Abstract
In the 1980s, Shiio and coworkers demonstrated using random mutagenesis that the following three phenotypes were effective for boosting lysine production by Corynebacterium glutamicum: (1) low-activity-level citrate synthase (CSL), (2) phosphoenolpyruvate carboxylase (PEPC) resistant to feedback inhibition by aspartic acid (PEPCR), and (3) pyruvate kinase (PYK) deficiency. Here, we reevaluated these phenotypes and their interrelationship in lysine production using recombinant DNA techniques.
The pyk deletion and PEPCR (D299N in ppc) independently showed marginal effects on lysine production, but both phenotypes synergistically increased lysine yield, demonstrating the importance of PEPC as an anaplerotic enzyme in lysine production. Similar effects were also found for glutamic acid production. CSL (S252C in gltA) further increased lysine yield. Thus, using molecular techniques, the combination of these three phenotypes was reconfirmed to be effective for lysine production. However, a simple CSL mutant showed instabilities in growth and lysine yield.
Surprisingly, the pyk deletion was found to increase biomass production in wild-type C. glutamicum ATCC13032 under biotin-sufficient conditions. The mutant showed a 37% increase in growth (based on OD660) compared with the ATCC13032 strain in a complex medium containing 100 g/L glucose. Metabolome analysis revealed the intracellular accumulation of excess precursor metabolites. Thus, their conversion into biomass was considered to relieve the metabolic distortion in the pyk-deleted mutant. Detailed physiological studies of various pyk-deleted mutants also suggested that malate:quinone oxidoreductase (MQO) is important to control both the intracellular oxaloacetic acid (OAA) level and respiration rate. These findings may facilitate the rational use of C. glutamicum in fermentation industries.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaplerotic pathway
- Citrate synthase
- Corynebacterium glutamicum
- Feedback inhibition
- Glutamic acid
- Lysine
- Malate:quinone oxidoreductase
- Phosphoenolpyruvate carboxylase
- Pyruvate kinase
- Respiration
1 Contribution of Anaplerotic Reactions to Lysine Yield: Phosphoenolpyruvate Carboxylase vs. Pyruvate Carboxylase
1.1 Introduction
The anaplerotic reaction that replenishes oxaloacetic acid (OAA) is important for fermentative production of lysine because OAA, a precursor metabolite for lysine biosynthesis, is continuously withdrawn from the tricarboxylic acid (TCA) cycle during lysine production. In other words, an efficient anaplerotic reaction is required to increase lysine yield. Two anaplerotic reactions operate in Corynebacterium glutamicum: the formation of OAA from phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxylase (PEPC) and that from pyruvic acid by pyruvate carboxylase (PC). The contributions of these two reactions to lysine production have been investigated in detail for over 3 decades. Here, a brief history of these studies is provided.
1.2 Phosphoenolpyruvate Carboxylase Reaction for Lysine Production
In the 1980s, Shiio and coworkers were the first to shed light on the PEPC reaction for lysine production [1]. Using repeated random mutagenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine (NTG) treatment, they demonstrated enhanced lysine yields in Brevibacterium flavum (later reclassified as C. glutamicum) mutants having three distinct phenotypes: (1) low-activity-level citrate synthase (CSL), (2) PEPC that is resistant to feedback inhibition by aspartic acid (PEPCR), and (3) a pyruvate kinase (PYK) defect, in either a feedback-inhibition-resistant aspartokinase (AKR) background or a null-activity homoserine dehydrogenase background, suggesting the contribution of PEPC to catalyzing an anaplerotic reaction in lysine production. A PYK-defective mutant, KL-18, derived from an AKR-type lysine producer, No. 2-190, exhibiting both CSL and PEPCR, is a representative example of the importance of PEPC as an anaplerotic enzyme for lysine production ([2], Table 1). As shown in Fig. 1, PEPCR may contribute to improved lysine production, presumably through a smooth supply of OAA from PEP. A mutation causing PYK deficiency may block the metabolism of PEP to pyruvic acid, thereby directing the conversion of PEP into OAA by PEPCR. On the other hand, CSL is expected to decrease the consumption of OAA, enabling more OAA and pyruvic acid to be used for lysine biosynthesis. However, as these studies were conducted using mutants derived randomly by repeated NTG treatment, the precise contribution of each mutation to lysine production and the interrelationship among these mutations remain to be elucidated.
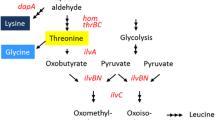
Metabolic pathways focusing on anaplerotic reactions and respiration-related reactions in Corynebacterium glutamicum. PEP phosphoenolpyruvate, PYK pyruvate kinase, PEPC phosphoenolpyruvate carboxylase, PEPCK phosphoenolpyruvate carboxykinase, PC pyruvate carboxylase, AAT aspartate aminotransferase, CS citrate synthase, LdhA lactate dehydrogenase, LldD quinone-dependent lactate dehydrogenase, MQO malate:quinone oxidoreductase, MDH malate dehydrogenase, NDH-II, NADH dehydrogenase-II, e - electron. The dashed line denotes feedback inhibition
1.3 Pyruvate Carboxylase as an Important Anaplerotic Enzyme for Lysine Production
From the early 1990s, the development of genetic engineering enabled more precise metabolic analysis in C. glutamicum. Moreover in 1997, the activity of PC, another anaplerotic enzyme, was detected for the first time in this species using a permeabilized cell preparation [3]. This promoted identification of the anaplerotic reaction that contributes to lysine production in this bacterium. Specifically, for lysine production, PC but not PEPC was found to be important as a major anaplerotic enzyme supplying OAA; knockout of the PEPC gene did not affect lysine production [4], overexpression of the PC gene enhanced it, while inactivation of the PC gene led to a decrease in lysine yield [5]. Moreover, the positive effects of PYK-defective mutation on lysine production as reported by Shiio and coworkers [2, 6] were not observed, and even negative effects were detected in three independent studies in which pyk-gene knockout mutants were generated by genetic engineering [7–9]. As listed in Table 1, introduction of a pyk knockout mutation to various lysine producers having either AKR or homoserine auxotrophy led to decreased lysine productivity, to approximately 50% of those of the corresponding parents. Under these conditions, PC has generally been recognized as an important anaplerotic enzyme for lysine production; the P458S mutation in pyc (enhanced PC activity) [10] has often been employed to construct lysine producers. Until recently, these contradictions concerning the effect of PYK mutation on lysine production were ignored. However, these discrepancies strongly suggested the importance of the coexistence of PYK defect and PEPCR for the enhancement of lysine production, as reported by Shiio and coworkers in their work using random mutagenesis [2, 6].
1.4 Refocusing on Phosphoenolpyruvate Carboxylase for Lysine Production
Although the PEPC reaction had been overlooked regarding lysine production, recently, PEPC was again demonstrated to be important for lysine production by a study in which its feedback inhibition by aspartic acid was deregulated [11]. Incidentally, a recent molecular technique study also demonstrated the effectiveness of decreased CS activities on lysine production in C. glutamicum [12]. Therefore, the interrelationships among mutations conferring the aforementioned phenotypes described by Shiio and coworkers, namely, CSL, PEPCR, and PYK defect, on lysine production need to be reevaluated using modern recombinant DNA techniques.
2 Effects of Pyruvate Kinase Mutation on Glutamic Acid and Lysine Production in the Presence or Absence of Phosphoenolpyruvate Carboxylase-Desensitizing Mutation
2.1 Introduction
As described in the previous section, pyk knockout or deletion [7–9] and PEPCR [11] generated by recombinant DNA techniques were independently identified to have negative and positive effects on lysine production, respectively. However, the combined effect of these mutations on lysine production had not been clarified. In addition, no reports have described the effect of either pyk deletion or PEPCR on glutamic acid production. Thus, we have investigated the effects of pyk deletion on glutamic acid and lysine production in the presence or absence of the PEPCR mutation. In addition, the impact of CSL on lysine production, which Shiio and coworkers [13] suggested to have a promoting effect, was also evaluated using recombinant DNA techniques.
2.2 Effects of pyk Deletion and Phosphoenolpyruvate Carboxylase Desensitization on Glutamic Acid Production
We derived a pyk-deleted mutant (strain D1) from wild-type C. glutamicum ATCC13032 using the double-crossover chromosome replacement technique and investigated its glutamic acid productivity under biotin-limited conditions [14]. When cultured in a 2-L jar fermentor using Medium F4 (complex medium with 100 g/L glucose and 3 μg/L biotin), strain D1 produced 25% more glutamic acid (32.0 g/L) than did ATCC 13032 (25.6 g/L) (Table 2). This was the first demonstration of the primary effects of pyk deletion in a defined C. glutamicum mutant.
An amino acid residue involved in PEPCR in C. glutamicum ATCC13032 was next identified based on the PEPC sequence information of C. glutamicum No. 70 [15]. A single amino acid substitution in PEPC, D299N, was found to relieve the feedback inhibition by aspartic acid. A simple mutant, strain R1, having this D299N substitution in PEPC was constructed from ATCC13032 using the same double-crossover chromosome replacement technique as in the case of strain D1. Strain R1 produced significantly more (21% more) glutamic acid (31.0 g/L) than did ATCC13032 in a jar fermentor culture under biotin-limited conditions in Medium F4 (Table 2), indicating a positive effect of PEPCR on glutamic acid production. This effect was also investigated in combination with pyk deletion [16]. For this purpose, strain DR1, having both a pyk deletion and PEPCR, was constructed using strain D1 as the parent. Glutamic acid production by strain DR1 was elevated up to 36.9 g/L (Table 2), which was 44% higher than that by ATCC13032 and significantly higher than those by D1 and R1. Thus, these two mutations were found to exhibit a synergistic effect on glutamic acid production in C. glutamicum.
2.3 Effects of pyk Deletion and Phosphoenolpyruvate Carboxylase Desensitization on Lysine Production
To clarify the effects of pyk deletion and PEPCR on lysine production, these mutations were introduced into lysine-producing C. glutamicum singly or in combination [17]. The C. glutamicum ATCC13032 mutant having AKR conferred by T311I amino acid substitution [10] was used as the parent strain (strain P). Strain P produced 9.36 g/L lysine from 100 g/L glucose in a jar fermentor culture. Under these conditions, while the simple mutant D2 with pyk deletion or R2 with PEPCR (D299N) showed marginally increased lysine yields (10.1 and 10.8 g/L, respectively; Table 2), the mutant strain DR2 having both mutations showed a synergistic increase in lysine production (38% higher, 12.9 g/L; Table 2).
2.4 Effect of Citrate Synthase Mutation on Lysine Production
The importance of CSL in lysine production was previously suggested by Shiio and coworkers [13]. However, no defined CS-defective mutant had been reported until recently, when C. glutamicum mutants with CSL created by a molecular approach were demonstrated to be effective for boosting lysine production [12]. We also identified a single amino acid substitution (S252C) responsible for CSL in C. glutamicum No. 70 [15]. This mutation was then introduced into the lysine-producing mutant strain DR2 having both pyk deletion and PEPCR mutations. The resulting strain, DRL2, showed a greater increase in lysine yield than did strain DR2 (22% higher, 15.7 g/L; Table 2). Thus, the CSL mutation was confirmed to be effective for lysine production, and these three mutations, pyk deletion, PEPCR, and CSL, were found to enhance lysine yield coordinately in C. glutamicum [17].
2.5 Decreased Phosphoenolpyruvate Carboxykinase Activity Associated with pyk Deletion
In both glutamic acid-producing and lysine-producing strains, alterations of the enzyme activities of the anaplerotic pathway were measured. In all of the pyk-deleted strains evaluated, D1, DR1, D2, and DR2, an increase in PEPC and a significant decrease in phosphoenolpyruvate carboxykinase (PEPCK) activities were detected compared with those in the corresponding pyk wild-type strains. As an example, changes in enzyme activities in strain D1 during culture for glutamic acid production in Medium F4 under biotin-limited conditions are shown in Table 3. The strain C1, whose pyk had been complemented by plasmid, showed similar PEPC and PEPCK activities to those in the wild-type strain, suggesting that the observed enzyme activity changes were caused by the pyk deletion. PEPCK activities in strains D2 and DR2 were 17% and 54% of those in the parental strain P and strain R2, respectively [17].
Since the enhanced PEPC activity and reduced PEPCK activity may boost anaplerotic flux from PEP to OAA, the pyk-deleted strains seemed to avoid PEP overaccumulation resulting from the absence of the PYK reaction. Aspartic acid formation in the culture medium under biotin-limited conditions (Table 2) also indicated increased OAA availability in the pyk-deleted mutants, as aspartic acid is synthesized via a one-step aminotransferase reaction from OAA (Fig. 1); the pyk-deleted mutants, strains D1 and DR1, produced 2.3 and 4.4 g/L aspartic acid, respectively, while the wild-type strain produced only 0.44 g/L.
2.6 Interrelationship Among pyk Deletion, Phosphoenolpyruvate Carboxylase Resistant to Feedback Inhibition by Aspartic Acid, and Low-Activity-Level Citrate Synthase
Notably, CSL and PEPCR mutations were simultaneously introduced when a prototrophic revertant, No. 15, was derived from a CS-defective glutamic acid auxotroph, B. flavum No. 214, by Shiio and coworkers [18]. Furthermore, the PYK-defective mutation was also simultaneously introduced when an S-(2-aminoethyl)-l-cysteine (AEC)-resistant lysine-producing mutant, No. 1-231, was derived from strain No. 15 harboring both CSL and PEPCR [1]. These findings suggested causal relationships between these mutations as the probability of two mutations occurring simultaneously is extremely low.
It was speculated that PEPCR may relieve the stress caused by CSL, such as the possible accumulation of PEP. In addition, the PYK-defective mutation seemed to be necessary to increase the intracellular lysine concentration by an enhanced OAA supply to competitively cancel out the toxic effect of a high concentration of AEC, a lysine analogue. PEPCR may also stabilize the effects of PYK-defective mutation by enabling the smooth conversion of PEP into OAA, thus avoiding the accumulation of toxic PEP.
The fact that the previously reported CSL mutants [12] were derived from a strain harboring the P458S mutation in pyc (enhanced PC activity, [10]) supported our speculation, as this mutation may relieve the metabolic constraints, for example, the possible accumulation of pyruvic acid, caused by CSL. We also experienced difficulty in introducing the CSL mutation (S252C) into strain P to obtain the simple CSL mutant (unpublished results). The obtained CSL mutant exhibited unstable growth and lysine productivity (lower than or comparable to that of strain P). Although this issue was not examined, it is possible that the CSL was compensated for by the expression of another enzyme possessing CS activity (e.g., methylcitrate synthase) [19], possibly leading to the observed instability. These findings suggest that the CSL mutant strains are stable only under conditions in which the anaplerotic reactions (PEPC or PC) have been enhanced.
2.7 Conclusion
All three mutations focused on by Shiio and coworkers during the 1980s in random mutagenesis studies, pyk deletion, PEPCR, and CSL, have been shown to have positive effects on lysine production using modern recombinant DNA techniques as well. The positive effect of pyk deletion on lysine production was more evident against a PEPCR background. Under lysine-producing (biotin-sufficient) conditions, PC (Fig. 1), but not PEPC, was regarded as the major anaplerotic enzyme supplying OAA. However, our results also showed the importance of the PEPC reaction as an alternative anaplerotic reaction when its feedback inhibition is deregulated. Thus, our results highlight the potential for pyk deletion in combination with PEPCR to enhance the anaplerotic reaction to improve lysine and glutamic acid production in C. glutamicum.
3 Enhanced Biomass Production in a pyk-Deleted Mutant of Corynebacterium glutamicum Cultured Under Biotin-Sufficient Conditions
3.1 Introduction
The previous section described evaluation of the fermentation characteristics of a simple pyk-deleted mutant, strain D1, under biotin-limited conditions, which revealed 25% higher glutamic acid yield than that in the wild-type strain [14]. This section summarizes the results of evaluating strain D1 under biotin-sufficient conditions. Surprisingly, strain D1 showed increased biomass production, which has not been described as a phenotype of C. glutamicum mutants during the long history of studies on this species.
3.2 Enhanced Biomass Production in a pyk-Deleted Mutant Under Biotin-Sufficient Conditions
Enhanced biomass production in a pyk-deleted mutant under biotin-sufficient conditions was evaluated in both complex Medium F5 and minimal CGXII medium, each containing 60 μg/L biotin [20]. Interestingly, strain D1 showed a 37% increase in growth in Medium F5 (based on OD660; Fig. 2A) compared with the wild-type ATCC13032. An increased rate of specific glucose consumption (consumed glucose (g) [dry cell weight (g)]−1 h−1) was also observed (Fig. 2B) (35% higher than that in the parent). In addition, increased biomass production was observed in the minimal CGXII medium, although the increase (16%, based on OD660) was smaller than that observed in Medium F5. The pyk-complemented strain, C1, showed the same growth level and glucose consumption rate as those of ATCC13032 (Figs. 2A, B), indicating that the observed phenotypes had been caused by the pyk deletion.
Profiles of growth and glucose consumption of the wild-type C. glutamicum ATCC13032 strain, pyk-deleted mutant (D1), and pyk-complemented strain (C1) cultured in Medium F5. Reproduction of (A) and (B) from the report by Sawada et al. [20]. (A) Growth and (B) glucose consumption. Symbols: circle, wild-type strain; triangle, D1 strain; square, C1 strain. Values are means of three independent experiments. Bars represent the standard deviations
3.3 Measurements of Anaplerotic Enzyme Activities in a pyk-Deleted Mutant
Both PEPC and PEPCK activities were measured in cells cultured in Medium F5 under biotin-sufficient conditions (Table 3). Strain D1 showed a 52% increase in PEPC activity and an 88% decrease in PEPCK activity compared with ATCC13032, which were similar to the changes observed in Medium F4 under biotin-limited conditions, although the changes were more pronounced in Medium F5 than in Medium F4. These simultaneous changes in PEPC and PEPCK activities seemed to represent an important strategy to avoid PEP overaccumulation caused by pyk deletion, irrespective of the biotin concentration. The pyk-complemented strain C1 also showed enzyme activities similar to those of the wild-type strain (Table 3).
3.4 Metabolome Analysis
Clarification of the mechanism underlying the increased biomass production appeared to be difficult. Eventually, we implemented a metabolome analysis using cells cultured in CGXII medium, which provided valuable information to explain the enhanced growth in the mutant [20]. As shown in Table 4, there was accumulation of many intermediate metabolites located upstream of the PYK reaction, in both the glycolytic pathway and the pentose phosphate pathway, some of which were precursor metabolites for biomass production. On the other hand, the levels of intermediate metabolites located downstream of the PYK reaction, including the TCA cycle metabolites, were decreased. The intermediate concentration ratios of D1 to ATCC13032 tended to increase at 11 h (early stationary phase) compared with those at 6 h (logarithmic growth phase) (Table 4), suggesting increased metabolic distortion toward early stationary phase culture in strain D1. The accumulated precursor metabolites seemed to be converted into biomass, which probably relieved the metabolic distortion in strain D1. Metabolic changes in strain D1 under biotin-sufficient conditions, deduced from the glucose consumption rate, changes in enzyme activity, transcriptional analysis, and metabolome analysis, are summarized in Fig. 3 [20].
Schematic model of carbon flux in C. glutamicum strain D1. The cross represents pyk deletion. Reproduction of Fig. 3 from the report by Sawada et al. [20]. The thick arrow line indicates increased flux and the dotted arrow line decreased flux, as deduced from enzyme activity measurements, transcriptional analysis, or rate analysis. Metabolites that increased in abundance as detected by metabolome analysis of strain D1 are shown in boldface. Asterisk indicates a precursor metabolite for biomass synthesis. Glucose 6-P glucose 6-phosphate, PTS, PEP carbohydrate phosphotransferase system, fructose 6-P fructose 6-phosphate, fructose1,6-BP fructose1,6-bisphosphate, GA3-P glyceraldehyde 3-phosphate, DHAP dihydroxyacetone phosphate, 3-PG glycerate 3-phosphate, 6-PGL 6-phosphogluconolactone, 6-PG 6-phosphogluconate, Ru5-P ribulose 5-phosphate, R5-P ribose 5-phosphate, S7-P sedoheptulose 7-phosphate, E4-P erythrose 4-phosphate. Other abbreviations are the same as shown in the legend of Fig. 1
3.5 Excretion of Glycolytic Intermediates in pyk-Deleted Lysine Producers
Notably, previously reported simple pyk-deleted lysine producers (Table 1) not only showed lower lysine yields than those of their parents, but also produced glycolytic intermediate-related compounds such as dihydroxyacetone in combination with either glycerol or glyceraldehyde [7–9]. These C3 metabolites may be derived from dihydroxyacetone phosphate or glyceraldehyde phosphate. For example, pyk-gene-disrupted Corynebacterium lactofermentum L124 produced both 3.5 g/L dihydroxyacetone and 14.7 g/L glyceraldehyde along with 15 g/L lysine, while its parent strain C. lactofermentum ATCC21799 produced 26.3 g/L lysine, without these C3 metabolites arising as by-products [7].
Under these conditions, our metabolome analysis strongly suggested that, in simple pyk-deleted lysine-producing mutants, the accumulation of glycolytic intermediates induced C3 metabolites as by-products, which reduced the lysine yield. On the other hand, in lysine producers such as strain DR2, having both pyk deletion and PEPCR, glycolytic intermediates seemed to be smoothly converted to OAA by the deregulated PEPC, leading to improved lysine production without C3 metabolites arising as by-products.
4 Alterations of Malate:Quinone Oxidoreductase Activity in a pyk-Deleted Mutant and Elucidation of its Physiological Roles
4.1 Introduction
Malate:quinone oxidoreductase (MQO) is a unique TCA cycle enzyme that catalyzes the conversion of malate to OAA with concomitant electron transfer to menaquinone in the respiratory chain [21]. During the characterization of pyk-deleted mutants, we found another interesting role of MQO in the regulation of carbon metabolism in C. glutamicum, which is described in this section.
4.2 Malate:Quinone Oxidoreductase as the Main Site of Entry for Electrons into the Respiratory Chain in Corynebacterium glutamicum
In C. glutamicum, it has been reported that MQO constitutes an NADH reoxidation system in coupling with malate dehydrogenase (MDH) that catalyzes the reverse reaction with concomitant oxidation of NADH. In our previous study [22], measurements of the activities of known enzymes involved in NADH reoxidation other than MQO and MDH, quinone-dependent lactate dehydrogenase (LldD) and lactate dehydrogenase (LdhA), both constituting a similar coupling system to MQO/MDH, and NADH dehydrogenase-II (NDH-II), identified the MQO/MDH coupling system as a major gate of electron transfer from NADH to the respiratory chain, judging from the far higher MQO activity than those of LldD and NDH-II. In line with these observations, an H+-ATPase-defective mutant of C. glutamicum, strain A-1, which showed enhanced glucose consumption and respiration, exhibited elevations in both MQO/MDH activities and their gene expression levels compared with those in the parent strain, ATCC13032 ([22]; Table 5).
4.3 Malate:Quinone Oxidoreductase as a Controller of the Respiration Rate in Corynebacterium glutamicum
The finding that MQO is involved in OAA metabolism prompted us to measure MQO activity in the simple pyk-deleted mutant, strain D1, during culture under biotin-sufficient conditions in Medium F5 [20]. In terms of the results, a dramatic decrease in MQO activity down to 37% of that in the parent ATCC13032 was found, with a concomitant decrease in the respiration rate to 43% of that in the parent (Tables 3 and 5). These changes recovered to the parental levels in the pyk-complemented strain C1 (Table 3). The decreased MQO activity was regulated at the transcriptional level [20]. Similar simultaneous decreases in both MQO activity and the respiration rate were also observed in the pyk-deleted lysine producer strain D2 (38% MQO activity/65% respiration rate compared with the parent strain P, Table 5; [17]). In both cases, no significant change in activity was observed in MDH (Tables 3 and 5). These positive correlations between MQO activities and respiration rates observed in both of the pyk-deleted mutants (strains D1 and D2) and the aforementioned H+-ATPase-defective mutant (strain A1) led us to conclude that MQO may be involved in respiration control in C. glutamicum. Measurements of respiration-related enzymes, namely MQO, LldD, SDH, and NDH-II, in the wild-type ATCC13032 cultured in Medium F5 again confirmed MQO as the major enzyme contributing to respiration (Table 3).
4.4 Malate:Quinone Oxidoreductase as a Fine-tuner of Oxaloacetic Acid Concentration
Apart from the roles played by MQO in respiration control in C. glutamicum, its possible role in carbon metabolism as a TCA cycle enzyme needs to be considered. Our study revealed that, in contrast to the case under biotin-sufficient conditions, such coordinated decreases in MQO activity and respiration rate were not observed in strain D1 when cultured under biotin-limited conditions in Medium F4 during glutamic acid production [20]. In pyk-deleted mutants, strains D1 and D2, cultured under biotin-sufficient conditions, development of an excessive OAA supply was apparent, which probably led to the decreased MQO activity to relieve OAA overaccumulation (Fig. 1). However, under biotin-limited conditions in Medium F4, OAA overaccumulation did not seem to take place as TCA cycle intermediates including OAA were consumed as precursors of glutamic acid biosynthesis, thereby probably rendering a decrease in MQO activity unnecessary. These observations led us to propose an additional role of MQO in carbon metabolism as a modulator or safety valve to fine-tune the OAA concentration, as well as its role in controlling the respiration rate (redox balance) in C. glutamicum [20]. The regulation of MQO gene expression in response to these metabolic constraints needs to be clarified in the future to obtain a clearer understanding of the roles of MQO.
5 Conclusions
Our studies on pyk-deleted mutants clarified the conditions under which the PEPC reaction is a useful anaplerotic reaction to supply OAA for lysine and glutamic acid production in C. glutamicum. Furthermore, detailed physiological studies suggested MQO to be important in controlling both carbon flow at the OAA node and respiration rate/redox balance. These findings may contribute to the rational use of this bacterium in fermentation industries.
References
Ozaki H, Shiio I (1983) Production of lysine by pyruvate kinase mutants of Brevibacterium flavum. Agric Biol Chem 47:1569–1576
Shiio I, Yokota A, Sugimoto S (1987) Effect of pyruvate kinase deficiency on L-lysine productivities of mutants with feedback-resistant aspartokinases. Agric Biol Chem 51:2485–2493
Peters-Wendisch PG, Wendisch VF, Paul S et al (1997) Pyruvate carboxylase as an anaplerotic enzyme in Corynebacterium glutamicum. Microbiology 143:1095–1103
Gubler M, Park SM, Jetten M et al (1994) Effects of phosphoenol pyruvate carboxylase deficiency on metabolism and lysine production in Corynebacterium glutamicum. Appl Microbiol Biotechnol 40:857–863
Peters-Wendisch PG, Schiel B, Wendisch VF et al (2001) Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol 3:295–300
Shiio I, Yoshino H, Sugimoto S (1990) Isolation and properties of lysine-producing mutants with feedback-resistant aspartokinase derived from a Brevibacterium flavum strain with citrate synthase- and pyruvate kinase-defects and feedback-resistant phosphoenolpyruvate carboxylase. Agric Biol Chem 54:3275–3282
Gubler M, Jetten M, Lee SH et al (1994) Cloning of the pyruvate kinase gene (pyk) of Corynebacterium glutamicum and site-specific inactivation of pyk in a lysine-producing Corynebacterium lactofermentum strain. Appl Environ Microbiol 60:2494–2500
Park SM, Sinskey AJ, Stephanopoulos G (1997) Metabolic and physiological studies of Corynebacterium glutamicum mutants. Biotechnol Bioeng 55:864–879
Becker J, Klopprogge C, Wittmann C (2008) Metabolic responses to pyruvate kinase deletion in lysine producing Corynebacterium glutamicum. Microb Cell Fact 7:8
Ohnishi J, Mitsuhashi S, Hayashi M et al (2002) A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant. Appl Microbiol Biotechnol 58:217–223
Chen Z, Bommareddy RR, Frank D et al (2014) Deregulation of feedback inhibition of phosphoenolpyruvate carboxylase for improved lysine production in Corynebacterium glutamicum. Appl Environ Microbiol 80:1388–1393
Ooyen J, Noack S, Bott M et al (2012) Improved L-lysine production with Corynebacterium glutamicum and systemic insight into citrate synthase flux and activity. Biotechnol Bioeng 109:2070–2081
Yokota A, Shiio I (1988) Effects of reduced citrate synthase activity and feedback-resistant phosphoenolpyruvate carboxylase on lysine productivities of Brevibacterium flavum mutants. Agric Biol Chem 52:455–463
Sawada K, Zen-in S, Wada M et al (2010) Metabolic changes in a pyruvate kinase gene deletion mutant of Corynebacterium glutamicum ATCC 13032. Metab Eng 12:401–407
Mori M, Shiio I (1984) Production of aspartic acid and enzymatic alteration in pyruvate kinase mutants of Brevibacterium flavum. Agric Biol Chem 48:1189–1197
Wada M, Sawada K, Ogura K et al (2016) Effects of phosphoenolpyruvate carboxylase desensitization on glutamic acid production in Corynebacterium glutamicum ATCC 13032. J Biosci Bioeng 121:172–177
Yanase M, Aikoh T, Sawada K et al (2016) Pyruvate kinase deletion as an effective phenotype to enhance lysine production in Corynebacterium glutamicum ATCC13032: redirecting the carbon flow to a precursor metabolite. J Biosci Bioeng 122:160–167
Shiio I, Ozaki H, Ujigawa-Takeda K (1982) Production of aspartic acid and lysine by citrate synthase mutants of Brevibacterium flavum. Agric Biol Chem 46:101–107
Radmacher E, Eggeling L (2007) The three tricarboxylate synthase activities of Corynebacterium glutamicum and increase of L-lysine synthesis. Appl Microbiol Biotechnol 76:587–595
Sawada K, Wada M, Hagiwara T et al (2015) Effect of pyruvate kinase gene deletion on the physiology of Corynebacterium glutamicum ATCC13032 under biotin-sufficient non-glutamate-producing conditions: enhanced biomass production. Metab Eng Commun 2:67–75
Molenaar D, van der Rest ME, Drysch A et al (2000) Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Corynebacterium glutamicum. J Bacteriol 182:6884–6891
Sawada K, Kato Y, Imai K et al (2012) Mechanism of increased respiration in an H+-ATPase-defective mutant of Corynebacterium glutamicum. J Biosci Bioeng 113:467–473
Acknowledgments
We thank the staff at the Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co., Inc., for their collaboration with this work. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (KAKENHI No. 23580096, to M.W.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yokota, A., Sawada, K., Wada, M. (2016). Boosting Anaplerotic Reactions by Pyruvate Kinase Gene Deletion and Phosphoenolpyruvate Carboxylase Desensitization for Glutamic Acid and Lysine Production in Corynebacterium glutamicum . In: Yokota, A., Ikeda, M. (eds) Amino Acid Fermentation. Advances in Biochemical Engineering/Biotechnology, vol 159. Springer, Tokyo. https://doi.org/10.1007/10_2016_31
Download citation
DOI: https://doi.org/10.1007/10_2016_31
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56518-5
Online ISBN: 978-4-431-56520-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)