, supine position with light reading permitted. A venous catheter was placed at 1415 h to later collect eight consecutive blood samples each for analyzing the ACTH and the cortisol concentration, respectively. After an accommodation time of 45 min, two blood samples were taken before an intravenous injection of 100 µg CRH was administered at 1501 h (15 and 1 min before injection). Afterwards, six more blood samples were collected at fifteen-minute intervals (15, 30, 45, 60, 75, 100 min post-injection). Hormonal data confirm the cortisol stress response and are reported in our previous paper (Petrowski et al. 2012).
Instruments
Psychometrics
The psychopathological burden and the severity of the panic-agoraphobia symptoms were measured by two instruments: (1) the Symptom-Check-List [SCL; (Derogatis 1977; Franke and Derogatis 2002] consisting of 90 items with a five-point rating scale (range: 20-80) to evaluate the psychological and physical impairment and (2) the Panic and Agoraphobia- Scale (PAS; (Bandelow 1997) consisting of 13 items rated on a five-point rating-scale (range: 0–52) to assess the symptom severity for phobic anxiety. Additionally, the depressive symptoms were evaluated using (3) the Beck-Depression-Inventory (BDI; (Beck et al. 1987; Hautzinger et al. 1994) which consists of 21 symptoms rated in terms of intensity from 0 to 3 (range: 0–63). All instruments were administered in German.
HRV Assessment
The HR and HRV parameters were calculated over 5-minuteintervals during a pre-TSST resting period, after the mental arithmetic task, and during a post-TSST recovery period. The HR analysis was performed by means of the Polar ® watch system (S810, Polar, Finland) as previously described in detail (Radespiel-Tröger et al. 2003) RR-intervals were recorded automatically by means of a receptor belt and stored using a wrist sensor unit and transferred by means of an interface to a microcomputer. The S810 recorded in the sampling frequency of 1.000 Hz, giving a temporal solution of 1 ms for each RR period. The Polar S software corrects for artefacts, using an error filter and beat protection function. The Polar S software was used for the HRV analysis. To evaluate the HRV, the following parameters were used: the root mean square successive differences (RMSSD) were calculated for a time domain parameter of the HRV. The RMSSD captures changes in the HR in response to respiration (Berntson et al. 2005). Further, the high frequency (HF; 0.15–0.40 Hz), the low frequency power (LF; 0.04–0.15 Hz) and the LF/HF ratio were assessed by means of a fast Fourier transformation algorithm to specify the HRV. The HRV parameters (not HR) were subject to log transformations.
Statistical Analyses
A power analysis showed that expecting a medium effect size of Cohen´s f = .025 and using an ANOVA for repeated measures as statistic test to prove in-between interactions with three groups, n = 8 repetitions and a significance level of p = .05, that a total sample size of n = 21 study participants per group was needed. Thus, the sample in the present study was large enough for testing our hypotheses. The data were analyzed according to the normality of distributions and were, in case of not normally distributed data, subjected to natural log transformations.
The course of saliva cortisol release was analyzed by a repeated measures ANCOVA with group [2] as between-subject factor and time [8] as within-subject factor, and with baseline saliva cortisol as a covariate. This served as a manipulation check for stress induction and was carried out for both the TSST and DEX–CRH test. The degree of freedom was adjusted with the Greenhouse-Geisser approach, taking sphericity into account.
For the analysis of the effects on the HRV during the TSST, we conducted a 2 (group: patients with PD, healthy control participants) x 3 (time: pre-TSST resting period, post-mental arithmetic task, post-TSST recovery period) ANOVA for repeated measures for HR, RMSSD, HF, LF and LF/HF ratio. As group comparisons revealed a significant difference in antidepressant medication intake, and since tricyclic antidepressants are known to decrease the HRV (Kemp et al. 2010), ANOVA for repeated measures were repeated excluding the PD patients who reported antidepressant medication intake. Further, cigarettes smoked per day were added as additional covariate since groups differed significantly in the number of cigarettes per day. The pre-TSST resting period was defined as 5 min resting interval before TSST instruction. The post-mental arithmetic interval is considered as a measure of HRV during TSST. The post-TSST recovery period was defined as 5 min resting interval after cessation of the TSST. Again, participants rested in supine position with light reading permitted.
For the analysis of the effects on the HRV during the DEX, we conducted a 2 (group: patients with PD, healthy control participants) × 3 (time: pre-DEX resting period, post-injection, post-DEX recovery period) ANOVA for repeated measures for HR, RMSSD, HF, LF and LF/HF ratios. As group comparisons revealed significant differences in antidepressant treatment and cigarettes smoked per day, cigarettes smoked per day was added as additional covariate, and ANCOVA for repeated measures were repeated excluding the PD patients who reported antidepressant medication intake from the analyses. The pre-DEX resting period was defined as 5 min resting interval at the end of the accommodation time before CRH-injection. The participants rested in supine position with light reading permitted. A 5 min interval immediately after CRH injection (and before the 15 min blood sample) is considered as a measure of HRV during DEX–CRH test. The post-DEX-injection was defined as 5 min interval after cessation of blood sample collection. The homogeneity of variances was controlled by Mauchly’s test of spherecity. The ANOVA results were corrected by Greenhouse-Geisser whenever necessary.
Results
Participant Characteristics
The participants’ characteristics are described in Table 1. PD patients matched the healthy control participants in demographic and biological variables, except for significant differences in cigarettes smoked per day and antidepressant medication intake (p’s ≤ .05), whereby six PD patients were on antidepressant treatment as compared to none in the healthy control group (two patients were taking tricyclic antidepressants). Comorbid mental disorders were depressive disorders (n = 25) and specific phobia (n = 1).
With regard to the clinical measures, significant differences between the patients with PD and the healthy control participants could be shown for the BDI-score (p ≤ .001). On a descriptive level, patients with PD showed higher scores in the symptom severity index on the panic and agoraphobia-scale, the phobic-anxiety-scale, and the Global Severity Index (GSI) of the SCL-90 (Table 1). Eight patients showed a borderline severity of panic and agoraphobic symptoms, n = 8 patients showed a mild, n = 14 patients a moderate severity, n = 8 patients with PD showed a severe psychopathology on the Panic and Agoraphobia-Scale, respectively. The mean age at the onset of the PD was 27.48 ± 11.21 years of age, and the mean duration of the PD was 6.10 ± 9.34 years. Sixteen of the patients had shown a first manifestation of the symptomatology during the previous two years.
Manipulation Check
Saliva cortisol levels were found to increase following the TSST (F 1.662;83.119 = 20.522, p ≤ .001). ANCOVA revealed neither a group effect (F 1;50 = 1.120, p = .295) nor a time x group interaction effect (F 1.662;83.119 = 1.043, p = .346). Also in regard to the DEX–CRH testing, saliva cortisol levels were found to increase as already reported in our previous paper (Petrowski et al. 2012).
TSST HRV Parameters
There were no significant differences between groups in baseline pre-TSST HRV parameters (p’s ≥ .292). During the TSST, the following results were noted: as shown in Table 2, significant main effects of time (p’s ≤ .01; see Table 2) on all cardiovascular parameters (mean HR, HF total and relative power, LF/HF-ratio, RMSSD), except for LF total and relative power, were observed. No significant main effects of group were observed (p’s ≥ .101). Further, ANCOVA revealed a significant time x group interaction effect (p’s ≤ .05). Post hoc analyses showed an increase in mean HR during the TSST and a decrease at post-TSST towards pre-TSST level, while the increase in mean HR was greater in the healthy control participants. The same was true when PD patients who reported antidepressant treatment were excluded (data not shown). The difference in the BDI sum score remained significant after excluding these patients (mean (S.D.) PD patients: 9.66 (6.53), healthy control participants: 4.74 (6.09); F 1;54 = 8.014, p ≤ .05).
DEX–CRH HRV Parameters
There were no significant differences between groups in baseline pre-DEX HRV parameters (p’s ≥ .123). During the DEX–CRH test, the following results were noted: as shown in Table 3, significant main effects of time (p’s ≤ .05; see Table 3) were observed for (mean HR, HF total and relative power, LF relative power and the RMSSD). No significant group effects were observed (p’s ≥ .238). Further, ANCOVA revealed a significant time x group interaction effect on LF/HF-ratio (p ≤ .05). Post hoc analyses revealed that LFHF-ratio decreased after CRH-injection and increased during recovery in PD patients, while in healthy control participants LFHF-ratio increased after injection and stayed at the same level during recovery. Basically, the same results were observed when PD patients who reported antidepressant treatment were excluded from the analyses (data not shown).
Discussion
The HRV is a measurement of the fluctuations of the HR and is of cardinal importance to human health (Task Force 1996). Lowered HRV is a marker of autonomic inflexibility (Task Force 1996). Different HRV parameters in the time-domain (RMSSD) and frequency-domain (HF, LF, LF/HF-ratio) are established markers in the detection of autonomic inflexibility in various clinical disorders (Bloomfield et al. 1997; Ponikowski et al. 1997).
The present study applied the TSST protocol and the DEX–CRH test to patients diagnosed with PD and healthy control participants. We hypothesized that patients with PD would show a decreased HRV both after psychosocial stress induction using the TSST and interoceptive stress induction using the DEX injection compared to healthy control participants. During the psychosocial stress condition, mean HR and LFHF-ratio increased and predominantly vagally mediated HRV-components decreased (the HF component and the RMSSD) in both patients with PD as well as healthy control participants. Similar alterations of cardiovascular parameters were observed following the CRH-injection: mean HR increased, while HF, LF relative power and the RMSSD decreased in both study groups. It is interesting that both, the PD patients and the healthy control participants, showed comparable HRV profiles. The results of our investigation cannot be interpreted as specific to PD patients since the healthy control participants of our investigation showed similar HRV profiles. Thus our hypothesis was only partially confirmed in that the HRV components decreased indeed, but no significant group differences emerged. However, statistical analyses revealed significant interaction effects in regard to mean HR (TSST), and LF/HF-ratio (DEX–CRH test). The healthy control participants showed more pronounced alterations in both parameters. In healthy control participants the LFHF-ratio increased after CRH-injection and remained at the same level during recovery, as compared to the PD patients in whom the LFHF-ratio decreased after injection and increased during recovery. Concerning mean HR during the TSST, both study groups showed an increase of mean HR during the TSST and a decrease after the TSST, but increase in mean HR was greater in the healthy control participants. However, these significant interactions may have been chance findings, considering the otherwise negative findings, and possibly reflect type I errors.
Previous research demonstrated mixed findings on HRV parameters in patients with PD.
Specifically, a depressed HRV through vagal blocking and/or sympathetic activation were found in response to different stressors (orthostasis: (Friedman and Thayer 1998; Yeragani et al. 1993, 1997); lactate infusion: (Yeragani et al. 1994); isoproterenol: (Yeragani et al. 1995b). However, some authors reported no increase in the sympathetic activity upon orthostatic challenge (Phebe et al. 1997) or even an increase in the total HRV upon challenge (sympathomimentics: (Albus et al. 1992; Pohl and Yeragani 2001; Yeragani et al. 1992); psychosocial stress induction: (Cohen et al. 2000; Petrowski et al. 2010). Besides, there are investigations that failed to demonstrate any differences in the HR and HRV parameters between PD patients and healthy control study participants (Asmundson and Stein 1994; Stein and Asmundson 1994; Yeragani et al. 1990). Overall, our findings combine well with the results by the latter who also failed to find a distinct HRV profile in PD patients. Further, our finding of no baseline differences under resting conditions in HRV parameter conflict with previous research showing decreased HRV parameters at rest in PD patients (Klein et al. 1995; Middleton et al. 1994; Yeragani et al. 1990). However, healthy control participants showed more distinctive alterations in mean HR (TSST) and LFHF-ratio (DEX–CRH) as compared to the PD patients. This difference resembles the hypo-reactiveness in the cortisol stress response to the TSST seen in PD patients (Hollander et al. 1989; Levin et al. 1987; Petrowski et al. 2010; Sinha et al. 1999).
To our knowledge, this is the first study using two highly standardised procedures in a patient sample and a healthy control sample. On a descriptive level, the patients diagnosed with PD showed lower scores of vagally-mediated HRV parameters (HF, RMSSD) especially during the DEX–CRH test as compared to the healthy control participants. The patients with PD may have experienced the DEX–CRH test as an “interoceptive challenge” triggering stress and sympathetic activation. The DEX–CRH test induces hormonal stress and therefore might trigger one of a panic patient’s major fears. Patients with PD usually demonstrate a heightened level of fear of interoceptive sensations (McNally 2002), and report fear of medication intake and side effects. Due to this concern, patients with PD are engaged in attending to internal sensations that indicate panic and in monitoring these sensations. The DEX–CRH test requires the oral intake of Dexamethasone und the injection of 100 µg CRH. Anticipatory fear of uncomfortable bodily sensations may have increased the sympathetic control over the HR and thus depressed the HRV. We did not include an instrument which could have assessed the level of anxiety experienced by our study participants. Hence, we cannot know whether the PD patients experienced more discomfort in response to either one of the two interventions as compared to the healthy participants. Possibly, the stressors we used did not trigger any anxiety in the patients with PD as hyperventilation, orthostatic challenge, or lactate infusion had done of previous studies. Further, the specific symptoms and intensity of PD vary over the duration of the disorder, also including asymptomatic episodes. Most studies reported significant reductions in the HRV parameters in PD patients (Chalmers et al. 2014; Friedman 2007). It is unknown if HRV parameters normalized during asymptomatic intervals. In this context, emotion regulation abilities seem to be important (Wang et al. 2016). However, descriptive findings of decreased HRV in PD patients possibly reflect differences in task involvement. This explanation correlates with findings of decreased physiological arousal parameters in non-assertive women as compared to assertive women during an assertion challenge task (Lehrer and Leiblum 1981). Further, Backs et al. (2000) observed vagal withdrawal in a medium and high workload air traffic control task. Since we did not include an instrument which controls for task involvement, this explanation remains rather suggestive.
Limitations include the lack of a baseline condition. Previous research showed PD patients to be specifically reactive in response to stress anticipation or novelty cues and stress recovery (Abelson et al. 2007; Grillon et al. 2008). Possibly, the absence of a baseline condition in the present study may have masked differential HRV effects between study groups. A second major limitation of this study is that we did not assess the respiration rate. The control of the respiration rate is recommended since the HRV (especially the HF component and the RMSSD) differs with respiratory frequency (Berntson et al. 1997; Berntson et al. 2005). Further, it is critical that most of the PD patients suffered from a depressive disorder (64%), making it difficult to separate the impact of the PD alone on the HRV. Depression is also associated with a decreased HRV (Kemp et al. 2010). Future studies should include study groups of PD patients with and without comorbid depressive disorders to analyze the impact of comorbid depression on the HRV parameter in patients with PD as compared to PD patients and healthy control participants. Last, future studies should include a self-report instrument of state anxiety to test whether the stressors used trigger a patient’s particular anxiety.
In conclusion, our results indicate “normal” HRV profiles in patients diagnosed with PD. The HRV of PD patients seems no worse than that of healthy control participants. Future studies should address the impact of comorbid depression as well as the sympathetic or vagal origin of the LF component.
References
Abelson, J. L., Khan, S., Liberzon, I., & Young, E. A. (2007). HPA axis activity in patients with panic disorder: Review and synthesis of four studies. Depression and Anxiety, 24(1), 66–76.
Albert, C. M., Chae, C. U., Rexrode, K. M., Manson, J. E., & Kawachi, I. (2005). Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation, 111(4), 480–487.
Albus, M., Zahn, T. P., & Breier, A. (1992). Anxiogenic properties of yohimbine. European Archives of Psychiatry and Clinical Neuroscience, 241(6), 337–344.
Alvarenga, M. E., Richards, J. C., Lambert, G., & Esler, M. D. (2006). Psychophysiological mechanisms in panic disorder: A correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosomatic Medicine, 68(1), 8–16.
Asmundson, G., & Stein, M. B. (1994). Vagal attenuation in panic disorder: An assessment of parasympathetic nervous system function and subjective reactivity to respiratory manipulations. Psychosomatic Medicine, 56(3), 187–193.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders DSM-IV-TR fourth edition (text revision).
Backs, R. W., Navidzadeh, H. T., & Xu, X. Cardiorespiratory indices of mental workload during simulated air traffic control. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 2000 (Vol. 44, pp. 89–92). SAGE.
Bandelow, B. (1997). Panik-und Agoraphobie-Skala:(PAS). Göttingen: Hogrefe, Verlag für Psychologie.
Beck, A. T., Steer, R., & Brown, G. (1987). Beck depression inventory manual. San Antonio, TX: The Psychological Corporation. Harcourt Brace Jovanovich. Beck, AT, Ward, CH, Mendelson, M., Mock, J., & Erbaugh, J.(1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
Berntson, G. G., Bigger, J. T., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., et al. (1997). Heart rate variability: Origins, methods, and interpretative caveats. Psychophysiology, 34, 623–648.
Berntson, G. G., Lozano, D. L., & Chen, Y. J. (2005). Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology, 42(2), 246–252.
Billman, G. E. (2007). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. In Heart Rate Variability: Clinical Applications and Interaction between HRV and Heart Rate (p. 54).
Bloomfield, D. M., Kaufman, E. S., Bigger, J. T., Fleiss, J., Rolnitzky, L., & Steinman, R. (1997). Passive head-up tilt and actively standing up produce similar overall changes in autonomic balance. American Heart Journal, 134(2), 316–320.
Chalmers, J. A., Quintana, D. S., Abbott, M. J., & Kemp, A. H. (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in psychiatry, 5, 80.
Cohen, H., Benjamin, J., Geva, A. B., Matar, M. A., Kaplan, Z., & Kotler, M. (2000). Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Research, 96(1), 1–13.
Dekker, J. M., Crow, R. S., Folsom, A. R., Hannan, P. J., Liao, D., Swenne, C. A., et al. (2000). Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes The ARIC Study. Circulation, 102(11), 1239–1244.
Derogatis, L. (1977). SCL-90: Administration and Procedures Manual-I for the R (evised) Version. Baltimore: Clinical Psychometrics Research.
Eckberg, D. L. (1997). Sympathovagal balance a critical appraisal. Circulation, 96(9), 3224–3232.
Erhardt, A., Ising, M., Unschuld, P. G., Kern, N., Lucae, S., Pütz, B., et al. (2006). Regulation of the hypothalamic–pituitary–adrenocortical system in patients with panic disorder. Neuropsychopharmacology, 31(11), 2515–2522.
Fleet, R., Lavoie, K., & Beitman, B. D. (2000). Is panic disorder associated with coronary artery disease? A critical review of the literature. Journal of Psychosomatic Research, 48(4), 347–356.
Franke, G. H., & Derogatis, L. R. (2002). SCL-90-R: Symptom-Checkliste von LR Derogatis: Deutsche version: Manual. Weinheim: Beltz Test.
Friedman, B. H. (2007). An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199.
Friedman, B. H., & Thayer, J. F. (1998). Autonomic balance revisited: panic anxiety and heart rate variability. Journal of Psychosomatic Research, 44(1), 133–151.
Goldstein, D. S., Bentho, O., Park, M. Y., & Sharabi, Y. (2011). Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Experimental Physiology, 96(12), 1255–1261.
Goodwin, R. D., Faravelli, C., Rosi, S., Cosci, F., Truglia, E., de Graaf, R., et al. (2005). The epidemiology of panic disorder and agoraphobia in Europe. European Neuropsychopharmacology, 15(4), 435–443.
Grillon, C., Lissek, S., Rabin, S., McDowell, D., Dvir, S., & Pine, D. S. (2008). Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry, 165(7), 898–904.
Hautzinger, M., Bailer, M., Worall, H., & Keller, F. (1994). Beck-depressions-inventar (BDI). Bearbeitung der deutschen Ausgabe. Testhandbuch. Bern: Huber.
Heuser, I., Yassouridis, A., & Holsboer, F. (1994). The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. Journal of Psychiatric Research, 28(4), 341–356.
Hollander, E., Liebowitz, M. R., Gorman, J. M., Cohen, B., Fyer, A., & Klein, D. F. (1989). Cortisol and sodium lactate—Induced Panic. Archives of General Psychiatry, 46(2), 135–140.
Katerndahl, D. A. (2008). The association between panic disorder and coronary artery disease among primary care patients presenting with chest pain: An updated literature review. Primary Care Companion to The Journal of Clinical Psychiatry, 10(4), 276–285.
Kemp, A. H., Quintana, D. S., Gray, M. A., Felmingham, K. L., Brown, K., & Gatt, J. M. (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074.
Kirschbaum, C., Pirke, K.-M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81.
Klein, E., Cnaani, E., Harel, T., Braun, S., & Ben-Haim, S. A. (1995). Altered heart rate variability in panic disorder patients. Biological Psychiatry, 37(1), 18–24.
Lehrer, P., & Leiblum, S. (1981). Physiological, medical, and cognitive measures of assertiveness and assertion anxiety. Behavioral Counseling Quarterly, 1(261), 274.
Levin, A. P., Doran, A. R., Liebowitz, M. R., Fyer, A. J., Gorman, J. M., Klein, D. F., et al. (1987). Pituitary adrenocortical unresponsiveness in lactate-induced panic. Psychiatry Research, 21(1), 23–32.
McNally, R. J. (2002). Anxiety sensitivity and panic disorder. Biological Psychiatry, 52(10), 938–946.
Middleton, H. C., Ashby, M., & Robbins, T. W. (1994). Reduced plasma noradrenaline and abnormal heart rate variability in resting panic disorder patients. Biological Psychiatry, 36(12), 847–849.
Pellegrino, P. R., & Schiller, A. M. (2015). Letter to the editor: Does low-frequency power of heart rate variability correlate with cardiac sympathetic tone in normal sheep? American Journal of Physiology-Heart and Circulatory Physiology, 308(2), H146.
Petrowski, K., Herold, U., Joraschky, P., Mück-Weymann, M., & Siepmann, M. (2010). The effects of psychosocial stress on heart rate variability in panic disorder. German Journal of Psychiatry, 13(2), 66–73.
Petrowski, K., Wintermann, G.-B., Kirschbaum, C., & Bornstein, S. R. (2012). Dissociation between ACTH and cortisol response in DEX–CRH test in patients with panic disorder. Psychoneuroendocrinology, 37(8), 1199–1208.
Phebe, T., Adamson, P., Scarborough, A., Williams, D., Groff, J., & McClean, H. (1997). Paroxetine increases heart rate variability in panic disorder. Journal of Clinical Psychopharmacology, 17(5), 370–376.
Pohl, R., & Yeragani, V. K. (2001). QT interval variability in panic disorder patients after isoproterenol infusions. The International Journal of Neuropsychopharmacology, 4(01), 17–20.
Ponikowski, P., Anker, S. D., Chua, T. P., Szelemej, R., Piepoli, M., Adamopoulos, S., et al. (1997). Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. The American journal of cardiology, 79(12), 1645–1650.
Porges, S. W. (2011). The Polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation (Norton series on interpersonal neurobiology). New York: WW Norton & Company.
Radespiel-Tröger, M., Rauh, R., Mahlke, C., Gottschalk, T., & Mück-Weymann, M. (2003). Agreement of two different methods for measurement of heart rate variability. Clinical Autonomic Research, 13(2), 99–102.
Schreiber, W., Lauer, C. J., Krumrey, K., Holsboer, F., & Krieg, J.-C. (1996). Dysregulation of the hypothalamic-pituitary-adrenocortical system in panic disorder. Neuropsychopharmacology, 15(1), 7–15.
Sinha, S. S., Coplan, J. D., Gorman, J. M., Pine, D. S., Martinez, J. A., & Klein, D. F. (1999). Panic induced by carbon dioxide inhalation and lack of hypothalamic–pituitary–adrenal axis activation. Psychiatry Research, 86(2), 93–98.
Smit, F., Cuijpers, P., Oostenbrink, J., Batelaan, N., de Graaf, R., & Beekman, A. (2006). Costs of nine common mental disorders: Implications for curative and preventive psychiatry. Journal of Mental Health Policy and Economics, 9(4), 193–200.
Smoller, J. W., Pollack, M. H., Wassertheil-Smoller, S., Jackson, R. D., Oberman, A., Wong, N. D., et al. (2007). Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Archives of General Psychiatry, 64(10), 1153–1160.
Spitzer, R. L., Williams, J. B., Gibbon, M., & First, M. B. (1992). The structured clinical interview for DSM-III-R (SCID): I: History, rationale, and description. Archives of General Psychiatry, 49(8), 624–629.
Stein, M. B., & Asmundson, G. J. (1994). Autonomic function in panic disorder: Cardiorespiratory and plasma catecholamine responsivity to multiple challenges of the autonomic nervous system. Biological Psychiatry, 36(8), 548–558.
Stein, P. K., & Kleiger, R. E. (1999). Insights from the study of heart rate variability. Annual Review of Medicine, 50(1), 249–261.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability. Standards of measurement, physiologic interpretation, and clinical use. Circulation, 93(5), 1043–1065.
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216.
Thayer, J. F., & Lane, R. D. (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33(2), 81–88.
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131.
Wang, S.-M., Lee, H.-K., Kweon, Y.-S., Lee, C. T., Chae, J.-H., Kim, J.-J., et al. (2016). Effect of emotion regulation training in patients with panic disorder: Evidenced by heart rate variability measures. General Hospital Psychiatry, 40, 68–73.
Winchell, R. J., & Hoyt, D. B. (1996). Spectral analysis of heart rate variability in the ICU: A measure of autonomic function. Journal of Surgical Research, 63(1), 11–16.
Wittchen, H.-U. (1993). Strukturiertes klinisches Interview für DSM-III-R: SKID: Beltz.
Woodward, S. H., Arsenault, N. J., Voelker, K., Nguyen, T., Lynch, J., Skultety, K., et al. (2009). Autonomic activation during sleep in posttraumatic stress disorder and panic: A mattress actigraphic study. Biological Psychiatry, 66(1), 41–46.
Yeragani, V. K., Balon, R., Pohl, R., & Ramesh, C. (1995a). Depression and heart rate variability. Biological Psychiatry, 38(11), 768–770.
Yeragani, V. K., Balon, R., Pohl, R., Ramesh, C., Glitz, D., Weinberg, P., et al. (1990). Decreased R-R variance in panic disorder patients. Acta Psychiatrica Scandinavica, 81(6), 554–559.
Yeragani, V. K., Berger, R., Pohl, R., Srinivasan, K., Balon, R., Ramesh, C., et al. (1992). Effects of yohimbine on heart rate variability in panic disorder patients and normal controls: A study of power spectral analysis of heart rate. Journal of Cardiovascular Pharmacology, 20(4), 609–618.
Yeragani, V. K., Berger, R., Songer, D. A., & Yeragani, S. (1997). Power spectrum of the QRS complex in patients with panic disorder and normal controls. Psychiatry Research, 66(2), 167–174.
Yeragani, V. K., Pohl, R., Berger, R., Balon, R., Ramesh, C., Glitz, D., et al. (1993). Decreased heart rate variability in panic disorder patients: A study of power-spectral analysis of heart rate. Psychiatry Research, 46(1), 89–103.
Yeragani, V. K., Pohl, R., Srinivasan, K., Balon, R., Ramesh, C., & Berchou, R. (1995b). Effects of isoproterenol infusions on heart rate variability in patients with panic disorder. Psychiatry Research, 56(3), 289–293.
Yeragani, V. K., Srinivasan, K., Balon, R., Ramesh, C., & Berchou, R. (1994). Lactate sensitivity and cardiac cholinergic function in panic disorder. American Journal of Psychiatry, 151(8), 1226–1228.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The original version of this article was revised: The co-author Gloria-Beatrice Wintermann was not listed in the author group. This has been corrected in this version.
An erratum to this article is available at http://dx.doi.org/10.1007/s10484-017-9357-1.
Rights and permissions
About this article
Cite this article
Petrowski, K., Wichmann, S., Siepmann, T. et al. Effects of Mental Stress Induction on Heart Rate Variability in Patients with Panic Disorder. Appl Psychophysiol Biofeedback 42, 85–94 (2017). https://doi.org/10.1007/s10484-016-9346-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-016-9346-9