Abstract
This review is a retrospective of ecological effects of bioactivities produced by biofilms of surface-dwelling marine/intertidal microbes as well as of the industrial and environmental biotechnologies developed exploiting the knowledge of biofilm formation. Some examples of significant interest pertaining to the ecological aspects of biofilm-forming species belonging to the Roseobacter clade include autochthonous bacteria from turbot larvae-rearing units with potential application as a probiotic as well as production of tropodithietic acid and indigoidine. Species of the Pseudoalteromonas genus are important examples of successful surface colonizers through elaboration of the AlpP protein and antimicrobial agents possessing broad-spectrum antagonistic activity against medical and environmental isolates. Further examples of significance comprise antiprotozoan activity of Pseudoalteromonas tunicata elicited by violacein, inhibition of fungal colonization, antifouling activities, inhibition of algal spore germination, and 2-n-pentyl-4-quinolinol production. Nitrous oxide, an important greenhouse gas, emanates from surface-attached microbial activity of marine animals. Marine and intertidal biofilms have been applied in the biotechnological production of violacein, phenylnannolones, and exopolysaccharides from marine and tropical intertidal environments. More examples of importance encompass production of protease, cellulase, and xylanase, melanin, and riboflavin. Antifouling activity of Bacillus sp. and application of anammox bacterial biofilms in bioremediation are described. Marine biofilms have been used as anodes and cathodes in microbial fuel cells. Some of the reaction vessels for biofilm cultivation reviewed are roller bottle, rotating disc bioreactor, polymethylmethacrylate conico-cylindrical flask, fixed bed reactor, artificial microbial mats, packed-bed bioreactors, and the Tanaka photobioreactor.
Graphical Abstract

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
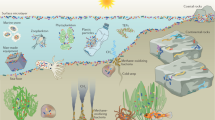
Surface colonization is a universal phenomenon in marine systems. Microorganisms frequently live as biofilm communities which are consortia of cells having high density, contained in an extracellular matrix, and possessing microcolony structures or other multicellular arrangements [1]. The intertidal zone, occupying the upper margin of the world’s coastline, covers 1,600,000 km and comprises rocky shores, sandy beaches, mudflats, estuaries, salt marshes, mangrove forests, coral reefs, and manmade civil structures. This region is an important coastal habitat in terms of biological productivity and economic value [2]. Intertidal microbial communities often exist as biofilms. Biofilms form protective microenvironments in the changing environments of intertidal regions and support a variety of microbial processes [3]. Complex and highly differentiated surface microbial communities arise due to close spatial proximity of microbial cells which produces specific intercellular communications. A highly competitive environment due to space and nutrient limitation has forced the surface-dwelling microbes to develop adaptive responses and antagonistic strategies to prevent potential competitors occupying their habitat. During surface colonization of eukaryotes, the function and composition of surface microbial consortia are significantly influenced by chemical communication and interactions between the microbes and their eukaryotic hosts. Therefore, multiple factors define the properties and composition of a microbial surface community. The worldwide demand for developing novel bioactives has projected the unique, interactive, and highly diverse environment of marine and intertidal surfaces as a potential space for biotechnological innovations [1].
Microbial ecology and biotechnology could be innately linked to each other: microbial ecology providing the scientific basis for the processes aimed to attain the realistic goals of biotechnology and biotechnological processes affording fascinating ecosystems for microbial ecologists to investigate and evolve their concepts and methodologies. One biological system in which there is a direct association between the knowledge of the lifestyle of the marine organisms and biotechnological applications therefrom is the biofilm. This two-way learning process has proved successful in developing new methods for the prevention of marine biofouling. This review is a retrospective of first, the investigations focused on marine microbes living on surfaces and their bioactivities that have made them successful colonizers, and second, the biotechnologies (industrial and environmental) developed exploiting the knowledge of efficacious biofilm formation.
2 Ecological Perspectives
Microbial biofilms can be quite uneven in distribution in species as well as in chemical composition. Monospecific biofilms can have complex as well as positive or negative influences on marine flora and fauna attributed mainly to the production of antibiotic compounds and stimulatory chemical signals. Chemical compounds produced by microorganisms can alter biofilm structure by disrupting or enhancing the growth of existing biofilms. They can induce or inhibit eukaryotic larval settlement on living and nonliving surfaces [4]. In this section, some examples of these complex relationships have been provided from the Roseobacter clade and the genus Pseudoalteromonas. Nitrous-oxide—emitting marine biofilms that can have profound effects on global warming form a separate subsection.
2.1 Biofilm Formation and Bioactivities of the Roseobacter Clade
Bacteria belonging to the Roseobacter clade are very efficient colonizers and both attachment as well as biofilm formation has been associated with the ability of members of the clade to grow in a “multicellular rosette shape” [5, 6]. In this subsection some of the recent investigations on biofilm formation and the production of bioactive compounds by different species of the Roseobacter clade have been reviewed from the standpoint of microbial ecology.
Bruhn et al. demonstrated that the culture supernatant of Roseobacter 27-4 was inhibitory to the turbot egg yolk sac larval pathogens Vibrio anguillarum and Vibrio splendidus [5]. Known antibacterial compounds, thiotropocin or its strongly related precursor tropodithietic acid, were identified as the active principles. A thick biofilm characterized by “multicellular star-shaped aggregated cells” formed at the air–liquid interface when Roseobacter 27-4 was cultivated under static growth conditions. Antibacterial activity was associated with the appearance of a brownish pigment. Although aerated conditions increased cell yield 10-fold, cultures were nonpigmented, grew as single cells, and no antibacterial activity was observed. The star shape was critical for the organism to amass into a thick biofilm. Detection of 3-hydroxy-decanoyl homoserine lactone in cultures of Roseobacter 27-4 indicated quorum control of antibacterial production. Thus, attachment and biofilm-forming characteristics may be pivotal for survival in the marine environment. Roseobacter strain 27-4 was also obtained from aquaculture tank walls where the identical subtype remained over one year [7, 8]. Potential use of Roseobacter 27-4 as a probiotic culture should ensure that it is cultivated in stagnant or adhered states.
Bruhn et al. developed a real-time PCR method that allowed direct quantification of bacteria on a surface. Cell densities were determined by plate counting and by real-time PCR. “Cycle threshold value (C T )” was the cycle at which fluorescence achieved an identified threshold. It corresponded to the cycle at which a statistically significant enhancement in fluorescence was first observed. Bruhn et al. noted that the number of cycles required for the amplification-associated fluorescence to attain a specific threshold level of detection (the C T value) was inversely related to the amount of nucleic acid present in the sample [9]. The quantity of nucleic acid in the sample was proportional to the number of cells in CFU/ml. Therefore, the lowest CFU/ml on agar plates having the lowest amount of nucleic acid gave the highest C T value and vice versa. Values of C T and CFU/ml were compared by linear regression and resulted in a linear correlation coefficient (R 2) of 0.991. The lowest C T value obtained by the minimum treatment without sodium dodecyl sulfate indicated the presence of the highest number of attached cells on the surface [9]. The authors concluded that by applying species-specific primers, this method should be useful in studying microbial surface colonization as well as in quantitative evaluation of novel antifouling surfaces or newly described cleaning and disinfection methods for removing attached bacteria.
Bruhn et al. further attempted to determine whether production of antibacterial compounds and biofilm formation were common phenotypes found in strains of the Roseobacter clade associated with the dinoflagellate, Pfiesteria piscicida, and whether such activity occurred under defined growth conditions (static or shaken cultures) and was associated with a specific rosette morphotype [10]. Silicibacter sp. TM1040 and Phaeobacter (formerly Roseobacter) strain 27-4 produced the highest amounts of antibacterial substances and their sterile filtered supernatants were lethal to many non-Roseobacter marine bacteria. On the other hand, Roseobacter strains were inhibited only when exposed to concentrated compounds, thus implying the role of these compounds in annihilating competitors. Silicibacter sp. TM1040 cells attached among themselves forming rosettes and produced antibacterial compounds when cultivated in a liquid medium under static conditions. A spontaneous Phaeobacter 27-4 mutant incapable of forming rosettes also could neither develop into a biofilm nor produce antibacterial compounds, suggesting the importance of rosette formation. In 8 of 14 Roseobacter clade strains examined, rosette formation was noted and was very high under static growth in 5 of these strains. Strains able to form rosettes were 13–30 times more efficient in attaching to glass compared to strains where rosette formation was not observed by Bruhn et al. [10].
The active compound of Phaeobacter strain 27-4 possessing antibacterial activity (Fig. 1) was tropodithietic acid (TDA) [5, 11]. Silicibacter sp. strain TM1040 also yielded an antibacterial compound and production in both strains was correlated with the elaboration of a brown pigment. TDA contains two sulfur atoms, which is of interest because bacteria of the Roseobacter clade can metabolize dimethylsulfoniopropionate (DMSP) produced by algae and dinoflagellates [12] which is linked to sulfur cycling in the ocean. DMSP was utilized through the demethylation pathway in Silicibacter sp. strain TM1040 and sulfur from DMSP may be employed in the synthesis of TDA [13]. The ability to synthesize TDA, a compound having an unusual seven-member aromatic tropolone ring backbone was demonstrated in several Roseobacter genera including Ruegeria and Phaeobacter species and is an effective model for Roseobacter secondary metabolite production [14, 15]. D’Alvise et al. [16] enquired if concentrations of bis-(3′- 5′)-cyclic dimeric guanosinmonophosphate (c-di-GMP) inside Ruegeria mobilis cells could be correlated with transitions between biofilm and planktonic modes of growth of this bacterium. In bacteria this compound generally functions as a second messenger regulating biofilm formation [17]. Through genome sequencing, plasmid-directed manipulation of genes, chromatography, and mass spectrophotomeric studies, D’Alvise et al. concluded that biofilm formation in R. mobilis and associated phenotypic characteristics, in particular, TDA production, were c-di-GMP controlled [16].
Porsby et al. demonstrated that Roseobacters displaying inhibitory activity against Vibrio anguillarum colonized a Danish turbot larval farm [18]. The production unit consisted of a fish tank, water cleaning system, and tank with copepod cultures. A bag containing the algal cultures also formed a part of the production unit; however, the significant difference was that the bag did not have a surface for attachment of cells that the other three components had. A total of 43 samples collected from the Danish turbot larval farm were screened and 100 isolates were found to have antagonistic activity against V. anguillarum. There were 54 isolates that showed antagonism in both spot and well diffusion assays, 51 of which were identified as members of the Roseobacter clade. The remaining 46 isolates showed inhibitory activity in spot assay only and 38 of them were acknowledged as Vibrio spp. Through phylogenetic analyses it was shown that Phaeobacter inhibens and Phaeobacter gallaeciensis-like strains were encountered in fish tanks, water cleaning systems, and tanks with copepod cultures of the production site, whereas Ruegeria mobilis or Ruegeria pelagia were present in algal cultures kept in bags. Ruegeria species were naturally occurring in the water from the Danish fiord and not present as a contaminant or inoculated in the bags containing the algal culture. Phaeobacter sp. demonstrated greater inhibitory activity against 17 microbes of the larval unit than Ruegeria sp. When cultivated under shaking and static conditions, Phaeobacter sp. produced TDA and a brown pigment and inhibited Vibrio anguillarum. Ruegeria sp., however, exhibited these three properties only under a static condition similar to the studies [5, 10]. Phaeobacter sp. reported in this study would be more suitable for practical application as a fish probiotic organism than the Phaeobacter strain 27-4 [5, 10] as few regions of an aerated fish tank would have static fluid conditions [18]. The subtypes of Roseobacter isolates of the production site (Pheobacter) differed from the isolates of the bags with algal cultures (Ruegeria) which established the fact that there was a tendency to grow and inhabit specific niches by particular subtypes or they may have been established randomly and continued to grow at that site [18].
Roseobacters are generally recognized to synthesize a multitude of secondary metabolites [5, 19–22]. However, genomic investigations by Newton et al. [23] showed that most sequenced Roseobacters lacked the TDA biosynthesis pathway usually considered as the Roseobacter secondary metabolite production model pathway [14, 15]. Against this background, Cude et al. [24] reported for the first time, synthesis of the blue pigment indigoidine (Fig. 2) by Phaeobacter sp. strain Y41 through a nonribosomal peptide synthase (NRPS)-based biosynthetic pathway coded by a sequence of linked genes, igiBCDFE. Cude et al. correlated indigoidine production by Y41 to growth inhibition of Vibrio fischeri, a hitherto unknown bioactivity of indigoidine [24]. This property provided Y41 a competitive advantage over V. fischeri during surface colonization. When cells were grown planktonically, production of indigoidine was, however, ineffectual. Furthermore, pleiotropic effects of the pigment were demonstrated through indigoidine offering protection to Phaeobacter sp. strain Y41 from reactive oxygen species as well as contributing to swimming motility and surface colonization. Phenotypic observations were supported by gene expression studies, in particular, upregulation of igiD when Y41 grew as biofilms compared to planktonic cultures.
2.2 Biofilm Formation and Bioactivity of the Pseudoalteromonas Genus
Species of the genus Pseudoalteromonas are generally found associated with marine eukaryotes and display antibacterial, agarolytic, and algicidal activities. Several Pseudoalteromonas isolates specifically deter the settlement of common marine fouling organisms. Production of a variety of compounds lethal against many competitor organisms is a distinctive characteristic of this genus. Thus, Pseudoalteromonas cells are advantaged in their contest for nutrients and space and colonization of surfaces, and are protected against predators grazing at surfaces [25].
The most widely investigated species in the genus Pseudoalteromonas is the green-pigmented Gram-negative gamma-proteobacterium P. tunicata. Surfaces of the marine plant Ulva lactuca are colonized by this bacterium and it produces at least five novel inhibitory compounds [26]. A 190-kDa multisubunit antibacterial protein named AlpP was produced by a marine bacterium D2 (P. tunicata) that was isolated from the surface of the ascidian larvae, Ciona intestinalis. The protein was effective against Gram-negative and Gram-positive bacteria occurring in a variety of environments. AlpP protein was shown to be released during the stationary growth phase of D2. The protein contained at least two subunits (60 and 80 kDa), which were linked together by noncovalent bonds [27]. Rao et al. noted that expression of iron uptake and AlpP in P. tunicata was controlled by a ToxR-like regulon [26]. AlpP was autoinhibitory to P. tunicata itself, therefore its ecological role was questionable. Rao et al. contemplated that during biofilm growth of P. tunicata, AlpP might provide a competitive edge over competitors [26].
Rao et al. further investigated whether P. tunicata was a superior competitor in comparison to other bacteria isolated from U. lactuca [26]. In pure culture and within 72 h, all marine isolates developed into biofilms containing microcolonies. P. tunicata in mixed-species biofilm with the competitor, Pseudoalteromonas gracilis, coexisted with P. gracilis until the competitor was insensitive to AlpP. Microcolony formation may improve an organism’s capacity to compete against P. tunicata and its persistence [26]. Roseobacter gallaeciensis, however, demonstrated potent inhibitory activity against P. tunicata, outcompeted it and produced a biofilm. The AlpP (minus) mutant of P. tunicata was less competitive when placed into pre-established biofilms, signifying that AlpP played a role during competitive biofilm formation [26].
Barja et al. [28] reported that Alteromonas species (later reclassified as Pseudoalteromonas) isolated from seaweed produced low-molecular-weight (molecular weights less than 2 kDa) thermolabile inhibitors whereas strains isolated from seawater produced high-molecular-weight antibiotics such as a glycoprotein (molecular weight 90 kDa) purified from strain P-31. These compounds presented broad-spectrum antagonistic activity against medical and environmental isolates and their role in the prevention of surface-colonizing competitors was implicated [28].
It may be anticipated that chemical defenses in biofilms would offer vital protection against predators. Planktonic populations would be expected to depend on defense mechanisms such as cell morphology or escape and chemical defenses would be less important [29]. Matz et al. compared the occurrence and effectiveness of chemical defenses in biofilms versus planktonic populations of marine bacteria [29]. By examining growth and survival of two common bacterivorous nanoflagellates (Cafeteria roenbergensis and Rhynchomonas nasuta), Matz et al. demonstrated that chemically mediated defense against protozoan predators was common among marine bacterial biofilms. The authors further showed that the purple pigment violacein (Fig. 3), an L-tryptophan—derived alkaloid consisting of three structural units, 5-hydroxyindole, 2-pyrrolidone, and oxindole was responsible for the antiprotozoal activity of Pseudoalteromonas tunicata biofilm [29]. Antiprotozoal activity was also elicted by violacein derivative deoxyviolacein that was produced at a 3-fold lower concentration compared to violacein. As discussed by Matz et al. [29], cellular extracts of the β-proteobacterium Chromobacterium violaceum were the first described source of violacein [30]. An 8-kb region coding a presumed biosynthetic gene cluster comprising five open reading frames in the P. tunicata genome showed high predicted amino acid sequence similarity to the violacein operon vioABCDE of C. violaceum [31, 32].
Franks et al. tested the ability of P. tunicata to inhibit fungi [33]. During surface colonization of the green alga Ulva australis, P. tunicata was afforded a competitive advantage over the marine yeast Rhodosporidium sphaerocarpum through production of an antifungal compound. This yellow-pigmented broadspectrum inhibitory compound (YP1) disrupted an already established fungal biofilm by reducing the number of surface-attached yeast cells. Franks et al. [33] further reported that a long-chain fatty acid-coenzyme A ligase was implicated in its production. YP1 produced by P. tunicata possesses a 2, 2-bipyrrole ring system with an unsaturated 12-carbon alkyl chain. It is a member of the tambjamine class of compounds and was the first reported natural tambjamine with an unsaturated alkyl chain. YP1 was previously isolated from eukaryotes in the marine environment and was reported to exhibit antimicrobial activities. It may be considered that activation of long-chain fatty acids was essential for the elaboration of an active antifungal metabolite. The yeast test strains were of industrial, agricultural, and medical importance and their growth inhibition by P. tunicata opens possibilities for their biotechnological applications.
To determine the level of antifouling activities and the production of bioactive compounds within the genus Pseudoalteromonas, 10 Pseudoalteromonas species derived mostly from various host organisms were examined in a number of biofouling assays [34]. The growth of the initial fouling organisms (bacteria and fungi) on marine surfaces was assayed in the presence of the 10 Pseudoalteromonas species. Further assays included the settlement of invertebrate larvae (Hydroides elegans and Balanus amphitrite) and settlement of Ulva lactuca and Polysiphonia sp.spores. P. tunicata inhibited all target fouling organisms whereas Pseudoalteromonas haloplanktis and Pseudoalteromonas nigrifaciens demonstrated feeble activity in the bioassays.
Production of bioactive compounds was correlated with the expression of pigment. The antibacterial compound produced by P. tunicata was a high molecular mass protein (190 kDa in size) consisting of two subunits and the effect was bactericidal, not bacteriostatic. Pseudoalteromonas luteoviolecea, P. tunicata, and Pseudoalteromonas aurantia were able to inhibit most of the Pseudoalteromonas species as well as other marine epiphytic bacteria and nonmarine strains [34]. The strong antagonistic activity displayed by the three species may provide a competitive edge in the colonization of habitats in comparison to the other Pseudoalteromonas species. Six out of the ten species of Pseudoalteromonas encouraged the attachment of H. elegans larvae. Three species partially inhibited larval settlement, and P. tunicata showed complete inhibition of the larval surface attachment. The antilarval compound obtained from P. tunicata was less than 500 Da in size, heat stable, and polar. Four Pseudoalteromonas species strongly inhibited the settlement of B. amphitrite larvae and none of the species stimulated larval settlement. In a study by Egan et al., 56 marine isolates were tested for their ability to prevent spore settlement of U. lactuca [35]. Inhibitory activity was demonstrated by biofilms of 13 isolates and 3 of these isolates, including P. tunicata, completely hindered spore settlement. Biofilms of Pseudoalteromonas species were more effective against the settlement of Polysiphonia spores compared to U. lactuca spores as demonstrated by Holmström et al. [34]. Bacteria showing activity against the settlement of U. lactuca spores were generally active against Polysiphonia sp. spores as well. This suggested that analogous antialgal component(s) from different bacterial species targeted more than one alga [34]. It is generally presumed that bacteria possessing antibacterial activity also have the ability to produce antifouling compounds. However, it is not known if these properties are related and if an exploration for antibacterial activity can be considered a surrogate for searching antifouling activity [36]. Bernbom et al. [36] reported the highest antifouling activity in the biofilms of Pseudoalteromonas piscicida, Pseudoalteromonas tunicata, and Pseudoalteromonas ulvae. P. piscicida killed the test strain Pseudoalteromonas S91 in suspension cultures, whereas P. tunicate, P. ulvae, and P. aliena were not bactericidal against S91 but prevented its adhesion. Thus, the authors concluded that antibacterial activity was not a substitute for the antifouling effect. The alpP gene, which is responsible for antifouling, was present only in P. tunicata, therefore the authors speculated that there may be other molecules or mechanisms through which the other Pseudoalteromonas strains displayed antifouling activity. Bernbom et al. [36] further reported that during their one-year screening program for bioactive bacteria at 11 Danish coastal locations they found the numbers as well as the natures of bacteria showing bioactivity to differ with respect to season and niche.
A survey of surface-dwelling antibiotic-producing bacteria from seaweed and subsequent study of their antimicrobial potential against Staphylococcus, Alcaligenes, Pseudomonas, Vibrio, Pasteurella, and Achromobacter were carried out by Lemos et al. [37]. From five species of green and brown marine algae, 224 bacterial strains were isolated and screened for antibiotic production. Antimicrobial activity was displayed by 38 strains and Enteromorpha intestinalis was the superior source of producer strains. All epiphytic bacteria possessing antibiotic activity were classified in the Pseudomonas–Alteromonas group. Antagonism was demonstrated among the isolates: one producer strain inhibiting growth of other producers and other nonactive strains isolated from seaweed. Preliminary characterization of the antimicrobial substances showed that they were low-molecular-weight compounds, thermolabile, anionic, and resistant to proteolytic enzymes. Furthermore, Lemos et al. showed that the antibiotic substances remained strongly bound in the periplasmic space after excretion [37]. Ecologically, fast discharge of antibiotic substances by producer epiphytic bacteria would not confer any competitive benefit to them as the inhibitors would be instantly washed away by the surrounding seawater. In contrast, if antibiotics remained bound to cells, they would be excreted gradually and continually to the immediate environment, thus inhibiting colonization by competitors on the algal surface.
Bacterium–bacterium interactions occur at micrometer spatial scales and antagonism is an interaction in such microenvironments. Long et al. developed a model system on the antibiotic-producing Alteromonas isolate (SWAT5) obtained from a marine particle and its dominant antibiotic, 2-n-pentyl- 4-quinolinol (PQ) to investigate the significance of this antimicrobial in antibiosis and carbon cycling on particles [38]. Production of PQ by SWAT5 was observed only on surfaces and when the isolate was cultivated in polysaccharide matrices. PQ diffused within the matrices but not into the proximate seawater. Lemos et al. concluded that SWAT5 possibly created a localized zone of high antimicrobial concentration on particles suspended or sinking through seawater similar to the previous inference [37]. Bacterial respiration of exterminated bacteria was most sensitive to PQ, whereas a higher concentration of PQ was required to inhibit DNA and protein synthesis as well as bacterial motility. The structure of the bacterial community that colonized and developed in the model particle system was influenced by PQ. Long et al. noted that the particle-attached bacterium, such as SWAT5 may reduce the “biochemical influence” that competing bacteria may show enzymatically on a particle’s organic matter [38]. In the pelagic ocean, antibiosis may also play a role in distribution of bacterial species at the microscale level [39].
Results of the investigation on antibiotic production by Pseudoalteromonas rubra (previously Alteromonas rubra, isolated from the Mediterranean waters off Nice, France) indicated that maximum antimicrobial activities were observed on hydrophilic surfaces, and the number of attached cells was higher on hydrophobic surfaces [40]. Ivanova et al. demonstrated that the degree of substratum hydrophobicity influenced the production of antibacterial metabolites [41]. The highest antimicrobial activity was observed on hydrophilic surfaces notwithstanding the abundance of attached Pseudoalteromonas cells on hydrophobic surfaces. In response to environmental variables and stimuli present outside the cell, the elaboration of antimicrobial activity may be increased or decreased. Holmström and Kjelleberg [25] supported the conclusions of Ivanova et al. [41].
Before beginning the next subsection, it would be interesting to note the dissimilarities in colonization strategies demonstrated by the epiphytic bacteria of the Roseobacter clade and Pseudoalteromonas genus. Biofilm-forming marine bacteria Pseudalteromonas tunicata and Roseobacter gallaeciensis are often found associated with the Ulva australis surface. They are believed to protect the host plant against common fouling organisms by producing inhibitory compounds. Rao et al. [42] investigated the factors influencing the surface attachment and colonization of U. australis by P. tunicata and R. gallaeciensis. Rao et al. further studied the competitive interactions occurring between the two bacteria and other isolates of U. australis during biofilm formation on the plant surface. Although R. gallaeciensis was able to colonize U. australis under all conditions tested, colonization by P. tunicata was specifically enhanced by high cell densities, dark inoculation, interactions with a natural seawater community, and presence of cellobiose. It may be noted that cellulose is the main surface polymer of U. australis. The epiphytic habitation of R. gallaeciensis was attributed to its selective utilization of a number of carbon sources unavailable to competing strains as well as to the production of antibacterial compounds and signaling molecules. When a pre-established biofilm was challenged with P. tunicata, it resulted in the cohabitation of competitors partially due to the defensive activity of microcolonies that resisted invasion. Metabolically active cells at the outer edge of the microcolony died whereas cells in the deeper regions of the biofilm were protected from the antibacterial activity as a result of the diffusion gradient in microcolonies. Coexistence of competing strains was also due to limited nutrients on the U. australis surface that led them to occupy distinct niches on the plant. R. gallaeciensis, on the other hand, was not hindered by low-nutrient conditions and was able to attack and annihilate competing strains, indicating that its antibacterial substance was able to spread through microcolonies.
2.3 Nitrous-Oxide (N2O): Emitting Biofilms
Following carbon dioxide and methane, nitrous oxide (N2O) is the third most important greenhouse gas. In their article, Heisterkamp et al. [43] noted that the atmospheric concentration of N2O is swiftly increasing, contributing appreciably to global warming as observed by the Intergovernmental Panel on Climate Change [44] and to the depletion of the stratospheric ozone layer as noted by Ravishankara et al. [45]. Heisterkamp et al. [43] commented that fertilized soils and coastal areas that are characterized by high input and turnover rates of inorganic nitrogen are considered as principal sites of N2O emission [46]. Heisterkamp et al. [43] further observed that in coastal sediments and rock biofilms, due to high riverine input of nitrogen coupled with microbe-mediated nitrogen conversions, N2O production is further increased [47]. Heisterkamp et al. [43] noted that nitrous oxide is also emitted by earthworms and freshwater invertebrates [48], and a dense population of filter- and deposit-feeding invertebrates [49] with exposure to high nitrate concentrations [50] that make coastal marine sediments active regions of N2O emission. In their study, Heisterkamp et al. [43] investigated N2O emission capacities of marine invertebrate species found in the coastal sediments of the North Sea and Baltic Sea and of the aquacultured shrimp Litopenaeus vannamei. Animal-associated N2O production was strongly related to body weight, habitat, and exoskeletal biofilms. Heisterkamp et al. [43] reported that N2O emission by the snail Hinia reticulata with an intact exoskeletal biofilm was approximately 3.5 times more than by a snail without the biofilm. Thus, N2O production associated with marine invertebrates was not always due to gut denitrification, but may originate from microbial activity on the external surfaces of the animal. The microbial pathway for biofilm-associated N2O production was not identified. Heisterkamp et al. [43] remarked that oxygen availability inside the biofilm would determine if nitrification or denitrification or both would contribute to the production of N2O [51]. Ammonium from animal excretion or nitrate from the water column, or both could be the sources of N2O production in the exoskeletal biofilm. Furthermore, nitrification and denitrification would probably be coupled if an oxic–anoxic transition zone prevailed in the biofilm [52].
The next study by this group showed that 18–94 % of the total animal-associated N2O emission arose from shell biofilms of Mytilus edulis, Littorina littorea, and Hinia reticulata possessing different lifestyles [53]. Nitrification and denitrification contributed equally to N2O emission from shell biofilms and both processes occurred in biofilms as a result of heterogeneous oxygen distribution. N2O production in shell biofilms of the three mollusc species were supported by ammonium, nitrite, and nitrate. The animals provided a nutrient-enriched microenvironment, in particular ammonium excretion, that stimulated growth and sustained N2O production of the shell biofilm. Heisterkamp et al. [53] demonstrated that when H. reticulata was still living inside the shell, biofilm on the shell surface exhibited the highest N2O emission rates. N2O production originated from the shell biofilm of M. edulis whereas N2O production by gut denitrification was negligible although M. edulis is known to be a very proficient filter-feeder that ingests large numbers of bacteria and is capable of high N2O production in its gut [48]. Heisterkamp et al. [53] reasoned that most of the ingested bacteria may be digested by the high gut lysozyme activity of M. edulis and N2O would not be produced due to inhibition of denitrification.
Svenningsen et al. [54], using the freshwater bivalve Dreissena polymorpha (zebra mussel) as the model organism, quantified the biofilm-derived N2O production and the mechanism(s) thereof. Svenningsen et al. [54] reported that the shell biofilm contributed approximately 25 % to the total N2O emission from this species. The mussel gut is oxygen depleted, so denitrification would be induced by denitrifiers whereas ammonia oxidation would be repressed by ammonia oxidizers in the gut. As mussel biofilms were relatively thin and presumably fully oxic, N2O would therefore be produced mainly by nitrification, and denitrification would be repressed. However, high nir gene (coding for nitrite reductase) abundance implied that denitrification might contribute to N2O production if anoxic microsites developed within the biofilm.
2.4 Antimicrobial and Auxin-Producing Biofilms
Wilson et al. tested whether antimicrobial activity of biofilm cultures is directed towards competing bacteria found in those biofilms [55]. Fourteen of the 105 marine isolates collected from marine invertebrate, algae, and gravel surfaces were found to possess antimicrobial activity when cultivated as biofilms. The strength and spectrum of activity were greater when isolates were grown as biofilms compared to cultivation as shaken cultures. Supernatants of biofilm cultures from 11 of the 13 isolates demonstrated activity in organic phases of varying polarity signifying the presence of multiple antibiotic molecules of different polarities. Six isolates showed activity against Shewanella sp. in hexane, dichloromethane, and ethyl acetate extracts and five isolates displayed activity against Shewanella sp. in the aqueous phase. Wilson et al. [55] thus concluded that biofilm-forming marine bacteria were active against competing bacteria in biofilms.
Two species of gliding bacteria were isolated from a marine biofilm and identified as members of the genus Cytophaga [56]. Colony expansion of one isolate (RB1058) was inhibited by the other (RB1057) through production of an extracellular inhibitor that prevented adhesion of RB1058 to substrata and its gliding activity. Burchard and Sorongon [56] characterized the inhibitor as a glycoprotein with 60-kDa apparent molecular mass. The metabolic cost of synthesis and export of a 60-kDa glycoprotein by RB1057 would be substantial and thus its biosynthesis should be of high ecological significance. The high-molecular-weight substance would diffuse relatively slowly through the biofilm’s channels and would therefore more likely be retained in the proximity of the producing bacteria than a low-molecular-weight secondary metabolite. Similar mechanisms for antimicrobial substances to be ecologically effective were demonstrated in Pseudoalteromonas [37, 38].
Kerkar et al. [57] noted that the natural auxin produced by plants, algae, mosses, and lichens is indole-3-acetic acid (IAA). Soil rhizosphere bacteria associated with plants are known to produce IAA. Physiological effects of IAA on plants and its role in plant–microbe interaction has been studied by Patten and Glick [58]. However, few studies have been carried out on IAA-producing bacteria found in association with aquatic biofilms and their contribution towards growth of biofilms [57]. Kerkar et al. reported rapid growth of biofilm mats formed in salterns of the Indian west coast during the monsoon. The heterogeneous population of the biofilm comprised mainly green algae, blue green algae, Euglenophyceae, and diatoms. Four types of green algae (viz. Pediastrum duplex, Oedogonium sp., Cladophora sp., and Spirogyra exiles), three blue green algae (Phormidium sp. (corium), Phorimidium sp. (ambigeum), and Oscillatoria sp.), and one type of Euglenophyceae (i.e., Phacus sp.) were reported. The diatoms comprised Pleurosigma sp. and Navicula sp. Among the 125 bacteria recovered from biofilms, 16 produced IAA whereas four isolates therefrom, in the presence of tryptophan, consistently produced high amounts of IAA. The IAA-producing bacteria were Aeromonas aquariorum, Pseudomonas alcaliphila, Vibrio diazotrophicus, and Pseudomonas pachastrellae. IAA produced by biofilm-associated bacteria functions as a chemical messenger between microorganisms [59].
3 Industrial and Environmental Bioprocesses
It is generally observed that surfaces of inanimate structures (buildings, ships) immersed in the sea swiftly become covered by a microbial biofilm. This is followed by colonization by larger organisms leading to macrofouling of the surfaces. The majority of marine organisms, although covered with a thin film of epibiotic bacteria, are not fouled by macrorganisms. These epibionts impart protection to the host organism by releasing compounds that prevent macroorganisms from contaminating the surface. Interestingly, these epibionts may also have industrial and medical applications such as the production of antimicrobial or antifouling compounds [60]. Furthermore, a shift in the mode of culture from suspension to surface culture affects the type and quantity of compounds produced. Armstrong et al. noted that surface-grown bacteria released bioactive compounds with higher activity against target strains in comparison to those obtained from the same strain cultivated in the planktonic mode [60]. Thus, surface-dwelling bacteria of seaweed may produce greater amounts of compounds protecting the seaweed exterior from further fouling. The enhanced expression of biosynthetic genes is responsible for bioactive compound production, in a manner similar to increased extracellular polysaccharide gene expression which is also necessary for bacterial surface colonization. Wilson et al. [55], citing the literature [1, 60, 61], noted that the molecules produced by successful bacterial surface colonizers that prevent attachment, growth, and survival of competing organisms hold antifouling or antimicrobial properties. Colonization of competing organisms is hindered by antifouling molecules, whereas antimicrobial action results in the death of competing bacteria. Thus, ecological considerations directed the exploration of biotechnological applications of biofilm-forming microbes for antifouling and antimicrobial activities, with which this section begins. Applications of marine and intertidal biofilms in production of antimicrobial and cytotoxic compounds, exopolysaccharides, enzymes, melanin, riboflavin, and aquaculture feedstock as well as in bioremediation and microbial fuel cells are also described in the following section.
3.1 Production of Antifouling and Biocontrol Agents
Ortega-Morales et al. [62] observed that biofouling is the reason for massive damage to oceanic infrastructures such as ship hulls and offshore platforms, contributing to financial losses [63]. Undesirable environmental effects of applying broadspectrum biocides such as tributyl tin (TBT)—containing paints on marine structures as well as unacceptable performance of antifouling coatings have stimulated the search for natural, environmentally friendly products to tackle biofouling [62, 63]. The International Maritime Organisation (IMO) agreed at its London Assembly in November 1999 that TBT would be phased out between 2003 and 2008. According to Ortega-Morales et al. [62], although marine-algae—derived natural compounds have been projected as novel antifoulants [64], it was demonstrated that certain epibiotic bacteria living in relationship with higher organisms (including algae) yield inhibitory extracellular compounds that inhibit colonization of common fouling organisms and are thus helpful to host survival [42]. Thus, within a microorganism–macroorganism association, chemical protection is provided to the host by the epibiotic biofilm without innate chemical defense afforded by the host [62].
Ortega-Morales et al. screened biofilm bacteria able to produce novel natural antifoulants [62]. Marine biofilm bacteria were isolated from the surfaces of turtle grass leaves and limestone fragments. The nine isolates were cultivated in planktonic condition in shake flasks containing yeast extract broth. The bacterial biomasses as well as the fermentation broths were subjected to solvent extraction to obtain the antifouling compounds. The common fouling bacterium, Halomonas marina, and a crustacean, Artemia salina, were applied as test organisms to assay antifouling activity of extracts of nine representative strains isolated from various surfaces. Antimicrobial as well as toxic activities were detected in most of the organic extracts. Molecular phylogeny revealed that the isolates were relatives of Bacillus mojavensis and Bacillus firmus. Bioactive lipopeptides surfactin A, mycosubtilin, and bacillomycin D were identified as the active factors. As stated by Ortega-Morales et al. [62], B. mojavensis was originally found in the soil of the Mojave Desert, United States [65], and also reported to be an endophyte [66]. Therefore, Ortega-Morales et al. [62] speculated that this bacterium might have been washed off into the coasts by terrestrial run-off. Halotolerance and exopolymer synthesis pointed towards an intertidal habitat of Bacillus mojavensis.
Bernbom et al. [67] examined the antifouling potential of different Pseudoalteromonas species in a system that simulated the natural marine environment. The bacteria were further included into ship paints and their prospective antifouling property was verified in a field situation. The authors [67] reported that P. piscicida strains B39bio and A38q-4a and P. tunicata strain J38a-5a survived as biofilms for 53 days in sterile seawater, although a 2.5-log reduction in CFU numbers over time was observed. On the other hand, P. tunicata strains existed for only 7 days and none of the strains were detectable after 53 days. The authors [67] further noted that after 7 days, the counts of culturable bacteria attaching to the Pseudoalteromonas-precoated surfaces were higher compared to the control surfaces. In spite of this, after 53 days, seven of eight Pseudoalteromonas strains under study had lowered bacterial adhesive capacity compared to the control. P. piscicida, P. antarctica, and P. ulvae were detected on the surface as was found initially, and P. tunicata was undetectable. P. tunicata strain J36q-4a prevented the attachment of the test strain (S91) significantly, although the other three P. tunicata strains had minute antifouling effect against S91. The authors [67] concluded that subject to the model organisms selected, marine bacteria may either deter or draw other bacteria. Next, the authors included suspensions of P. piscicida and P. tunicata into ship paints applied on plates at a test site in Jyllinge Harbor, Denmark. No disparities were noted between control and treated plates during the first 4 months. However, after 5–6 months fouling was observed on the control plates but not on the plates coated with the Pseudoalteromonas-based paint. It was concluded that antifouling effects were difficult to ascertain through laboratory studies only. For better assessment of the prospective antifouling capacity of novel agents or organisms, a blend of laboratory and field-based studies was recommended [67].
Holmström et al. [68] screened 40 marine bacterial isolates against laboratory-reared barnacle larvae (Balanus amphitrite) and ascidian larvae (C. intestinalis) to find bacteria with antifouling properties. A facultative, anaerobic, Gram-negative bacterium (D2, mentioned previously) was isolated from the surface of C. intestinalis. Biofilm was the source of the larvicidal activity and stationary-phase biofilms possessed higher activity than developing biofilms. The biologically active factor was a heat stable, <500 Da, polar, neutral, nonprotein compound contained or bound to carbohydrate moieties.
Antagonistic activity of marine biofilm bacteria against terrestrial fungal plant pathogens was studied by Ortega-Morales et al. [69]. Colletotrichum gloeosporioides ATCC 42374 was inhibited by close relatives of Bacillus mojavensis and Bacillus firmus. The active bacterial isolates were further challenged against C. gloeosporioides, Colletotrichum fragariae, and Fusarium oxysporum in different bacterial growth phases and cultivated in varying fungal nutritional conditions. Susceptibility of the pathogens was dependent on fungal nutrition and time of bacterial colonization. Bacillus sp. MC3B-22 proved to be a better antifungal agent than the commercially available biocontrol strain Bacillus subtilis G03, which indicated its biotechnological potential, specifically, prevention of fungal colonization on mango leaves. Epiphytic biofilms of Thalassia testidinum, a marine sea grass, was the source of the most active isolate whereas the rest were isolated from epilithic biofilms of the intertidal habitat. B. subtilis MC3B-22 was antagonistic to C. gloesporioides independent of the colonization time (early, simultaneous, and late). Ortega-Morales et al. [69] also established that the principal polymeric material forming surface attachment structures is an extracellular heteropolysaccharide previously known to be a metabolite of B. subtilis MC3B-22. The authors concluded that terrestrial plant pathogens could be inhibited by biofilm bacteria isolated from the fluctuating physical and chemical environments of intertidal regions.
3.2 Production of Antimicrobial and Cytotoxic Compounds
Marine bacteria growing on algal surfaces and derived from intertidal regions of Japan, were investigated as potential sources of novel bioactive compounds [70]. Kanagasabhapathy et al. [70] reported that several strains were active against pathogenic as well as fouling bacteria. Molecular phylogenetic analysis identified the epibiotic bacteria as members of the Bacillus genus similar to the results of Ortega-Morales et al. [62]. Surface attachment was a significant factor affecting the metabolism of marine epibiotic bacteria. Vandevivere and Kirchmann found that addition of sand to shake flask cultures stimulated exopolymer synthesis by some surface-attached bacteria and that attached cells produced higher amounts of exopolymer than planktonic cells [71]. Yan et al. [72] observed that shaken flask cultivation did not provide the correct conditions of antimicrobial production by surface-growing bacteria. The authors opined that the common laboratory method of agitated suspension cultures rendered artificial growth conditions to the organism contrary to the natural environment from where the bacteria are sourced. Thus, seemingly inactive surface isolates may be induced to produce bioactive compounds through novel cultivation strategies by simulating the natural habitats of microorganisms in “niche-mimicking” bioreactors. To this end, Yan et al. modeled the epibiotic growth conditions in a “modified roller bottle” culture method [72]. Two epibiotic marine strains (preliminarily identified as Bacillus species) isolated from the surface of marine alga Palmaria palmata were grown as a biofilm and shown to produce antimicrobial substances (Fig. 4). The antibiotic spectrum varied when the isolates were cultivated in the biofilm and suspension modes. Yan et al. assumed that sustained production of the antimicrobial compounds was linked to periodic exposure of the biofilm to the liquid medium and air.
Roller-bottle cross-section as described by Yan et al. [72] for cultivation of marine epiphytic bacteria. Reprinted with permission from Springer
Yan et al. [73] designed one more novel reactor, the “air–membrane surface” (AMS) bioreactor for attached growth of bacteria as a biofilm in contact with air. A surface-growing bacterium (Bacillus licheniformis EI-34-6) demonstrated antimicrobial activity (identified as bacitracin) in the AMS reactor but not in shake flask cultures. Surface-grown cells produced an unidentified red pigment not detected in planktonically grown cells. Bacillus subtilis strain DSM10T, applied in cross-species induction experiments, and Bacillus pumilus strain EI-25-8, another epibiotic isolate, were cultivated in the AMS reactor. Interestingly, spent media obtained from beneath the membrane of the reactor after growing these two strains, could stimulate production of antimicrobial compounds and a red pigment in suspension cultures of B. licheniformis isolate EI-34-6, but was not observed with shake flask culture spent media of DSM10T and EI-25-8. Yan et al. [73] conjectured that compounds inducing bacitracin and red pigment production diffused into the medium below the membrane and some of the compounds were retained in the biofilm which facilitated accumulation of inducer compounds. Accretion of inducer compounds in shake flask cultures was unlikely. Yan et al. further established that ferric iron, glycerol, and air–membrane interfacial biofilm growth were essential for production of bacitracin and red pigment by EI-34-6, although the antimicrobial compound itself was not an inducer. Yan et al. [73] surmised that the physical environment of the AMS bioreactor was crucial for production of inducer compounds, which promoted bacitracin and red pigment synthesis. The physical environment was no longer required once the inducer compounds attained a threshold level.
Yan et al. [73] stated that the biofilm state is the preferred growth mode of surface-dwelling bacteria in natural environments. Yan et al. maintain that heterogeneous growth conditions may be expected in a biofilm [74] and microcolonies within the biofilm can have pH gradients [75]. Yan et al. [73] further attest that uneven bacterial starvation may occur due to constraints in substrate transport into the biofilm [76, 77], phenomena not observed in suspension culutues. Consequentially, biofilm bacterial metabolism is very dissimilar from that of suspension culture [78]. Cell density-dependent signaling and gene expression mechanisms may be presumed within biofilms as cell densities on the order of 1012 CFU/cm3 are frequently reached. Following the accepted model of bacterial quorum sensing, Yan et al. [73] reasoned that an increase in concentration of signal molecules above a threshold limit stimulated a change in gene expression that led to an altered phenotype [37, 79].
Yang et al. [80] investigated the effect of agitation on the production of the antimicrobial violacein by Pseudoalteromonas luteoviolacea, a surface-residing marine bacterium isolated from the marine sponge Acanthella cavernosa. Static growth conditions elicited the highest amounts of violacein whereas production decreased with increasing agitation speed. It was also noted [80] that cells formed clusters under stagnant conditions and higher agitation progressively separated clusters into single cells, suggesting that bacterial aggregation may be essential for violacein production by P. luteoviolacea. Yang et al. concluded that bacterial films formed under static conditions of the culture broth had higher cell densities on surfaces that might have triggered gene expression of certain inducers, as suggested by Boettcher and Ruby [81] in a related fashion to that expressed during extracellular polysaccharide production once bacteria attach to a surface [82]. The conclusion of this study is similar to that of Yan et al. [72, 73] where changes in the mode of culture, from suspension to biofilm affected the nature and amount of compounds produced by biofilm-forming bacteria.
Nannocystis exedens, a gliding bacterium belonging to the δ-Proteobacteria class and isolated from the intertidal region of Crete, produced phenylnannolones as secondary metabolites [83]. Phenylnannolone A possessed an unusual chemical structure comprising an ethyl-substituted polyene chain linked to a pyrone moiety on one side and to a phenyl ring on the other. The compound had promising anticancer activity. Biofilm-based bioprocesses on this intertidal-dwelling organism would be of interest given the exciting advancements made in biofilm cultivations described earlier [62, 70, 72, 73].
As mentioned by Sarkar et al. [84], marine actinomycetes may be supposed to have features different from terrestrial actinomycetes as the marine habitat is completely dissimilar to the terrestrial environment. Novel chemical entities have emerged as a result of extensive screening based on culture-dependent, culture-independent cultivation methods combined with the application of bioinformatics. The culture-dependent approach of bioprospecting is based upon microbe isolation from various geographic locations using taxon-selective isolation media and sediment pretreatments, preliminary characterization, and dereplication of isolates to avoid redundancy in screening. The culture-independent bioprospecting strategy has relied on extraction of total bacterial or actinobacterial DNA from the environment, cloning into surrogate hosts, identification of gene or gene cluster, sequencing, dereplication, and community profiling with single-strand conformation polymorphism, denaturing gradient gel electrophoresis or terminal restriction fragment length polymorphism, expression of the target gene or gene cluster, and characterization of the desired activity. The bioinformatics-based strategy that can be applied to explore novel compounds having actinobacterial properties are taxonomy as a road map to genes and the discovery of three-dimensional taxonomic space. According to Sarkar et al. [84] establishment of new marine genera, Salinospora and Marinophilus, which are taxonomically distinctive members of marine actinomycetes and the discovery of novel secondary metabolites therefrom, have given new directions to marine natural product research [85–88]. Indian investigators have identified the Bay of Bengal as a potential source of marine-derived bacterial bioactive compounds [84]. Mukherjee and coworkers isolated several microbes with novel bioactivities [89 and related references therein] from the Sundarbans, the world’s largest tidal mangrove forest, off the Bay of Bengal. Sarkar et al. [84] also noted that in the research domain of marine bacterial antibiotic production, bioreactor engineering and design of bioprocesses have been neglected. Although a plethora of novel compounds are being reported, most cultivations were performed in shake flasks leading to poor understanding of mechanisms underlying antibiotic production processes, thus impeding prospects of commercial scale-up [90].
Inspired by the researches of Yan et al. [72, 73] and Yang et al. [80], a novel reactor system, the “rotating disk bioreactor” (RDBR), was applied by Sarkar et al. [84] to simulate the niche environment of three halotolerant estuarine actinobacteria isolated from the Sundarbans. Designed on the concept of a rotary biological contactor (RBC), generally used in wastewater treatment (Fig. 5), the RDBR supports the growth of surface-attached biofilms. The shaft of the RDBR on which 10 discs are coaxially mounted was rotated at an ultra-low speed of one revolution per day, 1,440 times lower than the 1.0-rpm speed used by Yan et al. [72]. When the shaft is rotated with a half-filled tank with a liquid medium, any given point on the discs would be exposed to air and submerged in the medium alternatively for 12 h. The reactor thus mimicked the intertidal environment of the location from where the actinobacteria were collected. Actinomycin D was produced within a shorter time in the RDBR compared to the time required in a conventional stirred-tank bioreactor (STBR). Similar results were noted for the other two strains and Sarkar et al. [84] reasoned that surface attachment of the microbes and biofilm formation were pivotal factors for the enhanced production of antimicrobials by the intertidal actinobacteria.
Schematic of the ultralow- speed rotating disk bioreactor as described in Sarkar et al. [92]: 1 air pump, 2 rotameter, 3 air filter, 4 electrical motor and reducing gear train, 5 sampling port, 6 temperature sensor, 7 antifoam port, 8 inoculation and medium addition port, 9 acid port, 10 pH sensor, 11 alkali port, 12 DO sensor, 13 reactor vessel, 14 rotating coaxial disks, 15 shaft, 16 sparger, 17 drain, 18 base plate. Reprinted with permission from Springer
In furtherance of this work, Sarkar et al. [91] introduced the parameters’ “peak activity attainment rate” (PAAR) defined as the ratio of the “peak antibiotic activity” (PAA) and the time taken to attain this peak value, to determine the effect of environmental/operating parameters on actinomycin-D production by the biofilm-forming estuarine isolate Streptomyces sp. MS 310 in small-scale shake flask cultures, as well as in the RDBR. The most favorable pH and temperature for antibiotic production were ascertained through designed experiments in shake flasks. Subsequently, RDBR operating conditions were investigated employing a statistical experimental design where aeration and disk submergence were considered at three levels maintaining the rotational speed at 1.0 rev/day. The highest aeration rate in the niche-mimic condition was found to be most suitable for antibiotic production as PAA and PAAR simultaneously attained their highest values. Sarkar et al. further attributed the high actinomycin-D production by Streptomyces sp. MS 310 in the RDBR to biofilm formation owing to the substantial surface area (per unit volume of culture) of their reaction vessel [91].
In another study, Sarkar et al. [92] further examined the application of the RDBR for the cultivation of Streptomyces sundarbansensis [93], an actinomycete producing 2-allyloxyphenol, by first investigating the effect of nutrition and cultivation conditions on biofilm formation vis-à-vis antimicrobial production in small-scale experiments. Sarkar et al. [92] used the data thus obtained to examine the effect of medium pH, degree of disc submergence, and aeration rate in the RDBR on biofilm formation and antimicrobial activity of S. sundarbansensis. The maximum antimicrobial activity in the RDBR was attained under true intertidal conditions, 12 h periods of immersion and emersion. In the ideal niche-mimic condition, biofilm density was highest at the maximal aeration rate, where planktonic growth was also maximum and dissolved oxygen was rapidly utilized. Sarkar et al. [92] reckoned that high cell concentration during planktonic growth allowed more cells to be recruited for biofilm formation, following which low oxygen tension reduced biofilm growth permitting the film to strengthen and attain a higher density [94]. The RDBR has been noted as the first model reactor system for in vitro process simulation of the intertidal/estuarine environment [95].
3.3 Production of Exopolysaccharides
Wave action, temperature and dessication stresses, ultraviolet exposure, and nutrient depletion create a highly variable or “poikilotrophic” environment [96] in intertidal rocky shores. Ortega-Morales et al. [97] observed that an ecological strategy of microbial biofilms to cope with this harsh environment is through the production of profuse amounts of highly hygroscopic extracellular polymeric substances (EPS), the production of which is induced by desiccation [98]. The physicochemical properties of EPS of intertidal biofilms accelerate the generation of severe geochemical gradients, offering protection to the microbial cells, thus providing incredible pliancy to the biofilm during periods of stress [3, 99]. This ecological function makes EPS molecules potentially valuable as gelling, stabilizing, emulsifying, chelating, thickening, and film-making agents, which would be of significant importance in chemical and food industries as well as environmental bioremediation. Two EPS-producing biofilm bacteria from a rocky intertidal shore of the southern Gulf of Mexico were studied [97]. The major compound of the EPS synthesized by Microbacterium sp. MC3B-10 was a glycoprotein, whereas the polymer produced by Bacillus sp. MC6B-22 was an anionic polysaccharide. The biopolymer produced by Microbacterium sp. MC3B-10 was nonionic, had high emulsifying activity and stability at elevated temperature, and salinity. These properties may be practical in bioremediation applications. The chemical composition of polymer MC6B-22 suggested its potential utilization in tissue regeneration [2, 97].
The polychaete Alvinella pompejana isolated from a hydrothermal vent in the East Pacific Rise housed a heterotrophic and mesophilic marine bacterium on its surface. The bacterium was assigned to the genus Alteromonas and produced large amounts of an acidic polysaccharide in batch cultures during the stationary phase of growth [100]. Alteromonas macleodii, the single member of the Alteromonas genus, was isolated from a hydrothermal vent in north Fiji and was found to produce a polysaccharide having unusual high molecular weight in batch cultures. The viscosity of this exopolysaccharide is similar to that of xanthan, another bacterial polysaccharide of commercial interest, thus proving its biotechnological potential [101]. A novel bacterium, Paracoccus zeaxanthinifaciens subsp. payriae isolated from a microbial mat, “kopara” in French Polynesia produced water-soluble high-sulfate—containing exopolysaccharides. Potential cosmetic applications were envisaged [102]. Guézennec et al. [103] projected further applications of EPS-producing microbes of the kopara microbial mats in detergent, textile, adhesives, paper, paint, food, beverage, and pharmaceutical industries, specifically in cancer therapies and drug delivery systems. The EPS can be gainfully employed for metal recovery in oil, mining industry, and industrial waste. Cell culture media can also be formulated using the EPS. Microbe-derived exopolysaccharides are used for the preparation of polysaccharide gels that are constituents of microbial cell culture media. Generally, agar, which has a high melting point of about 60–97 °C and a low solidifying point of about 32–40 °C is very useful in the preparation of a microbial culture medium. Nowadays, other compounds such as gellan or gelrite have drawn the attention of researchers as well as the industry as a substitute for agar. Gellan can be integrated into microbial culture media that allow better growth of microorganisms compared to agar-containing media. Moreover, thermophiles get a growth benefit due to the high temperature stability of gelrite [104].
Particulate material obtained from seawater and ice from the Antartica southern ocean yielded two psychrophilic Pseudoalteromonas species from sea-ice microbial populations. The bacteria produced highly anionic extracellular polymers containing neutral sugars and uronic acids with sulphates [105]. Extracellular polymeric substances were purified from Oceanobacillus iheyensis BK6, isolated from a marine natural biofilm from a coastal region of India, the first report on the occurrence of EPS in the genus Oceanobacillus [106]. Antibiofilm activity of the EPS against pathogenic Staphylococcus aureus was observed by Kavita et al. [106]. The physicochemical properties of the polymer make it suitable for pharmaceutical and industrial applications.
3.4 Production of Enzymes
Identification and characterization of biofilm-forming bacteria through culture-based methods was performed by Iijima et al. [107]. Among three genera studied, Pseudoalteromonas, Vibrio, and Halomonas, the first was found to form active biofilms. To compare the protease activity under biofilm-based as well as planktonic cultivation the isolated bacteria were grown at standing and shaking conditions, respectively. Expression analysis of the biofilm metalloprotease I (bmpI) gene of Pseudoalteromonas sp. SB-B1 by reverse transcriptase PCR at different cultivation conditions revealed that biofilm-based cultivation stimulated bmpI gene expression which was responsible for enhanced protease activity. Application of the beneficial properties in fish farming was also considered. It was believed that Pseudoalteromonas bacteria of the biofilm community partially contributed to the elimination of excess proteins from fish farm sediment sludge. Foods and feces of fish liberate copious amounts of organic matter that result in oxygen depletion by aerobes, sulfide and methane production by anaerobes, and occurrence of pathogens [108]. Therefore, Pseudoalteromonas strains isolated from fish farms probably utilized bmpI-induced protease through biofilm formation and contributed towards removal of excess nutrients.
Sarkar et al. [109] designed a novel shaking flask the “conico-cylindrical flask” (CCF; Fig. 6), that promoted biofilm formation, had provision for assessment of aeration requirements, allowed the use of diverse internal surface materials, and could be easily placed in a typical rotary shaker for regular small-scale studies (US patent application number US2012/0295293A1, published November 22, 2012). This flask was applied for protease production by a biofilm-forming bacterium, an intertidal gamma-Proteobacterium (DGII) isolated from the Sundarbans, India. Protease activity during cultivation in the CCF with a hydrophilic (glass) surface was compared to that with a hydrophobic (PMMA) surface. The CCF with hydrophobic (PMMA) surface contained an “inner arrangement” comprising eight equidistantly arranged rectangular bars in a radial fashion on a circular disk. Because of the higher surface area, the “inner arrangement” of the vessel promoted formation of microbial biofilm on its hydrophobic surface. The second vessel, CCF with the hydrophilic (glass) surface contained 16 autoclaved glass slides that were affixed to both sides of the “inner arrangement” of PMMA–CCF with a nontoxic glue. The comparative study of protease production was done with the above-mentioned PMMA–CCF, GS–CCF, and a standard 500-ml Erlenmeyer flask (EF). The CCF allowed 30 % higher protease production and 20 % higher biomass accumulation in comparison to the standard Erlenmeyer flask. The CCF with a hydrophobic surface generated higher protease yields as well as biomass compared to the CCF with a hydrophilic surface. Sarkar et al. [109] concluded that cell growth and protease production were favored in the vessel configuration and design that supported higher cell attachment and ensuing biofilm formation. The conclusions of this study were similar to those of Yan et al. [72].
a Conico-cylindrical flask (CCF) described in Sarkar et al. [109] and US patent application number US2012/0295293A1 and b components of the CCF: 1 lower cylindrical portion, 2 inner arrangement, 3 upper funnel portion, 4 neck for joining top lid, 5 top lid for provision for aeration. Reprinted with permission from Springer
Mitra et al. [110] commented in their article on biofilm-based enzyme production by fungi that enzyme production and polyaromatic hydrocarbon oxidation were potential biotechnological applications of filamentous fungi found in intertidal estuarine regions [111, 112]. Mitra et al. [110] highlighted the proposal of a new fermentation category named “surface-adhesion fermentation” (SAF) by Gutierrez-Correa and Villena [113]. The CCF was employed to test the hypothesis if surface attachment of intertidal fungi could increase bioactive metabolite production. Cellulase production by Chaetomium crispatum (obtained from estuarine sediments of the Weser River, Germany) was compared in a CCF with hydrophobic surface (PMMA–CCF), CCF with hydrophilic glass surface (GS–CCF), and a standard unbaffled Erlenmeyer flask (EF) [110]. Growth of C. crispatum as well as endo-β-1,4-glucanase and FPase (filter paper degradation) activities increased 3.5- and 2.6-fold, respectively, in the PMMA–CCF compared to the other vessels. Additionally, Mitra et al. [110] studied biofilm development with a confocal laser scanning microscope (CLSM) over 6 days through two-channel fluorescence detection of EPS and whole cells. Results demonstrated 100 % increase of biovolume (an estimation of biofilm biomass), 25 % increase of thickness, and 62.5 % increase of both substratum coverage as well as the total spreading of C. crispatum biofilm on the hydrophobic surface (Fig. 7).
Typical confocal laser scanning micrograph of C. crispatum biofilm on PMMA surface as described in Mitra et al. [110]. Exopolysaccharides (EPS) are stained green and whole cells are stained red. a Attachment of EPS on PMMA surface b recruitment of cells, and c development of mature biofilm. Reprinted with permission from Springer
Fungal biofilm formation is initiated by active attachment to a surface by adhesive substances secreted by germinating spores and active germlings. Subsequently, microcolony formation occurs by apical elongation and hyphal branching. Hyphal ramification ensues across surfaces and a monolayer forms. Firm attachment of the growing colony to the substrate is ensured through production of a polymeric extracellular matrix [114]. Two basic processes, adhesion and successive differential gene expression, characterize fungi as regular biofilm-forming organisms. Upon attachment, fungi acquire new and discrete phenotypes diverse from those of free living conditions [113, 115]. Elements describing filamentous fungal biofilms along with a basic model depicting the different stages of biofilm development were proposed [114]. Through the investigation of Mitra et al. [110], successful satisfaction of the three proposed criteria [114] was demonstrated: first, intricate growth of the fungus by cellular or hyphal aggregation on a surface; second, cells inlaid in an extracellular self-released polymeric matrix; and third, modified gene expression causing increased or decreased enzyme production. Biofilm architectural parameters of C. albicans, such as biovolume, mean thickness, roughness coefficient, and surface area/volume ratio were obtained through CLSM image analyses and calculated by COMSTAT mathematical modeling [116, 117]. Multichannel analysis as performed by Mitra et al. [110] using the PHLIP image analysis software [118] was more informative compared to single-channel analysis (e.g., COMSTAT) as this method could distinguish various biofilm components.
3.5 Production of Melanin
Melanin has significant relevance in the progress of organic conducting polymers (2000 Nobel Prize in Chemistry). It has applications in sunscreen cosmetics as an UV absorber, paint emulsion stabilizer, and antioxidant in coatings [40]. The CCF mentioned above was further applied for melanin production by Shewanella colwelliana (isolated from estuarine oyster water of Lewes, United States, previously known as Alteromonas colwelliana) [40]. A comparative study of melanin production in three different vessels (1) PMMA–CCF having a hydrophobic surface (2) GS–CCF having a hydrophilic surface, and (3) a standard Erlenmeyer flask (EF) was peformed [40]. Compared to the other vessels, melanin production in the hydrophobic PMMA–CCF was higher by at most 33.5 % and growth of S. colwelliana was higher by at most 309.2 %. Reactor surface area, surface hydrophobicity, and planktonic cell growth, as well as biofilm formation were positively linked to melanin synthesis. Mitra et al. [40] noted a dual effect of enhanced surface area and hydrophobicity of the PMMA–CCF to be accountable for increased melanin activity in the hydrophobic vessel. The PMMA–CCF that supported attached growth, the inherent natural mode of growth of S. collwelliana, was more suitable for cell growth and melanin production. Mitra et al. [40] reasoned that biofilm cells (as higher biofilm biomass was attained in the PMMA–CCF) or positive cooperation among planktonic cells (as a higher concentration of quorum sensing molecules was observed) could be the cause for increased production. Acyl homoserine lactone molecules have been reported as quorum sensing molecules in Shewanella species as noted by Tait et al. [119].
3.6 Production of Riboflavin
Chemical riboflavin (vitamin B2) production is eventually being replaced by microbial processes. The largest chemical company in the world, BASF (German: Badische Anilin- und Soda-Fabrik, English: Baden Aniline and Soda Factory) has installed a plant in South Korea that produces riboflavin using Ashbya gossypii. Current approaches to improve industrial productivity in Candida famata, a naturally occurring overproducer of riboflavin, include selection of antimetabolite-resistant mutants, enhancing medium iron concentration, and adding biosynthetic precursors [120]. It may be conjectured that surface attachment and biofilm formation by the intertidally derived C. famata, isolated from the estuarine waters of Rio de Janerio, Brazil, can enhance vitamin production. The CCF was again used by Mitra et al. [121] to compare riboflavin production between CCFs with hydrophobic or hydrophilic surfaces and Erlenmeyer flasks. Mitra et al. reported a 22-fold increase in riboflavin production in the hydrophilic GS–CCF and a 4-fold increase both in the EF and PMMA–CCF when C. famata was grown as “biofilm-induced” cultures in comparison to the traditional suspension culture [121]. Planktonic growth was suppressed in cultivations showing higher biofilm formation and vitamin production was related to biofilm formation. Similar to the previous CLSM study by Mitra et al. [110], early development of a mature stable biofilm on glass in contrast to a PMMA surface was demonstrated. Mitra et al. [121] concluded that the genetically modified C. famata strains recently developed by Dmytruk et al. [122] may be explored for further enhancement of production by switching to the biofilm cultivation mode as it is known that all species of Candida are able to form biofilms [123]. Primary cell separation processes may be eliminated in biofilm cultivation thus lowering downstream processing costs.
3.7 Biofilm-Based Bioremediation Processes
Integration of a denitrifying step to the nitrification process leads to conversion of nitrate to nitrogen gas, thus allowing complete removal of polluting nitrogen in discharge water of recirculating aquaculture systems [124]. Van Rijn et al. remarked that the anaerobic ammonium oxidation (anammox) process can be successfully applied in this situation [125]. Under anaerobic conditions, ammonia is oxidized in the presence of nitrite in the anammox process which is performed by autotrophic bacteria belonging to the order Planctomycetales [126]. This process offers two advantages. First, in comparison to heterotrophic denitrification, complete autotrophic nitrogen removal occurs with no requirement of an organic electron source. Second, as ammonia oxidation demands less oxygen than that consumed by the conventional nitrification–denitrification process, the anammox process is economically attractive [127] as noted by Tal et al. [124]. Occurrence and functioning of anammox bacteria in aerobic and anaerobic fixed-film biofilters as well as anaerobic waste sludge sections of a marine recirculating aquaculture system were examined for the first time by Tal et al. [124]. Through community DNA analysis, Planctomycetales were found to be of ubiquitous occurrence and the anaerobic denitrifying biofilters contained one clone showing high levels of sequence identity to known anammox bacteria. Results were confirmed by fluorescence in situ hybridization studies.
Xenobiotic compounds derived from wastewater generated by engine rooms of ships, “bilge water,” and by washing oil tanks, “slops” persist and accumulate in the marine ecosystem. The wastewater generated on the order of millions of tons per year is a major worldwide disposal problem. For the treatment of slops, Mancini et al. [128] appraised the applicability of a biological process with acclimatized microorganisms. Fitch et al. specifically considered the bioregeneration of the exhaust “granular activated carbons” [129] discharged with slops and a biofilm membrane bioreactor for secondary treatment of light-pretreated slops. Positive results were obtained by Fitch et al. [129].
High concentrations of organic compounds and salinity typified wastewater released by a factory processing marine products. Lysis of the organisms in the saline environment limited biological treatment of this wastewater in conventional systems. Gharsallah et al. [130] adapted a specific flora from a fish-processing industry by gradually increasing the salt concentration and accomplished the treatment process in a continuous fixed biofilm reactor (Fig. 8). Experiments were performed with different organic loading rates (OLR) and maximal removal efficiencies were attained at low OLR [130]. During the adaptation phase, microflora of the biofilm comprised small flocs and dispersed Gram-negative bacteria. After approximately 50 days, protozoa happened to be the predominant species, whereas under steady-state conditions, the microbial community was made up of rotifers, different ciliates, and some nematodes.
Schematic diagram of the fixed-bed reactor described in Gharsallah et al. [130] and used for biological treatment of saline wastewaters. Reprinted with permission from Wiley
A novel method of bioremediation using microbial mats was proposed as a pragmatic, cost-effective, and environmentally acceptable treatment option appropriate for high biodiversity areas, such as coastal marine environments [131]. Consisting of vertically differentiated, interdependent layers of multiple microbial communities, microbial mats are physiologically diverse and are capable of performing heterotrophic, chemotrophic, and phototrophic metabolism [132] as mentioned by Zamora-Castro et al. [131]. The nature of the environment and characteristics of the waste discharge determine the choice of support material for the construction of microbial mats [133]. Microbial mats were developed on low-density polyester for ex situ bioremediation of NH4 + –N (ammonium nitrogen), NO2 − -N (nitrite nitrogen), NO3 − -N (nitrate nitrogen) and PO4 3− -P (orthophosphate) [131]. Wastewater from a municipal treatment plant releasing into the beach of Todos Santos Bay, Mexico was successfully treated using this system (Fig. 9). Bacteria, microalgae, and cyanobacteria grew as self-forming and self-sustaining communities on a polyester support consuming various N and P substrates in the wastewater. Cyanobacterial genera such as Chroococcus sp., Lyngbya sp., bacteria of the subclass Proteobacteria, and the eukaryote Nitzschia sp. were dominant species of the microbial mat.
Conceptual drawing of a photobioreactor packed with constructed microbial mats as described in Zamora-Castro et al. [131]. Reprinted with permission from Springer
Low ambient temperature prevails for the major part of the year in the northern hemisphere. As it is not practical to heat the water for allowing mesophilic microbes to be active, cold-adapted microorganisms may be effective in microbial treatment processes in cold climates. The effectiveness of a suspended carrier biofilm process in attaining denitrification at low temperatures was investigated by Welander and Mattiasson [134]. Interestingly, the denitrification rate showed poor dependence on medium temperature, thus establishing a useful alternative for low-temperature denitrification.
Labelle et al. [135] noted that there is limited knowledge of biofilm processes mediating treatment of saline wastewater, especially denitrification of high sulfate-containing aquarium and aquaculture seawater in closed circuit systems. High sulfate content in seawater obscures denitrification in biofilm processes by encouraging growth of sulfate-reducing bacteria in the anaerobic deep layer of the biofilm and residual nitrate should be made available to deter this process [134] as explained by Labelle et al. [135]. The carbon source necessary for denitrification is utilized by unwanted sulfate reduction which in turn generates sulfides that prevent the transformation of nitrous oxide to nitrogen. Labelle et al. [135] remarked that agitation methods applied in some moving-bed biofilm reactors (MBBR) were not acceptable as large dead mixing zones developed with resultant excessive biomass growth thus promoting sulfate reduction [136]. To circumvent this problem, Labelle et al. [135] designed a pilot-scale submerged MBBR at the Montreal Biodome, Canada and eventually scaled up to a commercial MBBR. Using methanol as a carbon source at various C/N ratios, seawater denitrification in a 3.25-million-liter closed-circuit mesocosm was investigated. The MBBR was partially filled with “positively buoyant” spherical polyethylene carriers representing 35 % of the total surface area. To deoxygenate the seawater prior to denitrification, pretreatment was done in a recirculated fixed bed. The carriers were maintained submerged by a conical grid and circulated through the downflow jet of an eductor (Fig. 10). Denitrification stoichiometric values corresponded to methanol consumption and sulfate reduction was not observed. Labelle et al. further noted that the C/N ratio was correlated to rates of denitrification and concentration of effluent residual dissolved organic carbon. Carrier fouling could be avoided by the downflow jet current of the denitrification unit. Low biofilm thickness was maintained during maximal denitrification activity [135].
Schematic diagram of the experimental setup as described in Labelle et al. [135]. A packed-bed biofilter containing 63-mm random plastic carriers occupying 80 % of total filter volume is the first-stage pretreatment deoxygenation unit. Denitrification unit comprising a submerged MBBR designed to reduce media fouling and dead mixing zones is the second stage. Reprinted with permission from Elsevier
In an attempt to maintain nitrate concentration within acceptable limits, the Montreal Biodome established a methanol-fed denitrification reactor to treat 3-million-liter seawater [137]. A denitrifying biofilm on the fluidized bed of plastic carriers was formed through colonization by naturally occurring microorganisms from seawater effluent in this completely mixed reactor. Through 16S rRNA gene sequencing the culturable isolates were established as members of alpha-Proteobacteria. The nonculturable ones were related to the Methylophaga members of the Piscirickettsia family (gamma-Proteobacteria) and other bacterial nitrifiers [138]. In their next study, Auclair et al. further detected functional genes coding for different denitrification reductases of the bacterial denitrifiers and their expression [139]. Quantitative PCR was applied to determine the concentrations of the different nitrate reductase gene sequences (narG, napA, nirS, and nirK) to ascertain the presence of denitrifiers and nitrate-reducing bacteria in the biofilm [139]. Sequences were found to be identical to the corresponding genes found in Hyphomicrobium sp. NL23 and Methylophaga sp. JAM1. Auclair et al. also demonstrated the predominance of Methylophaga sp. JAM1 and Hyphomicrobium sp. NL23 among the denitrifiers of the biofilm and indicated that the latter could use the nitrite generated by the former [139].
Biological fixed-film processes are advantageous for total ammonia nitrogen (TAN) removal in recirculated water systems (RAS) because of ease of operation, enhanced process stability to shock loads, and no possibility of bacterial wash off as reviewed by Fitch et al. [129] and noted by Rejish Kumar et al. [140]. However, despite the advantages, immobilized nitrifiers in RAS have demonstrated poor performance and require long start-up times [140]. To overcome these limitations a specialized nitrifying packed-bed bioreactor (PBBR) immobilized with ammonia-oxidizing and nitrite-oxidizing bacteria was developed. This reactor required a short start-up time and could be easily integrated into existing hatchery designs to operate under closed recirculating mode [141]. Microbial community analysis by fluorescent in situ hybridization (FISH) detected the presence of nitrifiers of the genera Nitrosococcus, Nitrobacter, and Nitrospira [142]. Nitrification in the PBBR integrated into a marine Penaeus monodon maturation system was analyzed for 70 days [140]. Instant nitrification was observed and TAN as well as NO2-N removal were significant. FISH analysis of the biofilms showed presence of beta-ammonia oxidizers, Nitrosospira species, halophilic Nitrosomonas species, and Nitrospira species. Rejish Kumar et al. [140] observed that the biofilm was dominated by autotrophic nitrifiers when the reactor system was operated with saturated oxygen and low concentrations of TAN and further stated that nitrifying biofilms could be excellently established on plastic bead carrier material (Fig. 11).
Packed-bed bioreactor connected to a shrimp maturation system as described in Kumar et al. [140]. AS aeration supply, AT aeration tube, CT collecting tank, FB filter bags, OHT overhead tank, PB polystyrene beads, P pump, R1–R6 reactors, V valves. Reprinted with permission from Wiley
Although a large number of investigations have been done on bacterial biofilm-mediated bioremediation processes, fungal biofilm processes, in contrast, have not received much attention. Mitra et al. [143] studied the influence of surface attachment of Cunninghamella elegans and niche intertidal conditions simulated in a bioreactor on the biotransformation of fluoranthene by this filamentous fungus. To this end, Mitra et al. [143] applied the CCF described earlier [40, 109, 110, 121] to compare fluoranthene biotransformation between biofilm and planktonic cultures as well as between hydrophobic and hydrophilic surfaces of biofilm attachment. Biofilm cultures showed more enhanced growth as well as fluoranthene transformation than did planktonic cultures with concomitant cytochrome P450 gene expression. Stable biofilm developed on the hydrophobic surface in comparison to the hydrophilic surface, with greater colocalization of fluoranthene in the extracellular polymeric substances as observed through three-channel confocal laser scanning microscopy. The RDBR used previously [84, 91, 92] was employed to provide six-hourly submergence and aerial exposure, thus mimicking the semidiurnal intertidal conditions from where C. elegans was obtained. Compared to a process not simulating the niche environmental conditions, growth, fluoranthene transformation, and cytochrome P450 gene expression were higher in the process mimicking the intertidal conditions. Investigators concluded that in both small and large systems, biofilm formation was higher than planktonic cultures with a corresponding higher concentration of biofilm exopolysaccharides. This condition permitted enhanced movement of fluoranthene inside the biofilm with a resultant elevated gene expression.
3.8 Biofilms in Microbial Fuel Cells
About 20 years ago, microbial fuel cells employing immobilized Proteus vulgaris cells were used to generate continuous electric current. Improved mass-transfer kinetics resulting from the proximity of the immobilized bacteria to the electrode surface allowed increased efficiency compared to suspended cells [144]. The electrochemically active (EA) microorganisms termed “electricigens” are able to form biofilms on the electrode surface and oxidize organic compounds to CO2 with simultaneous direct transfer of electrons to the electrode in measurable quantities [145]. Using acetate as the substrate, electrochemically active biofilms were developed on graphite anodes under constant polarization versus saturated calomel reference (SCE) [146]. Different microbial samples, from natural biofilm formed on a floating bridge (located in the Atlantic coastal port of La Tremblade, France) surface, marine sediments collected directly under the floating bridge, and beach sand were used to inoculate the cells. Erable et al. [146] obtained higher current densities with the biofilm inoculum compared to the other samples. Bacteria related to Bacteroidetes, Halomonas, and Marinobacterium were recovered from the EA biofilm, and species related to Mesoflavibacter were prevalent on sediment biofilm. The coulombic efficiency of acetate consumption was improved by progressively adapting the anode to acetate utilization by serial additions of the substrate. After 8 days of biofilm formation, a maximal current density of 7.9 A/m2 was attained with 10 mM acetate, the highest value as claimed by Erable et al. [146]. The authors further observed that microorganisms gradually colonized the anode surface with the progressive increase in anodic current. Microbial surface growth as well as current production was stimulated by acetate addition. Current density decreased rapidly after reaching the maximum value, possibly due to acetate exhaustion. On the other hand, control experiments with seawater without biofilm inoculum did not yield any current, irrespective of acetate addition [146].
The efficiency of the cathodic reduction process in microbial fuel cells is low [147] as noted by Erable et al. [148]. Although platinum can improve the efficiency, its application is limited by high cost and anodic poisoning. Therefore, microbial cathodes have potential as a cheap and sustainable alternative to platinum. Erable et al. [148] remarked that oxygen reduction on stainless steels has been observed to be catalyzed by biofilms formed in natural seawater [149], opening up a possibility for cheap microbial cathodes for fuel cells. Erable et al. [148] further commented that current densities up to 1.89 A/m2 were achieved with a biofilm-covered cathode in 2005 [150] and implementation of marine microbial cathodes in MFCs in a sea environment were later sought [151, 152]. However, the necessity of exposure of stainless steel under constant polarization for several days in large volumes of seawater with continuous renewal [153] posed a hindrance in developing efficient seawater biofilms [148]. Erable et al. attempted to develop electrochemically active (EA) biofilms in closed electrochemical vessels that could be conveniently handled [148]. A mechanistic understanding of biocatalysis was offered by identifying EA seawater biofilm-forming microbial strains and the electrochemical efficiency of each isolate was ascertained. Wild EA biofilms were developed by immersing stainless steel in open sea water with monitoring of the current generated. The film was then removed by scraping, resuspended in seawater, and applied as inoculum in closed 0.5 L electrochemical reactors. A 20-fold improvement in the current density was attained by continuously feeding an open reactor with filtered seawater. Erable et al. [148] reasoned that seawater filtration prevented indigenous common strains from competing with the EA strains. Among the biofilm formers, Winogradskyella poriferorum and Acinetobacter johsonii yielded modest current densities and the importance of synergistic effects occurring in the biofilm was pointed out [148].
3.9 Aquaculture Feedstock Production
Avendaño-Hererra and Riquelme [154] explained that primary colonization of surfaces by bacteria precedes the maturation of a mixed biofilm formed of diatoms and other microorganisms [155]. The authors [154] further discussed that production of extracellular polysaccharides which function at cellular and intercellular levels enables the formation of strong irreversible bonds with the surface [156] and succession of macroorganisms follows colonization by microorganisms [157]. The bacteria of the biofilm can have stimulatory or inhibitory effects on the late-colonizing microalgae, thus influencing the microalgal population [158] as cited in [154]. Larval settlement is of vital importance in the artificial production of Argopecten purpuratus (Peruvian scallop). The possibility of improving postlarval settlement of A. purpuratus using a substrate, “cultch”, pretreated with native diatom biofilm was investigated. Avendaño-Hererra et al. [159] showed an increase of spatfall and production of larger settlement were attained by the addition of diatom biofilms. An innovative microalgal culture system was used for the production of Navicula veneta (diatom) biofilm by addition of bacteria [154]. NC1 (Halomonas sp.) was found to be the best growing strain utilizing the extracellular products of N. veneta in small-scale studies conducted in Petri plates. In the Tanaka photobioreactor (Fig. 12), the diatom produced the highest yields when cocultivated with NC1 (Halomonas sp.) in six replicate cultures running in three cycles lasting 7 days each. Chlorophyll a concentration (indicator of diatom growth) and bacterial cell mass were positively correlated. The Tanaka photobioreactor can be gainfully exploited for the mass production of diatom–bacteria mixed biofilms. The biofilms can find application in the settlement of mass cultures of marine invertebrates of commercial importance as well as aquaculture food production. Avendaño-Hererra and Riquelme [154] further remarked that the biofilm could be used to provide nutrition for abalone or scallop juvenile stages and/or to colonize the larvae settlement substrata, thus reducing the process time [160].
Schematic of the Tanaka photobioreactor as described in Avendaño-Herrera and Riquelme [154]. Top of the reactor allows introduction of starting inoculum, air, and bristles, and culture is harvested through the side-arm effluent tube. Reprinted with permission from Elsevier
Similar to the observations of Yan et al. [72] and Yan et al. [73], Silva-Aciares and Riquelme [161] noted that traditionally used suspension microalgal culture systems were not suitable for benthic diatoms that have characteristic surface-attached lifestyles. Larval settlement and metamorphosis of Pinctada maxima (pearl oyster) in response to both natural (bacterial biofilms) and artificial inducers (K+, Ca2+, and NH4 + and seven types of neuroactive compounds such as 3-isobutyl-1-methylxanthine, γ-aminobutyric acid, choline chloride, acetylcholine chloride, serotonin hydrochloride, 3-(3,4-dihydroxyphenyl)-L-ananine and dopamine) were investigated by Zhao et al. [162]. Natural bacterial biofilms supported larval settlement. Zhao et al. [162] noted that some pharmacological agents acted as potent artificial inducers of P. maxima larval settlement. These ecological observations, however, were not transformed into large-scale production of algal biofilms [161]. Mass culture of six benthic diatom species in a bristles photobioreactor (PBB; Fig. 13) were undertaken by Silva-Aciares and Riquelme [161]. The reactor holds polyvinyl chloride bristles providing a surface for attachment of adhesive diatoms and constant water movement was afforded by an airlift system [161]. Performance of this reactor was compared with that attained by cultivating the diatom species in a bubble column photobioreactor without support bristles (PBC), while maintaining a turbulent hydrodynamic regime through a strong current of air bubbles. The PBB proved to be a superior reactor for adhesive diatoms Amphora sp., Amphora sp. 2, Navicula sp., and Nitzschia ovalis. The PBC system, on the other hand, was more suitable for the lesser adhesive diatoms Nitzschia sp. and Cylindrotheca closterium which grew better in suspension mode. Higher bacterial counts were obtained in the systems having maximum microalgal populations. The positive correlation between the bacterial concentration and the number of microalgae in photobioreactors is mutually beneficial. Diatoms are recognized for secretion of organic compounds with high amounts of carbohydrates [163] that supply nutrients to heterotrophs [164]. Several studies suggest that during the bacterial–microalgal interactions, various bacterial species secrete organic compounds which support the microalgal growth [154, 158]. These organic compounds may be converted to carbon dioxide by the bacteria that is utilized for photosynthesis by the phototrophic microalgae [165].
Diagram of the bristles photobioreactor (PBB) as described in Silva-Aciares and Riquelme [161]. A general view of the PBB: 1 top of the tube with a PVC cap, 2 air inlet PVC connector, 3 clear acrylic tube, 4 base coupling, 5 ball valve, 6 PVC bristles in a “bottle brush” with a metal axis coated by plastic, 7 PVC “bubbling column,” and 8 plastic compressed airline. Reprinted with permission from Elsevier
At least seven mechanisms of attachment in solitary or colonial arrangement have been described for diatoms [166] as cited in [161]. Diatoms grow in two-dimensional or multiple three-dimensional layers governed by their motility and adhesive force. The high degree of adhesion and slow movement that typifies “type B” growth was observed on the PVC bristles but not in the water column microalgae. Diatoms that yielded higher concentrations and biomass in the PBC systems, and developed satisfactorily in the water column forming biofilm microaggregates, had “type A” growth distinguished by weak substrate adhesion and fast movement [166].
4 Conclusions
This chapter reviews the research done on the ecological perspectives of marine and intertidal surface-attached microbes and their bioactivities as well as the industrial and environmental bioprocesses dependent on biofilm formation. Biofilm formation and bioactivities of the Roseobacter clade and the Pseudoalteromonas genus are described in detail. Nitrous-oxide (N2O)—emitting biofilms, antimicrobial- and auxin-producing biofilms are also illustrated. The chapter discusses biofilm-mediated production of antifouling, antimicrobial, and cytotoxic compounds, exopolysaccharides, enzymes, melanin, riboflavin, and aquaculture feedstock. Bioremediation processes and operation of microbial fuel cells through application of marine and intertidal surface-attached microbes are important components of the chapter. From a biotechnological perspective, the production of bioactive compounds for competition and defense by surface-associated marine and intertidal microorganisms represent an unmatched pool for the discovery of new molecules, with medical, industrial, and environmental applications.
Through the nexus of ecology and engineering it is important to comprehend the fundamental characteristics concerning the behavior of cells in a biofilm such as cell physiology and mobility of established biofilms. Development and progression of biofilms can be controlled to make them productive through understanding of biofilm growth mechanisms against an ecological background. With an improved knowledge of these physicochemical properties of biofilms, further optimization of process conditions and design of reactors based on fundamental information can be visualized. The prospects of multispecies marine/intertidal biofilms in bioprocess development need to be researched. A potentially interesting field of research would be to understand the role of multiple species catalyzing a series of reactions in a cascade. Comprehension of the basis of interspecies dependencies of microbial communities requires further fundamental research [167, 168].
Future research should be directed to target new areas for the use of marine/intertidal biofilms in productive catalysis. Possibilities exist for applications of biofilms in biotransformations involving solvents or other daunting reactants as biofilms display increased resistance to toxic substrates and products. Biofilms might prove useful for the production of low-value bulk chemicals because they can provide the required catalyst concentration (biomass) to achieve an efficient transformation of the substrate. Another emerging area of research is the prospective use of extracellular enzymes produced by biofilms, such as hydrolases for use in biofuel production. Christenson and Sims noted all microalgal cultivations for biofuel production involve substantial challenges of biomass harvesting that can account for up to 30 % of total costs [169]. Because of the high centrifugation costs associated with harvesting suspended microalgae, there is interest in using surface-attached algal biofilm systems that are naturally concentrated and more readily harvestable. Another promising sector for biofilm applications is the pharmaceutical industry. Biofilm reactors can support continuous processing of enzyme cascades within whole cells that may be used to synthesize complex pharmaceuticals, for example, those containing multiple chiral centers [170].
References
Egan S, Thomas T, Kjelleberg S (2008) Curr Opin Microbiol 11:219
Ortega-Morales BO, Chan-Bacab MJ, De la Rosa-García SDC, Camacho-Chab JC (2010) Curr Opin Biotechnol 21:346
Decho AW (2000) Cont Shelf Res 20:1257
Bowman JP (2007) Mar Drugs 5:220
Bruhn JB, Nielsen KF, Hjelm M, Hansen M, Bresciani J, Schulz S, Gram L (2005) Appl Environ Microbiol 71:7263
Ruger HJ, Hofle MG (1992) Int J Syst Bacteriol 42:133
Hjelm M, Bergh Ø, Riaza A, Nielsen J, Melchiorsen J, Jensen S, Duncan H, Ahrens P, Birkbeck H, Gram L (2004) Syst Appl Microbiol 27:360
Hjelm M, Riaza A, Formoso F, Melchiorsen J, Gram L (2004) Appl Environ Microbiol 70:7288
Bruhn JB, Haagensen JAJ, Bagge-Ravn D, Gram L (2006) Appl Environ Microbiol 72:3011
Bruhn JB, Gram L, Belas R (2007) Appl Environ Microbiol 73:442
Brinkhoff T, Bach G, Heidorn T, Liang L, Schlingloff A, Simon M (2004) Appl Environ Microbiol 70:2560
Moran MA, González JM, Kiene RP (2003) Geomicrobiol J 20:375
Miller TR, Belas R (2004) Appl Environ Microbiol 70:3383
D’Alvise PW, Melchiorsen J, Porsby CH, Nielsen KF, Gram L (2010) Appl Environ Microbiol 76:2366
Geng H, Bruhn JB, Nielsen KF, Gram L, Belas R (2008) Appl Environ Microbiol 74:1535
D’Alvise PW, Magdenoska O, Melchiorsen J, Nielsen KF, Gram L (2013) Environ Microbiol (in press)
Smith KD, Shanahan CA, Moore EL, Simon AC, Strobel SA (2011) Proc Natl Acad Sci USA 108:7757
Porsby CH, Nielsen KF, Gram L (2008) Appl Environ Microbiol 74:7356
Brinkhoff T, Giebel HA, Simon M (2008) Arch Microbiol 189:531
Buchan A, González JM, Moran MA (2005) Appl Environ Microbiol 71:5665
Lafay B, Ruimy R, De Traubenberg CR, Breittmayer V, Gauthier MJ, Christen R (1995) Int J Syst Bacteriol 45:290
Wagner-Döbler I, Biebl H (2006) Annu Rev Microbiol 60:255
Newton RJ, Griffin LE, Bowles KM, Meile C, Gifford S, Givens CE, Howard EC, King E, Oakley CA, Reisch CR, Rinta-Kanto JM, Sharma S, Sun SL, Varaljay V, Vila-Costa M, Westrich JR, Moran MA (2010) ISME J 4:784
Cude WN, Mooney J, Tavanaei AA, Hadden MK, Frank AM, Gulvik CA, May AL, Buchan A (2012) Appl Environ Microbiol 78:4771
Holmström C, Kjelleberg S (1999) FEMS Microbiol Ecol 30:285
Rao D, Webb JS, Kjelleberg S (2005) Appl Environ Microbiol 71:1729
James SG, Holmström C, Kjelleberg S (1996) Appl Environ Microbiol 62:2783
Barja JL, Lemos ML, Toranzo AE (1989) Antimicrob Agents Chemother 33:1674
Matz C, Webb JS, Schupp PJ, Phang SY, Penesyan A, Egan S, Steinberg P, Kjelleberg S (2008) PLoS ONE 3:e2744
Riveros R, Haun M, Campos V, Duran N (1988) Arq Biol Technol 31:475
August PR, Grossman TH, Minor C, Draper MP, MacNeil IA, Pemberton JM, Call KM, Holt D, Osburne MS (2000) J Mol Microbiol Biotechnol 2:513
Sánchez C, Braña AF, Méndez C, Salas JA (2006) ChemBioChem 7:1231
Franks A, Egan S, Holmström C, James S, Lappin-Scott H, Kjelleberg S (2006) Appl Environ Microbiol 72:6079
Holmström C, Egan S, Franks A, McCloy S, Kjelleberg S (2002) FEMS Microbiol Ecol 41:47
Egan S, James S, Holmström C, Kjelleberg S (2001) FEMS Microbiol Ecol 35:67
Bernbom N, Ng YY, Kjelleberg S, Harder T, Gram L (2011) Appl Environ Microbiol 77:8557
Lemos ML, Toranzo AE, Barja JL (1985) Microb Ecol 11:149
Long RA, Qureshi A, Faulkner DJ, Azam F (2003) Appl Environ Microbiol 69:568
Long RA, Azam F (2001) Aquat Microb Ecol 26:103
Mitra S, Sarkar S, Gachhui R, Mukherjee J (2011) Appl Microbiol Biotechnol 90:321
Ivanova EP, Nicolau DV, Yumoto N, Taguchi T, Okamoto K, Tatsu Y, Yoshikawa S (1998) Mar Biol 130:545
Rao D, Webb JS, Kjelleberg S (2006) Appl Environ Microbiol 72:5547
Heisterkamp IM, Schramm A, De Beer D, Stief P (2010) Mar Ecol Prog Ser 415:1
IPCC (Intergovernmental Panel on Climate Change) (2007) Climate change 2007, the physical science basis—summary for policy makers. Cambridge University Press, Cambridge
Ravishankara AR, Daniel JS, Portmann RW (2009) Science 326:123
Bange HW (2006) Atmos Environ 40:198
Magalhães CM, Wiebe WJ, Joye SB, Bordalo AA (2005) Estuaries 28:592
Stief P, Poulsen M, Nielsen LP, Brix H, Schramm A (2009) Proc Natl Acad Sci USA 106:4296
Philippart CJM, Beukema JJ, Cadée GC, Dekker R, Goedhart PW, Van Iperen JM, Leopold MF, Herman PMJ (2007) Ecosystems 10:95
Van Beusekom JEE, Weigelt-Krenz S, Martens P (2008) Helgol Mar Res 62:49
Meyer RL, Allen DE, Schmidt S (2008) Mar Chem 110:68
Jenkins MC, Kemp WM (1984) Limnol Oceanogr 29:609
Heisterkamp IM, Schramm A, Larsen LH, Svenningsen NB, Lavik G, De Beer D, Stief P (2013) Environ Microbiol 15:1943
Svenningsen NB, Heisterkamp IM, Sigby-Clausen M, Larsen LH, Nielsen LP, Stief P, Schramm A (2012) Appl Environ Microbiol 78:4505
Wilson GS, Raftos DA, Nair SV (2011) Microbiol Res 166:437
Burchard RP, Sorongon ML (1998) Appl Environ Microbiol 64:4079
Kerkar S, Raiker L, Tiwari A, Mayilraj S, Dastager S (2012) Biologia 67:454
Patten CL, Glick BR (2002) Appl Environ Microbiol 68:3795
Kogure K, Simidu U, Taga N (1979) J Exp Mar Biol Ecol 36:201
Armstrong E, Yan L, Boyd KG, Wright PC, Burgess JG (2001) Hydrobiologia 461:37
Tait K, Sutherland IW (2002) J Appl Microbiol 93:345
Ortega-Morales BO, Chan-Bacab MJ, Miranda-Tello E, Fardeau ML, Carrero JC, Stein T (2008) J Ind Microbiol Biotechnol 35:9
Callow ME, Callow JA (2002) Biologist 49:10
Steinberg PD, De Nys R, Kjelleberg S (2001) Marine chemical ecology. In: McClintock JB, Baker BJ (ed). CRC, Florida, pp 355–388
Roberts MS, Nakamura LK, Cohan FM (1994) Int J Syst Bacteriol 44:256
Bacon CW, Hinton DM (2002) Biol Control 23:274
Bernbom N, Ng YY, Olsen SM, Gram L (2013) Appl Environ Microbiol 79:6885
Holmström C, Rittschof D, Kjelleberg S (1992) Appl Environ Microbiol 58:2111
Ortega-Morales BO, Ortega-Morales FN, Lara-Reyna J, De La Rosa-García SC, Martínez-Hernández A, Montero-M J (2009) Mar Biotechnol 11:375
Kanagasabhapathy M, Sasaki H, Haldar S, Yamasaki S, Nagata S (2006) Ann Microbiol 56:167
Vandevivere P, Kirchman DL (1993) Appl Environ Microbiol 59:3280
Yan L, Boyd KG, Burgess JG (2002) Mar Biotechnol 4:356
Yan L, Boyd KG, Adams DR, Burgess JG (2003) Appl Environ Microbiol 69:3719
Wimpenny J, Manz W, Szewzyk U (2000) FEMS Microbiol Rev 24:661
Vroom JM, De Grauw KJ, Gerritsen HC, Bradshaw DJ, Marsh PD, Watson GK, Birmingham JJ, Allison C (1999) Appl Environ Microbiol 65:3502
Beuling EE, Van Den Heuvel JC, Ottengraf SP (2000) Biotechnol Bioeng 67:53
James GA, Korber DR, Caldwell DE, Costerton JW (1995) J Bacteriol 177:907
Evans LV (2000) Biofilms: recent advances in their study and control. Harwood Academic, Marston, Amsterdam, The Netherlands
Boyd KG, Adams DR, Burgess JG (1999) Biofouling 14:227
Yang LH, Xiong H, Lee OO, Qi SH, Qian PY (2007) Lett Appl Microbiol 44:625
Boettcher KJ, Ruby EG (1995) J Bacteriol 177:1053
Davies DG, Chakrabarty AM, Geesey GG (1993) Appl Environ Microbiol 59:1181
Ohlendorf B, Leyers S, Krick A, Kehraus S, Wiese M, König GM (2008) ChemBioChem 9:2997
Sarkar S, Saha M, Roy D, Jaisankar P, Das S, Gauri Roy L, Gachhui R, Sen T, Mukherjee J (2008) Mar Biotechnol 10:518
Fiedler HP, Bruntner C, Bull AT, Ward AC, Goodfellow M, Potterat O, Puder C, Mihm G (2005) Antonie Van Leeuwenhoek 87:37
Jensen PR, Mincer TJ, Williams PG, Fenical W (2005) Antonie Van Leeuwenhoek 87:43
Bull AT, Stach JEM, Ward AC, Goodfellow M (2005) Antonie Van Leeuwenhoek 87:65
Imada C (2005) Antonie Van Leeuwenhoek 87:59
Mitra A, Santra SC, Mukherjee J (2008) Appl Microbiol Biotechnol 80:685
Marwick JD, Wright PC, Burgess JG (1999) Mar Biotechnol 1:495
Sarkar S, Mukherjee J, Roy D (2009) Biotechnol Bioprocess Eng 14:775
Sarkar S, Roy D, Mukherjee J (2010) Bioprocess Biosyst Eng 33:207
Arumugam M, Mitra A, Pramanik A, Saha M, Gachhui R, Mukherjee J (2011) Int J Syst Evol Microbiol 61:2664
Ahimou F, Semmens MJ, Haugstad G, Novak PJ (2007) Appl Environ Microbiol 73:2905
Zhang Y, Arends JBA, Van de Wiele T, Boon N (2011) Biotechnol Adv 29:312
Kirkwood AE, Buchheim JA, Buchheim MA, Henley WJ (2008) Microb Ecol 55:453
Ortega-Morales BO, Santiago-García JL, Chan-Bacab MJ, Moppert X, Miranda-Tello E, Fardeau ML, Carrero JC, Bartolo-Pérez P, Valadéz-González A, Guezennec J (2007) J Appl Microbiol 102:254
Ortega-Morales BO, López-Cortés A, Hernández-Duque G, Crassous P, Guezennec J (2001) Methods Enzymol 336:331
Mancuso Nichols CA, Guezennec J, Bowman JP (2005) Mar Biotechnol 7:253
Vincent P, Pignet P, Talmont F, Bozzi L, Fournet B, Guezennec J, Jeanthon C, Prieur D (1994) Appl Environ Microbiol 60:4134
Raguenes G, Pignet P, Gauthier G, Peres A, Christen R, Rougeaux H, Barbier G, Guezennec J (1996) Appl Environ Microbiol 62:67
Raguenes G, Moppert X, Richert L, Ratiskol J, Payri C, Costa B, Guezennec J (2004) Curr Microbiol 49:145
Guézennec J, Moppert X, Raguénès G, Richert L, Costa B, Simon-Colin C (2011) Process Biochem 46:16
Sutherland IW (1996) Extracellular polysaccharides. In: Rehm HJ, Reed G (eds) Biotechnology: products of primary metabolism. Verlag Chemie, Weinheim, Germany, pp 613–657
Mancuso Nichols CA, Garon S, Bowman JP, Raguénès G, Guézennec J (2004) J Appl Microbiol 96:1057
Kavita K, Singh VK, Mishra A, Jha B (2014) Carbohyd Polym 101:29
Iijima S, Washio K, Okahara R, Morikawa M (2009) Microb Biotechnol 2:361
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Microbiol Mol Biol Rev 64:655
Sarkar S, Roy D, Mukherjee J (2011) Bioresour Technol 102:1849
Mitra S, Banerjee P, Gachhui R, Mukherjee J (2011) Bioprocess Biosyst Eng 34:1087
Niture SK, Pant A (2007) World J Microbiol Biotechnol 23:1169
Da Silva M, Cerniglia CE, Pothuluri JV, Canhos VP, Esposito E (2003) World J Microbiol Biotechnol 19:399
Gutiérrez-Correa M, Villena GK (2003) Rev Peru Biol 10:113
Harding MW, Marques LLR, Howard RJ, Olson ME (2009) Trends Microbiol 17:475
Wimpenny J, Manz W, Szewzyk U (2000) FEMS Microbiol Rev 24:661
Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S (2000) Microbiology 146:2395
Da Silva WJ, Seneviratne J, Samaranayake LP, Del Bel Cury AA (2010) J Biomed Mater Res B Appl Biomater 94:149
Mueller LN, De Brouwer JFC, Almeida JS, Stal LJ, Xavier JB (2006) BMC Ecol 6:1
Tait K, Williamson H, Atkinson S, Williams P, Cámara M, Joint I (2009) Environ Microbiol 11:1792
Stahmann KP, Revuelta JL, Seulberger H (2000) Appl Microbiol Biotechnol 53:509
Mitra S, Thawrani D, Banerjee P, Gachhui R, Mukherjee J (2012) Appl Biochem Biotechnol 166:1991
Dmytruk KV, Yatsyshyn VY, Sybirna NO, Fedorovych DV, Sibirny AA (2011) Metab Eng 13:82
Malm A, Chudzik B, Piersiak T, Gawron A (2010) Ann Agric Environ Med 17:115
Tal Y, Watts JEM, Schreier HJ (2006) Appl Environ Microbiol 72:2896
Van Rijn J, Tal Y, Schreier HJ (2006) Aquac Eng 34:364
Terada A, Zhou S, Hosomi M (2011) Clean Technol Envir 13:759
Jetten MSM, Wagner M, Fuerst J, Van Loosdrecht M, Kuenen G, Strous M (2001) Curr Opin Biotechnol 12:283
Mancini G, Cappello S, Yakimov MM, Polizzi A, Torregrossa M (2012) Chem Eng Transac 27:37
Fitch MW, Pearson N, Richards G, Burken JG (1998) Water Environ Res 70:495
Gharsallah N, Khannous L, Souissi N, Nasri M (2002) J Chem Technol Biotechnol 77:865
Zamora-Castro J, Paniagua-Michel J, Lezama-Cervantes C (2008) Mar Biotechnol 10:181
Stolz JF (2000) Structure of microbial mats and biofilms. In: Riding RE, Awramik SM (eds) Microbial sediments. Springer, Heidelberg, pp 1–8
Ebeling JM, Rishel KL, Sibrell PL (2005) Aquac Eng 33:235
Welander U, Mattiasson B (2003) Water Res 37:2394
Labelle MA, Juteau P, Jolicoeur M, Villemur R, Parent S, Comeau Y (2005) Water Res 39:3409
Tal Y, Watts JEM, Schreier SB, Sowers KR, Schreier HJ (2003) Aquaculture 215:187
Parent S, Morin A (2000) Water Res 34:1846
Labbé N, Juteau P, Parent S, Villemur R (2003) Microb Ecol 46:12
Auclair J, Parent S, Villemur R (2012) Microb Ecol 63:726
Kumar VJR, Joseph V, Vijai R, Philip R, Singh ISB (2011) J Chem Technol Biotechnol 86:790
Kumar VJR, Achuthan C, Manju NJ, Philip R, Singh ISB (2009) J Ind Microbiol Biotechnol 36:355
Kumar VJR, Joseph V, Philip R, Singh ISB (2010) Water Sci Technol 61:797
Mitra S, Pramanik A, Banerjee S, Haldar S, Gachhui R, Mukherjee J (2013) Appl Environ Microbiol 79:7922
Allen RM, Bennetto HP (1993) Appl Biochem Biotechnol 39–40:27
Lovley DR (2006) Nat Rev Microbiol 4:497
Erable B, Roncato MA, Achouak W, Bergel A (2009) Environ Sci Technol 43:3194
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) J Power Sources 180:683
Erable B, Vandecandelaere I, Faimali M, Delia ML, Etcheverry L, Vandamme P, Bergel A (2010) Bioelectrochemistry 78:51
Scotto V, Cintio RD, Marcerano G (1985) Corros Sci 25:185
Bergel A, Féron D, Mollica A (2005) Electrochem Commun 7:900
Dumas C, Mollica A, Féron D, Basséguy R, Etcheverry L, Bergel A (2007) Electrochim Acta 53:468
Dumas C, Mollica A, Féron D, Basséguy R, Etcheverry L, Bergel A (2008) Bioresour Technol 99:8887
Faimali M, Chelossi E, Garaventa F, Corrà C, Greco G, Mollica A (2008) Electrochim Acta 54:148
Avendaño-Herrera RE, Riquelme CE (2007) Aquac Eng 36:97
Allison DG, Gilbert P (1992) Sci Prog 76:305
Wetherbee R, Lind JL, Burke J, Quatrano RS (1998) J Phycol 34:9
Wahl M (1989) Mar Ecol Prog Ser 58:175
Avendaño RE, Riquelme CE (1999) Aquac Res 30:893
Avendaño-Herrera R, Riquelmes C, Silva F, Avendañod M, Irgang R (2003) J Shellfish Res 22:393
Lebeau T, Robert JM (2003) Appl Microbiol Biotechnol 60:612
Silva-Aciares FR, Riquelme CE (2008) Aquac Eng 38:26
Zhao B, Zhang S, Qian PY (2003) Aquaculture 220:883
Myklestad SM (1995) Sci Total Environ 165:155
Lancelot C (1983) Mar Ecol Prog Ser 12:115
Pisman TI, Galayda YV, Loginova NS (2005) Adv Space Res 35:1579
Kawamura T (1996) The role of benthic diatoms in the early life stages of the Japanese abalone (Haliotis discus hannai). In: Watanabe Y, Yamashita Y, Oozeki Y (eds) Survival strategies in early life stages of marine resources. A. A Balkema, Rotterdam, The Netherlands, pp 355–367
Cheng KC, Demirci A, Catchmark JM (2010) Appl Microbiol Biotechnol 87:445
Halan B, Buehler K, Schmid A (2012) Trends Biotechnol 30:453
Christenson LB, Sims RC (2012) Biotechnol Bioeng 109:1674
Rosche B, Li XZ, Hauer B, Schmid A, Buehler K (2009) Trends Biotechnol 27:636
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Mitra, S., Sana, B., Mukherjee, J. (2014). Ecological Roles and Biotechnological Applications of Marine and Intertidal Microbial Biofilms. In: Muffler, K., Ulber, R. (eds) Productive Biofilms. Advances in Biochemical Engineering/Biotechnology, vol 146. Springer, Cham. https://doi.org/10.1007/10_2014_271
Download citation
DOI: https://doi.org/10.1007/10_2014_271
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09694-0
Online ISBN: 978-3-319-09695-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)