Abstract
Diols are compounds with two hydroxyl groups and have a wide range of appealing applications as chemicals and fuels. In particular, five low molecular diol compounds, namely 1,3-propanediol (1,3-PDO), 1,2-propanediol (1,2-PDO), 2,3-butanediol (2,3-BDO), 1,3-butanediol (1,3-BDO), and 1,4-butanediol (1,4-BDO), can be biotechnologically produced by direct microbial bioconversion of renewable materials. In this review, we summarize recent developments in the microbial production of diols, especially regarding the engineering of typical microbial strains as cell factory and the development of corresponding bioconversion processes.

Graphical Abstract
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
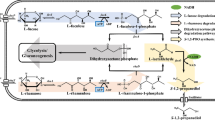
At the beginning of the twentieth century, before petroleum was introduced as raw material, the chemical industry had to rely on coal and renewable resources. Until 1930, the most important bulk products of that time, such as fuels (ethanol, butanol), organic acids (acetic acid, citric acid, lactic acid) and other basic chemicals, were biotechnologically produced from biomass. With the development of the petroleum industry, many of these biotechnological processes were replaced by chemical synthesis routes based on petroleum or natural gas. Nowadays, over 80 million tons of industrial chemicals are manufactured globally each year from fossil-based feedstocks [1]. These petrochemicals, which encompass building blocks, intermediate chemicals, and derived final products like polymers, are valued at over $2 trillion and provide the materials and products that impact and enable virtually every aspect of our daily existence [1]. However, these great benefits historically have come at great cost. While the chemicals themselves play a positive role in society, the petroleum-based processes used to manufacture chemicals engender challenges that can jeopardize the economy, the environment, and overall global security. Nowadays, the rapid advances in plant biotechnology, molecular biology, and new tools and concepts such as systems and synthetic biology, and biorefinery of renewable biomaterials have created new opportunities and markets for many biotechnologically produced (bio)chemicals. Many chemicals, which could only be produced by chemical processes in the past, could potentially be generated biologically from renewable resources. The microbial production of diols is a prominent example of success of the so-called white or industrial biotechnology in recent years. Diols are compounds with two hydroxyl groups which have a wide range of important applications as chemicals and fuels. They are considered as platform green chemicals for many industries. In particularly, the microbial production of 1,3-propanediol (1,3-PDO), 1,2-propanediol (1,2-PDO), 2,3-butanediol (2,3-BDO) and, more recently 1,4-butanediol (1,4-BDO) and 1,3-butanediol (1,3-BDO) has received much interest in industrial biotechnology. These diols can be produced from different renewable feedstocks and even waste materials from biofuel production (Fig. 1) [2].
Major routes for bioproduction of diols from different feedstocks (modified after [2]). 3-HPA 3-hydroxypropionaldehyde, GAP glyceraldehyde 3-phosphate, DHAP dihydroxyacetone phosphate
The production of 1,3-PDO and 1,4-BDO has reached commercial scales. They are especially useful as biomonomers for the polyesters polypropylene terephthalate (PPT) and polybutylene terephthalate (PBT). Both PPT and PBT have the potential to steal market share from the classic polyester polyethylene terephthalate (PET) [3]. Pilot plant-scale production of 1,2-PDO and 2,3-BDO has also been reported. All these diols are of immense industrial interests because they are either established chemicals presently produced from fossil resources in large production volumes (e.g. more than 1.5 Mio. t per year for 1,2-PDO and about 1.3 Mio. t per year for 1,4-BDO), or large market potentials [2]. Despite their large impact, relatively few publications are available for the biotechnological production of 1,2-PDO, 1,3-BDO, and 1,4-BDO. On the other hand, 1,3-PDO and 2,3-BDO have been most intensively studied in the last few years and several comprehensive reviews for the microbial production of these diols have been published [2, 4–7]. In this review article, microbial factories for the different diols and the pathways involved are illustrated. The current state of the art of strain improvement including synthetic pathways is also summarized.
2 Butanediol Production
Butanediol is a four-carbon diol having its hydroxy groups at various positions. 2,3-BDO is the only naturally occurring BDO, produced by several facultative and anaerobic bacteria. On the other hand, no natural metabolic pathways or micro-organisms are known which can produce 1,4-BDO or 1,3-BDO from sugar or other biomass. Although the market for synthetic 2,3-BDO is presently still very small, there is a shift towards the use of biobased 2,3-BDO. 1,4-BDO, the most widely used BDO compound, is currently produced from fossil fuel feedstocks. In the following, we summarize recent development in the microbial production of the different types of butanediol.
2.1 2,3-Butanediol
2,3-Butanediol (2,3-BDO) is one of the promising bulk chemicals which exhibits a wide range of potential applications [8–10]. It is used as the starting material for bulk chemicals such as methyl ethyl ketone, gamma-butyrolactone, and 1,3-butadiene [11]. Nowadays, the manufacture of 2,3-BDO is growing by an annual rate of 4–7 % due to the increased demand for many of its derivatives [12, 13]. 2,3-BDO is widely used in chemical, food, fuel, aeronautical, and other fields. Due to the presence of two chiral centres, 2,3-BDO has three isomers: levo (2R, 3R) and dextro (2S, 3S) forms with optical activity and the meso-form with no optical activity.
The optically active forms of 2,3-BDOs are very valuable chemicals in the directed asymmetric synthesis of chiral chemicals using boronic esters. Moreover, chiral compounds are especially important to provide chiral groups in drugs, in high-value pharmaceutical or for liquid crystals manufacture [14, 15]. The various applications of this polymer are summarized elsewhere [5, 16].
Although the first commercial production of 2,3-BDO was biotechnological one operated in Germany in the middle of the last century, currently the commercialized process for its synthesis is based entirely on a chemical route. However, the synthetic (petroleum-based) 2,3-BDO does not have a very large market due to its unique structure and costly chemical synthesis. Also, there is no efficient method to convert the intermediate into downstream derivatives such as butadiene, methyl ethyl ketone, and butenes. Therefore, 2,3-BDO has not been produced on a large scale and is currently available as a laboratory chemical and is being sold as a small-volume intermediate for some niche applications like food flavouring. Moreover, its high price led also to inadequate development of its application [17]. Therefore, biobased 2,3-BDO is considered to be a highly attractive market and is expected to provide immense opportunities to the main players involved in the market.
2.1.1 Micro-organisms of Potential Significance for 2,3-BDO Production
Bacteria effectively producing 2,3-BDO belong mainly to the Enterobacteriaceae family. Their representative species are Klebsiella pneumoniae, K. oxytoca, and E. aerogenes. Pseudomonas chlororaphis and Paenibacillus polymyxa belonging to the families Pseudomonadaceae and Paenibacillaceae, respectively, have received attention due to the formation of a pure optically active stereoisomer (L-form) in plant rhizospheres. In general, the highest 2,3-BDO concentrations were obtained with pathogen (risk group 2) micro-organisms (Table 1) and thus not desirable for industrial-scale production. Interestingly, Jurchescu et al. [18] reported recently the production of 2,3-BDO by Bacillus licheniformis DSMZ 8785 grown on glucose in fed-batch cultivation. The maximum 2,3-BDO concentration obtained was 144.7 g/L, which was comparable to that achieved by the risk group 2 strains. Moreover, by using thermophilic B. licheniformis strains, high concentrations (103–115 g/L) of 2,3-BDO could be produced either from glucose [19, 20] or from plant polysaccharide inulin in a simultaneous saccharification and fermentation process [20]. Advantages of the thermophilic process include less contamination risk at high temperature and more efficient utilization of the plant substrate by simultaneous saccharification [19]. Indeed, species of Bacillus or Paenibacillus appear to be more suitable for commercial 2,3-BDO production. While a mixture of levo and meso (1:1 ratio) was formed by B. licheniformis, P. polymyxa has the ability to form almost exclusively the levo-isomer (over 98 %) when grown under anaerobic conditions [13, 21–25]. Recently, Fu et al. [26] showed that NADH played a vital role for chirally pure d-2,3-BDO production in Bacillus subtilis grown under limited oxygen conditions. Although the final concentrations in the 2,3-BDO fermentation are lower than those of B. licheniformis, the optical purity of the produced diol could be of interest for the fine chemical industry and specific synthesis. Under microaerobic conditions, the 2,3-BDO productivity of this bacterium is higher, but the optical purity decreases, since the meso-form is increasingly formed [23].
2.1.2 Metabolic Pathways of 2,3-BDO Biosynthesis
Several bacteria, yeasts, or even algae have the capability to produce 2,3-BDO, but the observed yields are often quite different [5, 34, 35]. The 2,3-BDO biosynthetic pathway has been intensively studied in bacteria (Fig. 2). 2,3-BDO synthesis is typically a part of a mixed-acid fermentation pathway observed under anaerobic or microaerobic growth conditions of different micro-organisms (Fig. 2). In addition to 2,3-BDO and depending on the micro-organism and cultivation conditions, other end products are formed, such as ethanol, acetate, lactate, formate, and succinate. In order to enhance the 2,3-BDO yield (theoretical maximum yield 0.5 g/g on glucose), most of the work done was concentrated on an efficient channelling of pyruvate to 2,3-BDO and not to the different by-products. The formation and selectivity of 2,3-BDO stereoisomers and in particular the control of their purity have not been completely understood. Consequently, various metabolic pathways have been proposed (Fig. 3).
Mechanisms of the formation of 2,3-BDO stereoisomers (modified after [5])
Acetoin is the precursor of 2,3-BDO and is formed in bacteria from pyruvate through several enzymatic reactions. Under anaerobic conditions, α-acetolactate synthase catalyses the condensation of two pyruvate molecules with a single decarboxylation to form α-acetolactate that is converted to acetoin by α-acetolactate decarboxylase. Under low oxygen concentration, α-acetolactate can undergo a spontaneous decarboxylation, leading to the formation of diacetyl. Subsequently, a NADH-linked diacetyl reductase converts the latter to acetoin. Finally, 2,3-BDO of different isomeric forms is formed from acetoin by the action of different acetoin reductase enzymes with different stereospecificities, or by a cyclic pathway (the so-called butanediol cycle), the existence of which has been reported in different bacteria as shown in Fig. 3 [14, 36, 37]. Recently, autotrophic 2,3-BDO synthesis from CO2 and/or CO plus H2 was shown to exist in different acetogenic Clostridium species [35, 38]. Wood–Ljungdahl pathway was shown to be involved in which CO and/or CO2 feeds the methyl and carbonyl branches of the pathway. In the methyl branch, CO or CO2 is fixed in a sequence of tetrahydrofolate (THF)- and cobalamin-dependent reactions into a methyl group, which is then combined with CO (used either directly or after enzymatic reduction of CO2) to form acetyl-CoA, in which the latter is catalysed by the CODH/ACS (carbon monoxide dehydrogenase/acetyl-CoA synthase) complex. Acetyl-CoA serves as a precursor for growth and 2,3-BDO production [35] (Fig. 2).
In the BDO cycle (Fig. 2), acetoin is oxidized to diacetyl by acetoin dehydrogenase, and then, 2 diacetyl molecules are converted to acetylacetoin and acetate by the enzyme acetylacetoin synthase. Acetylacetoin is further reduced to acetylbutanediol with different stereospecificities by either NAD(P)H- or NADH-linked acetylacetoin reductase. Different 2,3-BDO stereoisomers are then formed by the action of acetylbutanediol reductase. Through this butanediol cycle, 2 forms of stereoisomers are formed in B. cereus as reported by Ui et al. [39]. Interestingly, in P. polymyxa grown under microaerobic conditions, diacetyl is converted to S-acetoin by a NAD(P)H-linked diacetyl reductase. Anaerobically, this bacterium produces 98 % of the levo-form through the catabolic 2,3-BDO formation route (Figs. 2, 3). Moreover, an acetoin racemase catalysing the conversion between the different forms of acetoin was proposed for the same bacterium [39]. Recently, Chen et al. [14] elaborated the mechanism of the different stereoisomer formation in K. pneumonia. They reported that glycerol dehydrogenase exhibited 2R,3R-butanediol dehydrogenase activity and was responsible for levo-butanediol synthesis from R-acetoin. This enzyme also contributed to meso-2,3-butanediol synthesis from S-acetoin. Butanediol dehydrogenase was the only enzyme that catalyses the conversion of diacetyl to S-acetoin and further to dextro-butanediol (Fig. 3).
2.1.3 Pathway Engineering and Synthetic Pathway for 2,3-BDO Formation
Despite the intensive research done on enhancing 2,3-BDO production by its native risk group 2 bacteria (e.g. see [11, 40, 41]), the concerns associated with the utilization of potential pathogenic bacteria and/or the inefficient utilization of cellulosic sugars have led many scientists to engineer more safer strains. Oliver et al. [42] have developed a 2,3-BDO biosynthetic pathway in the photosynthetic cyanobacterium Synechococcus elongatus. The strain still has a limited productivity (2.38 g/L 2,3-BDO), and more research is needed to reach a desirable titre suitable for industrial application. Efforts were also done to enhance the production of optically active 2,3-BDO in native strains. A mutant of P. polymyxa with constitutive synthesis of the α-acetolactate synthase was constructed [21]. The mutant obtained grew more slowly than the wild type but produced fourfold more 2,3-BDO. By knocking out some by-product-producing genes in Enterobacter cloacae, Li et al. [11] were able to produce 119 g/L of enantiomerically pure 2,3-BDO using lignocellulosic hydrolysates.
Moreover, E. coli was extensively used as a host for many metabolic engineering studies for the production of 2,3-BDO, especially for the production of optically active one. Until recently, the synthetic pathways constructed in E. coli for enantiomerically pure 2,3-BDO using different stereospecific dehydrogenases from diverse species gave relatively low concentration of 2,3-BDO [5, 43]. Recently, applying a systematic metabolic engineering approach, Xu et al. [17] optimized the production of 2,3-BDO in recombinant E. coli strains. 2,3-BDO biosynthesis gene clusters were cloned from several native 2,3-BDO producers, including B. subtilis, B. licheniformis, K. pneumoniae, Serratia marcescens, and E. cloacae, inserted into the expression vector pET28a, and compared for 2,3-BDO synthesis. The best strain was then studied in fed-batch fermentation and was found to produce 74 g/L within 62 h [17].
Since no natural producers for the dextro-2,3-BDO (2S,3S) have been found, biosynthesis of this diol enantiomer has been achieved using engineered E. coli [32, 44]. Li et al. [32, 44] obtained 26.8 g/L of highly pure (>99 %) (2S,3S)-2,3-BDO in a fed-batch culture from diacetyl. Moreover, through introducing NADH regeneration enzymes into E. coli, a higher product titre (31.7 g/L) of (2S, 3S)-2,3-BDO was obtained [32].
Many industrial biotechnological processes are moving towards the use of yeast as a platform. Engineered yeast strains were also reported that are capable of producing 100 g/L of enantiomerically pure levo-2-3-BDO from a mixture of glucose and galactose with a yield over 70 % of the theoretical value [33, 45]. The high titre and yield of the optically active 2,3-BDO produced make the engineered yeast strain promising hosts for a cost-effective production of biobased 2,3-BDO.
2.2 1,4-Butanediol
1,4-Butanediol (1,4-BDO) is an important commodity chemical used to manufacture over 2.5 million tons of valuable products annually. The major use of 1,4-butanediol is in the production of tetrahydrofuran (THF) and PBT [46]. THF is used to produce spandex fibres and other performance polymers, resins, solvents, and printing inks for plastics. PBT is an engineering-grade thermoplastic that combines excellent mechanical and electrical properties with robust chemical resistance. The automotive and electronics industries heavily rely on PBT to produce connectors, insulators, wheel covers, gearshift knobs, and reinforcing beams. There is also growing demand in the apparel industry for renewable, biobased spandex. 1,4-BDO is also used as a plasticizer (e.g. in polyesters and cellulosics), as a carrier solvent in printing ink, a cleaning agent, an adhesive (in leather, plastics, polyester laminates, and polyurethane footwear), in agricultural and veterinary chemicals, and in coatings (in paints, varnishes, and films). 1,4-butanediol is also reportedly used as a solvent in cosmetic formulations and as a humectant in pharmaceuticals [47]. Recently, Diaz et al. [48] reviewed the various biodegradable polymers that can be synthesized from 1,4-BDO and dicarboxylic acids. Application of a series of polymers that cover a wide range of properties, namely materials from elastomeric to rigid characteristics that are suitable for applications such as hydrogels, soft tissue engineering, drug delivery systems, and liquid crystals, is reported.
In nature, no metabolic pathway and no micro-organisms are found so far that can produce 1,4-BDO from sugar or other biomass. Therefore, fossil fuel-based feedstocks such as acetylene, butane, propylene, and butadiene are the current sources for its production. Recently, using genome-scale metabolic model of E. coli and biopathway prediction algorithms, the company Genomatica has established unnatural synthetic pathways and correspondingly engineered E. coli strains for 1,4-BDO bioproduction from sugars such as glucose, xylose, sucrose, and biomass-derived mixed-sugar streams [46, 49]. In one pathway, sugar is first converted into succinyl-CoA which is then further converted into 1,4-BDO over 4-hydroxybutyrate and other intermediates (Fig. 4), and a strain capable of producing 18 g/L 1,4-butanediol was engineered. The engineered E. coli has an enhanced anaerobic operation of the oxidative tricarboxylic acid cycle, thereby generating more reducing power to drive the synthetic 1,4-BDO pathway. According to Genomatica, they have done extensive work to optimize the yield and the rate of 1,4-BDO production, to minimize the by-products, and to enhance the 1,4-BDO tolerance of the engineered strain. Yim et al. [46] proposed that by rising the rates of key steps in the pathway, removing metabolic inefficiencies and substantially reducing by-products may increase the titre further. Burk [1] stated that the commercial production of 1,4-BDO from sugar will require much less energy and release significantly less carbon dioxide and is expected to have a substantial cost advantage relative to the current petrochemical process. Indeed, systems biology and fermentation process engineering approaches can identify and address bottlenecks that are obstacles to commercialization like achieving higher cell densities with improved specific productivity [46]. Recently, strains able to produce 30–40 g/L of 1,4-BDO in a continuous bioreactor were developed and patented by Genomatica [50].
1,4-BDO biosynthetic pathways introduced in E. coli (modified after Yim et al. [46]). Solid lines show reactions occurring naturally in E. coli, whereas dotted lines represent introduced synthetic reaction steps
2.3 1,3-Butanediol
1,3-Butanediol (1,3-BDO) is used as a chemical intermediate in the manufacture of polyester plasticizers, as a solvent for flavouring, and as a humectant in pet foods and tobacco. Its uses in cosmetics have been reviewed by the Cosmetic Ingredient Review which concluded that 1,3-butanediol is safe as normally used in cosmetics [51]. (R)-1,3-BDO, a non-natural alcohol, is a valuable building block for the synthesis of various optically active compounds such as pheromones, fragrances, and insecticides by direct incorporation into the target molecules, or is used as chiral template in the Lewis acid-mediated reactions of acetals with nucleophiles [52]. (R)-1,3-BDO is especially interesting as a starting material of chiral azetidinone derivatives and key intermediate of penems and carbapenems for industrial synthesis of ß-lactam antibiotics. Because these antibiotics are the mostly used antibacterial agents in clinical practice worldwide, the demand for R-1,3-BDO has been drastically increased, and as a consequence, the production method of R-1,3-BDO has been intensively studied [53–55]. So far, 1,3-BDO has been synthesized as a racemic mixture of R- and S-forms, mainly from petroleum-based chemicals such as a prochiral precursor, 4-hydroxy-2-butanone. Moreover, Eguchi and Mochida [56] attempted a kinetic resolution of 1,3-BDO by lipase-catalysed diacylations in organic solvent, resulting in (R)-1,3-diacetoxybutane with 23.4 % yield and 98.6 % enantiomeric purity. Using whole cells of recombinant E. coli expressing exogenous dehydrogenase from Candida parapsilosis, Daicel Chemical Industries Ltd. produce R-1,3-BDO with 48.4 % yield and 95 % enantiomeric purity [57]. Recently, Kataoka et al. [53] constructed an effective synthetic production route of 1,3-BDO from glucose in E. coli (Fig. 5). The high demand on reducing equivalents and cofactors for the production of 1,3-BDO (Fig. 5) reflects the importance of the aerobic catabolism of glucose for reducing equivalent regeneration. Hence, Kataoka et al. [54] optimized 1,3-BDO in an engineered E. coli by strict regulation of the overall oxygen transfer coefficient (k L a) during the cultivation. With optimized fermentation conditions, this recombinant E. coli strain was able to produce up to 9 g/L of 98.5 % enantiomeric purity of R-1,3-BDO. Although the titre reported by Kataoka et al. [53] was more than 8-fold higher than that reported in the patent published earlier in 2009 [58], still much work has to be done to reach an acceptable concentration suitable for commercialization.
3 Propanediol Production
Propanediol is a three-carbon diol having its hydroxy groups, at the first and the last carbon atom, in case of 1,3-PDO, or at the first and the second carbon atom in 1,2-PDO. 1,2-PDO is a chiral molecule and mostly available as a racematic mixture. Both 1,3-PDO and 1,2-PDO offer broad application spectra, either directly as solvents or as platform chemicals for a broad product spectrum. Even though a chemical synthesis is possible, the interest in biological production of propanediols increases. Fermentation processes need less pressure, ambient temperature, and no expensive catalysts. Furthermore, they allow a sustainable process by transferring waste streams of biodiesel production or lignocellulosic residues into valuable side products.
3.1 1,3-Propanediol
Because of the attractive physical and chemical properties, and hence the various applications of 1,3-PDO, the interest in such polymer increased significantly in the last few years. On the one hand, it is used directly as solvent and antifreeze component in varnish, adhesives, or resins [6], as polyglycol-type lubricant, and in cosmetic products [59]. On the other hand, it is a very suitable monomer for synthetic reactions like polycondensation. 1,3-PDO is well known for the production of polytrimethylene terephthalate (PTT), biodegradable polyester which is utilized fibre not only in textiles and carpets but also in coatings. Furthermore, it can be used for the production of other biodegradable plastics polyesters, polyethers, and polyurethanes [60].
Till recently, biotechnology could not economically compete with the chemical synthesis of 1,3-PDO. In 2004, however, DuPont constructed a biochemical plant in Loudon for manufacturing 1,3-propanediol using E. coli with a synthetic pathway from glucose. The plant was commissioned in November 2006. Very recently, the two companies METabolic Explorer (France) and SK Chemicals (South Korea) recently announced a joint agreement to manufacture 1,3-propanediol from crude glycerol. Together, they will market it in Europe and Asia to fulfil the expanding global demand for 1,3-PDO (www.metabolic-explorer.com, www.skchemicals.com, 2014). Biotechnological plants for 1,3-PDO from glycerol were also built in China. It is not clear if any of these plants are in operation.
3.1.1 Micro-organism of Potential Significance for 1,3-PDO Production
1,3-PDO is one of the natural products of the anaerobic degradation of glycerol in many bacteria. Therefore, and as by-products of biodiesel industry, crude glycerol was intensively used for the production of 1,3-PDO. However, the productive strains should be used that can tolerate impurities normally found in crude glycerol (salts, free fatty acids, and methanol [61]). The production of 1,3-PDO from glycerol is mainly performed by micro-organisms of the families Clostridiaceae and Enterobacteriaceae, and several species of Klebsiella, Clostridia, Citrobacter, and Enterobacter are known to convert glycerol to 1,3-PDO under anaerobic conditions. The most-studied and well-known species are K. pneumoniae and Clostridium butyricum, because of their high substrate tolerance as well as high yield and productivity. Although C. butyricum is strictly anaerobic and K. pneumonia is facultative anaerobic (easier to handle), species of Clostridia are more interesting for industrial application. K. pneumoniae is classified as an opportunistic pathogen, and hence, special safety precautions are needed to use K. pneumonia for fermentation. Recently, in a cocultivation of cyanobacteria with K. pneumoniae, Wang et al. reported the production of 1,3-PDO from CO2 [62]. Moreover, it was shown that 1,3-PDO can be produced in an unsterile process from raw glycerol using either mixed culture [63] or pure culture of C. butyricum [64] and C. pasteurianum [65]. This new development makes it economically very competitive. Moreover, the incorporation of the 1,3-PDO production into a biorefinery concept can further increase the ecological advantage and the commercial chance of the glycerol-based process. Friedmann and Zeng [66] proposed to use a mixed culture to produce 1,3-PDO and methane from glycerol. This concept was successfully demonstrated within a European 7th Framework research project (www.propnergy.eu) in laboratory and pilot scale. The basic idea was to use acidogenic and methanogenic bacteria for converting the by-products simultaneously into methane. Alternatively, the by-products can be degraded in a following biogas bioreactor. Formerly, a theoretical and metabolic flux study of syntrophic-like growth of C. butyricum and Methanosarcina mazei, a methanogenic archeon, under anaerobic conditions was carried out to analyse the several possible scenarios, especially to examine the preference of M. mazei in scavenging acetate and formate under conditions of different substrate availability, including methanol as a cosubstrate in biodiesel-derived raw glycerol [67]. Zhou et al. [68] studied the bioconversion of glycerol to 1,3-PDO with a mixed population in a microbial bioelectrochemical system (BES). Though the mixed population used in this study was less effective, the use of BES system for delivering the necessary reducing power for 1,3-PDO production represents an interesting development. More recently, Choi et al. [69] showed that C. pasteurianum, a promising 1,3-PDO producer as mentioned above, can directly use electrons from cathode for the regeneration of reducing power in glycerol fermentation. However, the electron flow from the cathode was relatively low and the effect on the glycerol fermentation was not significant. In fact, microbial electrochemical processes for biosynthesis are still poorly understood [70, 71]. The use of a mixed culture in BSE is even more complicated. In general, it is essential to better understand the regulation and metabolic interactions and to control the dynamics of microbial consortia suitable for such processes and to inhibit the 1,3-PDO degradation.
Mixtures of glucose and glycerol have also been used for the production of 1,3-PDO by using members of Lactobacillaceae. Lactobacilli have only the reductive conversion and need an additional substrate for the growth and generation of the reducing equivalents. L. reuteri, L. brevis, L. buchneri, L. collonoides, and L. panis were reported to produce 1,3-PDO in mixed substrate fermentation [72]. Pflügl et al. [73] reported the production of 42 g/L 1,3-PDO from glycerol by L. diolivorans. However, after the addition of glucose, the 1,3-PDO production increased up to 74 g/L [73]. Recently, Sabra et al. [74] reported the simultaneous production of 1,3-PDO and n-butanol in mixtures of glucose and glycerol in different ratios using C. pasteurianum. On the other hand, with glucose as monosubstrate, several approaches with genetically modified organisms have been reported (see Sect. 4.1.2). An overview of the potential 1,3-PDO productive strains is given in Table 2.
3.1.2 Biosynthetic Pathways and Pathway Engineering of 1,3-PDO
The natural pathway for the production of 1,3-PDO in different micro-organisms is shown in Fig. 6. Generally, the pathway is divided into two parallel routes, a reductive route for the production of 1,3-PDO (A) and an oxidative route (B) where glycerol is metabolized via glycolysis into pyruvate and energy is produced. Only about 5 % of the glycerol is used for biomass production, when it is the sole carbon source [59].
In the reductive route, glycerol is dehydrated into 3-hydroxypropionaldehyde (3-HPA) by glycerol dehydratase. 3-HPA is subsequently reduced to 1,3-PDO by 1,3-propanediol oxidoreductase (PDOR) under consumption of nicotinamide adenine dinucleotide (NADH2). This reducing equivalent is generated in the oxidative route through the synthesis of pyruvate and the transformation of pyruvate into acetyl-CoA. Different micro-organisms convert pyruvate into different by-products (Fig. 6). Indeed, the yield of 1,3-PDO per glycerol depends on the availability of NADH2. The availability is not only determined by the micro-organism itself but also dependent on the process conditions of the fermentation [89]. Hence, the yield of 1,3-PDO depends on the combination and stoichiometry of the reductive and oxidative pathways. Consequently, the maximum yield of 1,3-PDO formation from glycerol in clostridia represents 0.67 mol/mol and is achieved under conditions where acetic acid is the main by-product and not butyric acid, ethanol, or butanol [89–91]. If no hydrogen and butyric acid are produced at all during the fermentation, the theoretical yield can be further increased to 0.72 mol/mol [89, 90, 92]. The 1,3-PDO yield from glycerol can be additionally enhanced with an in vitro approach using crude enzymes from different organisms [93]. These systems feature several biomanufacturing advantages, such as fast reaction rate, easy product separation, broad reaction condition and tolerance to toxic substrates or products [94]. Nevertheless, the cost and stability of enzyme and coenzymes restrict the use of such systems in industrial scale.
Intensive work has been done to genetically modify micro-organisms to convert glucose to 1,3-PDO in one micro-organism. In the DuPont PDO process, a synthetic pathway was successfully developed to produce PDO from glucose, in which the glycerol synthesis pathway from S. cerevisiae (catalysed by glycerol 3-phosphate dehydrogenase (DAR1) and glycerol 3-phosphate phosphatase (GPP1/2) and the metabolic pathway of converting glycerol to PDO from K. pneumonia (glycerol dehydratase, encoded by the genes dhaB1, dhaB2, and dhaB3) were integrated into E. coli (Fig. 7, [95]). The last step, the formation of 1,3-PDO is realized by a 1,3-propanediol oxidoreductase isoenzyme from E. coli (YqhD). Continuous strain development was made by DuPont/Genencor, and the most fundamental changes done were probably the elimination of d-glucose transport by the phosphotransferase system (PTS) and the downregulation of glyceraldehyde 3-phosphate dehydrogenase (gap) together with reactivation of tpi. Finally, the yield could be increased to 135 g/L with a productivity of 3.5 g/L h [95].
Still, in such a production system, the substrate suicide of glycerol dehydratase (GDHt) that could limit the productivity has to be overcome [97]. Recently, Chen et al. [96] constructed a new non-glycerol-derived synthetic 1,3-PDO synthesis in E. coli (Fig. 7). With protein engineering of glutamate dehydrogenase, they extended the pathway of homoserine, a natural intermediate of cellular amino acid metabolism. At first, homoserine is converted by deamination into 4-hydroxy-2-ketobutyrate, followed by decarboxylation into 3-hydroxypropionaldehyde (3HPA). Like in the conventional pathway, 3HPA is subsequently transformed by alcohol dehydrogenase into 1,3-PDO. The theoretical maximum yield (1.5 mol 1,3-PDO/mol glucose) of the new 1,3-PDO pathway is the same as that of the DuPont route. Since homoserine synthesis is a common pathway in most of the bacteria, the proposed route can be engineered into selected hosts with the more favourable ability to utilize different and cheap sugars. Moreover, the proposed pathway does not utilize GDHt and thus can avoid the serious problems associated with vitamin B12 and substrate suicide. This non-natural pathway is thus very appealing for 1,3-PDO production.
3.2 1,2-Propanediol
1,2-propanediol (1,2-PDO), generally called propylene glycol, is a major commodity chemical with a global demand estimated to be around 1.36 Mio. t/a for several industries [98]. It appears as a colourless hygroscopic liquid with low volatility and an oily consistency. This industrial important compound is mainly utilized as solvent, antifreeze, de-icer, and heat transfer fluids [99]. Furthermore, it could be applied as colour compound and flavour and fragrance carrier in foods, beverages, cosmetics, and pharmaceuticals, or even as tobacco humectants [100]. The interest in 1,2-PDO increases since it is less toxic than products based on ethylene glycol for humans and animals. The US Food and Drug Administration (FDA) has determined 1,2-PDO to be “generally recognized as safe” for use in food, cosmetics, and medicines [98].
3.2.1 Microbial Cell Factories for the Production of 1,2-PDO
The biological route for producing 1,2-PDO from sugars is known since many years. Early studies on Thermoanaerobacterium thermosaccharolyticum [101, 102], Bacteroides ruminicola [103], C. sphenoides [104], L. Buchneri [105]), and E. coli [99] have demonstrated 1,2-PDO formation. In comparison with other diols, the 1,2-PDO yields are much lower, either from sugars or from glycerol [92]. The biosynthesis of 1,2-PDO requires the conversion of the main carbon source into DHAP with the glycolytic pathways (Fig. 8). Therefore, due to higher reduction degree of glycerol, the yield of 1,2-PDO is higher than that from glucose (theoretical maximum yield of 0.63 and 0.72 g/g from glucose and glycerol, respectively). In either way, the biosynthesis consumes redox equivalent and ATP [99].
a Metabolic pathways of deoxy sugar fermentation into 1,2-propanediol by E. coli and b the various routes of the conversion of methylglyoxal to 1,2-PDO in different micro-organisms (modified after [106])
There are two possible pathways for the biosynthesis of 1,2-PDO. The first one metabolizes deoxy sugars (methyl pentoses (Fig. 8a)), whereas the second one converts DHAP into methylglyoxal (Fig. 8b) and further to 1,2-PDO. The deoxy pathway is well studied in E. coli and is reviewed by Bennett and San [106]. At first, L-rhamnose is converted into L-rhamnulose-1-phosphate, which is subsequently split into dihydroxyacetone phosphate and S-lactaldehyde by the enzyme RhaD (L-rhamnose dehydrogenase). Fucose, on the other hand, is first isomerized into L-fuculose and transformed into L-fuculose-1-phosphate by the enzyme L-fuculose kinase. Another enzyme, fucA (L-fuculose-1-phosphate aldolase), cleaves it into dihydroxyacetone phosphate and L-lactaldehyde. Depending on the redox conditions, the lactaldehyde can be either reduced to 1,2-PDO or oxidized to lactic acid. Anaerobic conditions lead to conversion into S-1,2-PDO, catalysed by a NAD-oxidoreductase fucO (S)-1,2-propanediol oxidoreductase [106]. Since deoxy sugars are quite expensive as substrate, the deoxy pathway is considered to be uneconomical as an industrial process.
In T. thermosaccharolyticum, the second pathway is found. At first, DHAP is produced from glucose, xylose, mannose, or cellobiose. DHAP is then converted with methylglyoxal synthase into methylglyoxal (MG). Subsequently, MG is reduced to R-1,2-PDO with aldose reductase or glycerol dehydrogenase. If glucose is fermented only into R-1,2-PDO, acetate, and CO2, a theoretical yield of 0.42 g R-1,2-PDO/g glucose is possible. In E. coli, MG is converted into acetol with the NADPH- or NADH-dependent lactaldehyde oxidoreductase, and alcohol or aldehyde dehydrogenases. E. coli also converts MG into R-lactaldehyde with NADH-dependent glycerol dehydrogenase. On the contrary, the yeast S. cerevisiae produces S-lactaldehyde from MG, which is subsequently converted into S-1,2-PDO by a NADPH-dependent aldose reductase. However, the MG production in S. cerevisiae is non-enzymatic and spontaneous, and the final 1,2-PDO titre is quite low [101].
Recently, Clomburg and Gonzales [99] developed a new strain of E. coli with increased production of 1,2-PDO. The functional pathway was engineered by combining different strategies (Fig. 9a): (I) to ensure DHAP availability, they changed the PEP-dependent DHAK (dihydroxyacetone kinase) with the ATP-dependent DHAK from Citrobacter freundii; (II) they overexpressed the genes for 1,2-PDO synthesis from DHAP; and (III) competitive pathways for acetate and lactate were deleted. Other side products were maintained to ensure the necessary redox balance and ATP generation. The recombinant E. coli strain produced 5.6 g/L 1,2-PDO with a yield of 0.21 g/g glycerol [99]. More recently, Koch et al. [107] established a recombinant E. coli to enhance 1,2-PDO production from several carbon sources with three newly integrated and highly expressed enzymes. This new pathway avoids the toxic intermediate methylglyoxal and use the natural formation of lactate. The latter one is transferred into lactyl-CoA by lactate-CoA transferase and then into lactaldehyde by lactyl-CoA reductase. In the last step 1,2-PDO is formed with the help of lactaldehyde reductase (also 1,2-propanediol oxidoreductase). Due to this, a maximum yield of 0.55 g PDO/glycerol and additional ATP can be achieved.
Furthermore, in a more sustainable approach, Li and Liao [108] described a photosynthetic conversion of carbon dioxide with a newly engineered cyanobacterium S. elongatus PCC 7942 (Fig. 9b). For the production of 1,2-PDO, genes for methylglyoxal synthase (mgsA), glycerol dehydrogenase (gldA), and aldehyde reductase (yqhD) from E. coli have been inserted. Additionally, the alcohol dehydrogenase (sADHs) from C. beijerinkii is induced into the cyanobacterium. The NADPH pool of S. elongates itself was taken into account for the 1,2-PDO production that requires many reducing equivalents. Therefore, the NADPH-specific secondary alcohol dehydrogenase was newly implemented in the pathway, resulting in the production of 150 mg/L 1,2-PDO from environmental CO2 and light. Despite the progresses made in the implementation of metabolic engineering strategies and developing different new strains, the low reaction rate and product concentration are the most important barriers in its industrial production.
4 Recovery of Diols
The production of diols suitable for chemical or pharmaceutical applications is only achievable by using suitable separation and purification steps (downstream processing) after the fermentation. The downstream processing is one of the most influencing factors, contributing up to 50–70 % of the total product costs [109]. Thus, to have a suitable downstream process is of major interest for an economic and sustainable production of biobased diols. Xiu and Zeng [109] reviewed extensively the downstream processing of 2,3-butanediol and 1,3-propanediol fermentation broths. In the following, the main steps and challenges of recovering diols are briefly mentioned and some of the recent studies are then highlighted.
4.1 Recovery of Butanediol
The principle process of product recovery is almost the same for all diols. After cultivation, the final fermentation broth is a multicomponent mixture not only consisting of water, residual substrate and salts, and side products (e.g. alcohols, organic acids), but also consisting of cells and cell debris in addition to the target product. The initial step is the separation of biomass, which can be performed via centrifugation, filtration, or flocculation [110]. Residual salts may cause fouling on heating devices or inactivation of catalysts. Therefore, they may need to be removed, e.g., by electrodialysis, salting out, or ion exchange chromatography. The excess amount of water in the broth can be reduced by evaporation. The last step to obtain high purification grades is mainly conducted via distillation.
Difficulties in the recovery of butanediol-like 2,3-BDO are mainly caused by the high boiling point (180 °C for 2,3-BDO) and high hydrophilicity. Extractive separation is hampered by its low selectivity and a relatively low distribution coefficient towards extracting solvents. Promising solvents studied include ethyl acetate, tributyl phosphate, diethyl ether, n-butanol, dodecanol, and oleyl alcohol. For example, Anvari and Kayati used the non-toxic oleyl alcohol for an in situ extraction, but separated only 68 % of the total 2,3-BDO produced by K. pneumonia [111]. Improvement of extraction methods represents the combination of solvent extraction and salting-out techniques. The salting-out technique is based on a system of two aqueous phases: one with a hydrophilic solvent and one with highly concentrated salts. The increased ionic strength in the salt phase forces more diols to dissolve in the solvent phase, which is in this case an extractant. Li et al. [112] use a mixture of 32 % (w/w) ethanol and 16 % (w/w) ammonium sulphate to recover 91.7 % of 2,3-BDO next to 99.7 % of cells and 91.2 % of proteins. With 34 % (w/w) 2-propanol and 20 % (w/w) ammonium sulphate, Sun et al. [113] separated 93.7 % of 2,3-BDO. Also here, 99 % of the cells could be removed and reused for a new inoculation, which has a positive effect on the process economics. With butanol and potassium, phosphate salts up to 99 % of the 2,3-BDO can be separated, as revealed by an Aspen Plus simulation performed by Birajdar et al. [114]. However, the 2,3-BDO has to be separated again from the extractants by evaporation, which means additional downstream units with additional costs. The purification of 2,3-BDO directly by distillation is hampered due to the high boiling, and it might be only used to enhance the concentration. Qureshi et al. [115] described a vacuum membrane distillation process, where the membrane retains the 2,3-BDO and let the more volatile compounds (water, ethanol) pass through. The concentration could be increased from 40 to 430 g/L. However, medium components caused membrane fouling, and the water flux decreased at higher 2,3-BDO concentrations [115]. A newly developed process combines reactive extraction and reactive distillation. Li et al. [116] used n-butyraldehyde (BA) as reactant and extractant at the same time. It reacts with 2,3-BDO to 2-propyl-4,5-dimethyl-1,3-dioxolane (PDD), which is extracted by BA itself. Both BA and PDD are transferred into a reactive distillation column, where the catalysts sulphuric acid and hydrochloric acid cleave PDD again into BA and 2,3-BDO. Li et al. were able to recover 90 % of the 2,3-BDO with purity higher than 99 %.
4.2 Recovery of 1,3-Propanediol and 1,2-Propanediol
The downstream processing of 1,2-PDO and 1,3-PDO from fermentation broth is similar, but could be even more challenging than the recovery of butanediol because of their higher boiling points (188 and 233 °C, respectively).
Liquid–liquid extraction could be more advantageous for PDO because it is selective and more energy efficient than distillation. Malinowsky tested different solvents, such as the series of pentanol until nonanol and hexanal until decanal, and other organic solvent. The best results were achieved with aliphatic alcohols and aldehydes, but the distribution of PDO in the solvents has been very low. Thus, large amounts of solvents would be required [117]. Li et al. [118] described the extraction and salting-out method using ethanol and sodium carbonate. They could separate 97.9 % of the 1,3-PDO and were able to separate 99.1 % of cells and other fermentation products, such as organic acids, in one step with this method. A combination of methanol and dipotassium hydrogen phosphate leads to a slightly higher 1,3-PDO recovery of 98.1 % [119]. In addition, the main side product 2,3-BDO, as well as organic acids, could also be recovered. Müller et al. [120] used ionic liquids as extractants in combination with phosphate salts. Despite the fact that high distribution coefficients for 1,3-PDO could be achieved, extraction with ionic liquids is too expensive and not available for in situ processes due to their high toxicity for the bacteria. Another possibility is the reactive extraction of 1,3-PDO with formaldehyde or acetaldehyde into 2-methyl-1,3-dioxane. The extraction of the product is enabled by the organic solvent extractants ο-xylene, toluene, or ethylbenzene [121]. In a recent approach, Matsumoto et al. [122] use 1-butanal as reactant and toluene as diluent together with a hydrophobic acidic ionic liquid as a catalyst for the acetalization of 1,3-PDO into a dioxan. With this method, 96 % of the 1,3-PDO could be converted and extracted [122]. Possible drawbacks of this method are undesired reactions of reactant and fermentation by-products, forming further undesired components and causing loss of reactant. The reactive extraction of 1,2-PDO from aqueous environment was described by Broekhuis et al. [123]. 1,2-PDO reacts with acetaldehyde to form 2,4-dimethyl-1,3-dioxolane. In the next step, dioxalan is cleaved via hydrolysis into 1,2-PDO and acetaldehyde. Again, the last step in purification comprises a distillation column. Separation and purification combined in one operation unit can be realized in adsorption processes. With a sulphonate exchange resin, Hilaly and Binder were able to separate 95 % of the 1,3-PDO with a purity of 87 %. This process, however, had a high water demand, resulting in high energy cost [124]. For further cost reduction, Wang et al. [125] used a low-cost cation exchange resin based on polystyrene with high adsorption capacity to recover 1,3-PDO from fermentation broth. Other possibilities are adsorption on silica resin [126] or on beta zeolites [127]. In general, they are very selective, exhibit a simple design, are easy to operate, and are environmentally friendly because the absorbance material can be recovered [125]. However, they are difficult to be implemented in large-scale processes, due to high exchange surfaces and subsequent large pressure loss, together with high tendencies for fouling [109]. In addition, every adsorption process also requires a desorption step with additional costs.
5 Concluding Remarks
As summarized above, significant progresses have been made in the biosynthesis of different diols from various substrates. Quite clearly, for some of the diols, the microbial inherent weaknesses, such as the low product yield, slow reaction rate, high separation cost, and intolerance to toxic products, are the largest obstacles to the cost-competitive biotechnological production (e.g. 1,2-PDO and 1,3-BDO). A more profound comprehension of cell factories’ physiology and stress responses would necessarily offer improved tools (at either genetic, metabolic, or system levels) to favour high diol yield and high-quality production. In the past few years, steps taken towards these goals enhanced the bioproduction process economics of some diols significantly. Biobased 1,3-PDO, 1,4-BDO, and 2,3-BDO are successful examples. Still, providing low-cost production process limits the competitiveness of some processes, and hence, much R&D efforts are further needed which may include:
-
1.
Production of high-quality diols suitable for high-value products. This requires system-level understanding of the synthetic pathways to target the formation of desired isomers of diols within cell factories. Pure compounds of optically active 2,3-BDO, 1,3-BDO, 1,4-BDO, or 1,2-PDO are considered as high-value products. It is worth mentioning that chiral synthesis or separation remains a costly step in chemical synthesis, and hence, using enzymes or cells to synthesize compounds with high enantiomeric purity represents an alternative and effective approach.
-
2.
Formation of multiproduct in a biorefinery approach will reduce the process costs significantly. Hence, several conversion technologies (thermochemical, biochemical, etc.) are combined together to reduce the overall cost, as well as to have a better flexibility in product generation and to provide its own power. Examples are the simultaneous production of 1,3-PDO and biogas in unsterile process or the coproduction of 1,3-PDO and butanol.
-
3.
Development of robust microbial cell factories with wide substrate utilization specificities that can dominate in wide number of niches. Lignocellulosic residues that are plentiful and cheap have been widely investigated but their recalcitrance to degradation challenge the production of diols biotechnologically. Hence, adapted cell factories to inhibitors and environmental stresses in such raw substrates are then crucial for forthcoming diol production.
-
4.
Exploring new derivatives or uses of diols that will open new markets. Whereas bioprocesses for 2,3-BDO are well established in terms of productivity, yield, and titre, the market size for 2,3-BDO itself is still relatively small.
-
5.
Downstream processing of diols is technically feasible, and a relatively high purification grade can be achieved, though the costs could be rather high. As a cost-effective method, in situ product recovery integrated with the fermentation process should gain more attention in the future.
Designing new-generation bioprocesses increasingly depend on engineering process-compatible cell factories. The latter, whether through genetic or physiological manipulations, can be greatly assisted by metabolic engineering. To achieve these goals, more fundamental knowledge is needed about metabolic pathways, control mechanisms, and process dynamics to optimally design integrated systems. Chemical engineers, metabolic engineers, and microbial physiologist will have to work for such integrated process. We argue that only by developing cost-efficient processes through integration of fermentation and downstream processing, the microbial production of diols can fulfil their potentials as platform chemicals.
References
Burk MJ (2010) Sustainable production of industrial chemicals from sugars. Int Sugar J 112:30–35
Zeng AP, Sabra W (2011) Microbial production of diols as platform chemicals: recent progresses. Curr Opin Biotechnol 22:749–757
Adkins J, Pugh S, McKenna R, Nielsen DR (2012) Engineering microbial chemical factories to produce renewable “biomonomers”. Front Microbiol 3:313
Celinska E (2010) Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol Adv 28:519–530
Sabra W, Dai JY, Quitmann H, Zen, Zeng AP, Xiu ZL (2011) Microbial production of 2, 3-butanediol. Industrial Biotechnology and Commodity Products. Compr Biotechnol 3:87–97. doi:10.1016/B978-0-08-088504-9.00161-6
Saxena RK, Anand P, Saran S, Isar J (2009) Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnol Adv 27:895–913
Tjahjasari D, Kaeding T, Zeng AP (2011) 1,3-Propanediol and Polytrimethyleneterephthalate. Compr Biotechnol 3:229
Ma C, Wang A, Qin J, Li L, Ai X, Jiang T, Tang H, Xu P (2009) Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl Microbiol Biotechnol 82:49–57
Petrov K, Petrova P (2009) High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl Microbiol Biotechnol 84:659–665
Qin J, Xiao Z, Ma C, Xie N, Liu P, Xu P (2006) Production of 2,3-butanediol by Klebsiella pneumoniae using glucose and ammonium phosphate. Chinese J Chem Eng 14:132–136
Li L, Li K, Wang Y, Chen C, Xu Y, Zhang L, Han B, Gao C, Tao F, Ma C (2015) Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab Eng 2015(28):19–27
Li L, Su F, Wang Y, Zhang L, Liu C, Li J, Ma C, Xu P (2012) Genome sequences of two thermophilic Bacillus licheniformis strains, efficient producers of platform chemical 2,3-butanediol. J Bacteriol 194:4133–4134
Li J, Wang W, Ma Y, Zeng AP (2013) Medium optimization and proteome analysis of (R, R)-2,3-butanediol production by Paenibacillus polymyxa ATCC 12321. Appl Microbiol Biotechnol 97:585–597
Chen C, Wei D, Shi J, Wang M, Hao J (2014) Mechanism of 2,3-butanediol stereoisomer formation in Klebsiella pneumoniae. Appl Microbiol Biotechnol 98:4603–4613
Xiao Z, Lv C, Gao C, Qin J, Ma C, Liu Z, Liu P, Li L, Xu P (2010) A novel whole-cell biocatalyst with NAD+ regeneration for production of chiral chemicals. PLoS ONE 5:e8860
Ji XJ, Huang H, Ouyang PK (2011a) Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol Adv 3:351
Xu Y, Chu H, Gao C, Tao F, Zhou Z, Li K, Li L, Ma C, Xu P (2014) Systematic metabolic engineering of Escherichia coli for high-yield production of fuel bio-chemical 2,3-butanediol. Metab Eng 23:22–33
Jurchescu IM, Hamann J, Zhou X, Ortmann T, Kuenz A, Prusse U, Lang S (2013) Enhanced 2,3-butanediol production in fed-batch cultures of free and immobilized Bacillus licheniformis DSM 8785. Appl Microbiol Biotechnol 97:6715–6723
Li L, Zhang L, Li K, Wang Y, Gao C, Han B, Ma C, Xu P (2013) A newly isolated Bacillus licheniformis strain thermophilically produces 2,3-butanediol, a platform and fuel bio-chemical. Biotechnol Biofuels 6:123
Li L, Chen C, Li K, Wang Y, Gao C, Ma C, Xu P (2014) Efficient simultaneous saccharification and fermentation of inulin to 2,3-butanediol by thermophilic Bacillus licheniformis ATCC 14580. Appl Environ Microbiol 80:6458–6464
Celinska E, Grajek W (2009) Biotechnological production of 2,3-butanediol—current state and prospects. Biotechnol Adv 27:715–725
Magee RJ, Kosaric N (1987) The microbial production of 2,3-butanediol. Adv Appl Microbiol 32:89–161
Nakashimada Y, Kanai K, Nishio N (1998) optimization of dilution rate, pH and oxygen supply on optical purity of 2,3 butanediol produced by Paenibacillus polymyxa in chemostat culture. Biotechnol Lett 20:1133–1138
Nakashimada Y, Marwoto B, Kashiwamura T, Kakizono T, Nishio N (2000) Enhanced 2,3-butanediol production by addition of acetic acid in Paenibacillus polymyxa. J Biosci Bioeng 90:661–664
Yu B, Sun J-B, Bommareddy R, Song LF, Zeng AP (2011) A novel (2R,3R)-2,3-Butanediol Dehydrogenase from an industrially potential strain Paenibacillus polymyxa ATCC12321. Appl Environ Microbiol 77:4230 (accepted)
Fu J, Wang Z, Chen T, Liu W, Shi T, Wang G, Tang YJ, Zhao X (2014) NADH plays the vital role for chiral pure D-(-)-2,3-butanediol production in Bacillus subtilis under limited oxygen conditions. Biotechnol Bioeng 111:2126
Zeng AP, Biebl H, Deckwer WD (1991) Production of 2,3-butanediol in a membrane bioreactor with cell recycle. Appl Microbiol Biotechnol 34:463–468
Wang A, Xu Y, Ma C, Gao C, Li L, Wang Y, Tao F, Xu P (2012) Efficient 2,3-butanediol production from cassava powder by a crop-biomass-utilizer, Enterobacter cloacae subsp. dissolvens SDM. PLoS ONE 7:e40442
Ji XJ, Huang H, Du J, Zhu JG, Ren LJ, Li S, Nie ZK (2009) Development of an industrial medium for economical 2,3-butanediol production through co-fermentation of glucose and xylose by Klebsiella oxytoca. Bioresour Technol 100:5214–5218
Zhang L, Sun J, Hao Y, Zhu J, Chu J, Wei D, Shen Y (2010) Microbial production of 2,3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30. J Ind Microbiol Biotechnol 37:857–862
Li L, Li K, Wang K, Chen C, Gao C, Ma C, Xu P (2014) Efficient production of 2,3-butanediol from corn stover hydrolysate by using a thermophilic Bacillus licheniformis strain. Bioresour Technol 170:256–261
Wang Y, Li L, Ma C, Gao C, Tao F, Xu P (2013) Engineering of cofactor regeneration enhances (2S,3S)-2,3-butanediol production from diacetyl. Sci Rep 2013(3):2643
Lian J, Chao R, Zhao H (2014) Metabolic engineering of a Saccharomyces cerevisiae strain capable of simultaneously utilizing glucose and galactose to produce enantiopure (2R,3R)-butanediol. Metab Eng 23:92–99
Xiao Z, Xu P (2007) Acetoin metabolism in bacteria. Crit Rev Microbiol 33:127–140
Köpke M, Mihalcea C, Liew F, Tizard JH, Ali MS, Conolly JJ, Al-Sinawi B, Simpson SD (2011) 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl Environ Microbiol 77:5467–5475
Yu EK, Deschatelets L, Louis-Seize G, Saddler JN (1985) Butanediol production from cellulose and hemicellulose by Klebsiella pneumoniae grown in sequential coculture with Trichoderma harzianum. Appl Environ Microbiol 50:924–929
Yu EK, Saddler JN (1982) Enhanced production of 2,3-butanediol by Klebsiella pneumoniae grown on high sugar concentrations in the presence of acetic acid. Appl Environ Microbiol 44:777–784
Köpke M, Gerth ML, Maddock DJ, Mueller AP, Liew F, Simpson SD, Patrick WM (2014) Reconstruction of an acetogenic 2,3-butanediol pathway involving a novel NADPH-dependent primary-secondary alcohol dehydrogenase. Appl Environ Microbiol 80:3394–3403
Ui S, Masuda T, Masuda H, Muraki H (1986) Mechanism for the formation of 2,3-butanediol stereoisomers in Bacillus polymyxa. J Ferment Technol 64:481–486
Ji XJ, Nie ZK, Huang H, Ren LJ, Peng C, Ouyang PK (2011) Elimination of carbon catabolite repression in Klebsiella oxytoca for efficient 2,3-butanediol production from glucose-xylose mixtures. Appl Microbiol Biotechnol 89:1119–1125
Zhang CY, Peng XP, Li W, Guo XW, Xiao DG (2014) Optimization of 2,3-butanediol production by Enterobacter cloacae in simultaneous saccharification and fermentation of corncob residue. Biotechnol Appl Biochem 61:501
Oliver JW, Machado IM, Yoneda H, Atsumi S (2013) Cyanobacterial conversion of carbon dioxide to 2,3-butanediol. Proc Natl Acad Sci USA 110:1249–1254
Yan Y, Lee CC, Liao JC (2009) Enantioselective synthesis of pure (R, R)-2,3-butanediol in Escherichia coli with stereospecific secondary alcohol dehydrogenases. Org Biomol Chem 7:3914–3917
Li L, Wang Y, Zhang L, Ma C, Wang A, Tao F, Xu P (2012) Biocatalytic production of (2S,3S)-2,3-butanediol from diacetyl using whole cells of engineered Escherichia coli. Bioresour Technol 115:111–116
Nan H, Seo SO, Oh EJ, Seo JH, Cate JH, Jin YS (2014) 2,3-Butanediol production from cellobiose by engineered Saccharomyces cerevisiae. Appl Microbiol Biotechnol 98:5757–5764
Yim H, Haselbeck R, Niu W, Pujol-Baxley C, Burgard A, Boldt J, Khandurina J, Trawick JD, Osterhout RE, Stephen R, Estadilla J, Teisan S, Schreyer HB, Andrae S, Yang TH, Lee SY, Burk MJ, Van DS (2011) Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat Chem Biol 7:445–452
Werawattanachai N, Towiwat P, Unchern S, Maher TJ (2007) Neuropharmacological profile of tetrahydrofuran in mice. Life Sci 80:1656–1663
Diaz A, Katsarava R, Puiggali J (2014) Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: from polyesters to poly(ester amide)s. Int J Mol Sci 15:7064–7123
Burk MJ, Burgard AP, Osterhout RE, Sun J (2012) Microorganisms for the production of 1,4-Butanediol. US Patent 8, 178, 327, B2. (13/009,813)
Pharkya P, Burgard AP, Van Dien SJ, Osterhout RE, Burk MJ, Trawick JD, Kuchinskas MP, Steer B (2015) Microorganisms and methods for production of 4-Hydroxybutyrate, 1,4-Butanediol and related compounds. US patent 20150148513
Cosmetic Ingredient Review (1985) Final report on the safety assessment of butylene glycol, hexylene glycol, ethoxydiglycol, and dipropylene glycol. J Am Coll Toxicol 5:223–248
Matsuyama A, Yamamoto H, Kawada N, Kobayashi Y (2001) Industrial production of (R)-1,3-butanediol by new biocatalysts. J Mol Catal B Enzym 11:513–521
Kataoka N, Vangnai AS, Tajima T, Nakashimada Y, Kato J (2013) Improvement of (R)-1,3-butanediol production by engineered Escherichia coli. J Biosci Bioeng 115:475–480
Kataoka N, Vangnai AS, Ueda H, Tajima T, Nakashimada Y, Kato J (2014) Enhancement of (R)-1,3-butanediol production by engineered Escherichia coli using a bioreactor system with strict regulation of overall oxygen transfer coefficient and pH. Biosci Biotechnol Biochem 78:695–700
Zheng RC, Ge Z, Qiu ZK, Wang YS, Zheng YG (2012) Asymmetric synthesis of (R)-1,3-butanediol from 4-hydroxy-2-butanone by a newly isolated strain Candida krusei ZJB-09162. Appl Microbiol Biotechnol 94:969–976
Eguchi T, Mochida K (1992) Lipase-catalyzed diacylation of 1,3-butanediol. Biotechnol Lett 15:955–960
Yamamoto H, Matsuyama A, Kobayashi Y (2002) Synthesis of (R)-1,3-butanediol by enantioselective oxidation using whole recombinant Escherichia coli cells expressing (S)-specific secondary alcohol dehydrogenase. Biosci Biotechnol Biochem 66:925–927
Okabayashi T, Nakajima T, Yamamoto H (2012) Recombinant microorganisms with 1,3-butanediol producing function and uses thereof. Daicel Corporation, editor, Osaka, Japan. US patent 13/504,391(US20120276606 A1)
Drozdzynska A, Leja K, Czaczyk K (2011) Biotechnological production of 1,3-Propanediol from crude glycerol. J Biotechnol Comput Biol Bionanotechnol 92:92–100
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Chatzifragkou A, Dietz D, Komaitis M, Zeng AP, Papanikolaou S (2010) Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnol Bioeng 107:76–84
Wang Y, Tao F, Ni J, Li C, Xu P (2015) Production of C3 platform chemicals from CO2 by genetically engineered cyanobacteria. Green Chem 17:3100–3110
Dietz D, Zeng AP (2014) Efficient production of 1,3-propanediol from fermentation of crude glycerol with mixed cultures in a simple medium. Bioprocess Biosyst Eng 37:225–233
Chatzifragkou A, Papanikolaou S, Dietz D, Doulgeraki AI, Nychas GJE, Zeng AP (2011) Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl Microbiol Biotechnol 91:101
Kaeding T, DaLuz J, Kube J, Zeng AP (2014) Integrated study of fermentation and downstream processing in a miniplant significantly improved the microbial 1,3-propanediol production from raw glycerol. Bioprocess Biosyst Eng 38:3
Friedmann H, Zeng A-P (2008) Process and apparatus for the microbial production of a specific product and methane. EU patent: DE 102007001614 WO 2006/021087 A1 3/2006
Bizukojc M, Dietz D, Sun J, Zeng AP (2010) Metabolic modelling of syntrophic-like growth of a 1,3-propanediol producer, Clostridium butyricum, and a methanogenic archeon, Methanosarcina mazei, under anaerobic conditions. Bioprocess Biosyst Eng 33:507–523
Zhou M, Chen J, Freguia S, Rabaey K, Keller J (2013) Carbon and electron fluxes during the electricity driven 1,3-propanediol biosynthesis from glycerol. Environ Sci Technol 47:11199–11205
Choi O, Kim T, Woo HM, Um Y (2014) Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci Rep 4:6961
Lovley DR (2012) Electromicrobiology. Annu Rev Microbiol 66:391–409
Rosenbaum MA, Franks AE (2014) Microbial catalysis in bioelectrochemical technologies: status quo, challenges and perspectives. Appl Microbiol Biotechnol 98:509–518
da Cunha MV, Foster MA (1992) Sugar-glycerol cofermentations in lactobacilli: the fate of lactate. J Bacteriol 174:1013–1019
Pflügl S, Marx H, Mattanovich D, Sauer M (2012) 1,3-Propanediol production from glycerol with Lactobacillus diolivorans. Bioresour Technol 119:133–140
Sabra W, Groeger C, Sharma PN, Zeng AP (2014) Improved n-butanol production by a non-acetone producing Clostridium pasteurianum DSMZ 525 in mixed substrate fermentation. Appl Microbiol Biotechnol 98:4267–4276
Günzel B, Yonsel S, Deckwer WD (1991) Fermentative production of 1, 3-propanediol from glycerol by Clostridium butyricum up to a scale of 2m3. Appl Microbiol Biotechnol 36:289–294
Saint-Amans S, Perlot P, Goma G, Soucaille P (1994) High production of 1,3-propanediol from glycerol by Clostridium butyricum VPI 3266 in a simply controlled fed-batch system. Biotechnol Lett 16:831–836
Abbad-Andaloussi S, Manginot-Durr C, Amine J, Petitdemange E, Petitdemange H (1995) Isolation and characterization of Clostridium butyricum DSM 5431 mutants with increased resistance to 1,3-propanediol and altered production of acids. Appl Environ Microbiol 61:4413–4417
Petitdemange E, Dürr C, Abbad-Andaloussi S, Raval G (1995) Fermentation of raw glycerol to 1,3-propanediol by new strains of Clostridium butyricum. J Ind Microbiol 15:498–502
Reimann A, Biebl H (1996) Production of 1, 3-propanediol by Clostridium butyricum DSM 5431 and product tolerant mutants in fedbatch culture: feeding strategy for glycerol and ammonium. Biotechnol Lett 18:827–832
Hartlep M, Zeng A-P (2002) Fedbatch-Verfahren für die mikrobielle Herstellung von 1, 3-Propandiol in Klebsiella pneumoniae und Clostridium butyricum. Chem Ing Tech 74:663–664
Szymanowska-Powalowska D (2014) 1,3-Propanediol production from crude glycerol by Clostridium butyricum DSP1 in repeated batch. Electron J Biotechnol 17:322–328
Himmi EH, Bories A, Barbirato F (1999) Nutrient requirements for glycerol conversion to 1,3-propanediol by Clostridium butyricum. Biores Technol 67:123–128
Gonzalez-Pajuelo M, Meynial-Salles I, Mendes F, Andrade JC, Vasconcelos I, Soucaille P (2005) Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol. Metab Eng 7:329–336
Hirschmann S, Koschik I, Baganz K, Vorlop K (2005) Development of an integrated bioconversion process for the production of 1,3-propanediol from raw glycerol waters. Landbauforschung Völkenrode 55:261–267
Jensen TO, Kvist T, Mikkelsen MJ, Westermann P (2012) Production of 1,3-PDO and butanol by a mutant strain of Clostridium pasteurianum with increased tolerance towards crude glycerol. AMB Express 2:44
Metsoviti M, Zeng A-P, Koutinas AA, Papanikolaou S (2013) Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J Biotechnol 163:408–418
Yang G, Tian J, Li J (2007) Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Appl Microbiol Biotechnol 73:1017–1024
Jolly J, Hitzmann B, Ramalingam S, Ramachandran KB (2014) Biosynthesis of 1,3-propanediol from glycerol with Lactobacillus reuteri: effect of operating variables. J Biosci Bioeng 118:188–194
Biebl H (1991) Glycerol fermentation of 1,3-propanediol by Clostridium butyricum. Measurement of product inhibition by use of a pH-auxostat. Appl Microbiol Biotechnol 35:701–705
Zeng A-P (1996) Pathway and kinetic analysis of 1,3-propanediol production from glycerol fermentation by Clostridium butyricum. Bioprocess Eng 14:169–175
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng Biotechnol 74:239–259
Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol 31:20–28
Rieckenberg F, Ardoa I, Rujananon R, Zeng AP (2014) Cell-free synthesis of 1,3-propanediol from glycerol with a high yield. Eng Life Sci 14:380–386
Zhang YH (2014) Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges. Biotechnol Adv. doi:10.1016/j.biotechadv.2014.10.009
Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol 14:454–459
Chen Z, Geng F, Zeng AP (2015) Protein design and engineering of a de novo pathway for microbial production of 1,3-propanediol from glucose. Biotechnol J 10:284–289
Celinska E (2015) Fully glycerol-independent microbial production of 1,3-propanediol via non-natural pathway: paving the way to success with synthetic tiles. Biotechnol J 10:242–243
Jung JY, Yun HS, Lee J, Oh MK (2011) Production of 1,2-propanediol from glycerol in Saccharomyces cerevisiae. J Microbiol Biotechnol 21:846–853
Clomburg JM, Gonzalez R (2011) Metabolic engineering of Escherichia coli for the production of 1,2-propanediol from glycerol. Biotechnol Bioeng 108:867–879
Rode CV, Ghalwadkar AA, Mane RB, Hengne AM, Jadkar ST, Biradar NS (2010) Selective hydrogenolysis of glycerol to 1,2-propanediol: comparison of batch and continuous process operations. Org Process Res Dev 14:1385–1392
Cameron DC, Cooney CL (1986) A novel fermentation: the production of (R)-1,2-propanediol and acetol by Clostridium thermosaccharolyticum. Biores Technol 4:651–654
Altaras N, Etzel M, Cameron DC (2001) Conversion of sugars to 1,2-propanediol by Thermoanaerobacterium thermosaccharolyticum HG-8. Biotechnol Prog 17:52–56
Turner KW, Roberton AM (1979) Xylose, arabinose, and rhamnose fermentation by Bacteroides ruminicola. Appl Environ Microbiol 38:7–12
Tran-Din K, Gottschalk G (1985) Formation of D (-)-1, 2-propanediol and D (-)-lactate from glucose by Clostridium sphenoides under phosphate limitation. Arch Microbiol 142:87–92
Oude Elferink SJ, Krooneman J, Gottschal JC, Spoelstra SF, Faber F, Driehuis F (2001) Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Appl Environ Microbiol 67:125–132
Bennett GN, San KY (2001) Microbial formation, biotechnological production and applications of 1,2-propanediol. Appl Microbiol Biotechnol 55:1–9
Koch D, Meurer S-J, Eck J (2014) New means and methods for producing propanediol. BRAIN Biotechnology Research and Information Network AG, editor, Zwingenberg, Germany. US patent: 14/125,409(US 2014/0178953 A1), p 1–12
Li H, Liao JC (2013) Engineering a cyanobacterium as the catalyst for the photosynthetic conversion of CO2 to 1,2-propanediol. Microb Cell Fact 12:4
Xiu ZL, Zeng A-P (2008) Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl Microbiol Biotechnol 78:917–926
Willke T, Vorlop K (2008) Biotransformation of glycerol into 1,3 propanediol. Eur J Lipid Sci Technol 110:831–840
Anvari M, Khayati G (2009) In situ recovery of 2,3-butanediol from fermentation by liquid-liquid extraction. J Ind Microbiol Biotechnol 36:313–317
Li Z, Teng H, Xiu Z-L (2010) Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/ammonium sulfate system. Proc Biochem 45:731–737
Sun LH, Jiang B, Xiu Z-L (2009) Aqueous two-phase extraction of 2,3-butanediol from fermentation broths by isopropanol/ammonium sulfate system. Biotechnol Lett 31:371–376
Birajdar SD, Rajagopalan S, Sawant JS, Padmanabhan S (2015) Continuous countercurrent liquid-liquid extraction method for the separation of 2,3-butanediol from fermentation broth using n-butanol and phosphate salt. Proc Biochem. doi:10.1016/j.procbio.2015.05.016
Qureshi N, Meagher MM, Hutkins RW (1994) Recovery of 2,3-Butanediol by vacuum membrane distillation. Sep Sci Technol 29:1733–1748
Li Y, Wu Y, Zhu J, Liu J, Shen Y (2013) Separating 2,3-butanediol from fermentation broth using n-butylaldehyde. J Saudi Chem Soc. doi:10.1016/j.jscs.2013.02.005
Malinowski J (1999) Evaluation of liquid extraction potentials for downstream separation of 1,3-propanediol. Biotechnol Tech 13:127–130
Li Z-G, Sun Y-Q, Zheng W-L, Teng H, Xiu Z-L (2013) A novel and environment-friendly bioprocess of 1,3-propanediol fermentation integrated with aqueous two-phase extraction by ethanol/sodium carbonate system. Biochem Eng J 80:68–75
Li Z, Teng H, Xiu Z (2011) Extraction of 1,3-propanediol from glycerol-based fermentation broths with methanol/phosphate aqueous two-phase system. Proc Biochem 46:586–591
Müller A, Gorak A (2012) Extraction of 1,3-propanediol from aqueous solutions using different ionic liquid-based aqueous two-phase systems. Sep Purif Technol 97:130–136
Malinowski J (2000) Reactive extraction for downstream separation of 1,3-Propanediol. Biotechnol Prog 16:76–79
Matsumoto M, Nagai K, Kondo K (2015) Reactive extraction of 1, 3-propanediol with aldehydes in the presence of a hydrophobic acidic ionic liquid as a catalyst. Solvent Extr Res Dev Jpn 22:209–213
Broekhuis RR, Lynn S, King CJ (1994) Recovery of propylene glycol from dilute aqueous solutions via reversible reaction with aldehydes. Ind Eng Chem Res 33:3230–3237
Hilaly A, Binder T (2002) Method of recovering 1,3-propanediol from fermentation broth. US Patent 6479716
Wang S, Dai H, Yan Z, Zhu C, Huang L, Fang B (2014) 1,3-Propanediol adsorption on a cation exchange resin: adsorption isotherm, thermodynamics and mechanistic studies. Eng Life Sci 14:485–492
Cho M-H, Im Joen S, Pyo S-H, Mun S, Kim J-H (2006) A novel separation and purification process for 1,3-propanediol. Proc Biochem 41:739–744
Wang Z, Wu Z, Tan T (2013) Sorption equilibrium, mechanism and thermodynamics studies of 1,3-propanediol on beta zeolite from an aqueous solution. Bioresour Technol 145:37–42
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sabra, W., Groeger, C., Zeng, AP. (2015). Microbial Cell Factories for Diol Production. In: Ye, Q., Bao, J., Zhong, JJ. (eds) Bioreactor Engineering Research and Industrial Applications I. Advances in Biochemical Engineering/Biotechnology, vol 155. Springer, Berlin, Heidelberg. https://doi.org/10.1007/10_2015_330
Download citation
DOI: https://doi.org/10.1007/10_2015_330
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49159-1
Online ISBN: 978-3-662-49161-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)