Abstract
The knowledge of the potential of transition metal-based complexes as catalysts for the reduction of CO2 has grown significantly over the last few decades. This chapter focuses on the progress made during recent years in the field of homogeneous iridium-catalyzed reduction of CO2 by using hydrogen and/or silicon hydrides as reducing agents, comparing them with homogeneous catalysts based on other transition metals.
The reported studies on iridium-catalyzed CO2 reduction processes show that an important point to keep in mind when designing a catalyst is the nature of the reducing agent (hydrogen, hydrosilanes, and/or hydrosiloxanes). Thus, iridium(III) half-sandwich complexes with 4,4′-dihydroxy-bipyridine (DHBP) or 4,7-dihydroxy-1,10-phenanthroline (DHPT) ligands, and iridium(III)-PNP pincer complexes have proven to be excellent catalysts for the hydrogenation of CO2 to formic acid. However, Ir(III)-NSiNMe (NSiN = fac-bis-(4-methylpyridine-2-yloxy)methylsilyl) and Ir(III)-NSiMe (NSiMe = 4-methylpyridine-2-yloxydimethylsilyl) species are not stable under hydrogen atmosphere but are effective catalysts for the reduction of CO2 with hydrosiloxanes to silylformate under solvent-free conditions and moderate CO2 pressures and temperatures. Moreover, while using iridium(III)-DHBP half-sandwich complexes, high CO2 and H2 pressures are required to achieve the catalytic CO2 hydrogenation to methanol; Ir-NSiMe species catalyze the reduction of CO2 to methoxysilane with hydrosiloxanes under low CO2 pressure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Carbon dioxide is an abundant, easily available, cheap, and low toxic chemical. On the other hand, during the last decades, the concentration of CO2 in the earth’s atmosphere has reached historical values, which is generally considered one of the main reasons for the global warming. Therefore, both for economic and environmental reasons the development of sustainable processes that allow the transformation of CO2 on an industrial scale into valuable chemicals could be considered one of the most important tasks for the sustainability of the modern chemical industry [1,2,3,4,5]. In this context, to achieve the goal of using CO2 as raw material of the chemical industry there are several difficulties to face, among which its great thermodynamic stability stands out.
Catalysis has proven to be essential to overcome the challenge of CO2 stability. Thus, in recent decades, great advances have been made in the field of catalytic CO2 transformation into value added chemicals [6,7,8,9,10,11,12,13,14,15,16]. Particularly, catalytic hydrogenation [6, 9, 10, 17,18,19,20,21] and/or hydrosilylation [22,23,24,25] of CO2 have proven to be efficient methodologies for its reduction to formate, formaldehyde, methanol, or methane level (Scheme 1). In this regard, it is remarkable that several homogeneous catalytic systems based on iridium complexes have shown high catalytic performance as CO2 reduction catalysts [18, 26,27,28]. This chapter will focus on the progress made during recent years in the field of iridium-catalyzed reduction of CO2 by using hydrogen and/or hydrosilanes as reducing agents.
2 Recent Advances on Iridium-Catalyzed CO2 Hydrogenation
During last decades, several examples of homogeneous catalysts effective for the hydrogenation of CO2 have been reported, most of them are based on ruthenium(II) complexes but some examples of highly active iridium(III) catalysts have also been described. Among them are iridium(III) half-sandwich complexes with 4,4′-dihydroxy-bipyridine (DHBP) or 4,7-dihydroxy-1,10-phenanthroline (DHPT) ligands, which are excellent catalysts for the hydrogenation of CO2 to formic acid and also have been used as catalysts for the direct hydrogenation of CO2 to methanol. Moreover, iridium(III)-PNP pincer complexes have also been used as effective catalysts for the hydrogenation of CO2 to formic acid. Conversely, the potential of iridium complexes as catalysts for the hydrogenation of CO2 to formaldehyde, methyl carbonate, and/or methyl formate remains a challenge.
2.1 Iridium-Catalyzed Formic Acid or Formate Preparation from CO2 and H2
Catalytic hydrogenation of CO2 to formic acid (FA) has been a research subject of great interest over the last decades [6, 9, 10, 18, 20, 21, 28]. The hydrogenation of CO2 is endergonic in the gas phase (ΔG°298 = 32.9 kJ mol−1), however, in water solution and in presence of a base (NH3), this reaction becomes thermodynamically favored (ΔG°298 = −35.4 kJ mol−1) [29].
The first studies of the potential of transition metal complexes as homogenous catalysts for the hydrogenation of CO2 to FA were reported by Inoue et al. in 1976 [30]. These studies revealed that using NEt3 water solutions under 50 atm of mixtures of CO2 and H2 (1:1) at r.t. the complex [IrH3(PPh3)3] catalyzes this transformation, however, its catalytic activity is low. Under the same conditions species [RuH2(PPh3)4] was found to be the most active of the studied catalyst precursors [30]. Some years later, Leitner et al. reported very efficient rhodium phosphane water soluble catalysts, which were able to promote the formation of FA in relatively high yields [31, 32]. After that, Noyori et al. described that the effectivity of ruthenium phosphane complexes as CO2 hydrogenation catalysts improves when using supercritical carbon dioxide [33, 34]. Few years after that, Joó, Laurenczy et al. reported that the performance of catalytic systems based on water soluble Ir, Rh, Ru, and Pd phosphane complexes as CO2 hydrogenation catalyst is strongly pH dependent [35]. In this regard, Jessop et al. found that using the complex [RuCl(O2CMe)(PMe3)4], which is soluble in supercritical CO2, as catalysts for the hydrogenation of CO2 to FA in presence of the appropriate amine and one alcohol that has an aqueous scale pKa below that of the protonated amine, it was possible to achieve an initial turnover frequency (TOF) for FA production of 95,000 h−1 [36]. Since then till the development of the highly active Himeda’s catalysts [37], based on half-sandwich bipyridine iridium complexes, most of the homogeneous catalysts effective for the hydrogenation of CO2 to FA were based on Ru- and Rh-phosphane complexes.
Early examples of highly active iridium CO2 hydrogenation catalysts were based on iridium half-sandwich complexes with 4,4′-dihydroxy-bipyridine (DHBP) or 4,7-dihydroxy-1,10-phenanthroline (DHPT) ligands (Scheme 2) [38]. These catalysts are highly efficient for the hydrogenation of carbonate, in situ generated from CO2 in basic KOH aqueous solutions, to formate. The oxyanions generated from the hydroxy group along the catalytic process play a key role on both the catalytic activity and water solubility of these catalysts (Scheme 2).
Initial turnover frequencies (TOF) of 42,000 and 35,000 h−1 were obtained for the Ir-DHPB and Ir-DHPT (Scheme 2) catalyzed reactions, respectively. The best performance was achieved heating at 120°C aqueous KOH (1.0 M) solutions of the corresponding iridium catalysts under 6 MPa of CO2/H2 (1:1). Moreover, these iridium catalysts could be reused for four cycles maintaining high catalytic performance [38].
Himeda et al. have extended their studies to iridium half-sandwich complexes with N,N-bidentate ligands different from bipyridine such as picolinamide- [39, 40], azole- [41] and pyridyl-pyrazole derivatives [42]. Mechanistic studies have found that these catalysts promote the activation of CO2 via an outer-sphere mechanism [41]. Interestingly, it has been found that using this type of iridium catalysts it is possible to achieve the pH-controlled reversible hydrogen storage [40, 42, 43].
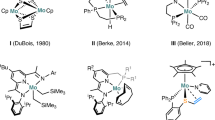
Further support to the relevant role of oxyanions in these type of catalysts comes from the studies reported from Peris et al. [44], which showed that using half-sandwich iridium(III) complexes with strong donor NHC ligands (Fig. 1) or bipyridine derivatives without hydroxy substituents, as catalysts precursors for the hydrogenation of CO2 lower activities (TOF = 1,600 h−1) were observed.
Iridium-pincer complexes have also found to be active catalysts for the homogeneous hydrogenation of CO2 to FA. The iridium(III)trihydride-PNP complex shown in Scheme 3 reached a TOF of 150,000 h−1 for the hydrogenation of CO2 to FA in basic medium. The performance of this catalytic system is strongly influenced by the nature of the base, the temperature and the presence of THF in the reaction medium. Thus, the best results were obtained at 200°C, using 1.0 M KOH aqueous solution and adding 0.1 mL of THF [45]. Mechanistic studies showed that two reactions pathways are possible, one of them involving a deprotonative dearomatization of the pyridinic ring and other a hydroxy-assisted hydrogenolysis as the rate determining step, respectively. Moreover, an outer-sphere mechanism has been found for the CO2 activation step (Scheme 3) [46].
Iridium-PNP catalysts showed the best performance in KOH aqueous solutions, however, under these conditions, the corresponding formate salt, not FA, is obtained as reaction product. Therefore, a neutralization step of the formate with a strong acid is required to obtain FA. Interestingly, when using amine derivatives as bases a simple distillation of the resulting ammonium formate allows separation of pure FA from the starting base. In this regard, Nozaki’s group has studied the effect of both using triethanolamine aqueous solution as base and having different substituents at the pyridinic ring on the activity of Ir-PNP catalysts (Fig. 2). They have found that under these conditions the dichlorohydride derivative with a p-MeO substituent is the most active catalyst, indeed, using this species as catalyst precursor in a 1.0 M triethanolamine aqueous solution, in presence of THF and heating at 150°C, a TON for the conversion of CO2 to FA of 160,000 (TOF = 12,000 h−1) was obtained [47].
On the other hand, Hazari and coworkers have studied the activity of Ir-PNHP (PNHP = bis{(2-diisopropylphosphanyl)ethyl}amine) pincer species as CO2 hydrogenation catalysts. They have shown that the insertion of CO2 into one of the Ir-H bonds of the trihydride derivative [Ir(PNHP)H3] gives the corresponding [Ir(PNHP)(HCO2)H2] species, which is stabilized by an intramolecular NH-OCO hydrogen bond (Scheme 4) [48]. This iridium-formate derivative catalyzes the hydrogenation of CO2, in 1 M aqueous KOH solution at 185°C, with a TON and TOF values of 348,000 and 18,780 h−1, respectively. DFT calculations show that the Ir-PNHP-catalyzed CO2 hydrogenation takes place through an outer-sphere mechanism (Scheme 4). The rate determining step of the overall catalytic process corresponds to the NH-assisted CO2 activation step [48].
2.2 Iridium-Catalyzed Methanol Preparation from Direct Hydrogenation of CO2
Methanol is commonly produced on an industrial scale using fossil fuel-based syngas as the principal feedstock. The annual demand for methanol has grown steadily over the last decade, consequently the CO2 emissions related to the industrial production of methanol have also grown [49, 50]. Therefore, the development of catalysts effective for the synthesis of methanol from renewable sources is attracting the interest of several research groups [50, 51]. In this regard, the production of methanol through carbon dioxide capture and recycling is one of the keys of the “Methanol Economy” concept [52]. The early example of a homogeneous catalyst effective for the direct hydrogenation of CO2 to methanol was reported by Tominaga et al. in 1993 [53, 54]. They used [Ru3(CO)12] as catalyst precursor, KI as additive to prevent the formation of metallic nanoparticles, and N-methylpyrrolidone as solvent at 240°C under 80 bar of a 1:3 mixture of CO2 and H2. In this regard, it should be mentioned that it is of great importance to avoid the decomposition of the homogeneous catalysts to colloidal or nanosized metallic particles, which may have different catalytic behavior than the parent homogeneous catalysts. Since then only few examples of catalytic systems effective for the direct hydrogenation of CO2 to methanol have been reported. The reason is that the direct conversion of CO2 to methanol is thermodynamically hampered at high temperatures due to the negative ΔH and ΔS values of this process.
The first examples of iridium homogeneous catalysts effective for the direct hydrogenation of CO2 to methanol were reported by Himeda, Laurenczy et al. in 2016. They found that the sulfate salt of the iridium half-sandwich cationic complex [IrCp*(DHBP)(OH2)][SO4] (DHBP = 4,4′-dihydroxy-2,2′-bypyridine) catalyzes the one pot hydrogenation of CO2 to methanol. This Ir-DHBP species catalyzes the quantitative hydrogenation of CO2 to formic acid in acidic media without any additives, and the subsequent disproportionation of the in situ generated formic acid to give methanol (96% selectivity; 47% yield; TON = 1,314), CO2 and H2O [55]. In this regard, it is important to be aware that whenever the hydrogenation of CO2 takes place in basic solution, the question arises whether the actual reactive partner of the catalysts is carbonate, bicarbonate, or (hydrated) CO2.
The activity of this iridium catalyst is higher than that reported for the Ru-(Triphos) (Triphos = 1,1,1-tris(diphenylphosphinomethyl)ethane) species (TON = 221) [56, 57], the ruthenium(II) species [Ru(PNP)(H)(H-BH3)(CO)] (PNP = {Bis[2-(diphenylphosphino)ethyl]amine}) [58, 59], Co-(Triphos) (TON = 50) [60] and Mn-(PNP) (TON = 36) [61]. Being surpassed by that of the complex Fe-(κ3-HTpm) (HTpm = tris(pyrazolyl)methane; 44% yield; TON = 2,283) [62].
2.3 Miscellaneous
Examples of homogenous catalysts effective for the hydrogenation of CO2 to other products, different of formic acid and/or methanol, are scarce. Indeed, to the best of our knowledge only few examples of ruthenium catalysts effective for the hydrogenation of CO2 to dimethyl ether [63], formaldehyde [64, 65], or methyl formate [66] have been reported. Therefore, the potential of iridium complexes as catalysts for these types of processes remains unexplored.
3 Recent Advances on Iridium-Catalyzed CO2 Hydrosilylation
The catalytic hydrogenation of CO2 with H2 requires high H2 and CO2 pressures and temperatures, as well as the addition of bases or other additives. Contrariwise, the catalytic reduction of CO2 with hydrosilanes features several advantages such as being a thermodynamically favored process and the fact that silanes are easier and safer to handle and to store than molecular hydrogen [22, 23, 25, 26, 67]. However, the utilization of silicon hydrides as reductants for large-scale reduction of CO2 faces some difficulties. One of them is the high price of hydrosilanes, which could be solved by using cheap hydrosiloxanes instead of hydrosilanes, another is the stoichiometric generation of siloxanes, which is unsustainable due to the challenge of Si-H regeneration from Si-O-Si bonds [24, 68]. Furthermore, differently to hydrogenation processes, the catalytic hydrosilylation cannot be performed in aqueous or alcoholic solutions since homogeneous hydrosilylation catalysts usually catalyzed the dehydrogenative hydrolysis and/or alcoholysis of silicon hydrides [69, 70].
The catalytic reaction of CO2 with silicon hydrides allows its selective reduction to the corresponding silylformate, bis(silyl)acetal or methoxysilane, and to methane [22, 23, 25, 26] (Scheme 5). In addition, the formation of methyl carbonates from the iridium-catalyzed reduction of CO2 with silicon hydrides has been recently reported (Scheme 5) [71].
The first examples of homogeneous catalytic reduction of CO2 using hydrosilanes as reductants were reported in the 1980s [72,73,74]. However, it was during the year 2012 that the breakthrough of this chemistry took place. Since then until today, the number of catalytic systems effective for the reduction of CO2 with hydrosilanes based on transition metal complexes as well as on metal-free catalysts or main elements derivatives that have proven to be effective in CO2 hydrosilylation processes has considerably grow up [8, 22,23,24,25, 67, 75]. Among them, catalysts based on iridium complexes stand out not only for their activity but also for their versatility that allows selectivity control by choosing proper ligands and/or tuning the reaction conditions. Furthermore, some examples of iridium-based CO2 hydrosilylation catalysts have proven to be effective under solvent-free conditions and using hydrosiloxanes as reductants.
3.1 Iridium-Catalyzed CO2 Hydrosilylation to Silylformate
The iridium complex [Ir(CN)(CO)(dppe)] (dppe = 1,2-bis(diphenylphosphino)ethane), reported in 1989 by Eisenschmid and Eisenberg, is the first example of a homogeneous iridium-based catalyst effective for the hydrosilylation of CO2. However, the catalytic activity and the selectivity of this iridium catalyst were low [74]. It was not until 2012 that an example of iridium catalyst, complex [Ir(CF3SO3)(NSiN)(SiR3)(NCMe3)] (NSiN = fac-bis-(pyridine-2-yloxy)methylsilyl; SiR3 = SiMe(OSiMe3)2), efficient for the hydrosilylation of CO2 to selectively give the corresponding silylformate was reported [76]. This catalytic system allows the solvent-free and gram-scale formation of silylformates under mild reaction conditions (3 bar, 298 K, TON = 97.5) but is slow (TON = 0.7 h−1) [76]. Interestingly, using species [Ir(CF3SO3)(NSiN)(H)(coe)] (coe = cis-cyclooctene, Scheme 6), which is easier to prepare than the abovementioned Ir-NSiN-acetonitrile derivative, under the same reaction conditions (3 bar, 298 K) produces an increase of the reaction rate (TOF = 1.2 h−1) [77, 78]. Further studies on the influence of reaction temperature [77] and CO2 pressure [78] on the catalytic performance of this catalytic system showed that the activity is directly proportional to the temperature; however, increasing the temperature reduces the selectivity to silylformate [77]. On the other hand, it is more difficult to generalize the CO2-pressure effect on the activity of the reaction. It is remarkable, that from the point of view of selectivity the CO2- pressure has proven to be a parameter to consider. Indeed, for each temperature an enhancement of the CO2-pressure results in increased the selectivity of the process [78]. Thus, using species [Ir(CF3SO3)(NSiN)(H)(coe)] as catalyst precursor the best reaction performance was achieved at 344 K and under 8 bar of CO2 (99.9% conversion, 89.7% purity (GC-MS), TOF = 138 h−1; TON = 87.5) (Scheme 6) [78].
The iridium(III) complex [Ir(H)(CF3CO2)(NSiNMe)(coe)] (NSiNMe = fac-bis-(4-methylpyridine-2-yloxy)methylsilyl), which contains a trifluoroacetate instead of a triflate ligand and a NSiNMe ligand with 4-methylated pyridinic rings (Fig. 3), has proven to be a highly effective CO2 hydrosilylation catalyst [79]. Using this Ir-trifluoroacetate-NSiNMe species as catalyst precursor for the hydrosilylation of CO2 to silylformate with HSiMe(OSiMe3)2 the best results were achieved at 328 K and under 8 bar of CO2 (100% conversion; 98.9% yield to SF by GC-MS; TOF = 99.3 h−1), at temperatures above 328 K a decrease in catalytic selectivity and activity was observed [79].
Mechanistic studies based on theoretical calculations at DFT level showed that while Ir-trifluoroacetate-NSiNMe species catalyzes the CO2 activation via an inner-sphere mechanism, an outer-sphere mechanism is favored for Ir-triflate-NSiNMe derivatives (Fig. 4) [80].
The presence of the Ir-silyl group of the NSiNR (R = H, Me) ligand trans-located to the trifluoroacetate (or triflate) ligand plays a key role on the catalytic activity of Ir-NSiNR catalysts. Based on this knowledge the catalyst precursor [Ir(CF3CO2)(κ2-NSiMe)2] (NSiMe = 4-methylpyridine-2-yloxydimethylsilyl), containing two Ir-Si bonds trans-located to the catalyst active positions was designed (Fig. 5) [81]. 1H NMR studies on the activity of [Ir(CF3CO2)(κ2-NSiMe)2] as CO2 hydrosilylation catalyst using HSiMe(OSiMe3)2 show that at 298 K under 4 bar of CO2 this catalyst is more active (TOF = 28.6 h−1) [81] than the previously reported Ir-NSiN species, which at 298 K independently of the CO2-pressure are low active with TOF values in the rage of 1.2–1.6 h−1 [78]. The higher activity of [Ir(CF3CO2)(κ2-NSiMe)2] allows the selective formation methoxysilane from CO2 and HSiMe(OSiMe3)2 as it is shown below [81].
Other iridium complex which have proven to be an active catalyst for the selective hydrosilylation of CO2 (3 bar) to silylformates is the zwitterionic iridium(III) half-sandwich species [IrClCp*{(MeIm)2CHCOO}] ((MeIm = 3-methylimidazol-2-yliden-1-yl; Cp* = pentamethylcyclopentadienyl) (Scheme 7) [82]. However, this catalytic system requires the use of acetonitrile as reaction solvent. It is relatively high active for the hydrosilylation of CO2 with HSiMe2Ph (TOF = 51 h−1), but under the same reaction conditions is not active when the hydrosiloxane HSiMe(OSiMe3)2 is used as reductant instead of HSiMe2Ph [82].
Other transition metal-based catalysts including Ru [83, 84], Co [85], Rh [86], Pd [87], Pt [88], Cu [89, 90], and Zn [91, 92] complexes effective for the selective hydrosilylation of CO2 to the formate level have been reported. Among them, the catalytic system based on the Pd-PAlP complex shown in Scheme 8 has proven to be the most active catalyst for CO2-hydrosilylation reported so far [87]. Indeed, using this Pd-PAlP catalyst in DMF as solvent in presence of CstBuCO2 (1.0 mol%) at 298 K, the selective reaction of CO2 with HSiMe2Ph to give HCO2SiMe2Ph (92%, TOF = 19,300 h−1) was achieved in 1 h (Scheme 8) [87].
Ir-NSiN and Ir-NSiMe species are comparatively less active than some of the abovementioned catalysts; however, they have the advantage of being active under solvent-free conditions and are highly effective when using hydrosiloxanes, instead of hydroorganosilanes, as reductants. Therefore, from the point of view of sustainability iridium species based on Ir-NSiN and Ir-NSiMe species could be considered promising for future applications of the catalytic reduction of CO2 with silicon hydrides.
3.2 Iridium-Catalyzed Reduction of CO2 to Methoxysilanes with Silicon-Hydrides
Only few examples of homogeneous catalysts effective for the reduction of CO2 to methanol level using silicon hydrides as reducing agents have been published to date. The first one was the abovementioned iridium complex [Ir(CN)(CO)(dppe)] (dppe = 1,2-bis(diphenylphosphino)ethane) [74]. This catalyst promotes the reduction of CO2 with HSiMe3 in C6D6 at 313 K to the corresponding methoxysilane, CH3OSiMe3. This reaction is slow, and 2 weeks are required to achieve the conversion of the starting hydrosilane into CH3OSiMe3. 13C NMR studies of this process using 13CO2 confirm that it entails in a stepwise progression with the initial formation of the corresponding silylformate HCO2SiMe3, which is further reduce to bis(silyl)acetal CH2(OSiMe3)2, the later finally reacts with one equivalent of HSiMe3 to give CH3OSiMe3 and O(SiMe3)2 (Scheme 9) [74].
The iridium(III) complex [Ir(CF3CO2)(κ2-NSiMe)2] (Fig. 5) has proven to be an effective catalyst for the reduction of CO2 with HSiMe(OSiMe3)2 to the methoxysilane CH3OSiMe(OSiMe3)2 under mild reaction conditions. 1H NMR studies of the reaction of CO2 (1 bar) with HSiMe(OSiMe3)2 in C6D6 at 298 K evidenced the selective formation of the corresponding methoxysilane after 16 h (99.0%; TON = 33.6; TOF = 2.1 h−1) [81]. Interestingly, increasing the CO2 pressure to 4 bar the reaction stops in the corresponding silylformate, which under 4 bar is the major reaction product (93%; TON = 93; TOF = 2.9 h−1) together with a 7% of CH3OSiMe(OSiMe3)2 after 3.5 h. 1H and 13C NMR studies and theoretical calculations at the DFT level on the Ir-NSiMe catalyzed CO2 reduction to methoxysilane with silicon hydrides, agree with an stepwise mechanism similar to that shown in Scheme 9.
The related complex [Ir(μ-CF3SO3)(κ2-NSiMe)2]2, which is a rare example of an iridium dinuclear species with triflate groups acting as bridges, catalyzed the reaction of CO2 (3 bar) with HSiMe(OSiMe3)2 in C6D6 at 323 K to afford, after 3 h, a mixture of the corresponding silylformate (65.2%), methoxysilane (8.1%) and methylsilylcarbonate (26.7%) (Scheme 10) [71].
1H and 13C NMR studies of the reaction shown in Scheme 10 evidenced that at 323 K, once all the starting hydrosilane is consumed; the methylsilylcarbonate is slowly transformed into the corresponding methoxysilane. These outcomes prove that the formation of methoxysilanes during the catalytic reduction of CO2 with silicon hydrides, which traditionally has been explained by the stepwise process shown in Scheme 9, could also be consequence of thermal decomposition of the corresponding methylsilylcarbonate (Scheme 11) [71].
Few examples of other homogeneous catalysts effective for the reduction of CO2 to methanol level using silicon hydrides as reductants have been described, which include the anionic rhenium complex [N(hexyl)4][ReO4] [93], the cationic zinc derivative [Zn(Me)(IDipp)][C6F5)3] (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) [94] and metal-free NHC-catalysts [95]. In this context, it is noteworthy that the activity of the Ir-trifluoroacetate-NSiMe catalyst is similar to that reported for these Re-, Zn-, and NHC-based catalytic systems.
3.3 Iridium-Catalyzed Reduction of CO2 to Methane with Silicon-Hydrides
The catalytic reduction of CO2 to methane using hydrosilanes as reducing agents remains a challenge. Examples of transition metal catalysts based on Zr [96, 97], Hf [97], Ir [98], Pd [99] and Pt [99] complexes as well as transition metal-free catalysts such as the frustrated Lewis pair B(C6F5)3/TMP (TMP = 2,2,6,6-tetramethylpyperidine) [100] and other Lewis acids and ionic pairs [101,102,103,104]. Among them stands out the iridium(III) cationic species [Ir(H)(η1-HSiR3)(POCOP)][B(C6F5)4] (POCOP = 2,6-bis((di-tert-butylphosphanyl)oxy)benzen-1-yl) reported by Brookhart et al. in 2012 [98], which has proven to be effective for the reduction of CO2 (1 bar, 296 K) to methane with different hydrosilanes (HSiEt3, HSiPh3, HSiMe2Et, HSiMe2Ph, and HSiEt2Me) using C6H5Cl as solvent. This catalytic system works reasonably well with HSiMe2Ph at 296 K (TOF = 115 h−1), moreover, increasing the temperature to 333 K produces a positive effect of the catalytic activity (TOF = 661 h−1) (Scheme 12) [98].
4 Concluding Remarks
This chapter illustrates the progress made during recent years in the field of iridium-catalyzed reduction of CO2 with hydrogen and/or silicon hydrides as reductants. It is difficult to draw general conclusions since not only the characteristics of the ligands but also the nature of the reducing agent (hydrogen, hydrosilanes, and/or hydrosiloxanes) strongly influences the reaction conditions and the mechanism. It has been observed that most of the iridium CO2 hydrogenation and hydrosilylation catalysts are based on Ir(III) species. The selectivity is one of the challenges of homogeneous catalytic CO2 reduction with hydrogen and silicon hydrides, this is because mixtures of different reduction products are frequently obtained. In this regard, it is worth mentioning that iridium(III) half-sandwich-DHBP species and iridium(III)-PNP pincer complexes have found to be highly efficient and selective CO2 hydrogenation catalysts and that Ir(III)-NSiN and Ir(III)-NSiMe species have proven to be highly selective CO2 hydrosilylation catalysts. From the point of view of the mechanism, it is difficult to establish a general behavior trend. Thus, although most of the reported homogeneous Ir(III) catalysts follow an outer-sphere CO2 activation mechanism, when using Ir-NSiN and Ir-NSiMe trifluoroacetate derivatives as CO2 hydrosilation catalysts, an inner-sphere CO2 activation mechanism is preferred. Therefore, it could be concluded that iridium(III) complexes have great potential as homogeneous CO2 reduction catalysts; however, there are still many mechanistic questions to answer and future applications to unveil.
Change history
26 June 2021
This book was inadvertently published without updating the following corrections:
References
Artz J, Müller TE, Thenert K, Kleinekorte J, Meys R, Sternberg A, Bardow A, Leitner W (2018) Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem Rev 118:434–504
Stahel WR (2016) Circular economy. Nature 531:435–438
Clark JH, Farmer TJ, Herrero-Davila L, Sherwood J (2016) Circular economy design considerations for research and process development in the chemical sciences. Green Chem 18:3914–3934
Grignard B, Gennen S, Jérôme C, Kleij AW, Detrembleur C (2019) Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem Soc Rev 48:4466–4514
Martens JA, Bogaerts A, De Kimpe N, Jacobs PA, Marin GB, Rabaey K, Saeys M, Verhelst S (2017) The chemical route to a carbon dioxide neutral world. ChemSusChem 10:1039–1055
Klankermayer J, Wesselbaum S, Beydoun K, Leitner W (2016) Selective catalytic synthesis using the combination of carbon dioxide and hydrogen: catalytic chess at the Interface of energy and chemistry. Angew Chem Int Ed 55:7296–7343
Liu Q, Wu L, Jackstell R, Beller M (2015) Using carbon dioxide as a building block in organic synthesis. Nat Commun 6:5933
Das Neves Gomes C, Jacquet O, Villiers C, Thuéry P, Ephritikhine M, Cantat T (2012) A diagonal approach to chemical recycling of carbon dioxide: organocatalytic transformation for the reductive functionalization of CO2. Angew Chem Int Ed 51:187–190
Jessop PG, Joó F, Tai C-C (2004) Recent advances in the homogeneous hydrogenation of carbon dioxide. Coord Chem Rev 248:2425–2442
Wang W-H, Himeda Y, Muckerman JT, Manbeck GF, Fujita E (2015) CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem Rev 115:12936–12973
Cokoja M, Bruckmeier C, Rieger B, Herrmann WA, Kühn FE (2011) Transformation of carbon dioxide with homogeneous transition-metal catalysts: a molecular solution to a global challenge? Angew Chem Int Ed 50:8510–8537
Peters M, Köhler B, Kuckshinrichs W, Leitner W, Markewitz P, Müller TE (2011) Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSusChem 4:1216–1240
Aresta M, Dibenedetto A, Angelini A (2014) Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem Rev 114:1709–1742
Goeppert A, Zhang H, Czaun M, May RB, Prakash GKS, Olah GA, Narayanan SR (2014) Easily regenerable solid adsorbents based on polyamines for carbon dioxide capture from the air. ChemSusChem 7:1386–1397
Sakakura T, Choi J-C, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107:2365–2387
Centi G, Quadrelli EA, Perathoner S (2013) Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energ Environ Sci 6:1711–1731
Ronda-Lloret M, Rothenberg G, Shiju NR (2019) A critical look at direct catalytic hydrogenation of carbon dioxide to olefins. ChemSusChem 12:3896–3914
Onishi N, Laurenzcy G, Beller M, Himeda Y (2018) Recent Progress for reversible homogeneous catalytic hydrogen storage in formic acid and in methanol. Coord Chem Rev 373:317–332
Jia J, Qian C, Dong Y, Li YF, Wang H, Ghoussoub M, Butler KT, Walsh A, Ozin GA (2017) Heterogeneous catalytic hydrogenation of CO2 by metal oxides: defect engineering – perfecting imperfection. Chem Soc Rev 46:4631–4644
Prieto G (2017) Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: thermodynamic and kinetic bounds and Progress with heterogeneous and homogeneous catalysis. ChemSusChem 10:1056–1070
Wang W, Wang S, Ma X, Gong J (2011) Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev 40:3703–3727
Fernández-Alvarez FJ, Oro LA (2018) Homogeneous catalytic reduction of CO2 with silicon-hydrides, state of the art. ChemCatChem 10:4783–4796
Fernández-Alvarez FJ, Aitani AM, Oro LA (2014) Homogeneous catalytic reduction of CO2 with Hydrosilanes. Cat Sci Technol 4:611–624
Chauvier C, Cantat T (2017) A viewpoint on chemical reductions of carbon-oxygen bonds in renewable feedstocks including CO2 and biomass. ACS Catal 7:2107–2115
Chen J, McGraw M, Chen EY-X (2019) Diverse catalytic systems and mechanistic pathways for hydrosilylative reduction of CO2. ChemSusChem 12:4543–4569
Fernández-Alvarez FJ, Iglesias M, Oro LA, Polo V (2013) CO2 activation and catalysis driven by iridium complexes. ChemCatChem 5:3481–3494
Iglesias M, Oro LA (2018) A leap forward in iridium-NHC catalysis: new horizons and mechanistic insights. Chem Soc Rev 47:2772–2808
Gunasekar GH, Park K, Jung K-D, Yoon S (2016) Recent development in the catalytic hydrogenation of CO2 to formic acid/formate using heterogeneous catalysts. Inorg Chem Front 3:882–895
Jessop PG, Ikariya T, Noyori R (1995) Homogeneous hydrogenation of carbon dioxide. Chem Rev 95:259–272
Inoue Y, Izumida H, Sasaki Y, Hashimoto H (1976) Catalytic fixation of carbon dioxide to formic acid by transition-metal complexes under mild conditions. Chem Lett 5:863–864
Graf E, Leitner W (1992) Direct formation of formic acid from carbon dioxide and dihydrogen using the [{Rh(cod)Cl}2]-Ph2P(CH2)4PPh2 catalyst system. J Chem Soc Chem Commun:623–624
Gassner F, Leitner W (1993) Hydrogenation of carbon dioxide to formic acid using water-soluble rhodium catalysts. J Chem Soc Chem Commun:1465–1466
Jessop PG, Ikariya T, Noyori R (1994) Homogeneous catalytic hydrogenation of supercritical carbon dioxide. Nature 368:231–233
Jessop PG, Hsiao Y, Ikariya T, Noyori R (1996) Homogeneous catalysis in supercritical fluids: hydrogenation of supercritical carbon dioxide to formic acid, alkyl formates, and formamides. J Am Chem Soc 118:344–355
Joó F, Laurenczy G, Nádasdi L, Elek J (1999) Homogeneous hydrogenation of aqueous hydrogen carbonate to Formate under exceedingly mild conditions – a novel possibility of carbon dioxide activation. Chem Commun:971–972
Munshi P, Main AD, Linehan JC, Tai C-C, Jessop PG (2002) Hydrogenation of carbon dioxide catalyzed by ruthenium trimethylphosphine complexes: the accelerating effect of certain alcohols and amines. J Am Chem Soc 124:7963–7971
Himeda Y (2007) Conversion of CO2 into formate by homogeneously catalyzed hydrogenation in water: tuning catalytic activity and water solubility through the acid-base equilibrium of the ligand. Eur J Inorg Chem:3927–3941
Himeda Y, Onozawa-Komatsuzaki N, Sugihara H, Kasuga K (2007) Simultaneous tuning of activity and water solubility of complex catalysts by acid-base equilibrium of ligands for conversion of carbon dioxide. Organometallics 26:702–712
Kanega R, Onishi N, Szalda DJ, Ertem MZ, Muckerman JT, Fujita E, Himeda Y (2017) CO2 hydrogenation catalysts with deprotonated picolinamide ligands. ACS Catal 7:6426–6429
Kanega R, Onishi N, Wang L, Murata K, Muckerman JT, Fujita E, Himeda Y (2018) Picolinamide-based iridium catalysts for dehydrogenation of formic acid in water: effect of amide N substituent on activity and stability. Chem A Eur J 24:18389–18392
Suna Y, Himeda Y, Fujita E, Muckerman JT, Ertem MZ (2017) Iridium complexes with proton-responsive azole-type ligands as effective catalysts for CO2 hydrogenation. ChemSusChem 10:4535–4543
Onishi N, Kanega R, Fujita E, Himeda Y (2019) Carbon dioxide hydrogenation and formic acid dehydrogenation catalyzed by iridium complexes bearing pyridyl-pyrazole ligands: effect of an electron-donating substituent on the pyrazole ring on the catalytic activity and durability. Adv Synth Catal 361:289–296
Hull JF, Himeda Y, Wang W-H, Hashiguchi B, Periana R, Szalda DJ, Muckerman JT, Fujita E (2012) Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat Chem 4:383–388
Sanz S, Benítez M, Peris E (2010) A new approach to the reduction of carbon dioxide: CO2 reduction to formate by transfer hydrogenation in iPrOH. Organometallics 29:275–277
Tanaka R, Yamashita M, Nozaki K (2009) Catalytic hydrogenation of carbon dioxide using Ir(III)-pincer complexes. J Am Chem Soc 131:14168–14169
Tanaka R, Yamashita M, Chung LW, Morokuma K, Nozaki K (2011) Mechanistic studies on the reversible hydrogenation of carbon dioxide catalyzed by an Ir-PNP complex. Organometallics 30:6742–6750
Aoki W, Wattanavinin N, Kusumoto S, Nozaki K (2016) Development of highly active Ir-PNP catalysts for hydrogenation of carbon dioxide with organic bases. Bull Chem Soc Jpn 89:113–124
Schmeier TJ, Dobereiner GE, Crabtree RH, Hazari N (2011) Secondary coordination sphere interactions facilitate the insertion step in an iridium(III) CO2 reduction catalysis. J Am Chem Soc 133:9274–9277
Pérez-Fortes M, Schöneberger JC, Boulamanti A, Tzimas E (2016) Methanol synthesis using captured CO2 as raw material: techno-economic and environmental assessment. Appl Energy 161:718–732
Ali KA, Abdullah AZ, Mohamed AR (2015) Recent development in catalytic technologies for methanol synthesis from renewable sources: a critical review. Renew Sustain Energy Rev 44:508–518
Hertrich MF, Beller M (2018) Metal-catalysed hydrogenation of CO2 into methanol. In: Dixneuf P, Soulé JF (eds) Organometallics for green catalysis. Topics in organometallic chemistry, vol 63. Springer, Cham
Olah GA (2005) Beyond oil and gas: the methanol economy. Angew Chem Int Ed 44:2636–2639
Tominaga K-I, Sasaki Y, Kawai M, Watanabe T, Saito M (1993) Ruthenium complex catalysed hydrogenation of carbon dioxide to carbon monoxide, methanol and methane. J Chem Soc Chem Commun:629–631
Tominaga K-I, Sasaki Y, Kawai M, Watanabe T, Saito M (1995) Homogeneous hydrogenation of carbon dioxide to methanol catalyzed by ruthenium cluster anions in the presence of halide anions. Bull Chem Soc Jpn 68:2837–2842
Sordakis K, Tsurusaki A, Iguchi M, Kawanami H, Himeda Y, Laurenczy G (2016) Carbon dioxide to methanol: the aqueous catalytic way at room temperature. Chem A Eur J 22:15605–15608
Wesselbaum S, vom Stein T, Klankermayer J, Leitner W (2012) Hydrogenation of carbon dioxide to methanol by using a homogeneous ruthenium–phosphine catalyst. Angew Chem Int Ed 51:7499–7502
Wesselbaum S, Moha V, Meuresch M, Brosinski S, Thenert KM, Kothe J, von Stein T, Englert U, Hölscher M, Klankermayer J, Leitner W (2015) Hydrogenation of carbon dioxide to methanol using a homogeneous ruthenium-triphos catalyst: from mechanistic investigations to multiphase catalysis. Chem Sci 6:693–704
Rezayee NM, Huff CA, Sanford MS (2015) Tandem amine and ruthenium-catalyzed hydrogenation of CO2 to methanol. J Am Chem Soc 137:1028–1031
Kar S, Sen R, Goeppert A, Prakash GKS (2018) Integrative CO2 capture and hydrogenation to methanol with reusable catalyst and amine: toward a carbon neutral methanol economy. J Am Chem Soc 140:1580–1583
Schneidewind J, Adam R, Baumann W, Jackstell R, Beller M (2017) Low-temperature hydrogenation of carbon dioxide to methanol with a homogeneous cobalt catalyst. Angew Chem Int Ed 56:1890–1893
Kar S, Goeppert A, Kothandaraman J, Prakash GKS (2017) Manganese-catalyzed sequential hydrogenation of CO2 to methanol via formamide. ACS Catal 7:6347–6351
Ribeiro APC, Martins LMDRS, Pombeiro AJL (2017) Carbon dioxide-to-methanol single-pot conversion using a C-scorpionate iron(II) catalyst. Green Chem 19:4811–4815
Thenert K, Beydoun K, Wiesenthal J, Leitner W, Klankermayer J (2016) Ruthenium-catalyzed synthesis of dialkoxymethane ethers utilizing carbon dioxide and molecular hydrogen. Angew Chem Int Ed 55:12266–12269
Siebert M, Seibicke M, Siegle AF, Kräh S, Trapp O (2019) Selective ruthenium-catalyzed transformation of carbon dioxide: an alternative approach toward formaldehyde. J Am Chem Soc 141:334–341
Seibicke M, Siebert M, Siegle AF, Gutenthaler SM, Trapp O (2019) Application of hetero-triphos ligands in the selective ruthenium-catalyzed transformation of carbon dioxide to the formaldehyde oxidation state. Organometallics 38:1809–1814
Westhues N, Belleflamme M, Klankermayer J (2019) Base-free hydrogenation of carbon dioxide to methyl formate with a molecular ruthenium-phosphine catalyst. ChemCatChem 11:5269–5274
Zhang Y, Zhang T, Das S (2020) Catalytic transformation of CO2 into C1 chemicals using hydrosilanes as reducing agents. Green Chem 22:1800–1820
Addis D, Das S, Junge K, Beller M (2011) Selective reduction of carboxylic acid derivatives by catalytic hydrosilylation. Angew Chem Int Ed 50:6004–6011
Garcés K, Fernández-Alvarez FJ, Polo V, Lalrempuia R, Pérez-Torrente JJ, Oro LA (2014) Iridium-catalyzed hydrogen production from hydrosilanes and water. ChemCatChem 6:1691–1697
Aliaga-Lavrijsen M, Iglesias M, Cebollada A, Garcés K, García N, Sanz Miguel PJ, Fernández-Alvarez FJ, Pérez-Torrente JJ, Oro LA (2015) Hydrolysis and methanolysis of silanes catalyzed by iridium(III) bis-N-heterocyclic carbene complexes: influence of the wingtip groups. Organometallics 34:2378–2385
Guzmán J, García-Orduña P, Lahoz FJ, Fernández-Alvarez FJ (2020) Unprecedent formation of methylsilylcarbonates from iridium-catalyzed reduction of CO2 with hydrosilanes. RSC Adv 10:9582–9586
Koinuma H, Kawakami F, Kato H, Hirai H (1981) Hydrosilylation of carbon dioxide catalysed by ruthenium complexes. J Chem Soc Chem Commun:213–214
Süss-Fink G, Reiner J (1981) Anionische Rutheniumcluster als Katalysatoren bei der Hydrosilylierung von Kohlendioxid. J Organomet Chem 221:C36–C38
Eisenschmid TC, Eisenberg R (1989) The iridium complex catalyzed reduction of carbon dioxide to methoxide by alkylsilanes. Organometallics 8:1822–1824
Motokura K, Nakagawa C, Pramudita RA, Manaka Y (2019) Formate-catalyzed selective reduction of carbon dioxide to formate products using hydrosilanes. ACS Sustainable Chem Eng 7:11056–11061
Lalrempuia R, Iglesias M, Polo V, Sanz Miguel PJ, Fernández-Alvarez FJ, Pérez-Torrente JJ, Oro LA (2012) Effective fixation of CO2 by iridium-catalyzed hydrosilylation. Angew Chem Int Ed 51:12824–12827
Jaseer EA, Akhtar MN, Osman M, Al-Shammari A, Oladipo HB, Garcés K, Fernández-Alvarez FJ, Al-Khattaf S, Oro LA (2015) Solvent-free iridium-catalyzed CO2 hydrosilylation: experiments and kinetic modeling. Cat Sci Technol 5:274–279
Oladipo HB, Jaseer EA, Julián A, Fernández-Alvarez FJ, Al-Khattaf S, Oro LA (2015) Effect of the CO2-pressure on the hydrosilylation of CO2 catalyzed by [Ir (NSiN)] species. J CO2 Utilization 12:21–26
Julián A, Jaseer EA, Garcés K, Fernández-Alvarez FJ, García-Orduña P, Lahoz FJ, Oro LA (2016) Tuning the activity and selectivity of iridium-NSiN catalyzed CO2 hydrosilylation processes. Cat Sci Technol 6:4410–4417
Julián A, Guzmán J, Jaseer EA, Fernández-Alvarez FJ, Royo R, Polo V, García-Orduña P, Lahoz FJ, Oro LA (2017) Mechanistic insights on the reduction of CO2 to silylformates catalyzed by Ir-NSiN species. Chem A Eur J 23:11898–11907
Guzmán J, García-Orduña P, Polo V, Lahoz FJ, Oro LA, Fernández-Alvarez FJ (2019) Ir-catalyzed selective reduction of CO2 to the methoxy or formate level with HSiMe(OSiMe3)2. Cat Sci Technol 9:2858–2867
Ojeda-Amador AI, Munarriz J, Alamán-Valtierra P, Polo V, Puerta-Oteo R, Jiménez MV, Fernández-Alvarez FJ, Pérez-Torrente JJ (2019) Mechanistic insights on the functionalization of CO2 with amines and hydrosilanes catalyzed by a zwitterionic iridium carboxylate-functionalized Bis-NHC catalyst. ChemCatChem 11:5524–5535
Jansen A, Görls H, Pitter S (2000) trans-[RuIICl(MeCN)5][RuIIICl4(MeCN)2]: a reactive intermediate in the homogeneous catalyzed hydrosilylation of carbon dioxide. Organometallics 19:135–138
Deglmann P, Ember E, Hofmann P, Pitter S, Walter O (2007) Experimental and theoretical investigations on the catalytic hydrosilylation of carbon dioxide with ruthenium nitrile complexes. Chem A Eur J 13:2864–2879
Scheuermann ML, Semproni SP, Pappas I, Chirik PJ (2014) Carbon dioxide hydrosilylation promoted by cobalt pincer complexes. Inorg Chem 53:9463–9465
Itagaki S, Yamaguchi K, Mizuno N (2013) Catalytic synthesis of silyl formates with 1 atm of CO2 and their utilization for synthesis of formyl compounds and formic acid. J Mol Catal A Chem 366:347–352
Takaya J, Iwasawa N (2017) Synthesis, structure, and catalysis of palladium complexes bearing a group 13 metalloligand: remarkable effect of an aluminum-metalloligand in hydrosilylation of CO2. J Am Chem Soc 139:6074–6077
Rios P, Díez J, López-Serrano J, Rodríguez A, Conejero S (2016) Cationic platinum(II) σ-SiH complexes in carbon dioxide hydrosilation. Chem A Eur J 22:16791–16795
Motokura K, Kashiwame D, Takahashi N, Miyaji A, Baba T (2013) Highly active and selective catalysis of copper diphosphine complexes for the transformation of carbon dioxide into silyl formate. Chem A Eur J 19:10030–10037
Zhang L, Cheng J, Hou Z (2013) Highly efficient catalytic hydrosilylation of carbon dioxide by an N-heterocyclic carbene copper catalyst. ChemCommun 49:4782–4784
Sattler W, Parkin G (2012) Zinc catalysts for on-demand hydrogen generation and carbon dioxide functionalization. J Am Chem Soc 134:17462–17465
Specklin D, Fliedel C, Gourlaouen C, Bruyere J-C, Avilés T, Boudon C, Ruhlmann L, Dagorne S (2017) N-heterocyclic carbene based tri-organyl-Zn–alkyl cations: synthesis, structures, and use in CO2 functionalization. Chem A Eur J 23:5509–5519
Morris DS, Weetman C, Wennmacher JTC, Cokoja M, Drees M, Kühn FE, Love JB (2017) Reduction of carbon dioxide and organic carbonyls by hydrosilanes catalysed by the perrhenate anion. Cat Sci Technol 7:2838–2845
Specklin D, Hild F, Fliedel C, Gourlaouen C, Veiros LF, Dagorne S (2017) Accessing two-coordinate ZnII Organocations by NHC coordination: synthesis, structure, and use as π-Lewis acids in alkene, alkyne, and CO2 hydrosilylation. Chem A Eur J 23:15908–15912
Riduan SN, Zhang Y, Ying JY (2009) Conversion of carbon dioxide into methanol with silanes over N-heterocyclic carbene catalysts. Angew Chem Int Ed 48:3322–3325
Matsuo T, Kawaguchi H (2006) From carbon dioxide to methane: homogeneous reduction of carbon dioxide with hydrosilanes catalyzed by zirconium−borane complexes. J Am Chem Soc 128:12362–12363
Luconi L, Rossin A, Tuci G, Gafurov Z, Lyubov DM, Trifonov AA, Cicchi S, Ba H, Pham-Huu C, Yakhvarov D, Giambastiani G (2019) Benzoimidazole-pyridylamido zirconium and hafnium alkyl complexes as homogeneous catalysts for tandem carbon dioxide hydrosilylation to methane. ChemCatChem 11:495–510
Park S, Bézier D, Brookhart M (2012) An efficient iridium catalyst for reduction of carbon dioxide to methane with trialkylsilanes. J Am Chem Soc 134:11404–11407
Mitton SJ, Turculet L (2012) Mild reduction of carbon dioxide to methane with tertiary silanes catalyzed by platinum and palladium silyl pincer complexes. Chem A Eur J 18:15258–15262
Berkefeld A, Piers WE, Parvez M (2010) Tandem frustrated Lewis pair/tris(pentafluorophenyl)borane-catalyzed deoxygenative hydrosilylation of carbon dioxide. J Am Chem Soc 132:10660–10661
Rauch M, Parkin G (2017) Zinc and magnesium catalysts for the Hydrosilylation of carbon dioxide. J Am Chem Soc 139:18162–18165
Khandelwal M, Wehmschulte RJ (2012) Deoxygenative reduction of carbon dioxide to methane, toluene, and Diphenylmethane with [Et2Al]+ as catalyst. Angew Chem Int Ed 51:7323–7326
Berkefeld A, Piers WE, Parvez M, Castro L, Maron L, Eisenstein O (2013) Decamethylscandocinium-hydrido-(perfluorophenyl)borate: fixation and tandem tris(perfluorophenyl)borane catalysed deoxygenative hydrosilation of carbon dioxide. Chem Sci 4:2152–2162
Chen J, Falivene L, Caporaso L, Cavallo L, Chen EY-X (2016) Selective reduction of CO2 to CH4 by tandem hydrosilylation with mixed Al/B catalysts. J Am Chem Soc 138:5321–5333
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fernández-Alvarez, F.J., Oro, L.A. (2020). Iridium-Catalyzed Homogeneous Hydrogenation and Hydrosilylation of Carbon Dioxide. In: Oro, L.A., Claver, C. (eds) Iridium Catalysts for Organic Reactions. Topics in Organometallic Chemistry, vol 69. Springer, Cham. https://doi.org/10.1007/3418_2020_52
Download citation
DOI: https://doi.org/10.1007/3418_2020_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69082-3
Online ISBN: 978-3-030-69083-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)