Abstract
Neurogenesis is the process by which new neurons are generated from neural stem cells (NSCs), which are cells that have the ability to proliferate and differentiate into neurons, astrocytes, and oligodendrocytes. The process is essential for homeostatic tissue regeneration and the coordination of neural plasticity throughout life, as neurons cannot regenerate once injured. Therefore, defects in neurogenesis are related to the onset and exacerbation of several neuropsychiatric disorders, and therefore, the regulation of neurogenesis is considered to be a novel strategy for treatment. Neurogenesis is regulated not only by NSCs themselves, but also by the functional microenvironment surrounding the NSCs, known as the “neurogenic niche.” The neurogenic niche consists of several types of neural cells, including neurons, glial cells, and vascular cells. To allow communication with these cells, transporters may be involved in the secretion and uptake of substrates that are essential for signal transduction. This chapter will focus on the involvement of polyspecific solute carriers transporting organic cations in the possible regulation of neurogenesis by controlling the concentration of several organic cation substrates in NSCs and the neurogenic niche. The potential therapeutic implications of neurogenesis regulation by these transporters will also be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: Neurogenesis, Neural Stem Cells, and the Neurogenic Niche
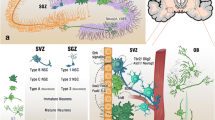
Neurons play pivotal roles in the fundamental functions of the brain, but do not have the ability to self-renew; consequently, neurons cannot regenerate themselves if injured. In contrast, neural stem cells (NSCs) generate new neurons and can thereby replenish damaged neurons (Johansson et al. 1999; Zhang et al. 2008; Knoth et al. 2010). NSCs exist in the subgranular zone (SGZ) of the hippocampal dentate gyrus and subventricular zone (SVZ) of the adult mammalian brain. NSCs have the capacity to proliferate and differentiate into neurons, astrocytes, and oligodendrocytes. The process of generating new neurons from NSCs is called neurogenesis. Neurogenesis contributes to homeostatic tissue regeneration and the coordination of neural plasticity throughout life (Zhang et al. 2008; Ming and Song 2011) and is affected by various factors in daily life, including exercise, sleep, food, and stress (LaDage 2015; Stankiewicz et al. 2017; Pons-Espinal et al. 2019).
In contrast, defects in neurogenesis are related to the onset and exacerbation of several neuropsychiatric disorders, such as major depressive disorder and Parkinson’s disease (PD) (Eisch and Petrik 2012; Le Grand et al. 2015; LaDage 2015). Therefore, the regulation of neurogenesis is considered a novel strategy for the treatment of neuropsychiatric disorders (Snyder et al. 2011; Kohl et al. 2012; Tuszynski et al. 2015; Alam et al. 2018). Indeed, there are a few neurogenic drugs under development. For example, the benzylpiperazine-aminopyridine neurogenic compound NSI-189 had positive effects on major depressive disorder in a Phase 1b clinical study (Fava et al. 2016), and a novel neurotrophic agent, T-817MA, preserved memory function and accelerated motor function recovery in brain-damaged animals (Fukushima et al. 2011; Abe et al. 2018). Moreover, clinically used drugs such as selective serotonin reuptake inhibitors (SSRIs) also promote neurogenesis (Banasr et al. 2004; Gur et al. 2007), which was recently proposed to be one of the mechanisms of their antidepressant activity.
There are considerably fewer NSCs (<1,200) than neurons (~70,000,000) and glial cells (approximately 10 times more than neurons) in the murine brain (Brazel and Rao 2004; Herculano-Houzel et al. 2006). This may lead to speculation that NSCs are regulated by crosstalk with cells other than NSCs. Histological analysis of the SGZ and SVZ revealed that many types of cells surround the NSCs (Bátiz et al. 2016; Bonzano et al. 2018; Lin et al. 2019). These include astrocytes, microglia, brain microvascular endothelial cells (BMECs), pericytes, and choroid plexus epithelial cells (CPECs). Neurogenesis is thought to be regulated by a “neurogenic niche,” which represents the functional microenvironment surrounding the NSCs. Thus, the cells surrounding the neurogenic niche may play regulatory roles in several functions of NSCs, including differentiation and proliferation. Neurogenesis is thought to be regulated by various neurotransmitters, growth factors, neurotrophins, cytokines, and exosomes secreted from astrocytes, microglia, BMECs, pericytes, neurons, and CPECs (Song et al. 2012; Bátiz et al. 2016; Wicki-Stordeur et al. 2016; Cope and Gould 2019; Lin et al. 2019; Lehtinen et al. 2011; Yanpallewar et al. 2010). For communication with other cells, membrane receptors are essential to receive and transduce signals, whereas transporters are involved in the secretion and uptake of these molecules for signal transduction (Song et al. 2012; Trujillo-Gonzalez et al. 2019; Pajarillo et al. 2019). Therefore, it is important to comprehend the expression and exact roles of transporters in NSCs and the neurogenic niche to understand the homeostasis of NSCs. In this chapter, we will focus on the regulation of neurogenesis by solute carriers (SLCs) transporting organic cations with broad specificity, which are defined as “OCTs,” in NSCs and other niche cells.
2 Organic Cations Transporting Solute Carriers Expressed in NSCs
2.1 OCTN1/SLC22A4 and Its Substrate Ergothioneine (ERGO)
Among OCTs, the expression of the carnitine/organic cation transporter OCTN1, OCTN2/SLC22A5, and OCTN3/SLC22A21 genes was detected in murine primary cultured NSCs, whereas the expression of OCT1/SLC22A1, OCT2/SLC22A2, OCT3/SLC22A3, multidrug and toxin extrusion protein 1 (MATE1), concentrative nucleoside transporter 2 (CNT2/SLC28A2), equilibrative nucleoside transporter 1 (ENT1/SLC29A1), and ENT4 was below the limit of detection (Ishimoto et al. 2014). The mRNA expression of OCTN1 was much higher than that of other genes (Ishimoto et al. 2014). No information is currently available on the expression of these genes in NSCs in higher animals, including humans. In cultured murine NSCs, immunocytochemical analysis showed that OCTN1 was expressed on the cell surface membranes of the NSC marker nestin-positive cells. In addition, the NSCs showed uptake of a typical substrate ERGO, which is a food-derived antioxidant, whereas NSCs derived from octn1 gene knockout mice (octn1−/−) minimally incorporated ERGO (Ishimoto et al. 2014), demonstrating that OCTN1 was functionally expressed in murine NSCs.
In the rodent brain, OCTN1 is expressed in neurons and their precursor cells NSCs, but not in glial cells such as astrocytes (Inazu et al. 2006; Nakamichi et al. 2012; Ishimoto et al. 2014, 2018). This may imply the involvement of OCTN1 in the differentiation of NSCs to neurons. Indeed, the transfection of OCTN1 siRNA into P19 embryonic carcinomas differentiated into neural stem-like cells (a model NSC cell line) suppressed their ability to differentiate into βIII-tubulin-positive cells (a neuronal marker) with a concomitant decrease in the functional expression of OCTN1 (Ishimoto et al. 2014), suggesting that this organic cation transporter may be involved in neuronal differentiation.
A typical OCTN1 substrate, ERGO, is present in serum in vivo and in the culture medium. Therefore, ERGO was considered to be involved, at least partially, in OCTN1-mediated neurogenesis. The exposure of NSCs to ERGO significantly increased the proportion of βIII-tubulin-positive cells and decreased that of GFAP-positive cells (an astrocyte marker) after the induction of differentiation, whereas ERGO did not affect the neuronal differentiation of NSCs derived from octn1−/−, suggesting that OCTN1-mediated ERGO uptake promoted neuronal differentiation (Ishimoto et al. 2019). Moreover, the uptake of ERGO by OCTN1 activated an amino acid sensor, mammalian target of rapamycin complex 1 (mTORC1), and neurotrophin 5/TrkB signaling prior to the promotion of neuronal differentiation, suggested the possible involvement of intracellular signaling in ERGO-mediated differentiation (Ishimoto et al. 2019).
Notably, despite the hydrophilic properties of ERGO, it has a tissue-to-plasma concentration ratio of more than two in the mouse brain, indicating active transport of this compound into the brain, whereas ERGO was not detected in the brain of octn1−/− mice (Kato et al. 2010; Nakamichi et al. 2016). This result implied that OCTN1-mediated ERGO uptake in the brain may have a significant role in brain function. Indeed, intake of ERGO-containing diet for 2 weeks in mice showed an increase in the newborn neuron marker doublecortin (Dcx)-positive cells in the SGZ of the hippocampus with the concomitant antidepressant-like effect of ERGO intake, as demonstrated in the forced swimming test (FST) and tail suspension test (TST) (Nakamichi et al. 2016). In addition, oral administration of ERGO enhanced cognitive function, as shown by a longer exploration time for novel objects in a novel object recognition test, a behavioral experiment for assessing memory function (Nakamichi et al. 2020). Oral administration of ERGO also protected against memory deficits induced by β-amyloid and D-galactose in mice (Yang et al. 2012; Song et al. 2014). This enhancement of memory function may be related to ERGO-induced neurogenesis as it is well known that neurogenesis positively regulates memory function (Kumar et al. 2019), although there are still other possible mechanisms, including the maturation of neurons and/or the antioxidant activity of ERGO (Yang et al. 2012; Song et al. 2014; Nakamichi et al. 2020).
In humans, there is no information regarding the involvement of OCTN1 in brain function; however, oral administration of ERGO-containing mushroom extract tablets for 8 and 12 weeks was reported to show improvement in cognitive function of healthy volunteers and patients with mild cognitive impairment (MCI) compared with that in the placebo group (Watanabe et al. 2020). The serum ERGO concentration in patients with PD and the blood ERGO concentration in patients with MCI were also reported to be significantly lower than those in healthy volunteers (Hatano et al. 2016; Cheah et al. 2016). Further epidemiological analysis is needed to clarify whether the lower ERGO concentration in brain may be a risk factor for PD and MCI.
2.2 OCTN2/SLC22A5 and Its Substrates Carnitine and Acetyl-L-Carnitine
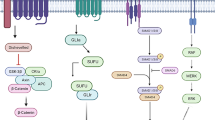
OCTN2 efficiently transports carnitine and acetyl-L-carnitine as endogenous substrates (Januszewicz et al. 2010; Kido et al. 2001; Ohashi et al. 1999; Tamai et al. 1998). Carnitine plays an essential role in the transport of fatty acids into the mitochondria for their subsequent β-oxidation, a process for generating energy (Frigeni et al. 2017). In humans, systemic carnitine deficiency, mainly caused by the dysfunction of OCTN2, leads to hypoketotic hypoglycemia, cardiomyopathy, and encephalopathy (Kimura and Amemiya 1990; Frigeni et al. 2017). In contrast, in the brain, the substrates carnitine and acetyl-L-carnitine are proposed to be involved in neurogenesis (Athanassakis et al. 2002; Cuccurazzu et al. 2013; Fathi et al. 2017; Singh et al. 2017).
The exposure of primary cultured NSCs to carnitine (40–200 μM) for 1 month resulted in the formation of a neural network that was positively stained with cresyl violet, whereas exposure to vehicle resulted in only neurospheres (Athanassakis et al. 2002). Moreover, the exposure of mesenchymal stem cells to carnitine (200 μM) induced gene expression of neurogenic markers, such as nerve growth factor (ngf), brain-derived neurotrophic factor (bdnf), and nestin, with a concomitant increase in the expression of proteins related to the protein kinase A (PKA) and Wnt/β-catenin pathways, such as β-catenin, low-density lipoprotein receptor-related protein (LRP) 5c, Wnt1, and Wnt3a. In contrast, the PKA inhibitor H89 suppressed neurogenic markers (Fathi et al. 2017). These results suggested that carnitine promotes neurogenesis, at least partially, through the Wnt/β-catenin and PKA pathways (Athanassakis et al. 2002; Fathi et al. 2017). At 40–200 μM of carnitine, the promotion of neurogenesis was observed, which is close to the endogenous carnitine concentration in the human brain (~0.05 μmol/g; Nakano et al. 1989), which corresponds to ~50 μM if the gravity of the brain is assumed to be unity, suggesting that carnitine may induce the promotion of neurogenesis in vivo.
As carnitine is known to restore mitochondrial membrane potential, Kim et al. tested whether supplementation with carnitine in vitro could reverse the defects in neuronal differentiation induced by mitochondrial dysfunction. Immunocytochemical analysis showed that exposure of adult SVZ-derived NSCs to an inhibitor of mitochondrial fission-promoting protein DRP1 decreased the number of βIII-tubulin-positive cells, whereas supplementation of carnitine at 50 nM fully reversed the decrease (Kim et al. 2015). Thus, even low concentrations of carnitine could recover the defects in neuronal differentiation induced by mitochondrial dysfunction.
Daily intraperitoneal administration of acetyl-L-carnitine (100 mg/kg) for a month restored the decrease in the number of Dcx-positive cells (a newborn neuron marker) in the SGZ of the hippocampus of rats with model PD induced by intrastriatal injection of 6-hydroxydopamine (Singh et al. 2017). Daily subcutaneous administration of acetyl-L-carnitine (100 mg/kg) for 21 days increased the number of cells with double-positive immunostaining for the neuronal marker NeuN and the cell proliferation marker BrdU in the SGZ of the hippocampus. In addition, behavioral assessment in the FST and TST showed antidepressant-like activity of acetyl-L-carnitine with an increase in protein expression of metabotropic glutamate receptor 2 receptor in the hippocampus (Cuccurazzu et al. 2013). Thus, not only carnitine, but also acetyl-L-carnitine, may be involved in the regulation of neurogenesis. The concentration of these compounds in the brain is at least partially regulated by OCTN2 expressed in BMECs, which act as the blood-brain barrier (Kido et al. 2001). Therefore, further studies are required to clarify whether OCTN2 expressed in BMECs may be associated with the effect of carnitine and/or acetyl-L-carnitine on neurogenesis. In contrast, OCTN2 was reported to be expressed in primary cultured NSCs and hippocampal neurons (Ishimoto et al. 2014; Lamhonwah et al. 2008). Therefore, in these neural cells, OCTN2 may also regulate the concentration of carnitine and acetyl-L-carnitine around the NSCs in the hippocampus.
2.3 SERT/SLC6A4 and Serotonin
SLCs transporting organic cations include various neurotransmitter transporters, notably the serotonin transporter SERT, the glutamate transporters (vGlut1-3 and EAAT1-3), and the GABA transporter GAT1, and were reported to be expressed in NSCs (Ren-Patterson et al. 2005; Sanchez et al. 2006; Benninghoff et al. 2012; Sánchez-Mendoza et al. 2017). Among these transporters, the involvement of SERT in neurogenesis has been relatively well characterized.
SERT is expressed in neurons, astrocytes, and NSCs (Ren-Patterson et al. 2005; Benninghoff et al. 2012; Malynn et al. 2013) and is believed to be a primary regulator of serotonin levels in the brain. Serotonin is a neurotransmitter related to the pathogenesis of several neuropsychiatric conditions, such as major depressive disorder, schizophrenia, and various anxiety disorders (Benninghoff et al. 2012). The brain serotonin level is thought to be relevant to neurogenesis owing to the following evidence. First, p-chlorophenylalanine, a serotonin synthesis inhibitor, significantly suppressed hippocampal neurogenesis by decreasing serotonin levels in the hippocampus (Jha et al. 2006). Second, inhibitors of SERT, some of which are SSRIs used clinically as antidepressants, increased the level of extracellular serotonin and caused hippocampal neurogenesis (Snyder et al. 2011; Li et al. 2008).
The expression of SERT in NSCs increases as NSCs become more differentiated into neuronal progenitor cells (Ren-Patterson et al. 2005), supporting the possible involvement of this transporter in neurogenesis. However, in sert gene knockout mice (sert−/−), the proliferative capacity of NSCs was higher than that in older wild-type mice (~14.5 months), whereas no significant difference in proliferation was observed in younger (~7 weeks) and adult (~3 months) mice between the two strains (Schmitt et al. 2007). Unexpectedly, there was minimal difference in the proliferative capacity of NSCs between sert−/− and wild-type mice, despite the large difference in serotonin concentration between the two strains. The serotonin concentration in the hippocampus of sert−/− was approximately three times lower than that of wild-type mice (Kim et al. 2005). Interestingly, the rate of serotonin synthesis in the brain of sert−/− was 1.5 times higher than that in wild-type mice (Kim et al. 2005), whereas the expression of OCT3, another serotonin transporter, in the hippocampus of sert−/− was ~1.4 times higher than that in wild-type mice (Schmitt et al. 2003; Baganz et al. 2008). Thus, alterations in the biosynthesis of serotonin and expression of OCT3 in sert−/− may be one of the possible compensation mechanisms.
The antidepressant effect induced by SSRIs generally requires repeated administration to patients for weeks to months. This requirement cannot be simply explained by direct inhibition of SERT by SSRIs; notably, more time-consuming processes, including neurogenesis, followed by the replenishment of damaged neurons are speculated to be involved in the pharmacology of these drugs (Warner-Schmidt and Duman 2006; Perera et al. 2008; Li et al. 2008; Gur et al. 2007; Han et al. 2011). Indeed, the antidepressant-like activity of an SSRI, fluoxetine, assessed using the TST, was diminished in the mice, and hippocampal neurogenesis was suppressed by conditional gene deletion of TrkB in NSCs (Li et al. 2008). Thus, the serotonin-induced promotion of neurogenesis may be a prerequisite, at least partly, for the antidepressant activity of SSRIs.
3 OCTs Expressed in the Neurogenic Niche
3.1 OCT2/SLC22A2 in the Hippocampus
The expression of OCTs in various neural cells is summarized in Table 1. In neurons, the expression of various OCTs, such as OCT2, 3, OCTN1-3, CNT2, and ENT1 and 4 were reported (Table 1). In particular, OCT2, 3, and OCTN1-3 are expressed in the hippocampus of rodents (Matsui et al. 2016; Vialou et al. 2008; Nakamichi et al. 2012; Lamhonwah et al. 2008). Immunohistochemical analysis revealed that OCT2 was detected by punctate, bouton-like staining in cholinergic, dopaminergic, and serotonergic axon terminals that were co-labeled with presynaptic neurochemical markers in mice (Matsui et al. 2016). OCT2 transports several neurotransmitters, such as acetylcholine and monoamines (Table 1, Matsui et al. 2016), and genetic deletion of oct2 in mice showed a significant reduction in concentrations of norepinephrine and serotonin in the hippocampus (Bacq et al. 2012), implying the possible regulation of neurotransmitters by OCT2 around the neuronal axon terminals.
Interaction between the axon terminals of neurons and NSCs has been proposed in SGZ and SVZ (Song et al. 2012; Pardal and López Barneo 2016). For example, the axon terminals of parvalbumin-positive GABAergic interneurons and NSCs are in proximity in the hippocampus, and the neurons regulate the fate of adult quiescent NSCs (Song et al. 2012). The synaptic regulator α-synuclein in dopaminergic nerve terminals is also essential for the maintenance of NSCs in the SVZ (Perez-Villalba et al. 2018). As in these examples, OCTs expressed on the axon terminals could potentially interact with NSCs in the neurogenic niche. OCTs show relatively lower affinity to neurotransmitters compared with the other neurotransmitter-specific transporters, and may play a role in the regulation of their extracellular concentration as neurotransmitters are present at high levels in the vicinity of neurons. For example, OCT2 expressed in the neuronal axon terminals proximate to NSCs in hippocampus might be involved in uptake of such neurotransmitters to maintain the appropriate concentration around the neurogenic niche.
3.2 OCT3/SLC22A3 in the Hippocampus
Among OCTs, OCT3/SLC22A3 is ubiquitously expressed in neurons, astrocytes, microglia, BMECs, and CPECs in the brain, whereas other OCTs, such as OCT1, OCT2, OCTN1-3, and ENT4, are expressed in a few types of cells (Table 1). Immunohistochemical analysis has shown that OCT3 is expressed in neurons and astrocytes of the hippocampus in mice (Vialou et al. 2008). In the hippocampus, neurons and astrocytes are in proximity to NSCs (Bonzano et al. 2018; Cope and Gould 2019). This may imply the possible regulation of NSCs by OCT3 expressed in neurons and astrocytes with NSCs, as OCT3 transports several neurotransmitters, including serotonin, dopamine, and histamine, which are known to activate neurogenesis (Backhouse et al. 1982; Banasr et al. 2004; Höglinger et al. 2004; Molina-Hernndez and Velasco 2008; Klempin et al. 2010; Veena et al. 2011; Masuda et al. 2012; Matthaeus et al. 2015; Saraiva et al. 2019). In neurons, OCT3 is localized on post-synaptic sites and may be involved in the reuptake of serotonin in the synaptic cleft. It is generally considered that OCT3 inhibition in neurons results in antidepressant activity via an increase in serotonin concentration in the synaptic cleft when the high-affinity transporters, including SERT, are saturated or inhibited in neurons (Daws 2009; Couroussé and Gautron 2015). An inhibitor of OCT3, decynium-22, enhanced the antidepressant activity of SSRI fluvoxamine in mice, possibly via inhibition of serotonin clearance (Horton et al. 2013, and see chapter “OCTs in Psychiatric Disorders”).
3.3 OCTN1/SLC22A4 in the Hippocampus
OCTN1 is expressed in the hippocampus in vivo, as shown by PCR and immunohistochemical analysis (Nakamichi et al. 2012; Lamhonwah et al. 2008) and is commonly detected in primary cultures of NSCs, neurons, and microglia in mice (Ishimoto et al. 2014, 2018; Nakamichi et al. 2012) (Table 1). In particular, microglia are very densely populated and proliferative in the neurogenic niche of SGZ and SVZ, and appear to be closely associated with NSCs (Mosher et al. 2012). It is well known that microglia communicate with NSCs via the secretion of growth factors and chemokines, which regulates neurogenesis (Matsui and Mori 2018; Osman et al. 2019).
Communication between NSCs and microglia via common substrates of common transporters can be speculated to involve ERGO and OCTN1. Both NSCs and microglia incorporated the antioxidant ERGO via OCTN1, to scavenge intracellular reactive oxygen species, followed by the regulation of their proliferation and cell hypertrophy, respectively (Ishimoto et al. 2014, 2018). In cultured microglia, OCTN1 negatively regulated the expression of the inflammatory cytokine IL-1β (Ishimoto et al. 2018), which promotes neuronal differentiation of NSCs (Park et al. 2018). The crosstalk between NSCs and microglia via OCTN1 needs to be further evaluated.
4 Possible Regulation of Neurogenesis by Clinically Used OCT Substrate Drugs
4.1 Metformin
OCTs transport several clinically used organic cation drugs. Brain function is affected by such drugs, especially those that are permeable through the blood-brain barrier, indicating high brain distribution.
The antidiabetic drug metformin is a substrate of OCT1-3, OCTN1, MATE1, and ENT4 (Table 1). Metformin has recently garnered attention because some clinical studies have suggested that metformin usage is associated with a reduced risk of dementia in humans (Campbell et al. 2018; Guo et al. 2014; Ng et al. 2014). In the mouse model of Alzheimer’s disease, 3xTg, metformin restored deficits in neurogenesis and spatial memory via activation of both atypical protein kinase C (aPKC) and CREB-binding protein (CBP) followed by a decrease in the expression of monoacylglycerol lipase (Wang et al. 2012; Syal et al. 2020). Treatment with metformin also restored the impairment of neurogenesis and spatial memory in an AlCl3-induced mouse model of neurodegeneration (Ahmed et al. 2017).
4.2 Ketamine
The anesthetic drug ketamine is an antagonist of the N-methyl-D-aspartate (NMDA) receptor and exhibits rapid antidepressant efficacy in patients with treatment-resistant depression (Kraus et al. 2017). This mode of action can be quite different from clinically used SSRIs, which take weeks to months to exert antidepressant activity. A single intraperitoneal ketamine administration (10 mg/kg) elevated the densities of neuronal progenitors and newborn granule cells in the ventral hippocampus related to emotion in adult mice, as shown by immunohistochemical analysis, and the FST showed antidepressant activity at 13 days post-injection (Yamada and Jinno 2019). Clarke et al. also showed that three ketamine injections (10 mg/kg for 2 weeks) reduced immobility time in FST both 2 and 8 days after the final injection and increased the number of Dcx-positive cells in the hippocampal dentate gyrus (Clarke et al. 2017). These results suggested that ketamine-induced neurogenesis was partially involved in its antidepressant activity. OCT3 is considered an important transporter involved in the disposition of ketamine (Keiser et al. 2018) and is expressed commonly in various neural cells (Table 1). It is possible that OCT3 may regulate ketamine concentration in the neurogenic niche, and consequently, ketamine-induced neurogenesis and antidepressant activity.
4.3 Memantine
Like ketamine, the anti-Alzheimer’s disease drug memantine is also an NMDA receptor blocker and has neurogenic activity. A single intraperitoneal injection of memantine (50 mg/kg) increased the number of BrdU-positive cells (a proliferating cell marker) in the dentate gyrus of the hippocampus of both 3- and 12-month-old mice (Maekawa et al. 2009). The mechanism underlying the neurogenic actions of memantine and ketamine has not yet been fully clarified, but the neurogenic activities may be caused by inhibition of NMDA receptors, as activation of NMDA receptors rapidly decreased the number of cells synthesizing DNA in the adult hippocampus, whereas inhibition of NMDA receptors rapidly increased the number of cells in the S phase, as identified with [3H]-thymidine (Cameron et al. 1995). Memantine was transported by OCT2 in in vitro transport studies (Busch et al. 1998; You and Morris 2014); however, the involvement of this transporter in the regulation of memantine concentration in the brain has not yet been demonstrated.
4.4 Gabapentin
The anti-epileptic drug gabapentin is a ligand of the α2δ-subunit of N-type calcium channels and increased the number of newborn mature neurons generated from adult hippocampal NSCs in vitro according to immunocytochemical analysis (Valente et al. 2012). Chronic intraperitoneal treatment with another α2δ ligand, pregabalin, at 10 mg/kg for 21 days significantly increased the number of adult-generated neurons positive for BrdU and NeuN double staining in the hippocampal region in mice, and the TST and FST showed the antidepressant-like activity of pregabalin in mice subjected to chronic restraint stress. Gabapentin is a substrate of OCTN1, and a polymorphism in the octn1 affects renal clearance of this drug (Urban et al. 2008). Pregabalin is also transported by OCTN1 (You and Morris 2014), although the association of this transporter with the induction of neurogenesis by these drugs is still unknown.
5 Future Perspectives
In this chapter, the possible involvement of OCTs and their substrates in neurogenesis has been reviewed. As neurogenesis plays pivotal roles in brain homeostasis, these transporters are useful in therapies targeting neurogenesis, which may be applicable to various neuropsychiatric disorders, and their neurogenic substrates are potential therapeutic and preventive agents for these disorders. Moreover, inhibition of these OCTs by clinically used drugs and other compounds expressed in NSCs and the neurogenic niche may affect neurogenesis by inhibiting the influx and efflux of neurogenic substrates. However, there is still limited evidence for the clinical relevance of transporter-mediated neurogenesis because of the difficulty of direct analysis of phenotypes in the human brain. To demonstrate OCT-mediated neurogenesis in humans, the use of postmortem brains, human iPS-NSCs, and biomarkers for neurogenesis would be helpful. Further analyses using these promising tools may enable the development of novel drugs targeted to OCTs for the treatment of neuropsychiatric disorders.
References
Abe H, Jitsuki S, Nakajima W et al (2018) CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science 360:50–57
Ahmed S, Mahmood Z, Javed A et al (2017) Effect of metformin on adult hippocampal neurogenesis: comparison with donepezil and links to cognition. J Mol Neurosci 62:88–98
Alam MJ, Kitamura T, Saitoh Y et al (2018) Adult neurogenesis conserves hippocampal memory capacity. J Neurosci 38:6854–6863
Athanassakis I, Zarifi I, Evangeliou A, Vassiliadis S (2002) L-Carnitine accelerates the in vitro regeneration of neural network from adult murine brain cells. Brain Res 932:70–78
Backhouse B, Barochovsky O, Malik C et al (1982) Effects of haloperidol on cell proliferation in the early postnatal rat brain. Neuropathol Appl Neurobiol 8:109–116
Bacq A, Balasse L, Biala G et al (2012) Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry 17:926–939
Baganz NL, Horton RE, Calderon AS et al (2008) Organic cation transporter 3: keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A 105:18976–18981
Banasr M, Hery M, Printemps R, Daszuta A (2004) Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29:450–460
Bátiz LF, Castro MA, Burgos PV et al (2016) Exosomes as novel regulators of adult neurogenic niches. Front Cell Neurosci 9:501
Benninghoff J, Van Der Ven A, Schloesser RJ et al (2012) The complex role of the serotonin transporter in adult neurogenesis and neuroplasticity. A critical review. World J Biol Psychiatry 13:240–247
Bonzano S, Crisci I, Podlesny-Drabiniok A et al (2018) Neuron-Astroglia cell fate decision in the adult mouse hippocampal neurogenic niche is cell-intrinsically controlled by COUP-TFI in vivo. Cell Rep 24:329–341
Brazel CY, Rao MS (2004) Aging and neuronal replacement. Ageing Res Rev 3:465–483
Busch AE, Karbach U, Miska D et al (1998) Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol 54:342–352
Cameron HA, McEwen BS, Gould E (1995) Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci 15:4687–4692
Campbell JM, Stephenson MD, de Courten B et al (2018) Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis 65:1225–1236
Cheah IK, Feng L, Tang RMY et al (2016) Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem Biophys Res Commun 478:162–167
Clarke M, Razmjou S, Prowse N et al (2017) Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology 112:210–220
Cope EC, Gould E (2019) Adult neurogenesis, glia, and the extracellular matrix. Cell Stem Cell 24:690–705
Couroussé T, Gautron S (2015) Role of organic cation transporters (OCTs) in the brain. Pharmacol Ther 146:94–103
Cuccurazzu B, Bortolotto V, Valente MM et al (2013) Upregulation of mGlu2 receptors via NF-κB p65 acetylation is involved in the proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology 38:2220–2230
Daws LC (2009) Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther 121:89–99
Eisch AJ, Petrik D (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338:72–75
Engel K, Zhou M, Wang J (2004) Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279:50042–50049
Fathi E, Farahzadi R, Charoudeh HN (2017) L-carnitine contributes to enhancement of neurogenesis from mesenchymal stem cells through Wnt/β-catenin and PKA pathway. Exp Biol Med 242:482–486
Fava M, Johe K, Ereshefsky L et al (2016) A phase 1B, randomized, double blind, placebo controlled, multiple-dose escalation study of NSI-189 phosphate, a neurogenic compound, in depressed patients. Mol Psychiatry 21:1372–1380
Frigeni M, Balakrishnan B, Yin X et al (2017) Functional and molecular studies in primary carnitine deficiency. Hum Mutat 38:1684–1699
Fukushima T, Nakamura A, Iwakami N et al (2011) T-817MA, a neuroprotective agent, attenuates the motor and cognitive impairments associated with neuronal degeneration in P301L tau transgenic mice. Biochem Biophys Res Commun 407:730–734
Gasser PJ, Hurley MM, Chan J et al (2017) Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Struct Funct 222:1913–1928
Guo M, Mi J, Jiang QM et al (2014) Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol 41:650–656
Gur TL, Conti AC, Holden J et al (2007) cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci 27:7860–7868
Han X, Tong J, Zhang J et al (2011) Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J Neurotrauma 28:995–1007
Hatano T, Saiki S, Okuzumi A et al (2016) Identification of novel biomarkers for Parkinson’s disease by Metabolomic technologies. J Neurol Neurosurg Psychiatry 87:295–301
Herculano-Houzel S, Mota B, Lent R (2006) Cellular scaling rules for rodent brains. Proc Natl Acad Sci U S A 103:12138–12143
Höglinger GU, Rizk P, Muriel MP et al (2004) Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735
Horton RE, Apple DM, Owens WA et al (2013) Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J Neurosci 33:10534–10543
Inazu M, Takeda H, Maehara K et al (2006) Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J Neurochem 97:424–434
Ishimoto T, Nakamichi N, Hosotani H et al (2014) Organic cation transporter-mediated ergothioneine uptake in mouse neural progenitor cells suppresses proliferation and promotes differentiation into neurons. PLoS One 9:e89434
Ishimoto T, Nakamichi N, Nishijima H et al (2018) Carnitine/organic cation transporter OCTN1 negatively regulates activation in murine cultured microglial cells. Neurochem Res 43:107–119
Ishimoto T, Masuo Y, Kato Y, Nakamichi N (2019) Ergothioneine-induced neuronal differentiation is mediated through activation of S6K1 and neurotrophin 4/5-TrkB signaling in murine neural stem cells. Cell Signal 53:269–280
Januszewicz E, Bekisz M, Mozrzymas JW, Nałecz KA (2010) High affinity carnitine transporters from OCTN family in neural cells. Neurochem Res 35:743–748
Jha S, Rajendran R, Davda J, Vaidya VA (2006) Selective serotonin depletion does not regulate hippocampal neurogenesis in the adult rat brain: differential effects of p-chlorophenylalanine and 5,7-dihydroxytryptamine. Brain Res 1075:48–59
Johansson CB, Momma S, Clarke DL et al (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96:25–34
Kato Y, Kubo Y, Iwata D et al (2010) Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm Res 27:832–840
Keiser M, Hasan M, Oswald S (2018) Affinity of ketamine to clinically relevant transporters. Mol Pharm 15:326–331
Kido Y, Tamai I, Ohnari A et al (2001) Functional relevance of carnitine transporter OCTN2 to brain distribution of L-camitine and acetyl-L-carnitine across the blood-brain barrier. J Neurochem 79:959–969
Kim DK, Tolliver TJ, Huang SJ et al (2005) Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 49:798–810
Kim HJ, Shaker MR, Cho B et al (2015) Dynamin-related protein 1 controls the migration and neuronal differentiation of subventricular zone-derived neural progenitor cells. Sci Rep 5:15962
Kimura S, Amemiya F (1990) Brain and liver pathology in a patient with carnitine deficiency. Brain Dev 12:436–439
Klempin F, Babu H, De Pietri TD et al (2010) Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci 3:14
Knoth R, Singec I, Ditter M et al (2010) Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 5:e8809
Kohl Z, Winner B, Ubhi K et al (2012) Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci 35:10–19
Kraus C, Rabl U, Vanicek T et al (2017) Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12
Kumar A, Pareek V, Faiq MA et al (2019) Adult neurogenesis in humans: a review of basic concepts, history, current research, and clinical implications. Innov Clin Neurosci 16:30–37
LaDage LD (2015) Environmental change, the stress response, and neurogenesis. Integr Comp Biol 59:243–250
Lamhonwah AM, Hawkins CE, Tam C et al (2008) Expression patterns of the organic cation/carnitine transporter family in adult murine brain. Brain Dev 30:31–42
Le Grand JN, Gonzalez-Cano L, Pavlou MA, Schwamborn JC (2015) Neural stem cells in Parkinson’s disease: a role for neurogenesis defects in onset and progression. Cell Mol Life Sci 72:773–797
Lehtinen MK, Zappaterra MW, Chen X et al (2011) The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905
Li Y, Luikart BW, Birnbaum S et al (2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59:399–412
Li B, Gu L, Hertz L, Peng L et al (2013) Expression of nucleoside transporter in freshly isolated neurons and astrocytes from mouse brain. Neurochem Res 38:2351–2358
Lin R, Cai J, Kenyon L et al (2019) Systemic factors trigger vasculature cells to drive notch signaling and neurogenesis in neural stem cells in the adult brain. Stem Cells 37:395–406
Maekawa M, Namba T, Suzuki E et al (2009) NMDA receptor antagonist memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus. Neurosci Res 63:259–266
Malynn S, Campos-Torres A, Moynagh P, Haase J (2013) The pro-inflammatory cytokine TNF-α regulates the activity and expression of the serotonin transporter (SERT) in astrocytes. Neurochem Res 38:694–704
Masuda T, Nakagawa S, Boku S et al (2012) Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through beta2 receptor. Prog Neuro-Psychopharmacol Biol Psychiatry 36:44–51
Matsui TK, Mori E (2018) Microglia support neural stem cell maintenance and growth. Biochem Biophys Res Commun 503:1880–1884
Matsui T, Nakata T, Kobayashi Y (2016) Localization of organic cation transporter 2 (OCT2) in monoaminergic and cholinergic axon terminals of the mouse brain. Neurosci Lett 633:118–124
Matthaeus F, Schloss P, Lau T (2015) Differential uptake mechanisms of fluorescent substrates into stem-cell-derived serotonergic neurons. ACS Chem Nerosci 6:1906–1912
Ming G-l, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702
Morris ME, Rodriguez-Cruz V, Felmlee MA (2017) SLC and ABC transporters: expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J 19:1317–1331
Molina-Hernndez A, Velasco I (2008) Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J Neurochem 106:706–717
Mosher KI, Andres RH, Fukuhara T et al (2012) Neural progenitor cells regulate microglia functions and activity. Nat Neurosci 15:1485–1487
Naganuma F, Yoshikawa T, Nakamura T et al (2014) Predominant role of plasma membrane monoamine transporters in monoamine transport in 1321N1, a human astrocytoma-derived cell line. J Neurochem 129:591–601
Nakamichi N, Taguchi T, Hosotani H et al (2012) Functional expression of carnitine/organic cation transporter OCTN1 in mouse brain neurons: possible involvement in neuronal differentiation. Neurochem Int 61:1121–1132
Nakamichi N, Nakayama K, Ishimoto T et al (2016) Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav 6:e00477
Nakamichi N, Nakao S, Nishiyama M et al (2020) Oral administration of the food derived hydrophilic antioxidant ergothioneine enhances object recognition memory in mice. Curr Mol Pharmacol 14:220–233
Nakano C, Takashima S, Takeshita K (1989) Carnitine concentration during the development of human tissues. Early Hum Dev 19:21–27
Nakata T, Matsui T, Kobayashi K et al (2013) Organic cation transporter 2 (SLC22A2), a low-affinity and high-capacity choline transporter, is preferentially enriched on synaptic vesicles in cholinergic neurons. Neuroscience 252:212–221
Ng TP, Feng L, Yap KB et al (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68
O’Donovan SM, Sullivan C, Koene R et al (2018) Cell-subtype-specific changes in adenosine pathways in schizophrenia. Neuropsychopharmacology 43:1667–1674
Ohashi R, Tamai I, Yabuuchi H et al (1999) Na+-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther 291:778–784
Osman AM, Rodhe J, Shen X et al (2019) The secretome of microglia regulate neural stem cell function. Neuroscience 405:92–102
Pajarillo E, Rizor A, Lee J et al (2019) The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: potential targets for neurotherapeutics. Neuropharmacology 161:107559
Pardal R, López Barneo J (2016) Mature neurons modulate neurogenesis through chemical signals acting on neural stem cells. Dev Growth Differ 58:456–462
Park SY, Kang MJ, Han JS (2018) Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol Brain 11:39
Perera TD, Park S, Nemirovskaya Y (2008) Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist 14:326–338
Perez-Villalba A, Sirerol-Piquer MS, Belenguer G et al (2018) Synaptic regulator α-synuclein in dopaminergic fibers is essentially required for the maintenance of subependymal neural stem cells. J Neurosci 38:814–825
Pons-Espinal M, Gasperini C, Marzi MJ et al (2019) MiR-135a-5p is critical for exercise-induced adult neurogenesis. Stem Cell Rep 12:1298–1312
Ren-Patterson RF, Kim D, Zheng X et al (2005) Serotonergic-like progenitor cells propagated from neural stem cells in vitro: survival with SERT protein expression following implantation into brains of mice lacking SERT. FASEB J 19:1537–1539
Sanchez JF, Crooks DR, Lee CT et al (2006) GABAergic lineage differentiation of AF5 neural progenitor cells in vitro. Cell Tissue Res 324:1–8
Sánchez-Mendoza EH, Bellver-Landete V, Arce C et al (2017) Vesicular glutamate transporters play a role in neuronal differentiation of cultured SVZderived neural precursor cells. PLoS One 12:e0177069
Saraiva C, Barata-Antunes S, Santos T et al (2019) Histamine modulates hippocampal inflammation and neurogenesis in adult mice. Sci Rep 9:8384
Schmitt A, Mössner R, Gossmann A et al (2003) Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res 71:701–709
Schmitt A, Benninghoff J, Moessner R et al (2007) Adult neurogenesis in serotonin transporter deficient mice. J Neural Transm 114:1107–1119
Singh S, Mishra A, Mishra SK, Shukla S (2017) ALCAR promote adult hippocampal neurogenesis by regulating cell-survival and cell death-related signals in rat model of Parkinson’s disease like-phenotypes. Neurochem Int 108:388–396
Snyder JS, Soumier A, Brewer M et al (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behavior. Nature 476:458–461
Song J, Zhong C, Bonaguidi MA et al (2012) Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489:150–154
Song TY, Lin HC, Chen CL et al (2014) Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with D-galactose. Free Radic Res 48:1049–1060
Stankiewicz AJ, McGowan EM, Yu L, Zhdanova IV (2017) Impaired sleep, circadian rhythms and neurogenesis in diet-induced premature aging. Int J Mol Sci 18:2243
Syal C, Kosaraju J, Hamilton L et al (2020) Dysregulated expression of monoacylglycerol lipase is a marker for anti-diabetic drug metformin-targeted therapy to correct impaired neurogenesis and spatial memory in Alzheimer’s disease. Theranostics 10:6337–6360
Tamai I, Ohashi R, Nezu JI et al (1998) Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 273:20378–20382
Trujillo-Gonzalez I, Wang Y, Friday WB et al (2019) MicroRNA-129-5p is regulated by choline availability and controls EGF receptor synthesis and neurogenesis in the cerebral cortex. FASEB J 33:3601–3612
Tuszynski MH, Yang JH, Barba D et al (2015) Nerve growth factor gene therapy activation of neuronal responses in Alzheimer disease. JAMA Neurol 72:1139–1147
Urban T, Brown C, Castro R et al (2008) Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther 83:416–421
Valente MM, Bortolotto V, Cuccurazzu B et al (2012) α2δ ligands act as positive modulators of adult hippocampal neurogenesis and prevent depression-like behavior induced by chronic restraint stress. Mol Pharmacol 82:271–280
Veena J, Rao BSS, Srikumar BN (2011) Regulation of adult neurogenesis in the hippocampus by stress, acetylcholine and dopamine. J Nat Sci Biol Med 2:26–37
Vialou V, Balasse L, Callebert J et al (2008) Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem 106:1471–1482
Wang J, Gallagher D, Devito LM et al (2012) Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11:23–35
Warner-Schmidt JL, Duman RS (2006) Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus 16:239–249
Watanabe N, Matsumoto S, Suzuki M et al (2020) Effect of ergothioneine on the cognitive function improvement in healthy volunteers and mild cognitive impairment subjects–A randomized, double-blind, parallel-group comparison study. Jpn Pharmacol Ther 48:685–697
Wicki-Stordeur XE, Sanchez-Arias XC, Dhaliwal J et al (2016) Pannexin 1 differentially affects neural precursor cell maintenance in the ventricular zone and Peri-infarct cortex. J Neurosci 36:1203–1210
Yamada J, Jinno S (2019) Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology 158:107710
Yang NC, Lin HC, Wu JH et al (2012) Ergothioneine protects against neuronal injury induced by β-amyloid in mice. Food Chem Toxicol 50:3902–3911
Yanpallewar SU, Fernandes K, Marathe SV et al (2010) α2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci 30:1096–1109
You G, Morris ME (2014) Drug transporters: molecular characterization and role in drug disposition, 2nd edn. Wiley, Hoboken
Zhang CL, Zou Y, He W et al (2008) A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451:1004–1007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ishimoto, T., Kato, Y. (2021). Regulation of Neurogenesis by Organic Cation Transporters: Potential Therapeutic Implications. In: Daws, L.C. (eds) Organic Cation Transporters in the Central Nervous System. Handbook of Experimental Pharmacology, vol 266. Springer, Cham. https://doi.org/10.1007/164_2021_445
Download citation
DOI: https://doi.org/10.1007/164_2021_445
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82983-4
Online ISBN: 978-3-030-82984-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)