Abstract
Autotrophic acetogenic bacteria are able to capture carbon (CO or CO2) through gas fermentation, allowing them to grow on a spectrum of waste gases from industry (e.g., steel manufacture and oil refining, coal, and natural gas) and to produce ethanol. They can also consume syn(thesis) gas (CO and H2) made from the gasification of renewable/sustainable resources, such as biomass and domestic/agricultural waste. Acetogenic gas fermentation can, therefore, produce ethanol in any geographic region without competing for food or land. The commercialization of the process is now at an advanced stage. The real potential of acetogens, however, resides in their capacity to produce chemicals and fuels other than ethanol. This requires the redesign and implementation of more efficient metabolic pathways, adapting them to high performing manufacturing processes. Respective species, their bioenergetics, the genetic tools developed for their metabolic engineering, culture techniques and fermenter set-ups, as well as the commercialization, are comprehensively described and discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The anaerobic conversion of CO2 and H2 to acetate in digested sludge was first described by Fischer and colleagues [1]. Later, Wieringa isolated a pure culture of Clostridium aceticum, which thus became the first known autotrophic acetogen [2,3,4]. As the organism seemed to be lost during World War II, the biochemical reactions of acetogenesis were elucidated using Moorella thermoacetica (formerly Clostridium thermoaceticum). In honor of the scientists mainly involved in this work, this metabolic feature was named the Wood–Ljungdahl pathway. In 1977, the sodium-dependent Acetobacterium woodii was isolated [5], and in 1981 a spore preparation of C. aceticum was found in a laboratory fridge of Barker (University of California Berkeley), which could be successfully revived [6]. Since then, numerous mesophilic and thermophilic autotrophic acetogens have been isolated and characterized [7,8,9,10]. Based on the presence of Wood–Ljungdahl pathway genes, more bacteria might possess the ability of autotrophic acetogenesis, but this needs to be verified experimentally.
The ability to capture carbon directly in the form of carbon monoxide (CO) and carbon dioxide (CO2), present in syn(thesis) gas (which is used for certain reactions in the chemical industry but is also a waste by-product of many industrial processes), through anaerobic fermentation gives many anaerobic acetogens great potential for use as microbial production platforms for a range of high value commodity chemicals and fuels. Thus, in recent years, acetogens have attracted significant attention as the process organism for the biotechnological production of fuels and chemicals from industrial waste gas streams [11]. This emerging technology allows sustainable, high volume production of fuels and commodities independent from food-based substrates. The technology has been successfully demonstrated by a few companies at pilot and demo scales and the first commercial units have been announced.
The development of these microbial production platforms has historically been hampered by a lack of available genomic sequences and genetic tools, although recent advances in sequencing technologies and subsequent implementation of genetic methods have made these organisms far more accessible for directed mutagenesis. Closed genome sequences have been published for A. woodii [12], M. thermoacetica [13], Clostridium ljungdahlii [14], Clostridium autoethanogenum [15], Clostridium carboxidivorans [16], C. aceticum [17], Eubacterium limosum [18], and Clostridium difficile [19].
Key to development of a microbial platform is the establishment of robust and reproducible procedures for DNA transfer into the organism. The delivery of plasmids into an organism whose genome sequence has been elucidated allows the development of targeted group II intron-based mutagenesis and directed homologous recombination strategies to facilitate individual gene knockout and complementation studies, as well as expression of heterologous genes.
2 Anaerobic Autotrophs
Many mesophilic and thermophilic autotrophic acetogens are currently known. However, not all have been investigated in detail and only a few are currently used in or for developing industrial applications. These latter ones are detailed in the following paragraphs.
2.1 Clostridium aceticum
C. aceticum was isolated in 1936 from sludge from a canal in the Netherlands and characterized with respect to morphology, nutritional requirements, growth pattern, and product formation. Its remarkable metabolic activity is the conversion of four molecules of hydrogen and two molecules of carbon dioxide into one molecule of acetate and two molecules of water. Heterotrophic substrates can also be used [2,3,4]. C. aceticum was the first autotrophic acetogen cultivated as a pure culture. CO can also serve as a carbon source [20]. After the war the culture seemed to be lost, but was later found in a laboratory culture collection in California [6]. At about the same time, attempts to re-isolate the organism were also successful [21]. The reason for various earlier failed attempts at its re-isolation was most probably the alkaline pH optimum of 8.3. The complete genome sequence of C. aceticum was recently published [17]. Interestingly, the bacterium does not contain genes for quinone synthesis [22] although cytochromes have been detected [6]. Thus, no electron transport chain via cytochromes and quinones is possible. Instead, C. aceticum harbors an Rnf (designation stems from Rhodobacter nitrogen fixation) complex, which might be acting as a proton pump in this organism [22] (see Sect. 3).
2.2 Acetobacterium woodii
A. woodii was isolated in 1977 from black sediment from an oyster pond in Woods Hole, MA [5]. It can grow on CO2 plus H2, producing acetate (as C. aceticum). The heterotrophic substrate range is rather narrow, being limited to some sugars, organic acids, and O-methylated aromatic compounds. The bacterium is Na+-dependent [23], uses an Na+-dependent ATPase [24], and generates an Na+-gradient across the cytoplasmic membrane by means of an Rnf complex [25]. Its genome has been completely sequenced [12] and its energy metabolism belongs to the best understood among acetogens [26]. A. woodii is the model organism for Na+-dependent autotrophic acetogens.
2.3 Clostridium ljungdahlii
C. ljungdahlii was isolated from chicken yard waste as an organism being capable of using syngas (mainly a mixture of CO plus H2) as sole carbon source [27]. Its genome was completely sequenced, indicating that this bacterium is one of the most versatile acetogens with respect to substrate utilization [14]. In addition to acetate, it produces large amounts of ethanol and smaller amounts of 2,3-butanediol and lactate [28]. The genes responsible for ethanol and 2,3-butanediol production have been identified as well as the function of the Rnf complex as a proton pump [29,30,31]. Together with C. autoethanogenum, C. ljungdahlii developed into a model organism for H+-dependent autotrophic acetogens. Strains of C. ljungdahlii are industrially used by INEOS Bio (see Sect. 7.2).
2.4 Moorella thermoacetica
Whereas all other bacteria mentioned in Sect. 2 are mesophilic, M. thermoacetica is a moderate thermophile (optimal growth temperature 55°C). It was isolated under heterotrophic conditions from horse manure [32]. M. thermoacetica served as the model organism for elucidation of the enzymology of the Wood–Ljungdahl pathway [8] (see Sect. 3). Much later it was discovered that the organism is also capable of autotrophic growth [33]. It does not contain an Rnf complex but instead possesses cytochromes and quinones as well as an energy-conserving hydrogenase (Ech) for generation of a proton gradient [13, 26, 34]. Nitrate and nitrite can be used as terminal electron acceptors [35, 36]. With nitrate, H2-dependent growth yields are higher than those with CO2. In the presence of nitrate, cytochrome synthesis is repressed [37].
2.5 Butyribacterium methylotrophicum
B. methylotrophicum is a catabolically versatile, mesophilic, spore-forming anaerobe that was isolated from a sewage digester in Marburg, Germany [38]. Heterotrophic growth is possible with sugars, organic acids, and C1-compounds such as methanol. Autotrophic growth relies on CO2 + H2 gas mixtures. An adapted strain, the so-called CO strain, can also grow on CO and syngas [39]. It is a mutant that expresses higher levels of ferredoxin: NAD+ oxidoreductase, which is not inhibited by NADH [40]. Products are acetate and butyrate, but, at decreasing pH values, increasing amounts of butanol and ethanol are formed [41, 42]. Lactate has also been described as a product [43]. In the EU, B. methylotrophicum is classified as risk group 2. A genome sequence is not currently available.
2.6 Eubacterium limosum
E. limosum was isolated on methanol as a substrate, inoculated with sheep rumen fluid and sewage sludge [44]. Products from methanol are acetate, butyrate, and caproate. Autotrophic growth with CO2 + H2 or CO as sole carbon and energy source is also possible. Under these conditions, no caproate is formed [45]. In defined media, butyrate is produced from CO [46, 47]. An energy conservation model has been presented, suggesting that the energetic benefit when growing on CO might be a reason that butyrate is only formed on CO and not on CO2 + H2 gas mixtures [48]. Complete and draft genomes are available for two E. limosum strains, the latter also producing butanol [18, 49].
2.7 Clostridium autoethanogenum
C. autoethanogenum was isolated from rabbit feces using CO as sole carbon and energy source. It produces acetate, ethanol, and CO2 from CO [50]. Other natural products are lactate and 2,3-butanediol [28]. It can also grow well on syn(thesis) gas. This organism is used industrially for ethanol production from steel mill exhaust gases by LanzaTech (see Sect. 7.2). The genome sequence has been determined [51, 52].
2.8 Clostridium coskatii
C. coskatii was first described in a poster at the 60th annual Meeting of the Society for Industrial Microbiology in San Francisco, August 1–5, 2010 [53]. The bacterium was isolated from estuary sediment collected from the Coskata-Coatue Wildlife Refuge in Nantucket, MA. C. coskatii produces ethanol as a primary product from CO or CO2 + H2. The organism is closely related to C. autoethanogenum, C. ljungdahlii, and Clostridium ragsdalei. The organism has been patented by Coskata, Inc. for ethanol production from CO-containing gas mixtures [54, 55].
2.9 Clostridium ragsdalei
C. ragsdalei strain P11 produces acetate, ethanol, and butanol when using CO as a substrate. Under these conditions, ethanol is the major product. However, when grown on fructose, acetate is the dominant fermentation product and no butanol is formed [56]. Optimization of the trace elements nickel, zinc, selenium, and tungsten improved growth and ethanol production of C. ragsdalei [57]. The organism is also able to reduce certain fatty acids to their corresponding alcohols. Formation of propanol, butanol, pentanol, and hexanol from propionate, butyrate, pentanoate, and hexanoate has been reported. In addition to these primary alcohols, acetone could be reduced to isopropanol [58].
2.10 Clostridium carboxidivorans
C. carboxidivorans strain P7 was isolated from sediment of an agricultural settling lagoon at Oklahoma State University under a CO atmosphere [59]. The organism stained Gram-positive and produced acetate, ethanol, butyrate, and butanol from CO. Low partial pressure of CO in the headspace led to increased butanol and also hexanol production [60]. The genome sequence has been determined [16].
2.11 Thermoanaerobacter kivui (Formerly Acetogenium kivui)
T. kivui was isolated from sediment samples from Lake Kivu, Africa. The enrichment culture was incubated at 60°C under an atmosphere of 20% CO2 and 80% H2. Acetate was the sole product. Heterotrophic growth with mannose, glucose, fructose, and pyruvate was also possible. Formate allowed only weak growth [61]. CO as sole energy source did not allow growth, although in combination with H2 doubling times of 2.7 h were observed [33]. Although one report states the importance of Na+ for autotrophic growth of T. kivui [62], genome sequencing and analysis as well as respective experimentation revealed a proton-dependent bioenergetic system. An Rnf membrane complex is not present, but instead an energy-conserving hydrogenase (Ech) [63].
2.12 Alkalibaculum bacchi
A. bacchi was isolated from soil under a CO atmosphere in the presence of 2-bromoethanesulfate (for inhibiting methanogenesis). In addition to gas mixtures such as H2 + CO2 and CO + CO2, a number of heterotrophic substrates (sugars, organic acids, alcohols, trimethylamine) can be used for growth with ethanol and acetate as main products. The bacterium is remarkably alkali-tolerant (up to pH 10.5) [64]. In mixed culture with Clostridium propionicum, a syngas fermentation resulted in production of ethanol and propanol plus minor amounts of butanol [65]. Added carboxylic acids (propanoic acid, butyric acid, hexanoic acid) could be reduced to their corresponding primary alcohols [66].
2.13 Blautia producta (Formerly Peptostreptococcus productus and Ruminococcus productus)
A strain of B. producta (U-1) was isolated from anaerobic sewage digester sludge under an atmosphere of 50% CO. Under optimal conditions, a doubling time of 1.5 h was recorded. Autotrophic growth on CO2 + H2 (significantly slower) and heterotrophic growth on a variety of substrates was also possible. Acetate was the main fermentation product [67]. When grown on sugars, strain U-1 also produced lactate, succinate, and formate [68]. Another B. producta isolate (strain Marburg) was also able to grow on CO, although the type strain is unable to do so [69]. A number of Wood–Ljungdahl pathway enzyme activities could be measured [70]. The active carbon species deriving from CO oxidation is CO2 rather than bicarbonate [71].
2.14 Clostridium difficile
C. difficile is a dangerous pathogen, representing a considerable threat to the North American and European healthcare systems. Infection rates are still increasing and numerous nosocomial outbreaks have been reported [72]. Originally, all bacteria pathogenic against humans were considered heterotrophs [73]. It therefore came as a surprise that C. difficile and phylogenetically closely related isolates were capable of autotrophic growth on CO2 plus H2 [74, 75]. The arrangement of genes encoding the enzymes for the Wood–Ljungdahl pathway in C. difficile is identical to the operon structure in C. ljungdahlii [75]. C. difficile is thus the first known autotrophic human bacterial pathogen, but as a risk group 2 organism it is not considered for biotechnological applications.
3 Wood–Ljungdahl Pathway and Bioenergetics
The reductive acetyl-CoA- or Wood–Ljungdahl (WL) pathway is probably the oldest carbon assimilation pathway on Earth [12, 76, 77]. It is found in acetogens, methanogens, and strictly anaerobic sulfate-reducing bacteria and archaea [77]. It consists of two parts, the methyl and the carbonyl branch (Fig. 1). In the former, one molecule of CO2 (or CO) is bound to the coenzyme tetrahydrofolate (in bacteria) and then reduced in several steps to a methyl group, which is transferred to an iron-sulfur-corrinoid protein (FeSCo-P). FeSCo-P serves as the methyl donor for the key enzyme of the pathway, the nickel-containing bifunctional CO dehydrogenase/acetyl-CoA synthase. In the carbonyl branch, another molecule of CO2 is reduced to CO, which is also bound to acetyl-CoA synthase. There, methyl and carbonyl groups are fused to an acetyl residue and, combined with coenzyme A (CoA), are converted into acetyl-CoA. This compound can be used anabolically for biosyntheses or catabolically for formation of acetate by phosphotransacetylase (Pta) and acetate kinase (Ack), thereby yielding one molecule of ATP by substrate level phosphorylation. Looking at the energetics of the pathway, it becomes clear that one ATP is required for formyl-THF formation although only one ATP is gained from acetate formation. So, the net balance is zero, and additional energy-conserving mechanisms must exist.
For this purpose, different mechanisms are employed by acetogens. Although the biochemical reactions of the WL pathway have been elucidated using M. thermoacetica until the late 1980s, A. woodii was the first acetogen whose energetics were well-understood [26]. The breakthrough was the discovery of flavin-based and ferredoxin-dependent electron bifurcation that can be coupled to proton or Na+ gradient generation and thus to ATP formation [78, 79]. So far, four such flavin-based electron-bifurcating enzyme complexes have been discovered in autotrophic acetogens (Table 1). Four more such reactions are known from Clostridium kluyveri, Clostridium acidurici, and methanogenic archaea [86], which, however, might only be the tip of the iceberg, as several other reactions might be candidates for such a mechanism. In principle, respective enzymes couple the exergonic reduction of a substrate with NADH to the endergonic reduction of ferredoxin with NADH. Similarly, flavin-based electron confurcation has been demonstrated in A. woodii in the case of lactate utilization (Table 1). Endergonic lactate oxidation with NAD+ as oxidant is driven at the expense of simultaneous exergonic electron flow from reduced ferredoxin to NAD+ [85].
Key to energy conservation under autotrophic conditions is the generation of reduced ferredoxin from H2. This reduced ferredoxin can be reoxidized at the membrane-bound Rnf complex, simultaneously reducing NAD+ to NADH. This exergonic reaction is coupled to pumping of either H+ or Na+ across the membrane. A proton gradient is, for example, formed in C. ljungdahlii [29] and then used for ATP generation by an H+-dependent ATPase. On the other hand, the Rnf complex of A. woodii pumps a sodium cation [87] and uses the Na+ gradient by an Na+-dependent ATPase for ATP formation [24].
In M. thermoacetica, cytochromes and quinones are present, indicating the presence of an electron-transport chain across the membrane. Such a chain might also involve iron-sulfur and flavoproteins, which are present in M. thermoacetica as well. A tentative scheme for generation of a proton gradient has been proposed [34].
A third possibility for energy conservation is the presence of an energy-conserving hydrogenase (Ech) catalyzing the reversible oxidation of reduced ferredoxin with protons to hydrogen, thereby generating a proton or Na+ gradient. Such membrane-associated enzymes have been found in several H2-consuming, as well as H2-producing, archaea and bacteria [79], for example, in M. thermoacetica. As this organism also forms cytochromes and quinone, the physiological role of the two systems still requires elucidation. Are both contributing to the generation of an ion gradient or only one and, in that case, which one? A recent hypothesis proposed to separate acetogens based on the presence of either rnf or ech genes [26]. Although in M. thermoacetica this proposal cannot yet be clearly verified (cytochrome plus quinone and also ech genes), in T. kivui (a thermophilic autotrophic acetogen) only ech genes could be detected [63]. Further genome comparisons revealed that indeed the presence of rnf and ech genes in autotrophic acetogens seem to be mutually exclusive [22].
Finally, the reduction step from methylene-tetrahydrofolate (THF) to methyl-THF is highly exergonic and irreversible under physiological conditions (DG0′ = −22 kJ/mol) [88]. This methylene-THF reductase could thus be another site, which is coupled to electron transport or electron bifurcation, as speculated for M. thermoacetica [34] and C. ljungdahlii [14]. Indeed, electron bifurcation with endergonic reduction of a still unknown receptor has been shown in M. thermoacetica [89], whereas in A. woodii an additional energy conservation during this reaction has been excluded [26].
In general, autotrophic acetogens do not possess many possibilities for ATP generation and are operating at the thermodynamic limit of life. This is an important aspect for metabolic engineering of novel pathways in these organisms. Syntheses, demanding a high amount of ATP, are unlikely to function well in this group of bacteria.
4 Genetic Methods and Metabolic Engineering
The full potential of acetogens as a chassis for the production of a wide range of chemicals and biofuels may be realized through the implementation of metabolic engineering strategies. These are reliant on the availability of effective gene tools and gene transfer procedures. The first anaerobic acetogen to undergo genetic modification studies successfully was C. difficile [90], although at the time its classification as an acetogen was not known, and mutation studies were primarily concerned with elucidation of virulence pathways and mechanisms. Plasmid transfer by conjugative methods from Escherichia coli donors was demonstrated using an indigenous Gram-positive replicon (pCD6) from C. difficile [90]. Subsequently, a range of directed mutagenesis methods were developed and implemented in C. difficile, including the ClosTron [91, 92], which utilizes a directed mobile group II intron from the ltrB gene of Lactococcus lactis, generating targeted insertional mutants, selectable by acquisition of antibiotic resistance. The nature of the mechanism of insertion of the intron means that such insertional mutants are completely stable, and host antibiotic resistance can only occur through chromosomal insertion of the group II intron. ClosTron technology allows for the rapid generation of insertional gene knockouts and has been found to be widely applicable within the genus Clostridium, including the homoacetogens for which a DNA transfer method, electroporation, or conjugative plasmid transfer from E. coli donors, has been established. Implementation of the ClosTron has been demonstrated in both C. ljungdahlii [unpublished data] and C. autoethanogenum [89].
The first anaerobic acetogen for which a genetic modification system was specifically designed with a view to creating a microbial platform for chemical synthesis was C. ljungdahlii. A relatively low frequency electroporation transformation procedure was described using the Clostridium–E. coli shuttle vector pIMP1, and heterologous expression of the Clostridium acetobutylicum butanol synthesis pathway genes thlA, hbd, crt, bcd, adhE, and bdhA (encoding thiolase, 3-hydroxybutyryl-CoA dehydrogenase, crotonase, butyryl-CoA dehydrogenase, bifunctional butanol/butyraldehyde dehydrogenase, and butanol dehydrogenase, respectively) was demonstrated [14]. This recombinant strain was shown to be capable of producing butanol, albeit at a low (2 mM) concentration in the exponential growth phase in batch culture. The low concentrations of butanol observed were shown to be caused by the organism’s ability to metabolize 1-butanol to butyrate. Nevertheless, this proof of principle experiment represented an important step towards the establishment of the first acetogenic microbial platform, with a system capable of heterologous expression of metabolic pathway genes, although knockouts of native genes had not yet been demonstrated.
Subsequently, the transformation procedure was optimized, and frequency was improved to a level where homologous recombination methods for directed chromosomal knockouts using suicide plasmids became possible [30]. A chromosomal ‘clean’ deletion of the gene fliA, encoding a putative sigma factor involved in flagella biosynthesis, demonstrated the growing potential for C. ljungdahlii as a model platform organism for gas fermentation based systems. More recently, it was shown that heterologous genes could be introduced in a stable manner into the chromosome from a plasmid through isolation of double crossover mutants using homologous recombination cassettes, delivered by suicide vectors, in this instance encoding enzymes required for butyrate production as proof of principle [93]. After a number of metabolic pathway modifications, this recombinant strain developed was shown to redirect carbon and electron flow significantly towards the production of butyrate. Carbon and electron yields in butyrate were approximately 50% with H2 as the electron donor, and 70% with CO [93]. This development represents a crucial component of the genetics toolkit, as it allows the generation of production strains, with metabolic pathways modified through expression of synthetic and heterologous genes, which require no antibiotic selective pressure for maintenance.
A useful recent addition to the tools available for manipulation of genetic pathways was the implementation of an inducible promoter system originally shown to be effective in C. perfringens [94], and later in the solventogenic bacterium C. acetobutylicum [95], based around the native lactose operon present in Clostridium perfringens. Inducible promoters have a broad range of applications, including gene complementation studies, adjustable modulation of protein expression, and transposon mutagenesis studies. This system consists of the constitutively expressed transcriptional activator bgaR, encoding a protein that binds to and activates the bgaL (β-galactosidase) promoter when in the presence of lactose. Adaptation of this system from C. acetobutylicum involved the exchange of a Gram-positive replicon on the plasmid for one that had previously been shown to function well in C. ljungdahlii, and the system was exemplified through inducible upregulation of the native adhE1 gene (encoding a bifunctional ethanol/acetaldehyde dehydrogenase), such that expression was shown to be 30 times higher than in the wild type organism [96].
The combined ability to modify native pathways through directed clean mutagenesis, and introduce stable heterologous genes into the chromosome, alongside the development of an inducible promoter system, further reinforces the importance of C. ljungdahlii as a forerunner model platform acetogen for the production of high value chemicals from synthesis gas.
A. woodii was the first autotrophic acetogen to be investigated in detail, and as such the native organism is well-characterized, with its energy conservation pathways amongst the best understood of all acetogens [26, 97, 98]. Plasmid transfer has been demonstrated into A. woodii via both conjugative transfer from an E. coli host strain and by electroporation [99]. The electroporation procedure was improved through adaptation of the refined protocol for C. ljungdahlii [14], and plasmid-based heterologous expression of selected theoretical bottlenecks in the Wood–Ljungdahl pathway was employed to increase metabolic flow and thus yields of acetate produced by the first engineered strain of A. woodii [100]. In a pH-controlled batch process, acetate concentrations in the recombinant strains reached a maximum of 51 g/L after 3.7 days, compared to the reference strain whose acetate concentrations reached 44.7 g/L under equivalent conditions [100]. Further genetic tools are currently in development for this organism.
A comprehensive range of tools for the moderately thermophilic acetogen M. thermoacetica has also recently been implemented, making use of a uracil auxotrophic mutant strain as a screening tool for successful double crossover homologous recombination events, and consequent insertion of heterologous genes into the host chromosome [101]. The system was developed through elucidation of a successful electroporation strategy which allowed integration of a methylated vector containing a knockout cassette targeting the gene pyrF, part of the uracil biosynthesis pathway. A double crossover deletion mutant was obtained through serial screening of isolated single colonies, and its uracil auxotroph phenotype confirmed. This strain can become the base strain for chromosomal insertion of synthetic and heterologous genes using plasmids which couple the repair of pyrF and alleviation of uracil auxotrophy to the insertion of foreign DNA. A lactate dehydrogenase gene from Thermoanaerobacter pseudethanolicus was inserted into the chromosome of M. thermoacetica under control of a native promoter as a proof of concept experiment, and synthesis of lactate was observed in the organism for the first time. When grown on basal media supplemented with fructose, lactate concentrations of 6.8 mM were observed in batch culture, whereas the wild type organism was unable to produce a detectable concentration.
Subsequently, a novel strain of M. thermoacetica (Y72) was isolated [102], and its frequency of transformation was shown to be approximately 20 times that of the ATCC 39073 strain, hypothesized to be because of the reduced number of copies of the native restriction–modification system. More recently, a novel thermostable kanamycin resistance marker (kan R), derived from a plasmid harbored by Streptococcus faecalis, was shown to be functional within M. thermoacetica [103], further expanding the rapidly growing genetic toolkit available for those wishing to develop a thermophilic acetogenic platform.
A further method likely to figure prominently in acetogens in the coming years is a method, now termed Allele-Coupled Exchange (ACE), which allows the rapid insertion of heterologous DNA of any size or complexity into the genome [104]. The system is designed so that, following integration of the plasmid by single-crossover recombination, the desired second recombination event leads to a plasmid-borne allele becoming ‘coupled’ to a genome-located allele, and the creation of a new selectable allele that facilitates the isolation of double-crossover cells. The order of recombination events is dictated by the use of highly asymmetric homology arms. A long homology arm (e.g., 1,200 base pairs) directs the first recombination event (plasmid integration) and a much shorter homology arm (e.g., 300 base pairs) directs the second recombination event (plasmid excision). A number of different genetic loci may be used to insert heterologous DNA via ACE. One of the most useful exemplifications of the method exploits the native pyrE gene. During the procedure this gene is inactivated by replacement of the wild-type allele with a mutant allele lacking approximately 300 base pairs from the 3′ end of pyrE. The pyrE gene encodes orotate phosphoribosyltransferase, which, in common with PyrF, is an enzyme involved in pyrimidine biosynthesis. One of its most useful features is that it can be used as both a positive and a negative selection marker. This is because the presence of a functional allele is essential in the absence of exogenous uracil, whereas the presence of a non-functional allele renders cells sensitive to 5-fluoroorotate (FOA). Accordingly, a heterologous pyrE gene can be used as a counter-selection marker in a pyrE minus background in an equivalent manner to pyrF [105]. Its use as a counter-selection marker was demonstrated in two different strains of C. difficile using a heterologous pyrE allele from Clostridium sporogenes [106]. Crucially, however, the design of the created uracil auxotroph strain is such that its mutant pyrE allele can be rapidly restored (2 days in the case of C. difficile) to wild-type using an appropriate ACE correction vector. This allows any specific in-frame deletion mutant made to be characterized in a clean, wild-type background. Furthermore, this facility provides the parallel opportunity to complement the mutant at an appropriate gene dosage through insertion of a wild-type copy of the inactivated gene, under the control of either its native promoter or the strong Pfdx promoter (derived from the ferredoxin gene of C. sporogenes), concomitant with restoration of the pyrE allele back to wild-type [106]. The suite of ACE vectors needed for the manipulation of the genomes of C. ljungdahlii and C. autoethanogenum have now been assembled and exemplified in both acetogens [unpublished data].
5 Fermentation
5.1 Fermentation Overview and Routes
Gas fermentations are fundamentally different from sugar fermentations in that the gaseous substrate has to be supplied continuously at high rates, and cannot be added to the media before the start of a fermentation run. As such, gas fermentations are most suitable as fed-batch or continuous process, whereas sugar fermentations are typically operated as batch or fed-batch processes. Continuous sugar fermentations are typically hampered by contamination problems, with other microorganisms thriving on the sugar substrate. Given that only a few organisms can effectively grow on one-carbon substrates and CO is toxic or at least inhibitory to most microorganisms, the threat of microbial contamination does not pose as great a limitation for gas fermentations. The product spectrum of gas fermentations is dictated by some degree by which substrate combination is used.
5.1.1 CO, CO + H2, and CO/CO2 + H2
Most gas fermentation work to date has been carried out on CO-containing gas streams. The reduced substrate CO acts as both carbon and energy source, thus providing sufficient energy to synthesize even reduced products such as ethanol, butanol, 2,3-butanediol, or isopropanol.
5.1.2 CO2 + H2
In contrast to CO, CO2 can only act as carbon source but not as energy source, and H2 is required for fixation of CO2. Most reports on fermentations with CO2 and H2 describe acetic acid as sole fermentation product, but production of ethanol [89] or other products such as acetone [9] has also been described.
5.1.3 Microbial Electrosynthesis (MES)
CO2 fixation has also been demonstrated in the absence of hydrogen when an electric current is supplied. In this so-called microbial electrosynthesis (MES) concept, the bacteria grow on a cathode. This has been shown for several acetogenic species including C. ljungdahlii, C. aceticum, M. thermoacetica, and two Sporomusa species with a high efficiency of over 80% [107]. Acetobacterium woodii, which is sodium- rather than proton–dependent, was unable to consume current. There are several excellent reviews that cover all aspects of microbial electrosynthesis in detail [108,109,110,111].
5.1.4 Acetogenic Mixotrophy
As a route to very energy intense products (e.g., isoprene) and still having maximized carbon utilization, a concept called acetogenic (anaerobic, non-photosynthetic; ANP) mixotrophy has been proposed where gases and carbohydrates are consumed at the same time [112].
5.1.5 Carboxylic Acid Conversion
Acetogens such as C. autoethanogenum, C. ljungdahlii, and C. ragsdalei have been demonstrated to convert effectively a range of carboxylic acids as acetic acid, propionic acid, butyric acid, valeric acid, and caproic acid into their respective alcohols in the presence of CO [113,114,115]. This may be integrated with a carboxylate fermentation platform [116].
5.2 Fermentation Control Parameters and Optimization
Parameters that can be used to monitor gas fermentations differ from those for aerobic fermentations. Although, in aerobic fermentations, dissolved oxygen (dO2) is a key parameter to monitor and control the process, this cannot be used in gas fermentations because of the lack of readily available technologies for the measurement of dissolved CO and routine indirect assays are arduous. Instead, one needs to rely on indirect gas measurements to monitor the fermentation in addition to biomass and metabolite as well as oxidation reduction potential (ORP) measurements to track the progress of a fermentation run.
Inlet and outlet gas measurements can give a direct indication of the fermentation status and show whether the microbes are readily utilizing the feed gas. As an example, in a fermentation of CO-rich gas to ethanol and acetate, the CO2/CO ratio can give an indication of the metabolic outcome of the supplied gas:
Gas supply to the culture can be altered by changing the parameters that control gas to liquid mass transfer, including gas feed rate, liquid agitation rate, or pressure. In addition, typical control parameters such as temperature and pH must be controlled to maintain the state of the fermentation and the metabolite profile.
5.2.1 pH
pH is one of the key parameters that needs to be controlled during a fermentation. Acetogens, as do other organisms, have a pH range in which growth is optimal and the cells are metabolically active. Given the phylogenetically diverse nature of acetogens [117], there are both acetogens that have a low pH optimum and those that prefer a higher pH range (Fig. 2).
Reported pH optimum for acetogens considered for industrial applications. C. autoethanogenum [50], C. ljungdahlii [27], C. ragsdalei (P11) [118], C. carboxidivorans (P7) [59], B. methylotrophicum [38], A. bacchi [64], E. limosum [119], A. woodii [5], M. thermoacetica [120], T. kivui [61], and C. aceticum [6]
Typically, solventogenic acetogens have a lower pH optimum than those that only produce acetic acid (homoacetogens), although this is not always true (see, e.g., A. bacchi). At lower pH, acetic acid is more toxic for the cells as more undissociated acid is present which can pass through the membrane and enter into the cell, where it can dissociate again and disrupt the proton gradient across the membrane. The maintenance of this proton gradient is required for energy conservation and several transport mechanisms.
As such, lowering the pH in the medium can lead to a shift from acidogenesis to solventogenesis, allowing increased production of ethanol and other highly reduced products [121, 122]. This was investigated by Gaddy and Clausen using C. ljungdahlii growing in a two-stage Continuous Stirred-Tank Reactor (CSTR) system, where the pH of the first reactor was set to pH 5 to promote cell growth and that of the second reactor to pH 4–4.5 to promote ethanol production [123]. A similar strategy was recently also investigated for C. autoethanogenum [124] and it has been demonstrated that a set-up with a smaller first stage and a larger second stage could also be a feasible option [125]. In the case of C. autoethanogenum, a pH around 4.75 was found optimal for ethanol formation [126, 127], whereas for C. ragsdalei a pH below pH 6 was not associated with high ethanol production [128].
Routine and continuous online monitoring the pH trend during the fermentation gives an instant understanding of the state of the fermentation process, as it is an indication of the metabolites the bacteria are producing. For example, a drop in the pH would indicate acetate production. Maintaining a relatively constant pH is important and adjustment of the fermentation pH is therefore critical to avoid a crash.
The pH range of the organism should also be considered when introducing and optimizing fermentation conditions for heterologous enzymes to match the pH optimum best.
5.2.2 Temperature
Temperature is also an important parameter as it influences the microbial activity as well as the gas solubility, which increases with decreasing temperature [128]. Most acetogens are mesophilic that grow best between 30 and 40°C, but there are also thermophilic acetogens such as M. thermoacetica [120] or T. kivui [61] that grow best between 55 and 75°C.
Lowering the temperature may also help to increase tolerance towards solvents. For C. ragsdalei (P11), ethanol production was higher at a temperature of 32°C than at its optimum growth temperature of 37°C [128].
5.2.3 Gas Supply
Both CO and H2 are not very soluble gases. Although different reactor designs are being developed to address the mass transfer issue (see Sect. 6), most fermentation development work is carried out either in bottles or in CSTRs. The gas supply in such systems can be increased via the gas feed rate or loading, agitation or shaking, or by pressure.
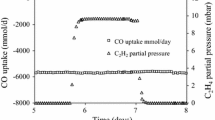
The partial pressure has a big influence on the microbial growth and metabolism [129]. In a study with C. carboxidivorans (P7), an increase in partial pressure of CO (PCO) from 0.35 to 2.0 atm led to a maximum cell concentration, an increase in ethanol production, and a decrease in acetate production [130]. In organisms that are more sensitive to CO, such as Blautia producta [131] and E. limosum [45], a prolonged doubling time has been observed when the partial pressure of CO was increased. Up to a pressure of 1.6 atm, a linear relationship between the reaction rate and CO partial pressure was observed, but at a pressure of 2.5 atm the culture failed to utilize CO after an initial period of CO uptake [132]. It was hypothesized that this might be because of CO toxicity, caused by insufficient cell concentrations resulting from a failure to keep the reaction at a mass transfer limit stage. Therefore, high CO pressure could be applied once a sufficient cell concentration is achieved. By gradually increasing the pressure applied to a culture, these researchers were able to achieve a CO partial pressure of 10 atm [132].
A model for hydrogen partial pressure (pH2) for syngas fermentation has been established for C. ragsdalei (P11) [133]. In A. woodii, the effect of hydrogen partial pressure on CO2/H2 fermentation was investigated. It was shown that acetate productivities increased linearly with pH2 between 400 and 1,700 mbar with a maximal acetate productivity of 1.6 gacetate/gcdw/day and a final acetate concentration of 44 g/L after 11 days [134]. In a follow up study, rates up to 147.8 g/L/day has been demonstrated in continuous fermentations at a dilution rate of 0.35/h [135].
5.2.4 Media Formulation
During gas fermentation, acetogens consume CO and CO2+ H2 as carbon and energy sources. Beside carbon, all bacteria need other elements such as nitrogen, sulfur, phosphorus, trace minerals and metals, vitamins, and reducing agents for synthesis of cell materials and products. Several media optimization studies have been carried out for acetogens including C. autoethanogenum [126, 136,137,138], C. ljungdahlii [122], C. ragsdalei (P11) [57, 128, 139,140,141,142,143,144], C. aceticum [145], and Moorella thermoacetica [146, 147] with the aim of increasing growth and product formation and establishing a defined or least-cost media.
A study by Phillips and Gaddy on C. ljungdahlii showed that, by reducing the B vitamin concentration and by eliminating yeast extract, a maximum concentration of 48 and 23 g/L of ethanol could be achieved in a CSTR with and without cell recycling, respectively [122].
Beside vitamins, trace metal concentrations were found to have a significant influence on growth and product formation as many of the enzymes involved in the Wood–Ljungdahl pathway and ethanol formation require metal co-factors including rare metals such as selenium and tungsten [148, 149]. Nickel, for example, is an important co-factor for enzymes such as CO dehydrogenase and acetyl-CoA synthase [150]. The use of nickel has been shown to improve CO uptake and ethanol production in a variety of acetogens [137]. The effect of various trace metal ions on growth and ethanol production in C. ragsdalei was investigated and it has been observed that the removal of Cu2+ from the medium and increasing concentrations of Ni2+, Zn2+, SeO4 2−, and WO4 2− had a positive effect on ethanol production [57].
As a low redox potential is required for strict anaerobes to grow, reducing agents such as titanium(III) citrate, cysteine, sodium sulfide, and sodium thioglycolate are commonly added to the fermentation medium and are shown to bring about an increase in solvent formation [127, 140, 151,152,153]. A study on C. ragsdalei showed that addition of methyl viologen promoted solventogenesis, where 1.3 g/L of ethanol was produced compared to 0.51 g/L without the addition of any reducing agent [140].
5.2.5 Inoculum
Inoculum preparation is important to ensure a quick start up and achieve maximum production rates as fast as possible and without a lag phase. For C. ljungdahlii, it was shown that pre-adaptation is important and the presence of gas in pre-adapted cultures led to better ethanol overall production [154] and in C. ragsdalei (P11) a positive effect on ethanol production was observed from heat shocking the cells prior to inoculation [155].
6 Mass Transfer and Reactor Optimization
Mass transfer is a major challenge in gas fermentation. Transferring the gaseous substrate to the reaction site in the cell is complex and involves a series of resistances at a micro scale: the resistance encountered when the gaseous substrate passes through the gas-liquid interface, during dispersion through the fermentation media, during the diffusion of the gaseous substrate through the microbial membrane, and the intracellular resistance through to the reaction site. The major mass transfer resistance for sparingly soluble gases such as CO, CO2, and H2 is encountered when diffusing through the gas–liquid interface [129]. This resistance can be overcome either by increasing the surface to volume ratio of gas bubbles or by reducing the resistance at the gas–liquid interface by minimizing the surface tension [131].
Surface tension can be reduced by addition of chemicals such as detergents, surfactants, solvents, or polymers [131, 156], and several studies have demonstrated that mass transfer can be enhanced by addition of functionalized nanoparticles or catalysts that can absorb and then release the CO to the fermentation broth [157, 158].
In addition, a variety of bioreactor configurations have been investigated to address these challenges. Techniques used in different reactor configurations to enhance mass transfer include optimizing pressure, fluid flow rates and patterns, the use of microbubbles, and the use of various impeller designs to facilitate shearing and break-up of gas bubbles. The main types of reactors currently being considered for gas fermentation include Continuous Stirred Tank Reactors (CSTR), Trickle Bed Reactors (TBR), Bubble Column Reactors (BCR), Membrane Bioreactors (MBR), and Moving Bed Biofilm Reactors (MMSB), which are discussed in detail below.
One main objective of bioreactor optimization is to enhance mass transfer rates, at the same time lowering operational costs to allow the process to be scaled up to commercially viable production levels. Therefore, the performance of a reactor design should be measured based on the volumetric mass transfer coefficient per unit power input (kLa/Pg). Several studies have modeled and compared performance of these reactor types for gas fermentation [132, 159,160,161] and a few studies have also explored use of a combination of different reactor types [125].
Further improvements can come from cell recycling to increase the number of cells in a reactor and gas recycling to utilize gas most efficiently. Cell recycling has been shown to be effective for increasing ethanol concentrations in gas fermentations [122], but also adds cost to the process, although gas recycling can increase the gas retention time and utilization efficiency [125].
6.1 Continuous Stirred-Tank Reactors (CSTR)
The CSTR uses a rotating impeller to break up gas bubbles, thus reducing the volume of individual bubbles and increasing the overall surface area of bubbles (the gas–liquid interfacial area). CSTRs are the most extensively used reactor type in gas fermentation. Although many studies have reported higher cell concentrations and product yield with increase in impeller speed, the high input of energy per unit volume in these reactors makes them economically challenging for large scale production processes.
6.2 Trickle Bed Reactors (TBR)
Trickle bed reactors are columns packed with inert packing material and fed with gas streams and media in either concurrent or counter flow configurations. Gas flow rate, liquid recirculation rate, and the packing material size are the main factors that affect mass transfer rates in TBRs.
6.3 Bubble Column Reactors (BCR)
BCRs employ gas sparging without mechanical agitation to achieve mass transfer. Because of the comparatively low capital and running costs associated with the operation of a BCR, these reactors are considered to be promising candidates for commercial scale operation of gas fermentation reactions. However, the conversion efficiency of the gas substrate is low in BCRs because of the short gas retention times.
6.4 Membrane Bioreactors (MBR) and Moving Bed Biofilm Reactors (MMSB)
MBRs are a class of reactors that employs membranes to facilitate the formation of a biofilm. A subclass of MBRs known as Modular Membrane Supported Bioreactors (MMSB) consists of multiple modules of hollow fibers (also known as Hollow Fiber membrane Reactors—HFR) made up of microporous or non-porous membranes. The substrate gases are introduced into the hollow compartments of the fibers and the microbial cells are attached to the outer surface of the membrane. These fibers are then immersed in growth media and contained within an outer shell. Because of their large surface area to volume ratio, MBRs have very efficient mass transfer rates, but a major disadvantage in this type of reactors is a phenomenon called pore wetting. This occurs when the media in contact with the outer surface of the hollow fibers enter into the lumen through the membrane because of a pressure drop within the fibers. This may be overcome by incorporating a liquid-impermeable layer, such as silicone coating, onto the membranes, stopping the liquid media from entering the fibers even when the inside pressure drops. Another disadvantage is that the cells first need to be immobilized.
7 Scale-Up and Commercialization
Most of the studies reported in the scientific literature were carried out on bench-top/lab-scale bioreactors which were less than 10 L in volume, with exception of a study with C. ragsdalei (P11) in a 100-L pilot scale fermenter fed by a gasifier at the Oklahoma State University [162]. In addition, three companies—INEOS Bio, Coskata, and LanzaTech—are operating gas fermentations at a larger scale and are working on commercialization of this new technology.
7.1 Process Integration
Several things need to be considered when scaling up a gas fermentation process. From integration with gas sources, through efficient reactor design (as discussed in Sect. 6), to integration with downstream processes as distillation or other separation technologies and the use of process water and bulk chemicals as well as water recycling.
A wide range of readily available gas sources can be considered as feedstock for gas fermentation, such as industrial waste gases such as off-gases from steel mills (>1.4 billion metric tonnes/year) or ferroalloys that are mainly composed of CO, reformed methane (biogas or natural gas; >180 Tera m3/year that is mainly composed of CO and H2), or syngas (composed of varying concentrations of CO, H2, and CO2) from biomass (>1.3 billion metric tonnes/year in the US only) or municipal solid waste MSW (>2 billion metric tonnes/year). These often contain trace amount of impurities such as different sulfur species (H2S, SO2, SOx, COS), nitrogen species (NH3, NOx), BTEX species (benzene, toluene, ethylbenzene, xylenes), methane, HCl, HCN, acetylene, naphthalene, phenol, light hydrocarbons, metal species (arsenic, vanadium, bromide, copper, iodide, chromium), and tar [163, 164]. Although acetogenic bacteria are generally much more tolerant to such impurities in the gases than chemical catalysts and can even utilize some of these impurities, such as certain sulfur, nitrogen, and metal species [165,166,167], it is important to track these and monitor the productivity of the fermentation process in response to contaminants in the gas streams. If certain impurities in the feed gas are present in too high concentrations, they have been shown to cause reduced cell growth, lower production rates, and even cell dormancy [168, 169].
Impurities such as NOx and acetylene are known to be potent irreversible inhibitors of hydrogenase enzyme activity [170, 171]. Any inhibition of the hydrogenase activity thus results in cells obtaining electrons from CO rather than H2, leading to reduced availability of CO as a carbon source for ethanol formation. CO itself is also known to be a competitive inhibitor of hydrogenase and it has been shown that in B. methylotrophicum the utilization of H2 is inhibited until CO is exhausted [43]. CO inhibition has also been investigated for the Hyt hydrogenase of C. autoethanogenum; the Ki for reduction of CO2 to formate was 0.3% CO [172].
Recent studies with C. carboxidivorans have shown the effects of inhibitors can be mitigated by cleaning the syngas using gas scrubbers or cyclones and a filter prior to introduction into the fermenter [169].
7.2 Commercial Projects
INEOS Bio, Coskata, and LanzaTech have all operated pilot and demonstration plants for extended periods of time and INEOS Bio and LanzaTech are currently scaling up their processes to a commercial scale.
INEOS Bio [173], a subsidiary of major chemical company INEOS (which acquired technology developed by gas fermentation pioneer James L. Gaddy of the University of Arkansas in Fayetteville in 2008), has built an 8 million gallons/year semi-commercial facility in Vero Beach, FL operated as New Plant Energy (NPE) Holding, LLC [174]. Construction of the $130 million project was completed in 2012 and, after commissioning, INEOS Bio declared mid-2013 that the plant was online and producing ethanol [175]. The facility uses MSW and generates 6 MW of electrical power. By the end of 2014 there had been reports and a statement from INEOS about problems with impurities such as HCN that were negatively impacting operations, and the commissioning of new equipment to address this problem [176].
LanzaTech [177], a start-up founded in Auckland, New Zealand in 2005 with its global headquarters in Chicago, IL, successfully operated a 100,000-gallon/year pre-commercial plant at one of Baosteel’s steel mills outside Shanghai, China in 2012. Using steel-making off-gases as substrate for the fermentation process, all productivity expectations were exceeded and all commercial milestones achieved [178]. In 2013, the company operated a second 100,000-gallon/year pre-commercial plant at a Shougang Steel mill near Beijing, China. LanzaTech’s process using steel mill waste gases at this facility has been certified by the Roundtable on Sustainable Biomaterials (RSB) [179]. In April 2015, China Steel Corporation out of Taiwan approved investment in a full LanzaTech commercial project. A 50,000 metric tonnes (17 million gallons)/year facility is planned for construction in Q4 2015, with the intention to scale up to a 100,000 metric tonnes (34 million gallons)/year commercial unit thereafter [180]. In July 2015, the company announced a second commercial project in partnership with ArcelorMittal, the world’s leading steel and mining company, and Primetals Technologies, a leading technology and service provider to the iron and steel industry. The 47,000-MT/year facility is to be built at ArcelorMittal’s flagship steel plant in Ghent, Belgium, is anticipated to commence later in 2017, with bioethanol production expected to start 2018. The intention is to construct further plants across ArcelorMittal’s operations. If scaled up to its full potential in Europe, the technology could enable the production of around 500,000 MT of bioethanol a year [181]. Although the initial product focus is to be industrial ethanol and gasoline additives, plans are for increased product diversity utilizing LanzaTech’s unique microbial capability. One example the company is working on is to produce jet fuel and a first demonstration flight in partnership with Virgin Atlantic and HSBC is being prepared [182]. Together with the world’s largest nylon producer Invista [183] and Korean energy and petrochemical company SK innovation [184], the company is working on new processes for the production of nylon and rubber precursor butadiene [185] and also has an agreement with major chemical company Evonik Industries for development of precursors to speciality plastics [186]. Evonik has recently announced the first successful production of PLEXIGLAS® precursor 2-hydroxyisobutyric acid from syngas [187].
Although Coskata [188], a start-up founded in 2006 in Warrenville, IL, has not yet announced any commercial project, the company has successfully operated a 40,000-gallon ethanol/year semi-commercial facility in Madison, PA over a 2-year period [189] and have recently announced that Elekeiroz, a Brazilian chemical company, has acquired technology rights on their butanol production processes [190].
7.3 Barriers to Market
Much of today’s legislation was written prior to the development of gas fermentation technologies and does not provide a clear framework for fuels produced from bacterial biomass through recycling waste carbon gases, such as those generated in the process of steel making [191]. Below, an overview is provided of some of the most relevant legislative framework.
7.3.1 European Union (EU) Waste Framework Directive 2008/98/EC (WFD)
This legislation is currently being transposed into member state law, and a proposal to revise the directive is pending withdrawal by the EU commission services. The current definition of waste in article 2(a) excludes gaseous effluents emitted into the atmosphere. The narrow scope of this definition does not allow for innovative solutions such as gas fermentation for fuel production from these gas emissions to benefit from advantages of recycling mentioned in the directive. CO/CO2 is valuable waste for CO2 reuse industries and, by including it into the waste definition, solutions such as carbon recycling can benefit from the waste hierarchy where prevention, reuse, and recycling are top priority. CO2 reuse technologies prevent pollution and at the same time reuse and recycling the carbon, so they fulfill key elements from the waste hierarchy.
7.3.2 Industrial Emissions Directive (IED)
The Industrial Emissions Directive (IED) has superseded the Waste Incineration Directive (WID) of 2000. It is intended to achieve a high level of protection for the environment as a whole from the harmful effects of industrial processes by applying the Best Available Techniques (BAT). Gas fermentation technologies should be recognized as such by offering an alternative to incineration of wastes, flaring of gases, or combustion for power generation at a steel mill.
7.3.3 European Union (EU) Carbon Capture and Storage Directive 2009/31/EC
To date, the CCS Directive from 2009 and the renewed strategy focus greatly on CCS, and carbon capture and utilization (CCU) technologies are becoming a reality. Therefore, any future CCS frameworks should also include and help the roll-out of CCU technologies in Europe.
A technology neutral approach is needed to provide a clear legislative framework for gas fermentation technologies in Europe today. Technologies should be qualified by sustainability results, for example by life-cycle assessment (LCA) data and environmental impact on land resources and biodiversity such as a recent report by E4 Tech and Ecofys that compared sustainability implications of different new routes to low carbon fuels [192].
References
Fischer F, Lieske R, Winzer K (1932) Biologische Gasreaktionen. II. Mitteilung: Über die Bildung von Essigsäure bei der biologischen Umsetzung von Kohlenoxyd und Kohlensäure mit Wasserstoff zu Methan. Biochem Z 245:2–12
Wieringa KT (1936) Over het verdwijnen van waterstof en koolzuur onder anaerobe voorwaarden. Ant Leeuwenhoek 3:263–273. doi:10.1007/BF02059556
Wieringa KT (1940) The formation of acetic acid from carbon dioxide and hydrogen by anaerobic spore-forming bacteria. Ant Leeuwenhoek J Microbiol Serol 6:251–262
Wieringa KT (1941) Über die Bildung von Essigsäure aus Kohlensäure und Wasserstoff durch anaerobe Bazillen. Brennst-Chem 14:161–164
Balch WE, Schoberth S, Tanner RS, Wolfe RS (1977) Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int J Syst Bacteriol 27:355–361. doi:10.1099/00207713-27-4-355
Braun M, Mayer F, Gottschalk G (1981) Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol 128:288–293. doi:10.1007/BF00422532
Drake HL, Küsel K, Matthies C (2006) Acetogenic prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, et al. (eds) The prokaryotes, 3rd edn. Springer, New York, pp. 354–420
Drake HL, Gössner AS, Daniel SL (2008) Old acetogens, new light. Ann N Y Acad Sci 1125:100–128. doi:10.1196/annals.1419.016
Schiel-Bengelsdorf B, Dürre P (2012) Pathway engineering and synthetic biology using acetogens. FEBS Lett 586:2191–2198. doi:10.1016/j.febslet.2012.04.043
Bengelsdorf FR, Straub M, Dürre P (2013) Bacterial synthesis gas (syngas) fermentation. Environ Technol 34:1639–1651. doi:10.1080/09593330.2013.827747
Dürre P, Eikmanns BJ (2015) C1-carbon sources for chemical and fuel production by microbial gas fermentation. Curr Opin Biotechnol 35:63–72. doi:10.1016/j.copbio.2015.03.008
Poehlein A, Schmidt S, Kaster A-K, et al (2012) An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7:e33439. doi:10.1371/journal.pone.0033439
Pierce E, Xie G, Barabote RD, et al (2008) The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ Microbiol 10:2550–2573. doi:10.1111/j.1462-2920.2008.01679.x
Köpke M, Held C, Hujer S, et al (2010) Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc Natl Acad Sci U S A 107:13087–13092. doi:10.1073/pnas.1004716107
Bruno-Barcena JM, Chinn MS, Grunden AM (2013) Genome sequence of the autotrophic acetogen Clostridium autoethanogenum JA1-1 strain DSM 10061, a producer of ethanol from carbon monoxide. Genome Announc 1:e00628–e00613. doi:10.1128/genomeA.00628-13
Li N, Yang J, Chai C, et al (2015) Complete genome sequence of Clostridium carboxidivorans P7T, a syngas-fermenting bacterium capable of producing long-chain alcohols. J Biotechnol 211:44–45. doi:10.1016/j.jbiotec.2015.06.430
Poehlein A, Bengelsdorf FR, Schiel-Bengelsdorf B, et al (2015) Complete genome sequence of Rnf- and cytochrome-containing autotrophic acetogen Clostridium aceticum DSM 1496. Genome Announc 3:e00786-15. doi:10.1128/genomeA.00786-15
Roh H, Ko H-J, Kim D, et al (2011) Complete genome sequence of a carbon monoxide-utilizing acetogen, Eubacterium limosum KIST612. J Bacteriol 193:307–308. doi:10.1128/JB.01217-10
Sebaihia M, Wren BW, Mullany P, et al (2006) The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet 38:779–786. doi:10.1038/ng1830
Lux MF, Drake HL (1992) Re-examination of the metabolic potentials of the acetogens Clostridium aceticum and Clostridium formicoaceticum: chemolithoautotrophic and aromatic-dependent growth. FEMS Microbiol Lett 74:49–56
Adamse A (1980) New isolation of Clostridium aceticum (Wieringa). Ant Leeuwenhoek 46:523–531
Poehlein A, Cebulla M, Ilg MM, et al (2015) The complete genome sequence of Clostridium aceticum: a missing link between Rnf- and cytochrome-containing autotrophic acetogens. mBio 6:e01168-15. doi:10.1128/mBio.01186-15
Heise R, Müller V, Gottschalk G (1989) Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium woodii. J Bacteriol 171:5473–5478
Müller V, Aufurth S, Rahlfs S (2001) The Na+ cycle in Acetobacterium woodii: identification and characterization of a Na+ translocating F1F0-ATPase with a mixed oligomer of 8 and 16 kDa proteolipids. Biochim Biophys Acta 1505:108–120
Biegel E, Müller V (2010) Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase. Proc Natl Acad Sci U S A 107:18138–18142. doi:10.1073/pnas.1010318107
Schuchmann K, Müller V (2014) Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12:809–821. doi:10.1038/nrmicro3365
Tanner RS, Miller LM, Yang D (1993) Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int J Syst Bacteriol 43:232–236
Köpke M, Mihalcea C, Liew F, et al (2011) 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl Environ Microbiol 77:5467–5475. doi:10.1128/AEM.00355-11
Tremblay P, Zhang T, Dar SA, et al (2012) The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio 4:e00406-12. doi:10.1128/mBio.00406-12
Leang C, Ueki T, Nevin KP, Lovley DR (2013) A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen. Appl Environ Microbiol 79:1102–1109. doi:10.1128/AEM.02891-12
Köpke M, Gerth ML, Maddock DJ, et al (2014) Reconstruction of an acetogenic 2,3-butanediol pathway involving a novel NADPH-dependent primary-secondary alcohol dehydrogenase. Appl Environ Microbiol 80:3394–3403. doi:10.1128/AEM.00301-14
Fontaine FE, Peterson WH, McCoy E, et al (1942) A new type of glucose fermentation by Clostridium thermoaceticum. J Bacteriol 43:701–715
Daniel SL, Hsu T, Dean SI, Drake HL (1990) Characterization of the H2- and CO-dependent chemolithotrophic potentials of the acetogens Clostridium thermoaceticum and Acetogenium kivui. J Bacteriol 172:4464–4471
Das A, Ljungdahl LG (2003) Electron-transport systems in acetogens. In: Ljungdahl LG, Adams MW, Barton LL, Ferry JG, Johnson MK (eds) Biochemistry and physiology of anaerobic bacteria. Springer, New York, pp. 191–204
Seifritz C, Daniel SL, Gössner A, Drake HL (1993) Nitrate as a preferred electron sink for the acetogen Clostridium thermoaceticum. J Bacteriol 175:8008–8013
Seifritz C, Drake HL, Daniel SL (2003) Nitrite as an energy-conserving electron sink for the acetogenic bacterium Moorella thermoacetica. Curr Microbiol 46:329–333. doi:10.1007/s00284-002-3830-6
Fröstl JM, Seifritz C, Drake HL (1996) Effect of nitrate on the autotrophic metabolism of the acetogens Clostridium thermoautotrophicum and Clostridium thermoaceticum. J Bacteriol 178:4597–4603
Zeikus JG, Lynd LH, Thompson TE, et al (1980) Isolation and characterization of a new, methylotrophic, acidogenic anaerobe, the marburg strain. Curr Microbiol 3:381–386. doi:10.1007/BF02601907
Lynd L, Kerby R, Zeikus JG (1982) Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol 149:255–263
Shen G-J, Shieh J-S, Grethlein AJ, et al (1999) Biochemical basis for carbon monoxide tolerance and butanol production by Butyribacterium methylotrophicum. Appl Microbiol Biotechnol 51:827–832. doi:10.1007/s002530051469
Grethlein AJ, Worden RM, Jain MK, Datta R (1991) Evidence for production of n-butanol from carbon monoxide by Butyribacterium methylotrophicum. J Ferment Bioeng 72:58–60. doi:10.1016/0922-338X(91)90147-9
Worden RM, Grethlein AJ, Jain MK, Datta R (1991) Production of butanol and ethanol from synthesis gas via fermentation. Fuel 70:615–619. doi:10.1016/0016-2361(91)90175-A
Heiskanen H, Virkajärvi I, Viikari L (2007) The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzym Microb Technol 41:362–367. doi:10.1016/j.enzmictec.2007.03.004
Sharak Genthner BR, Davis CL, Bryant MP (1981) Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol 42:12–19
Sharak Genthner BR, Bryant MP (1982) Growth of Eubacterium limosum with carbon monoxide as the energy source. Appl Environ Microbiol 43:70–74
Chang IS, Kim BH, Kim DH, et al (1999) Formulation of defined media for carbon monoxide fermentation by Eubacterium limosum KIST612 and the growth characteristics of the bacterium. J Biosci Bioeng 88:682–685. doi:10.1016/S1389-1723(00)87102-9
Chang IS, Kim D, Kim BH, Lovitt RW (2007) Use of an industrial grade medium and medium enhancing effects on high cell density CO fermentation by Eubacterium limosum KIST612. Biotechnol Lett 29:1183–1187. doi:10.1007/s10529-007-9382-x
Jeong J, Bertsch J, Hess V et al (2015) A model for energy conservation based on genomic and experimental analyses in a carbon monoxide-utilizing, butyrate-forming acetogen, Eubacterium limosum KIST612. Appl Environ Microbiol 81:4782–4790. doi: 10.1128/AEM.00675-15
Song Y, Cho B-K (2015) Draft genome sequence of chemolithoautotrophic acetogenic butanol-producing Eubacterium limosum ATCC 8486. Genome Announc 3:e01564–e01514. doi:10.1128/genomeA.01564-14
Abrini J, Naveau H, Nyns EJ (1994) Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch Microbiol 161:345–351. doi:10.1007/BF00303591
Brown SD, Nagaraju S, Utturkar S, et al (2014) Comparison of single-molecule sequencing and hybrid approaches for finishing the genome of Clostridium autoethanogenum and analysis of CRISPR systems in industrial relevant clostridia. Biotechnol Biofuels 7:40. doi:10.1186/1754-6834-7-40
Utturkar SM, Klingeman DM, Bruno-Barcena JM, et al (2015) Sequence data for Clostridium autoethanogenum using three generations of sequencing technologies. Sci Data 2:150014. doi:10.1038/sdata.2015.14
Zahn JA, Saxena J, Do Y et al (2010) P155: Clostridium coskatii, sp. nov., an anaerobic bacterium that produces ethanol from synthesis gas. 60th annual meeting of the society for industrial microbiology, San Franscisco, CA, August 1st, 2010. https://sim.confex.com/sim/2010/webprogram/Paper16899.html. Accessed 26 Jun 2015
Zahn JA, Saxena J (2011) Novel ethanologenic Clostridium species, Clostridium coskatii. US Patent 20110229947 A1
Saxena J, Zahn JA (2012) A novel ethanologenic Clostridium species, Clostridium coskatii. Patent WO 2011116124 A3
Huhnke RL, Lewis RS, Tanner RS (2010) Isolation and characterization of novel clostridial species. US Patent 7704723 B2
Saxena J, Tanner RS (2011) Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J Ind Microbiol Biotechnol 38:513–521. doi:10.1007/s10295-010-0794-6
Isom CE, Nanny MA, Tanner RS (2015) Improved conversion efficiencies for n-fatty acid reduction to primary alcohols by the solventogenic acetogen “Clostridium ragsdalei”. J Ind Microbiol Biotechnol 42:29–38. doi:10.1007/s10295-014-1543-z
Liou JS-C, Balkwill DL, Drake GR, Tanner RS (2005) Clostridium carboxidivorans sp. nov., a solvent-producing Clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int J Syst Evol Microbiol 55:2085–2091. doi:10.1099/ijs.0.63482-0
Phillips JR, Atiyeh HK, Tanner RS, et al (2015) Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: medium development and culture techniques. Bioresour Technol 190:114–121
Leigh JA, Mayer F, Wolfe RS (1981) Acetogenium kivui, a new thermophilic hydrogen-oxidizing acetogenic bacterium. Arch Microbiol 129:275–280. doi:10.1007/BF00414697
Yang H, Drake HL (1990) Differential effects of sodium on hydrogen- and glucose-dependent growth of the acetogenic bacterium Acetogenium kivui. Appl Environ Microbiol 56:81–86
Hess V, Poehlein A, Weghoff MC, et al (2014) A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genomics 15:1139. doi:10.1186/1471-2164-15-1139
Allen TD, Caldwell ME, Lawson PA, et al (2010) Alkalibaculum bacchi gen. nov., sp. nov., a CO-oxidizing, ethanol-producing acetogen isolated from livestock-impacted soil. Int J Syst Evol Microbiol 60:2483–2489. doi:10.1099/ijs.0.018507-0
Liu K, Atiyeh HK, Stevenson BS, et al (2014) Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Bioresour Technol 152:337–346. doi:10.1016/j.biortech.2013.11.015
Liu K, Atiyeh HK, Stevenson BS, et al (2014) Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour Technol 151:69–77. doi:10.1016/j.biortech.2013.10.059
Lorowitz WH, Bryant MP (1984) Peptostreptococcus productus strain that grows rapidly with CO as the energy source. Appl Environ Microbiol 47:961–964
Misoph M, Drake HL (1996) Effect of CO2 on the fermentation capacities of the acetogen Peptostreptococcus productus U-1. J Bacteriol 178:3140–3145
Geerligs G, Aldrich HC, Harder W, et al (1987) Isolation and characterization of a carbon monoxide utilizing strain of the acetogen Peptostreptococcus productus. Arch Microbiol 148:305–313
Ma K, Wohlfarth G, Diekert G (1991) Acetate formation from CO and CO2 by cell extracts of Peptostreptococcus productus (strain Marburg). Arch Microbiol 156:75–80
Bott M, Thauer RK (1989) The active species of “CO2” formed by carbon monoxide dehydrogenase from Peptostreptococcus productus. Z Naturforsch C 44:392–396
Freeman J, Bauer MP, Baines SD, et al (2010) The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev 23:529–549. doi:10.1128/CMR.00082-09
Maier R, Pepper I, Gerba C (2009) Environmental microbiology. Academic Press, San Diego
Rieu-Lesme F, Dauga C, Fonty G, Dore J (1998) Isolation from the rumen of a new acetogenic bacterium phylogenetically closely related to Clostridium difficile. Anaerobe 4:89–94. doi:10.1006/anae.1998.0153
Köpke M, Straub M, Dürre P (2013) Clostridium difficile is an autotrophic bacterial pathogen. PLoS One 8:e62157. doi:10.1371/journal.pone.0062157
Russell MJ, Martin W (2004) The rocky roots of the acetyl-CoA pathway. Trends Biochem Sci 29:358–363. doi:10.1016/j.tibs.2004.05.007
Fuchs G (2011) Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu Rev Microbiol 65:631–658
Herrmann G, Jayamani E, Mai G, Buckel W (2008) Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol 190:784–791. doi:10.1128/JB.01422-07
Buckel W, Thauer RK (2013) Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim Biophys Acta 1827:94–113. doi:10.1016/j.bbabio.2012.07.002
Schuchmann K, Müller V (2012) A bacterial electron-bifurcating hydrogenase. J Biol Chem 287:31165–31171. doi:10.1074/jbc.M112.395038
Wang S, Huang H, Kahnt J, et al (2013) A reversible electron-bifurcating ferredoxin- and NAD-dependent [FeFe]-hydrogenase (HydABC) in Moorella thermoacetica. J Bacteriol 195:1267–1275. doi:10.1128/JB.02158-12
Huang H, Wang S, Moll J, et al (2012) Electron bifurcation involved in the energy metabolism of the acetogenic bacterium Moorella thermoacetica growing on glucose or H2 plus CO2. J Bacteriol 194:3689–3699. doi:10.1128/JB.00385-12
Bertsch J, Parthasarathy A, Buckel W, et al (2013) An electron-bifurcating caffeyl-CoA reductase. J Biol Chem 288:11304–11311. doi:10.1074/jbc.M112.444919
Wang S, Huang H, Kahnt J, et al (2013) An NADP-specific electron-bifurcating [FeFe]-hydrogenase in a functional complex with formate dehydrogenase in Clostridium autoethanogenum grown on CO. J Bacteriol 195:4373–4386. doi:10.1128/JB.00678-13
Weghoff MC, Bertsch J, Müller V (2015) A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ Microbiol 17:670–677. doi:10.1111/1462-2920.12493
Dürre P (2015) Clostridium. In: Goldman E, Green LH (eds) Practical handbook of microbiology, 3rd edn. CRC Press, Boca Raton, pp. 467–485
Müller V, Imkamp F, Biegel E, et al (2008) Discovery of a ferredoxin:NAD+-oxidoreductase (Rnf) in Acetobacterium woodii: a novel potential coupling site in acetogens. Ann N Y Acad Sci 1125:137–146. doi:10.1196/annals.1419.011
Wohlfarth G, Diekert G (1991) Thermodynamics of methylenetetrahydrofolate reduction to methyltetrahydrofolate and its implications for the energy metabolism of homoacetogenic bacteria. Arch Microbiol 155:378–381. doi:10.1007/BF00243458
Mock J, Zheng Y, Mueller AP, et al (2015) Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation. J Bacteriol 197(18):2965–2980. doi:10.1128/JB.00399-15
Purdy D, O’Keeffe TAT, Elmore M, et al (2002) Conjugative transfer of clostridial shuttle vectors from Escherichia coli to Clostridium difficile through circumvention of the restriction barrier. Mol Microbiol 46:439–452. doi:10.1046/j.1365-2958.2002.03134.x
Heap JT, Pennington OJ, Cartman ST, et al (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464. doi:10.1016/j.mimet.2007.05.021
Heap JT, Kuehne SA, Ehsaan M, et al (2010) The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55. doi:10.1016/j.mimet.2009.10.018
Ueki T, Nevin KP, Woodard TL, et al (2014) Converting carbon dioxide to butyrate with an engineered strain of Clostridium ljungdahlii. mBio 5:e01636–e01614. doi:10.1128/mBio.01636-14
Hartman AH, Liu HL, Melville SB (2011) Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens. Appl Environ Microbiol 77:471–478. doi:10.1128/Aem.01536-10
Al-Hinai MA, Fast AG, Papoutsakis ET (2012) Novel system for efficient isolation of Clostridium double-crossover allelic exchange mutants enabling markerless chromosomal gene deletions and DNA integration. Appl Environ Microbiol 78:8112–8121. doi:10.1128/Aem.02214-12
Banerjee A, Leang C, Ueki T, et al (2014) Lactose-inducible system for metabolic engineering of Clostridium ljungdahlii. Appl Environ Microbiol 80:2410–2416. doi:10.1128/Aem.03666-13
Dilling S, Imkamp F, Schmidt S, et al (2007) Regulation of caffeate respiration in the acetogenic bacterium Acetobacterium woodii. Appl Environ Microbiol 73:3630–3636. doi:10.1128/Aem.02060-06
Imkamp F, Müller V (2002) Chemiosmotic energy conservation with Na+ as the coupling ion during hydrogen-dependent caffeate reduction by Acetobacterium woodii. J Bacteriol 184:1947–1951. doi:10.1128/Jb.184.7.1947-1951.2002
Strätz M, Sauer U, Kuhn A, et al (1994) Plasmid transfer into the homoacetogen Acetobacterium woodii by electroporation and conjugation. Appl Environ Microbiol 60:1033–1037
Straub M, Demler M, Weuster-Botz D, et al (2014) Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii. J Biotechnol 178:67–72. doi:10.1016/j.jbiotec.2014.03.005
Kita A, Iwasaki Y, Sakai S, et al (2013) Development of genetic transformation and heterologous expression system in carboxydotrophic thermophilic acetogen Moorella thermoacetica. J Biosci Bioeng 115:347–352. doi:10.1016/j.jbiosc.2012.10.013
Tsukahara K, Kita A, Nakashimada Y, et al (2014) Genome-guided analysis of transformation efficiency and carbon dioxide assimilation by Moorella thermoacetica Y72. Gene 535:150–155. doi:10.1016/j.gene.2013.11.045
Iwasaki Y, Kita A, Sakai S, et al (2013) Engineering of a functional thermostable kanamycin resistance marker for use in Moorella thermoacetica ATCC39073. FEMS Microbiol Lett 343:8–12. doi:10.1111/1574-6968.12113
Heap JT, Ehsaan M, Cooksley CM, et al (2012) Integration of DNA into bacterial chromosomes from plasmids without a counter-selection marker. Nucleic Acids Res 40:e59. doi:10.1093/nar/gkr1321
Tripathi SA, Olson DG, Argyros DA, et al (2010) Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl Environ Microbiol 76:6591–6599. doi:10.1128/Aem.01484-10
Ng YK, Ehsaan M, Philip S, et al (2013) Expanding the repertoire of gene tools for precise manipulation of the Clostridium difficile genome: allelic exchange using pyrE alleles. PLoS One 8:e56051. doi:10.1371/journal.pone.0056051
Nevin KP, Hensley SA, Franks AE, et al (2011) Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol 77:2882–2886. doi:10.1128/AEM.02642-10
Rabaey K, Girguis P, Nielsen LK (2011) Metabolic and practical considerations on microbial electrosynthesis. Curr Opin Biotechnol 22:371–377. doi:10.1016/j.copbio.2011.01.010
Lovley DR, Nevin KP (2013) Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr Opin Biotechnol 24:385–390. doi:10.1016/j.copbio.2013.02.012
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31:1796–1807. doi:10.1016/j.biotechadv.2013.10.001
Tremblay P-L, Zhang T (2015) Electrifying microbes for the production of chemicals. Front Microbiol 6:1–10. doi:10.3389/fmicb.2015.00201
Fast AG, Schmidt ED, Jones SW, Tracy BP (2015) Acetogenic mixotrophy: novel options for yield improvement in biofuels and biochemicals production. Curr Opin Biotechnol 33:60–72. doi:10.1016/j.copbio.2014.11.014
Simpson SD, Collet C, Tran PL et al (2009) Microbial alcohol production process. US Patent 8119378 B2
Perez JM, Richter H, Loftus SE, Angenent LT (2013) Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol Bioeng 110:1066–1077. doi:10.1002/bit.24786
Xie B-T, Liu Z-Y, Tian L, et al (2014) Physiological response of Clostridium ljungdahlii DSM 13528 of ethanol production under different fermentation conditions. Bioresour Technol 177:302–307. doi:10.1016/j.biortech.2014.11.101
Richter H, Loftus SE, Angenent LT (2013) Integrating syngas fermentation with the carboxylate platform and yeast fermentation to reduce medium cost and improve biofuel productivity. Environ Technol 34:1983–1994. doi:10.1080/09593330.2013.826255
Müller V, Frerichs J (2013) Acetogenic bacteria. In: Encyclopedia of Life Sciences. doi: 10.1002/9780470015902.a0020086.pub2
Huhnke RL, Lewis RS, Tanner RS (2008) Isolation and characterization of novel clostridial species. Patent WO2008/028055
Sharak Genthner BR, Bryant MP (1987) Additional characteristics of one-carbon-compound utilization by Eubacterium limosum and Acetobacterium woodii. Appl Environ Microbiol 53:471–476
Baronofsky JJ, Schreurs WJ, Kashket ER (1984) Uncoupling by acetic acid limits growth of and acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol 48:1134–1139
Grethlein AJ, Worden RM, Jain MK, Datta R (1990) Continuous production of mixed alcohols and acids from carbon monoxide. Appl Biochem Biotechnol 24/25:875–884. doi:10.1007/BF02920301
Phillips JR, Klasson KT, Claussen EC, Gaddy JL (1993) Biological production of ethanol from coal synthesis gas. Appl Biochem Biotechnol 39(40):559–571
Gaddy JL, Claussen EC (1992) Clostridium ljungdahlii, an anaerobic ethanol and acetate producing microorganism. US Patent 5173429 A
Abubackar HN, Veiga MC, Kennes C (2015) Ethanol and acetic acid production from carbon monoxide in a Clostridium strain in batch and continuous gas-fed bioreactors. Int J Environ Res Public Health 12:1029–1043. doi:10.3390/ijerph120101029
Richter H, Martin ME, Angenent LT (2013) A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 6:3987–4000. doi:10.3390/en6083987
Guo Y, Xu J, Zhang Y, et al (2010) Medium optimization for ethanol production with Clostridium autoethanogenum with carbon monoxide as sole carbon source. Bioresour Technol 101:8784–8789. doi:10.1016/j.biortech.2010.06.072
Abubackar HN, Veiga MC, Kennes C (2012) Biological conversion of carbon monoxide to ethanol: effect of pH, gas pressure, reducing agent and yeast extract. Bioresour Technol 114:518–522. doi:10.1016/j.biortech.2012.03.027
Kundiyana DK, Wilkins MR, Maddipati P, Huhnke RL (2011) Effect of temperature, pH and buffer presence on ethanol production from synthesis gas by “Clostridium ragsdalei”. Bioresour Technol 102:5794–5799. doi:10.1016/j.biortech.2011.02.032
Vega JL, Holmberg VL, Clausen EC, Gaddy JL (1988) Fermentation parameters of Peptostreptococcus productus on gaseous substrates (CO, H2/CO2). Arch Microbiol 151:65–70. doi:10.1007/BF00444671
Hurst KM, Lewis RS (2010) Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem Eng J 48:159–165. doi:10.1016/j.bej.2009.09.004
Gaddy JL, Chen G (1998) Bioconversion of waste biomass to useful products. US Patent US 5821111 A
Klasson KT, Ackerson MD, Clausen EC, Gaddy JL (1991) Bioreactor design for synthesis gas fermentations. Fuel 70:605–614
Skidmore BE, Baker RA, Banjade DR, et al (2013) Syngas fermentation to biofuels: effects of hydrogen partial pressure on hydrogenase efficiency. Biomass Bioenergy 55:156–162. doi:10.1016/j.biombioe.2013.01.034
Demler M, Weuster-Botz D (2011) Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnol Bioeng 108:470–474. doi:10.1002/bit.22935
Kantzow C, Mayer A, Weuster-Botz D (2015) Continuous gas fermentation by Acetobacterium woodii in a submerged membrane reactor with full cell retention. J Biotechnol 212:11–18. doi:10.1016/j.jbiotec.2015.07.020
Cotter JL, Chinn MS, Grunden AM (2009) Influence of process parameters on growth of Clostridium ljungdahlii and Clostridium autoethanogenum on synthesis gas. Enzym Microb Technol 44:281–288. doi:10.1016/j.enzmictec.2008.11.002
Simpson SD, Warner IL, Fung JMY, Köpke M (2010) Optimised fermentation media. Patent WO 2010064932 A1
Abubackar HN, Veiga MC, Kennes C (2015) Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour Technol 186:122–127. doi:10.1016/j.biortech.2015.02.113
Babu BK, Atiyeh HK, Wilkins MR, Huhnke RL (2010) Effect of the reducing agent dithiothreitol on ethanol and acetic acid production by Clostridium strain P11 using simulated biomass-based syngas. Biol Eng Trans 3:19–35. doi:10.13031/2013.35924
Panneerselvam A, Wilkins MR, Delorme MJM, et al (2010) Effects of various reducing agents on syngas fermentation by “Clostridium ragsdalei”. Biol Eng 2:135–144. doi:10.13031/2013.34831
Kundiyana DK, Huhnke RL, Maddipati P, et al (2010) Feasibility of incorporating cotton seed extract in Clostridium strain P11 fermentation medium during synthesis gas fermentation. Bioresour Technol 101:9673–9680. doi:10.1016/j.biortech.2010.07.054
Phillips JR, Hall A, Remondet NM et al (2011) Designing syngas fermentation medium for fuels and bulk chemicals production. Am Soc Agr Biol Eng Meeting Louisville, Kentucky. doi:10.13031/2013.37400
Maddipati P, Atiyeh HK, Bellmer DD, Huhnke RL (2011) Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour Technol 102:6494–6501. doi:10.1016/j.biortech.2011.03.047
Saxena J, Tanner RS (2012) Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J Microbiol Biotechnol 28:1553–1561. doi:10.1007/s11274-011-0959-0
Sim JH, Kamaruddin AH (2008) Optimization of acetic acid production from synthesis gas by chemolithotrophic bacterium--Clostridium aceticum using statistical approach. Bioresour Technol 99:2724–2735. doi:10.1016/j.biortech.2007.07.004
Lundie LL, Drake HL (1984) Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol 159:700–703
Savage MD, Drake HL (1986) Adaptation of the acetogen Clostridium thermoautotrophicum to minimal medium. J Bacteriol 165:315–318
Ragsdale SW (2008) Enzymology of the Wood-Ljungdahl pathway of acetogenesis. Ann N Y Acad Sci 1125:129–136. doi:10.1196/annals.1419.015
Bender G, Pierce E, Hill J, et al (2011) Metal centers in the anaerobic microbial metabolism of CO and CO2. Metallomics 3:797–815. doi:10.1039/c1mt00042j
Ragsdale SW, Kumar M (1996) Nickel-containing carbon monoxide dehydrogenase/acetyl-CoA synthase. Chem Rev 96:2515–2540
Klasson KT, Ackerson MD, Clausen EC, Gaddy JL (1992) Bioconversion of synthesis gas into liquid or gaseous fuels. Enzym Microb Technol 14:602–608
Sim JH, Kamaruddin AH, Long WS (2008) Biocatalytic conversion of CO to acetic acid by Clostridium aceticum—medium optimization using response surface methodology (RSM). Biochem Eng J 40:337–347. doi:10.1016/j.bej.2008.01.006
Atiyeh HK, Hall A, Wilkins MR, Huhnke RL (2009) Effect of the reducing agent dithiothreitol on ethanol and acetic acid production by Clostridium strain P11 using simulated biomass-based syngas. 2009 Bioener Eng Conf, Seattle. BIO-097917. doi: 10.13031/2013.28893
Tirado-Acevedo O, Cotter J, Chinn M (2011) Influence of carbon source pre-adaptation on Clostridium ljungdahlii growth and product formation. J Bioprocess Biotechniq S2:001. doi:10.4172/2155-9821.S2-001
Ramachandriya KD, Delorme MJ, Wilkins MR (2010) Heat shocking of Clostridium ragsdalei to promote sporulation and ethanol production. Biol Eng 2:115–131
Bredwell MD, Telgenhoff MD, Barnard S, Worden RM (1997) Effect of surfactants on carbon monoxide fermentations by Butyribacterium methylotrophicum. Appl Biochem Biotechnol 63/65:637–647. doi:10.1007/BF02920462
Zhu H, Shanks BH, Heindel TJ (2008) Enhancing CO−water mass transfer by functionalized MCM41 nanoparticles. Ind Eng Chem Res 47:7881–7887
Kim YK, Park SE, Lee H, Yun JY (2014) Enhancement of bioethanol production in syngas fermentation with Clostridium ljungdahlii using nanoparticles. Bioresour Technol 159:446–450. doi:10.1016/j.biortech.2014.03.046
Bredwell MD, Srivastava P, Wordon RM (1999) Reactor design issues for synthesis-gas fermentations. Biotechnol Prog 15:834–844. doi:10.1021/bp990108m
Ungerman AJ, Heindel TJ (2007) Carbon monoxide mass transfer for syngas fermentation in a stirred tank reactor with dual impeller configurations. Biotechnol Prog 23:613–620. doi:10.1021/bp060311z
Orgill JJ, Atiyeh HK, Devarapalli M, et al (2013) A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour Technol 133:340–346
Kundiyana DK, Huhnke RL, Wilkins MR (2010) Syngas fermentation in a 100-L pilot scale fermentor: design and process considerations. J Biosci Bioeng 109:492–498. doi:10.1016/j.jbiosc.2009.10.022
Xu D, Tree DR, Lewis RS (2011) The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 35:2690–2696
Griffin DW, Schultz MA, Irving E, Road P (2012) Fuel and chemical products from biomass syngas: a comparison of gas fermentation to thermochemical conversion routes. Environ Prog Sustain Energy 31:219–224
Vega JL, Klasson KT, Claussen EC, Gaddy JL (1990) Sulphur gas tolerance and toxicity of CO-utilizing and methanogenic bacteria. Appl Biochem Biotechnol 24(25):329–340
Smith KD, Klasson KT, Ackerson MD, et al (1991) COS degradation by selected CO-utilizing bacteria. Appl Biochem Biotechnol 28-29:787–796
Grethlein AJ, Soni BK, Worden RM, Jain MK (1992) Influence of hydrogen sulfide on the growth and metabolism of Butyribacterium methylotrophicum and Clostridium acetobutylicum. Appl Biochem Biotechnol 34/35:233–246. doi:10.1007/BF02920548
Datar RP, Shenkman RM, Cateni BG, et al (2004) Fermentation of biomass-generated producer gas to ethanol. Biotechnol Bioeng 86:587–594. doi:10.1002/bit.20071
Ahmed A, Cateni BG, Huhnke RL, Lewis RS (2006) Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 30:665–672. doi:10.1016/j.biombioe.2006.01.007
Krasna AI, Rittenberg D (1954) The inhibition of hydrogenase by nitric oxide. Proc Natl Acad Sci U S A 40:225–227
Smith LA, Hill S, Yates MG (1976) Inhibition by acetylene of conventional hydrogenase in nitrogen-fixing bacteria. Nature 262:209–210
Wang S, Huang H, Moll J, Thauer RK (2010) NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. J Bacteriol 192:5115–5123. doi:10.1128/JB.00612-10
http://www.ineos.com/businesses/ineos-bio/news/ineos-bio-produces-cellulosic-ethanol/?business=INEOS+Bio; http://www.ethanolproducer.com/articles/10096/ineos-declares-commercial-cellulosic-ethanol-online-in-florida
http://www.ineos.com/businesses/ineos-bio/news/ineos-bio-provides-operational-update/?business=INEOS+Bio; http://www.biofuelsdigest.com/bdigest/2014/09/05/on-the-mend-why-ineos-bio-isnt-reporting-much-ethanol-production/
http://www.lanzatech.com/china-steel-corporation-approves-investment-lanzatech-commercial-project/
Köpke M, Havill A (2014) LanzaTech’s route to bio-butadiene. Catal Rev 27:7–12
http://corporate.evonik.com/en/media/search/pages/news-details.aspx?newsid=40331
http://www.coskata.com/company/media.asp?story=8377ADFF-9DFE-4901-876B-B39FD96B213F
http://www.coskata.com/company/media.asp?story=ED7648F9-8575-4643-A705-1950AE866773
Kircher M (2015) Sustainability of biofuels and renewable chemicals production from biomass. Curr Opin Chem Biol 29:26–31. doi:10.1016/j.cbpa.2015.07.010
Acknowledgments
Work in the authors’ laboratories was funded by the ERA-Net IB 5 project CO2CHEM. Work in PD’s laboratory was supported by grants from the BMBF Gas-Fermentation project (FKZ 031A468A), the ERA-IB 3 project REACTIF (FKZ 22029612), the MWK-BW project Nachhaltige und effiziente Biosynthesen (AZ 33-7533-6-195/7/9), and the European Union’s Seventh Framework Programme for research, technological development, and demonstration under grant agreement no 311815 (SYNPOL project). Work in NPM’s laboratory was additionally funded by the BBSRC sLoLa GASCHEM (Grant no. BB/K00283X/1), the BBSRC/EPSRC Synthetic Biology Research Centre (Grant no. BB/L013940/1), and a BBSRC China Partnership Award (Grant no. BB/L01081X/1). LanzaTech thanks the following investors in its technology: Sir Stephen Tindall, Khosla Ventures, Qiming Venture Partners, Softbank China, the Malaysian Life Sciences Capital Fund, Mitsui, Primetals, CICC Growth Capital Fund I, L.P., and the New Zealand Superannuation Fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing
About this chapter
Cite this chapter
De Tissera, S., Köpke, M., Simpson, S.D., Humphreys, C., Minton, N.P., Dürre, P. (2017). Syngas Biorefinery and Syngas Utilization. In: Wagemann, K., Tippkötter, N. (eds) Biorefineries. Advances in Biochemical Engineering/Biotechnology, vol 166. Springer, Cham. https://doi.org/10.1007/10_2017_5
Download citation
DOI: https://doi.org/10.1007/10_2017_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-97117-9
Online ISBN: 978-3-319-97119-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)