Abstract
Nitric oxide (NO) raises the intracellular 3′,5′-cyclic guanosine monophosphate (cGMP) level through the activation of soluble guanylate cyclase and, in the presence of reactive oxygen species (ROS), reacts with biomolecules to produce nitrated cGMP derivatives. 8-Nitro-cGMP was the first endogenous cGMP derivative discovered in mammalian cells (2007) and was later found in plant cells. Among the six nitrogen atoms in this molecule, the one in the nitro group (NO2) comes from NO. This chapter asserts that this newly found cGMP is undoubtedly one of the major physiological cNMPs. Multiple studies suggest that its intracellular abundance might exceed that of unmodified cGMP. The characteristic chemical feature of 8-nitro-cGMP is its ability to modify proteinous cysteine residues via a stable sulfide bond. In this posttranslational modification, the nitro group is detached from the guanine base. This modification, termed “protein S-guanylation,” is known to regulate the physiological functions of several important proteins. Furthermore, 8-nitro-cGMP participates in the regulation of autophagy. For example, in antibacterial autophagy (xenophagy), S-guanylation accumulates around invading bacterial cells and functions as a “tag” for subsequent clearance of the organism via ubiquitin modifications. This finding suggests the existence of a system for recognizing the cGMP structure on proteins. Autophagy induction by 8-nitro-cGMP is mechanistically distinct from the well-described starvation-induced autophagy and is independent of the action of mTOR, the master regulator of canonical autophagy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Discovery of 8-Nitro-cGMP
1.1 Detection of 8-Nitro-cGMP
Endogenous nitric oxide (NO) and reactive oxygen species (ROS) are implicated in the nitration of biomolecules such as amino acids and fatty acids. Guanine nitration at position 8 of the ring has also been investigated since the 1990s as an inflammation marker because inducible nitric oxide synthase (iNOS) is upregulated in inflammation (Yermilov et al. 1995; Spencer et al. 1996; Ohshima et al. 2006).
In the early 2000s, Akaike et al. speculated that 3′,5′-cyclic guanosine monophosphate (cGMP ), an important second messenger, may have an endogenous derivative with such a nitro group. Detailed analysis using chemically prepared authentic 8-nitro-cGMP was culminated in the discovery of endogenous 8-nitro-cGMP in cultured cells (Sawa et al. 2007) (Fig. 1).
Formation and metabolisms of 8-nitro-cGMP. Endogenous 8-nitro-cGMP forms from the guanine nucleotide pool by the action of nitric oxide (NO) and reactive oxygen species (ROS). This nucleotide either modifies cysteine-containing proteins via “S-guanylation” or transforms into 8-amino-cGMP, which is further converted into intact cGMP by NO and ROS. 8-Nitro-cGMP reacts with endogenous persulfides to produce 8-SH-cGMP, whereas the similar reaction with sulfhydryl anion (SH−) produces 8-amino-cGMP
The intracellular 8-nitro-cGMP concentration can be estimated by immunocytochemistry or mass spectrometry (MS). However, the accurate quantification of 8-nitro-cGMP in a biological sample was not trivial during the early research period because of its instability during sample preparation. Prior addition of isotopically (15N)-labeled 8-nitro-cGMP before extraction was found to minimize the loss of endogenous 8-nitro-cGMP in the sample (Fujii et al. 2010). This isotope-labeled 8-nitro-cGMP not only prevents the degradation of endogenous 8-nitro-cGMP present in smaller amounts but also serves as an internal standard for mass spectrometric analysis. Notably, the endogenous level of 8-nitro-cGMP was initially estimated to be less than 1% in all cGMPs (Sawa et al. 2007), but recent results using this isotope labeling technique showed that the intracellular 8-nitro-cGMP concentration is often higher than unmodified cGMP (Fujii et al. 2010). Moreover, 8-nitro-cGMP may be widely distributed in animal tissues because it was recently found in both mouse heart (Nishida et al. 2012) and brain (Kunieda et al. 2015).
It is now generally accepted that 8-nitro-cGMP is biosynthesized via the nitration of GTP and subsequent action of guanylate cyclase (Fujii et al. 2010; Ahmed et al. 2012). In studies using rat C6 cells activated by lipopolysaccharide (LPS), the direct formation of 8-nitro-cGMP from cGMP appeared unlikely, because the endogenous cGMP concentration (maximum 4 μM) was consistently lower than 8-nitro-cGMP (maximum 40 μM). Furthermore, peroxynitrite, a nitration agent, quickly reacts with guanosine phosphates in the order GTP>GDP>GMP>cGMP. In addition, 8-nitro-GTP is a good substrate of the soluble guanylate cyclase in vitro yielding 8-nitro-cGMP, and an inhibitor of soluble guanylate cyclase was shown to suppress intracellular 8-nitro-cGMP generation.
1.2 S-Guanylation: A Novel Posttranslational Modification (PTM) by 8-Nitro-cGMP
The analysis of endogenous 8-nitro-cGMP was difficult in earlier studies because of its instability in biological samples. Because the cyclic phosphate of 8-nitro-cGMP is resistant to hydrolysis by PDE1 and PDE5 (Sawa et al. 2007), other degradation pathways may account for its instability. An important clue to the identity of endogenous 8-nitro-cGMP degradation pathways was obtained in 2005. Akaike and our group noticed that the yellow color of 8-nitro-cGMP disappeared upon treatment with thiol-containing compounds. A model reaction with 8-nitro-cGMP and glutathione was performed in vitro at room temperature, and the reaction product was thoroughly investigated using two-dimensional nuclear magnetic resonance (NMR) techniques and MS. These analyses revealed a glutathione adduct at the 8 position of the guanine ring. This substitution of the nitro group with thiol had never been reported in the chemistry literature and so was quite astonishing. This reaction was named “protein S-guanylation” (Fig. 1).
Polyclonal and monoclonal antibodies specific to protein S-guanylation were developed for immunoblot analysis and immunohistochemistry. The antibodies demonstrated that this PTM is present in cells under basal conditions and that levels increase during inflammation. Regulatory mechanisms of this PTM are currently unclear because S-guanylation proceeds without enzymes. The reactivity of each cysteine residue varies considerably depending on the neighboring sequence. For example, Kelch-like ECH-associated protein (Keap1), which contains multiple reactive cysteine residues, was S-guanylated even in the presence of a 1,000-fold excess of glutathione (Sawa et al. 2007). Thus, the propensity for S-guanylation may depend, at least in part, on the chemical environment around cysteine residues.
As described in Sects. 3 and 4, protein S-guanylation is involved in several physiological processes. However, it is still uncertain if endogenous levels and distributions of NO and ROS alone can account for the spatiotemporal dynamics of this PTM. Similarly, the intrinsic reactivity of each cysteine residue may not be the only factor governing the selectivity of S-guanylation. An interesting topic for future studies is the possible participation of enzymes.
2 Metabolism: Reduction to Intact cGMP via 8-Amino-cGMP/8-SH-cGMP
To date, three metabolic pathways for 8-nitro-cGMP have been elucidated, including protein S-guanylation. Here we describe two additional pathways (Fig. 1).
2.1 Reductive Metabolic Pathway to Unmodified cGMP via 8-Amino-cGMP
18O-labeled 8-nitro-cGMP was chemically synthesized, and its metabolic fates in cultured cells were monitored by liquid chromatography–mass spectrometry (LC-MS). Along with the S-guanylation products containing glutathione or cysteine, a new nucleotide, 8-amino-cGMP (8-NH2-cGMP), was identified (Saito et al. 2012). Immunocytochemistry of LPS-activated mouse macrophage-like RAW264.7 cells using a specific 8-amino-cGMP antibody revealed that the endogenous degradation of 8-nitro-cGMP was accompanied by the formation of 8-amino-cGMP. Moreover, isotope-labeled 8-amino-cGMP was further converted to unmodified cGMP in LPS-treated cells. It is intriguing that both oxidative modification (guanine nitration) and reductive metabolism (cGMP formation) occur simultaneously under oxidative stress conditions.
2.2 Substitution of the Nitro Group by Persulfide Species: 8-SH-cGMP Formation
Based on the analogy to S-guanylation at thiols in cysteine residues, substitution with endogenous sulfur species may be expected. Hydrogen sulfide (H2S) is one such sulfur species attracting considerable attention as a gaseous signaling mediator. Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) are responsible for H2S biosynthesis. Knockdown of these enzymes resulted in increased 8-nitro-cGMP levels, indicating the regulation of 8-nitro-cGMP actions by sulfur species. Moreover, a plausible product of S-guanylation with H2S, 8-mercapto-cGMP (8-SH-cGMP), was identified in mammalian cell lysates by LC-MS analysis (Nishida et al. 2012).
However, the involvement of H2S as a precursor of 8-SH-cGMP was soon questioned because the reaction of 8-nitro-cGMP with the hydrogen sulfide anion (SH−) in vitro predominantly yields 8-amino-cGMP (8-NH2-cGMP) (Terzić et al. 2014), a previously identified metabolite of 8-nitro-cGMP (Saito et al. 2012). Thus, it is reasonable to conclude that endogenous H2S may serve as a reducing agent in the conversion of 8-nitro-cGMP to 8-amino-cGMP.
A recent paper from the Akaike group provided an alternative pathway for 8-SH-cGMP biosynthesis. They demonstrated that CBS and CSE produce persulfide species (e.g., RS-S-H) that are more nucleophilic than hydrogen sulfide. Specifically, persulfides of cysteine (Cys-S-S-H) and glutathione (GS-S-H) are produced that react with 8-nitro-cGMP to provide the substitution product (RS-S-cGMP), which is expected to yield 8-SH-cGMP via a thiol-disulfide exchange reaction (Ida et al. 2014; Terzić et al. 2014).
Treatment of 8-SH-cGMP in vitro with hydrogen peroxide (H2O2) or reactive nitrogen species provided intact cGMP (Nishida et al. 2012). Similarly, 8-amino-cGMP, another metabolite of 8-nitro-cGMP, has already been demonstrated to yield cGMP in LPS-stimulated cells. Thus, 8-SH-cGMP may also be converted to intact cGMP in cells.
3 Regulation of Protein Functions by S-Guanylation
3.1 Activation Nrf2-ARE Pathway via S-Guanylation of Keap1
Keap1 is a major sensor of oxidative stress. It contains 25 cysteine residues and was the first identified target of protein S-guanylation (Sawa et al. 2007). Keap1 facilitates Nrf2 proteasomal degradation by acting as an adaptor for Cullin3-based E3 ubiquitin ligase (Kobayashi et al. 2004). Conversely, S-guanylation of Keap1 cysteine residues at the Nrf2 interaction site causes Nrf2 to dissociate from Keap1 and translocate into the nucleus, leading to the transcriptional activation of cytoprotective genes (Fig. 2). The cytoprotective actions of 8-nitro-cGMP against oxidative stress have been directly demonstrated (Cosker et al. 2014).
Keap1 S-guanylation activates Nrf2-ARE pathway. The Keap1-Nrf2 pathway regulates more than hundreds of cytoprotective genes. Keap1 inactivation by S-guanylation at cysteine residues leads to Nrf2 nuclear translocation and induction of the cytoprotective machineries. Keap1 Kelch-like ECH-associated protein 1, Nrf2 nuclear erythroid 2-related factor 2, ARE antioxidant response element
It should be emphasized, however, that many other endogenous electrophiles also modify the cysteine residues of Keap1 and exhibit similar cytoprotective actions. Moreover, 8-nitro-cGMP is an electrophile with modest reactivity; therefore, its contribution to Keap1 modification and downstream anti-oxidative stress responses requires further study.
3.2 Cellular Senescence by S-Guanylation of H-Ras Protein in a Heart Failure Model
In the early stages of heart failure, iNOS is overexpresssed to trigger cytoprotective responses. On the other hand, iNOS also serves as a crucial factor for cardiac remodeling. To understand these apparently opposing effects, the actions of 8-nitro-cGMP on rat primary cardiomyocyte and cardiac fibroblast cultures were investigated (Nishida et al. 2012). Exogenous 8-nitro-cGMP treatment induced growth arrest and senescence, as measured by endogenous β-galactosidase activity. These effects were not observed with other cGMP derivatives. The accumulation of 8-nitro-cGMP in mouse heart after myocardial infarction (MI) also suggests a role in the pathogenesis of heart failure. The administration of low-dose sodium hydrosulfide (50 μmol/kg/day) reduced the accumulation of 8-nitro-cGMP and limited the dysfunction of the left ventricle after MI. Oncogenic H-Ras activation can also induce cellular senescence, and Cys184 S-guanylation is a crucial regulator because 8-nitro-cGMP did not induce senescence in a rat cardiac fibroblast mutant expressing Cys C184S H-Ras (Fig. 3).
S-Guanylation of oncogenic H-Ras activates Raf-dependent ERK and p38 MAPK pathways. Partitioning of H-Ras between raft and non-raft domains is affected by the S-guanylation at Cys184. Non-raft-resident GTP-bound H-Ras activates Raf1 and the downstream signaling pathways. Cys181 and Cys186 of H-Ras are modified by palmitoylation and isoprenylation, respectively
3.3 S-Guanylation in the Mitochondria and Its Effects on ROS Export
The identification of new S-guanylated proteins provides additional clues to the underlying functions of 8-nitro-cGMP. Two optimized procedures for “S-guanylation proteomics” were reported (Rahaman et al. 2014), each with specific merits and demerits. In the first procedure, the immunoaffinity capture and LC-MS/MS method, protein samples are digested directly, and the resulting S-guanylated peptides are collected by immunoaffinity capture, followed by identification using LC–tandem MS. This procedure generally requires relatively large protein samples (1 mg/analysis). In the other procedure, the protein samples are first separated by 2D-PAGE, and the S-guanylated spots are excised and digested for LC-MS/MS analysis. In this 2D-PAGE-based procedure, smaller samples can be used (0.1 mg protein/analysis), and the relative abundance of each S-guanylated protein spots is known before MS/MS analysis. However, 2D-PAGE is insensitive for lipophilic membrane proteins and basic proteins (pI > 8).
Using these procedures, several mitochondrial proteins, including Hsp60 and mortalin, were shown to be S-guanylated in cells after stimulation with LPS and cytokines. Subsequent studies indicated that these PTMs may be involved in mitochondrial permeability-transition pore (mPTP) opening and mPTP-mediated ROS release into the cytoplasm (Fig. 4).
3.4 8-Nitro-cGMP in Nerve Cells
Neuronal NO synthase (nNOS) is also involved in 8-nitro-cGMP formation (Kasamatsu et al. 2014). Calcium ionophore treatment of nNOS-transfected HEK-293 cells enhanced protein S-guanylation suggesting 8-nitro-cGMP formation by nNOS-derived NO and ROS in the nervous system. In a nerve terminal model (synaptosomes), S-guanylation of synaptosomal-associated protein 25 (SNAP25) was identified (Kunieda et al. 2015). SNAP25 is a member of a SNARE complex that regulates synaptic vesicle fusion for exocytosis. Cysteine 90 of SNAP25 was identified as the main site of S-guanylation, and this modification was shown to stabilize the SNARE complex (Fig. 5).
Protein S-guanylation stabilizes SNARE complex. Synaptosomal-associated protein 25 kDa (SNAP25) is a component of the SNARE complex, which mediates fusion of vesicles with membranes. Ca2+-dependent activation of neuronal NO synthase (nNOS) produces 8-nitro-cGMP, and the resulting S-guanylation of SNAP25 at Cys90 enhances the stability of SNARE complex. This stabilizing effect of the SNARE complex may promote synaptic vesicle fusion
Another study using primary mesencephalic cells showed that exogenous 8-nitro-cGMP protected dopaminergic neurons from cytotoxic 1-methyl-4-phenylpyridinium (MPP+) via heme oxygenase-1 formation as well as the canonical protein kinase G (PKG) signaling pathway. Elevated extracellular K+ concentration induced an endogenous formation of 8-nitro-cGMP and also resulted in neuroprotection (Kurauchi et al. 2013). These studies suggest that nNOS-derived 8-nitro-cGMP has an important cytoprotective function in neuronal cells.
3.5 8-Nitro-cGMP in Plants
As described elsewhere in this book, the roles of cGMP in plants are not well understood compared to those in animals. Recently, the existence of 8-nitro-cGMP in stomatal guard cells of Arabidopsis was described (Joudoi et al. 2013) (Fig. 6). 8-Nitro-cGMP and cGMP appear to have different effects on stomatal function. Although 8-nitro-cGMP induced stomatal closure, 8-Br-cGMP, a widely used membrane permeable analogue of cGMP, did not. In the dark, 8-Br-cGMP induced stomatal opening, but 8-nitro-cGMP did not. Judoi et al. showed that the effects of 8-nitro-cGMP signaling are mediated by Ca2+, cyclic adenosine-5´-diphosphate-ribose, and the SLOW ANION CHANNEL1 (SLAC1). The same research group recently reported that the 8-nitro-cGMP metabolite 8-SH-cGMP (8-mercapto-cGMP, Sect. 2.2) also induces stomatal closure (Honda et al. 2015). However, the direct involvement of protein S-guanylation in these effects has not been demonstrated.
8-Nitro-cGMP in plants. 8-nitro-cGMP and cGMP have different effects on regulation of stomatal function. 8-Nitro-cGMP causes stomatal closure, whereas 8-Br-cGMP, a widely used membrane permeable analogue of cGMP, does not. In the dark, 8-nitro-cGMP does not cause stomatal opening. SLAC slow anion channel
4 8-Nitro-cGMP in Autophagy Regulation
Voluminous evidence accrued over the past decade clearly indicates that 8-nitro-cGMP can covalently modify Cys-containing proteins and influence their function. Recently, autophagy induction was demonstrated to be another major function of 8-nitro-cGMP (Ito et al. 2013; Rawet-Slobodkin and Elazar 2013; Abada and Elazar 2014).
4.1 8-Nitro-cGMP as an Endogenous Autophagy Inducer
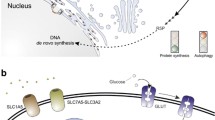
Autophagy is one of the two major cellular degradation systems conserved across eukaryotes (Fig. 7). A double-membrane vesicle called the autophagosome engulfs autophagic cargos and subsequently fuses with the lysosome (Mizushima and Komatsu 2011) for eventual degradation and recycling. In mammals, autophagy can be monitored by labeling the autophagosomal LC3 protein (homolog of yeast Atg8). Autophagy is a well-described response to nutrient starvation but is also induced by other stimuli. Damaged organelles, protein aggregates, and pathogens can be selectively destroyed by separate autophagic pathways that differ mechanistically from the canonical starvation-induced autophagic pathway.
Autophagy suppresses diseases via intracellular degradation. Autophagy is a cellular degradation system that plays a major role in human health and disease. Autophagosome surrounds and sequesters cytoplasmic cargos for lysosomal degradation. Autophagy is associated with suppression of diseases including cancer and neurodegenerative diseases
LPS is a component of bacterial cell membranes and a stimulator of innate immunity, eliciting a variety of inflammatory responses. LPS also induces autophagy, and, in this specific case, autophagy was shown to depend on NO and ROS (Yuan et al. 2009). Because the intracellular 8-nitro-cGMP level is upregulated by LPS-induced inflammation (Sawa et al. 2007), the involvement of this cGMP analogue in autophagy regulation was examined (Ito et al. 2013). Indeed, exogenous 8-nitro-cGMP treatment (50–100 μM) increased LC3-positive puncta in cells suggesting autophagy induction, and no cytotoxicity was observed. Considering the fact that cGMP does not penetrate cell membranes, the observed membrane permeability of 8-nitro-cGMP is noteworthy for future research and possibly for clinical applications.
An initial mechanistic study by Ito et al. demonstrated that 8-nitro-cGMP-induced autophagy does not employ target of rapamycin (TOR), the master regulator of canonical starvation-induced autophagy. Immunocytochemical analysis showed that S-guanylated proteins partly co-localized with LC3-positive autophagosomes, suggesting that 8-nitro-cGMP not only induces autophagy but also participates in selective clearance of its targets (S-guanylated proteins).
4.2 S-Guanylation as a Tag for Degradation in Antibacterial Autophagy
In contrast to the nonselective nature of canonical starvation-induced autophagy, autophagy for the clearance of intracellular pathogens or damaged organelles is considered highly selective. Because bacterial infection elevates the intracellular formation of 8-nitro-cGMP, the contribution of 8-nitro-cGMP to antibacterial autophagy was investigated using group A streptococcus (GAS) and murine macrophages. Autophagic clearance of GAS had previously been described in HeLa cells (Nakagawa et al. 2004). Invading GAS was efficiently cleared also from murine macrophages via autophagy, and this process required endogenous 8-nitro-cGMP generation (Ito et al. 2013) (Fig. 8). Exogenous addition of 8-nitro-cGMP to the culture media further accelerated GAS clearance.
Endogenous 8-nitro-cGMP accelerates selective autophagic clearance of invading group A streptococcus (GAS). (a) The innate immune system functions to trigger inflammation upon sensing of conserved microbial structures of invading pathogens. Enhanced formation of 8-nitro-cGMP during inflammation activates autophagy. (b) Ito et al. (2013) reported that accumulation of S-guanylated proteins around bacteria is the tag for subsequent ubiquitination and clearance. Involvement of the ubiquitin modification has been attracting attention as the degradation tag, but the correlation of S-guanylation and ubiquitination was demonstrated for the first time. Ub polyubiquitin chain
Selective autophagy is currently a field of extensive research, and autophagic cargo ubiquitination is also attracting attention because many “autophagy adaptors” have the ability to connect ubiquitinated cargos with LC3 protein on the autophagosomal membrane. An important question here is how specific autophagic cargos are recognized and selectively modified by ubiquitin ligases. We observed the accumulation of S-guanylation in GAS-containing autophagosomes (Fig. 8b). This S-guanylation of GAS cells was the tag required for subsequent modification with K63-linked polyubiquitin chains. Thus, S-guanylation is the degradation tag for antibacterial autophagy of GAS (Ito et al. 2013). Inhibitors of endogenous 8-nitro-cGMP generation could thus suppress the autophagic clearance of the GAS bacteria. Some of these S-guanylated bacterial proteins were identified by LC-MS analysis; however, host-derived S-guanylated proteins may also be involved in GAS recognition in autophagosomes.
As mentioned in previous sections, 8-nitro-cGMP can form in cells even in the absence of bacterial infection. K63-linked polyubiquitination was also involved in autophagy induction under these conditions (Ito et al. 2013), suggesting that S-guanylation is a general tag for autophagic degradation. However, many unanswered questions still exist. For example, can a protein be both S-guanylated and ubiquitinated? Or can a putative “S-guanylation recognition protein” bind to a S-guanylated protein and be ubiquitinated? Also, what are the major physiological substrates of this selective degradation?
Autophagy is believed to suppress a variety of human diseases; therefore, considerable efforts have been made to identify or create small molecular weight autophagy regulator (Levine et al. 2015; Rubinsztein et al. 2015). Among known autophagy inducers, 8-nitro-cGMP is the only compound that induces a selective degradation of its targets. Moreover, 8-nitro-cGMP is membrane permeable and resists hydrolysis by phosphodiesterase (Sawa et al. 2007). This nucleotide is thus a promising lead drug for a clinical activator of autophagy.
5 Preparative Methods of 8-Nitro-cGMP
As of late 2015, 8-nitro-cGMP is not available from commercial sources; therefore, researchers must prepare this nucleotide for their experiments. 8-Nitro-cGMP is believed to be generated in vivo via GTP nitration by reactive nitrogen species (e.g., peroxynitrate, Sect. 1.1). For preparative purposes in vitro, however, this nitration reaction is not practical because of its low yield. Instead, commercially available 8-bromo-cGMP (8-Br-cGMP) is used as the starting material, and nitration is performed with sodium nitrite (NaNO2) (Sawa et al. 2007). In our hands, this protocol yields an average of 10–20% 8-nitro-cGMP from a 100-mg scale reaction (Fig. 9). Unfortunately, this method is not easily scalable for more material because it requires HPLC purification of the product from unreacted 8-Br-cGMP. Thus, development of alternative, scalable synthetic procedures is in great demand for the pharmaceutical application of 8-nitro-cGMP in animal models. Organic chemists are also working on developing chemical tools such as fluorescent probes to study the functions of 8-nitro-cGMP (Saito et al. 2013; Samanta et al. 2014).
6 Future Problems
6.1 Regulatory Mechanism of Protein S-Guanylation
The majority of 8-nitro-cGMP effects described to date appear to be mediated by protein S-guanylation. However, no regulatory mechanism has been reported that governs the spatiotemporal selectivity of the modification. A current explanation for the selectivity is the cysteine acidity (pK a) of each site within each target protein. Neighboring amino acid residues are known to affect the acidity. Cysteine residues with low pK a dissociate to form sulfur anions that are more reactive with 8-nitro-cGMP, at least in vitro. The steric environment of each cysteine residue may also affect its reactivity.
However, it is unclear whether cysteine acidity alone can confer the requisite target specificity for intracellular signaling and autophagy regulation; therefore, the existence of enzymes that catalyze protein S-guanylation is a crucial future issue in this field.
6.2 Physiological Importance of 8-nitro-cGMP in PKG-Mediated Signaling Pathways
This chapter has focused on properties unique to the nitrated cGMP. However, it should be emphasized that like cGMP, 8-nitro-cGMP retains the capacity to activate PKG (Sawa et al. 2007). According to several studies, endogenous 8-nitro-cGMP levels are similar or even higher under some conditions (e.g., infection/inflammation) than cGMP levels (Fujii et al. 2010; Kunieda et al. 2015). The contribution of 8-nitro-cGMP to PKG-mediated signaling is worthy of further investigation. The metabolites of 8-nitro-cGMP (8-amino-cGMP and 8-SH-cGMP) also need to be considered as physiological PKG activators.
7 Concluding Remarks
8-Nitro-cGMP is the most recently discovered endogenous cNMP, and there is compelling evidence that endogenously produced 8-nitro-cGMP acts as a physiological modulator through the PTM of proteins and possibly also by PKG activation. It exists not only in mammals but also in higher plants, and the endogenous concentration often exceeds that of cGMP. This chapter is by no means a comprehensive discussion of all known or postulated physiological functions because a very large number of proteins can be modified by this cNMP. The possible pharmaceutical application of this nucleotide for a variety of autophagy-related human disorders, such as neurodegenerative diseases and cancer, is thus a promising avenue for future research.
References
Abada A, Elazar Z (2014) Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep 15:839–852
Ahmed KA, Sawa T, Ihara H, Kasamatsu S, Yoshitake J, Rahaman MM, Okamoto T, Fujii S, Akaike T (2012) Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: potential implications for ROS signalling. Biochem J 441:719–730
Cosker F, Lima FJ, Lahlou S, Magalhães PJ (2014) Cytoprotective effect of 1-nitro-2-phenylethane in mice pancreatic acinar cells subjected to taurocholate: putative role of guanylyl cyclase-derived 8-nitro-cyclic-GMP. Biochem Pharmacol 15:191–201
Fujii S, Sawa T, Ihara H, Tong KI, Ida T, Okamoto T, Ahtesham AK, Ishima Y, Motohashi H, Yamamoto M, Akaike T (2010) The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J Biol Chem 285:23970–23984
Honda K, Yamada N, Yoshida R, Ihara H, Sawa T, Akaike T, Iwai S (2015) 8-Mercapto-cyclic GMP mediates hydrogen sulfide-induced stomatal closure in arabidopsis. Plant Cell Physiol 56:1481–1489
Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T (2014) Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA 111:7606–7611
Ito C, Saito Y, Nozawa T, Fujii S, Sawa T, Inoue H, Matsunaga T, Khan S, Akashi S, Hashimoto R, Aikawa C, Takahashi E, Sagara H, Komatsu M, Tanaka K, Akaike T, Nakagawa I, Arimoto H (2013) Endogenous nitrated nucleotide is a key mediator of autophagy and innate defense against bacteria. Mol Cell 52:794–804
Joudoi T, Shichiri Y, Kamizono N, Akaike T, Sawa T, Yoshitake J, Yamada N, Iwai S (2013) Nitrated cyclic GMP modulates guard cell signaling in Arabidopsis. Plant Cell 25:558–571
Kasamatsu S, Watanabe Y, Sawa T, Akaike T, Ihara H (2014) Redox signal regulation via nNOS phosphorylation at Ser847 in PC12 cells and rat cerebellar granule neurons. Biochem J 459:251–263
Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24:7130–7139
Kunieda K, Tsutsuki H, Ida T, Kishimoto Y, Kasamatsu S, Sawa T, Goshima N, Itakura M, Takahashi M, Akaike T, Ihara H (2015) 8-Nitro-cGMP enhances SNARE complex formation through S-guanylation of Cys90 in SNAP25. ACS Chem Neurosci 6:1715–1725
Kurauchi Y, Hisatsune A, Isohama Y, Sawa T, Akaike KH (2013) Nitric oxide/soluble guanylyl cyclase signaling mediates depolarization-induced protection of rat mesencephalic dopaminergic neurons from MPP+ cytotoxicity. Neuroscience 231:206–215
Levine B, Packer M, Codogno P (2015) Development of autophagy inducers in clinical medicine. J Clin Invest 125:14–24
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T (2004) Autophagy defends cells against invading group A Streptococcus. Science 306:1037–1040
Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T (2012) Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8:714–724
Ohshima H, Sawa T, Akaike T (2006) 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal 8:1033–1045
Rahaman MM, Sawa T, Ahtesham AK, Khan S, Inoue H, Irie A, Fujii S, Akaike T (2014) S-guanylation proteomics for redox-based mitochondrial signaling. Antioxid Redox Signal 20:295–307
Rawet-Slobodkin M, Elazar Z (2013) 8-nitro-cGMP-a new player in antibacterial autophagy. Mol Cell 52:767–768
Rubinsztein DC, Bento CF, Deretic V (2015) Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J Exp Med 212:979–990
Saito Y, Sawa T, Yoshitake J, Ito C, Fujii S, Akaike T, Arimoto H (2012) Nitric oxide promotes recycling of 8-nitro-cGMP, a cytoprotective mediator, into intact cGMP in cells. Mol Biosyst 8:2909–2915
Saito Y, Ito C, Fujii S, Sawa T, Akaike T, Arimoto H (2013) Fluorescent probes for live cell imaging of endogenous Guanine nitration. Chembiochem 14:1068–1071
Samanta A, Thunemann M, Feil R, Stafforst T (2014) Upon the photostability of 8-nitro-cGMP and its caging as a 7-dimethylaminocoumarinyl ester. Chem Commun (Camb) 50:7120–7123
Sawa T, Zaki MH, Okamoto T, Akuta T, Tokutomi Y, Kim-Mitsuyama S, Ihara H, Kobayashi A, Yamamoto M, Fujii S, Arimoto H, Akaike T (2007) Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. Nat Chem Biol 3:727–735
Spencer JP, Wong J, Jenner A, Aruoma OI, Cross CE, Halliwell B (1996) Base modification and strand breakage in isolated calf thymus DNA and in DNA from human skin epidermal keratinocytes exposed to peroxynitrite or 3-morpholinosydnonimine. Chem Res Toxicol 9:1152–1158
Terzić V, Padovani D, Balland V, Artaud I, Galardon E (2014) Electrophilic sulfhydration of 8-nitro-cGMP involves sulfane sulfur. Org Biomol Chem 12:5360–5364
Yermilov V, Rubio J, Ohshima H (1995) Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett 376:207–210
Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA (2009) LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol 296:H470–H479
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Arimoto, H., Takahashi, D. (2017). 8-Nitro-cGMP: A Novel Protein-Reactive cNMP and Its Emerging Roles in Autophagy. In: Seifert, R. (eds) Non-canonical Cyclic Nucleotides. Handbook of Experimental Pharmacology, vol 238. Springer, Cham. https://doi.org/10.1007/164_2016_5000
Download citation
DOI: https://doi.org/10.1007/164_2016_5000
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52671-3
Online ISBN: 978-3-319-52673-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)