Abstract
Jatropha curcas seeds are rich in oil (28–32%), which can be converted to high quality biodiesel. The oil is non-edible due to the presence of toxic compounds, namely, phorbol esters (PEs). PEs have a number of agricultural/medicinal/pharmaceutical applications and hence their recovery generates a value added co-product towards the biodiesel production chain. This study aims to assess the effects of PE extraction on quality of both the residual oil and the biodiesel production from it. Two Approaches (1, use of an Ultra-turrax; and 2, use of a magnetic stirrer) were used with an effective treatment time of 2 and 5 min, resulting in 80 and 78% extraction of PEs, respectively. The phosphorus content was reduced by 70.2 and 75.8%, free fatty acids by 55.3 and 55.6%, and the fatty acid composition did not change in the residual oils. The peroxide value increased from 2.69 (untreated oil) to 3.01 and 3.49 mequiv O2/kg in the residual oils following Approach 1 and Approach 2, respectively. The biodiesel prepared from both residual oils met European (EN 14214:2008) and American biodiesel standard (ASTM D6751-09) specifications. Oxidative stability indices for both the biodiesels were well within the permitted limit. It is concluded that PEs could be isolated in active forms for various applications by either of the two methods with a high yield and the residual oil can be processed to produce high quality biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, biofuels have been getting considerable attention because of global emphasis on reducing greenhouse gases, conserving the environment and energy security. The use of biodiesel to (partially) replace fossil diesel has a significant potential for reducing pollution and creating socioeconomic benefits for farmers. Plant oils are usually converted into biodiesel by transesterification with short-chain alcohols, such as methanol, to bring their combustion properties closer to those of conventional fuels or mineral diesel. Biodiesel is currently being produced from grease, vegetable oils or animal fats. The use of edible plant oils for biodiesel production is under discussion as they compete with food crops for scarce agricultural land and water. Therefore, the focus is now moving towards the use of non-edible crops as feedstocks for biodiesel production, such as Jatropha and algae oil, used cooking oils, low-quality animal fats and side-streams from oil refining.
Jatropha curcas belongs to the Euphorbiaceae family and is commonly known as physic nut. It is believed to have originated in Central America and now it is widespread all over the tropical and subtropical world. The plant is perennial, drought resistant, and can grow in marginal lands, rocky lands, and even in saline soils. The seeds and kernels contain 22–31% and 51–59% oil, respectively, varying with different genotypes [1, 2]. The seeds are crushed and the oil is extracted using mechanical pressing. The press cake can then be solvent (hexane) extracted to optimize production yields. Both seed cake and oil are non-edible due to the presence of toxic compounds, namely, phorbol esters (PEs) [2–8].
PEs are diterpenes with a tigliane skeleton. They are hydrophobic, heat stable and oil soluble [8]. Six different types of PEs have been characterized from J. curcas oil. The concentration of PEs varies with genotype, ranging from 2 to 8 mg/g (unpublished data from our laboratory). PEs are potent inducers of a range of biological effects, including cocarcinogenicity and tumor growth [9]. PEs are used as a pharmacological tool for the investigation of biochemical processes such as carcinogenesis and also in many agricultural applications such as pesticides, molluscicides, insecticides, bacteriocides, and fungicides [8, 10, 11]. Isolated PEs could have a high commercial retail value ($2000 per gram according to Balandrin et al. [12]). Jatropha oil is a rich source of PEs and extraction of these esters as a co-product can increase the revenues for biodiesel industries. The extracted PEs could be used in various agricultural and pharmaceutical applications. The hypothesis in the present study was that the residual oil left after the extraction can be processed to high quality biodiesel, and that the isolated PEs are biologically active.
In this study, we demonstrate the quality of the residual Jatropha oil and the produced biodiesel, after extracting PEs from the crude oil, using optimized conditions as reported by Devappa, et al. [13], and the biological activity of the extracted phorbol esters.
Materials and Methods
Materials
J. curcas seeds (toxic Indian variety) were obtained from Jaipur in India. The phorbol 12-myristate-13-acetate standard was purchased from Sigma (St. Louis, USA). All other chemicals and solvents used in this study were of analytical grade.
Preparation of Jatropha Oil
J. curcas seeds were mechanically pressed in a screw press to obtain crude press oil. The oil was centrifuged at 3,150g for 20 min to remove solid residues and the supernatant was collected and stored in a refrigerator at 4–6 °C until further use.
Extraction of Phorbol Esters
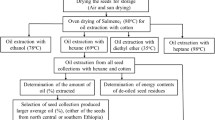
The optimization of the conditions for the extraction of PEs has been reported earlier by Devappa et al. [13]. The main purpose of study was to evaluate quality of the residual oil obtained after extraction of PEs and of the biodiesel produced from the residual oil. The process for extraction of PEs and biodiesel production is schematically presented in Fig. 1. The procedures adopted are described briefly.
Approach 1
Jatropha oil was mixed with methanol (1:2, w/v) at 23 °C and high shear mixed (T25 Ultra-turrax from IKA-Werke GmbH & Co, Staufen, Germany) at 13,000 rpm for 2 min. Thereafter, the mixture was centrifuged at 4,000g for 5 min to obtain upper methanolic and lower oily layers. After decantation, the extracted methanolic layer was recovered in a rotary evaporator and methanol removed under vacuum (300 mbar) at 55 °C to get a PE enriched fraction (PEEF-U). The oily layer was also rotary evaporated at 55 °C to remove residual methanol, and the oil was stored in a refrigerator (4–6 °C) until further use.
Approach 2
Jatropha oil was mixed with methanol (1:2, w/v) in a capped container and the contents were stirred at room temperature (23–25 °C) for 5 min using a magnetic stirrer (300 rpm). Thereafter, the mixture was centrifuged at 4,000g for 5 min to get upper methanolic and lower oily layers. After separation of the methanolic layer, methanol was removed under vacuum as stated in "Approach 1" to obtain a PE-enriched fraction (PEEF-M). The oily layer was also rotary evaporated at 55 °C to remove residual methanol, and the oil was stored in a refrigerator (4–6 °C) until further use.
Biodiesel Production
The residual oils obtained after extraction using approaches 1 and 2 were pre-treated (acid degumming, silica treatment and stripping) and transesterified according to the procedures described earlier by Makkar et al. [14].
Analytical Methods
The extracted oil and biodiesel samples were characterized using the following AOCS official methods and recommended practices [15]: water content (Ca 2e–84), free fatty acid content (Aa 6–38), peroxide value (Cd 8–53), para-anisidine value (Cd 18–90), fatty acid composition (Ce 1b–89 combined with Ce 1e–91), content of micro-elements (phosphorus, calcium, magnesium, sodium, potassium, and iron) using ICP (iCAP 6000 series, Thermo Scientific, Zellik, Belgium), acid value (Cd 3d–63) and oxidative stability index (ADM OSI, Omnion Inc., Rockland, MA, USA).
Phorbol Esters Analysis
PEs were determined at least in duplicate [2, 16]. Briefly, 0.5 g of oil sample was extracted four times with methanol. A suitable aliquot was loaded into a high-performance liquid chromatograph (HPLC) fixed with a reverse-phase C18 LiChrospher 100, 5 mm (250 × 4 mm i.d., from Merck (Darmstadt, Germany) column. The column was protected with a head column containing the same material. The separation was performed at room temperature (23 °C) and the flow rate was 1.3 ml/min using a gradient elution Makkar et al. [16]. The four phorbol ester peaks containing six PEs were detected at 280 nm and appeared between 25.5 and 30.5 min. Phorbol-12-myristate 13-acetate (PMA) was used as an external standard (appeared between 31 and 32 min). The area of the four phorbol ester peaks was summed and converted to phorbol-12-myristate 13-acetate equivalent by taking its peak area and concentration. PEs were analyzed in the residual oil obtained from approaches 1 and 2, respectively.
The PEEF-U and PEEF-M obtained following approaches 1 and 2 were taken further for the bioassay, based on snail toxicity (see below). For evaluation of the bioactivity of PEs present in these two fractions, PEs purified from untreated Jatropha oil were used as a positive control. The purification of PEs was done as described in Li et al. [9]. In brief, the untreated oil was extracted for PEs and subjected to HPLC as described by Makkar et al. [16] and PEs peaks were carefully collected at the retention time of 25.5 and 30.5 min. The collected fractions were in approximately 90% acetonitrile. The fractions were pooled and frozen at −20 °C. The top acetonitrile layer was separated from the frozen water layer to avoid any oxidation and the acetonitrile layer was further rotaevaporated to collect a colorless oily fraction designated as the purified phorbol esters fraction (PPEF). This fraction (PPEF) was redissolved in methanol and checked for purity and concentration by HPLC. The PPEF and PEEFs from Approaches 1 and 2 were evaluated for snail toxicity. The concentration of purified PEs and PEs in the PEEFs was expressed as equivalent to PMA.
Evaluation of Bioactivity using Snails
The bioactivity of PEEFs was assessed using snails (Physa fontinalis) as described by Devappa et al. [13]. In brief, all tests were carried out in deionized water at room temperature (23 °C). Stock solutions of PEEFs and PPEF were prepared in methanol and further diluted by water. Groups of ten snails were placed in glass containers with 400 ml of water containing the test substance. After 24 h of incubation with test sample, the snails were transferred to deionized water and maintained for another 48 h. Mortality was determined by the absence of movement and lack of reaction to irritation of the foot with a needle. The experiments were performed with the same quantity of methanol (negative control) in water as used for the test preparations and no mortality was recorded. All tests were independently repeated three times. Toxicity is expressed as LC100, referring to concentrations killing 100% of the snails.
Statistical Analysis
All bioassay data were subjected to a one-way analysis of variance (ANOVA) and the significance of the differences between means was tested using Duncan’s multiple range test (P < 0.05). The software used was SAS, Version 9.1 (Statsoft Inc., Tulsa, USA). Values are expressed as means ± standard deviation.
Results and Discussion
Evaluation of Phorbol ester Extraction Procedures
The initial concentration of PEs in the oil was 3.15 mg/g. Two effective tools, an Ultra-turrax (Approach 1) and a magnetic stirrer (Approach 2) were used for the maximum possible extraction of PEs with a minimum consumption of solvent and time (13). Approach 1 and Approach 2 reduced PE content in the oils by 80 and 78%, respectively. However, on using Approach 1, the color of the residual oil changed from golden yellow to greyish yellow color. Since both the approaches were effective in reducing PEs, the residual oils obtained were selected for biodiesel production.
Suitability of Extracted Residual Oil as Biodiesel Feedstock
The untreated Jatropha oil and the residual oils from both Approach 1 and Approach 2 were analyzed for feedstock quality before subjecting them to pre-treatment and transesterification procedures for biodiesel production. Various components could affect the transesterification process and final biodiesel quality. Higher concentration of metallic elements, especially phosphorous, can result in poor separation of biodiesel and glycerine after transesterification. There are no regulations for the presence of metals in feedstock materials, but these analyses are done to ensure that manufacturing process proceeds smoothly. The elemental content of the residual oils from both Approach 1 and Approach 2 are shown in Table 1. Both the approaches reduced the content of major elements by several fold. Phosphorus content was reduced by 70 and 76%; calcium by 13 and 16%; magnesium by 55 and 58%; sodium by 76 and 75%; and potassium by 93 and 97% for Approach 1 and Approach 2, respectively. The residual oil from Approach 1 had a higher iron content (increased by 3.7 times), which might be due to leaching out of rusted iron from the Ultraturrax probe used during extraction. However, the overall excessively high element content makes a degumming step prior to transesterification necessary.
The anisidine value (AnV) is a measurement of the secondary oxidation, and essentially reflects how oil has been handled and stored, while the peroxide value (PV) represents the status of the primary oxidation. For both AnV and PV, a lower number is better. Both the approaches for PE extraction did not substantially increase these values. The residual oils from Approach 1 and Approach 2 had 1.11 and 1.29 times higher PV than the control oil. AnV increased in Approach 1 (1.13 times) compared to the control oil and decreased to an undetectable level in Approach 2 (Table 1).
In oil, water is naturally present in small amounts. The control oil had a water content of 222 ppm, which was reduced by 83.3% in Approach 1 and was not detectable in the residual oil obtained from Approach 2 (Table 1). The substantial decrease in water content could be due to (a) solubility of water in methanol during the extraction of PEs, which was removed by phase separation in the subsequent step, and (b) removal of moisture together with methanol in the evaporation step (rotaevaporatory system, 55 °C, 270 mbar; generally this step took 30 min). For a complete transesterification reaction, a free fatty acid (FFA) value lower than 0.3% is needed. As the acidity of the oil increases, the efficiency of transesterification decreases, and moreover, the presence of FFA in biodiesel can do harm to the engine oil endangering the engine’s lubrication. The control oil had an FFA content of 2.93 (% as C18:1). After extraction of PEs, the FFA content was reduced by 55% in both Approach 1 and Approach 2 (Table 1). However, the residual FFA content of 1.3% was still too high and should be reduced by stripping prior to transesterification.
The fatty acid profile is another important characteristic for the evaluation of changes that occur during the extractions. The fatty acid composition of the control oil was compared with those of the residual oils from Approach 1 and Approach 2 (Table 2). The predominant fatty acids in Jatropha oil consist of monounsaturated (44.9%), followed by polyunsaturated (33.4%) and saturated (21.6%) fatty acids, which were similar for the control oil and the residual oils. The major fatty acids in Jatropha oil are oleic (44%), linoleic (33.3%), palmitic (14.7%) and stearic (6.7%) acids. The fatty acid composition observed in this study was similar to those observed in our earlier studies [14]. When compared to the control Jatropha oil: (a) the fatty acid composition of residual oil from both Approach 1 and Approach 2 did not change, and (b) there was no influence of the extraction of PEs on the fatty acid composition.
Quality of Oil after Pre-treatment
After degumming and stripping, the residual oil quality was excellent for producing biodiesel. During degumming, the phosphorous content was reduced from 32 to 40 ppm to below 2 ppm. Also the levels of Ca, Mg, Na, K, and Fe were reduced to acceptably low levels. Stripping resulted in FFA contents of the residual oils of below 0.05%. The water content after stripping was <110 ppm in both samples (Table 3).
Quality of Produced Biodiesel
The quality of the produced biodiesel depends on several factors such as genotype of the plant, soil type, plant health, maturity of seeds at harvest, seed storage conditions and duration, degree of unsaturation and fatty acid composition of oil, as well as the refining and transesterification process [14, 17]. These parameters are interrelated with several other fuel quality criteria, such as sulfated ash content, carbon residue, water content, FFA content, quantities of the chemicals used in the processing stages such as those of sodium/potassium hydroxides (catalysts) and alkaline earth metals (absorbents) [18–20]. The quality parameters of biodiesel prepared from both feedstocks (Approach 1 and Approach 2) were well within the limits of European Union and the United States biodiesel standards (EN 14214:2008 and ASTM D 6751-09) [21, 22]. The high quality biodiesel prepared from the residual oils obtained from both the approaches had low acid value, phosphorus, Na + K, Ca + Mg, and water content (Table 4).
The monoglycerides (0.67 and 0.65%), triglycerides (0.1 and 0.09%), free glycerol (0.005 and 0.012%) and total glycerol (0.225 and 0.194%) levels in the biodiesels meet the specifications of the European Union and United states biodiesel standards (Table 4). However, the biodiesel produced by following Approach 1 had a slightly higher diglyceride content (0.27%) than the European and United States biodiesel standards (0.2%) permit. The biodiesel prepared from residual oils obtained following Approach 1 and Approach 2 had higher OSI values (11.3 and 6.7 h, respectively). The reason for the higher OSI obtained for Approach 1 is not clear. However, both these values meet the EN 14214:2008 and ASTM D 6751-09 specifications [21, 22].
Bioactivity of PEEFs
Snails (Physa fontinalis) have been found to be highly sensitive to PEs (our unpublished data). In snails, both purified phorbol esters and methanol extract of PEEFs obtained from Approaches 1 and 2 were highly and equally lethal at a concentration of 0.5 and 1 ppm (LC100) indicating no influence of the extraction approaches on the activity of PEEFs (Table 5). The results suggest that PEEFs extracted either using Approach 1 or Approach 2 are biologically active and can be used as a suitable bio-control agent in agricultural and pharmaceutical applications. In addition, it may be noted that PEs present in PEEFs are completely biodegradable in soil (get degraded within 9 days (23% moisture and 32 °C), and the degradability increases with increases in temperature and moisture levels in the soil [23].
Conclusions
Approximately 80 and 77.7% of the PEs present in Jatropha oil could be extracted in 2 and 5 min using an Ultra-turrax (13,000 rpm, 23 °C) and a magnetic stirrer (300 rpm, 60 °C), respectively. The PEs bioactivity was not affected by the extraction procedures and thus, PEEFs could be used as a potential bio-control agent in various agricultural and pharmaceutical applications. The residual oil obtained after extraction of PEs is of good quality and could, after pre-treatment, be processed into high quality biodiesel, meeting the European and American biodiesel specifications.
References
Makkar HPS, Aderibigbe AO, Becker K (1998) Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem 62(2):207–215
Makkar HPS, Becker K, Sporer F, Wink M (1997) Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. J Agric Food Chem 45:3152–3157
Makkar HPS, Becker K, Schmook B (1998) Edible provenances of Jatropha curcas from Quintana Roo state of Mexico and effect of roasting on antinutrient and toxic factors in seeds. Plant Food Hum Nutr 52:31–36
Devappa RK, Bhagya S (2008) Biochemical and nutritional evaluation of Jatropha protein isolate prepared by steam injection heating for reduction of toxic and antinutritional factors. J Sci Food Agric 88:911–919
Becker K, Makkar HPS (2008) Jatropha curcas: a potential source for tomorrow’s oil and biodiesel. Lipid Technol 20:104–107
Makkar HPS, Becker K (2009) Challenges and opportunities for using byproducts from the production of biodiesel from Jatropha oil as livestock feed. Proceedings of Animal Nutrition Association World Conference (14–17 Feb). New Delhi. pp 168–170
Devappa RK, Darukeshwara J, Rathina Raj K, Narasimhamurthy K, Saibaba P, Bhagya S (2008) Toxicity studies of detoxified Jatropha meal (Jatropha curcas) in rats. Food Chem Toxicol 46(12):3621–3625
Makkar HPS, Becker K (2009) Jatropha curcas, a promising crop for the generation of biodiesel and value-added coproducts. Eur J Lipid Sci Technol 111:773–787
Li CY, Devappa RK, Liu JX, Makkar HPS, Becker K (2010) Toxicity of Jatropha curcas phorbol esters in mice. Food Chem Toxicol 48(2):620–625
Solsoloy AD, Solsoloy TS (1997) Pesticidal efficacy of formulated J. curcas oil on pests of selected field crops. In: Gübitz GM, Mittelbach M, Trabi M (eds) Biofuels and industrial products from Jatropha curcas. DBV, Graz, pp 216–226
Goel G, Makkar HPS, Francis G, Becker K (2007) Phorbol esters: structure, biological activity and toxicity in animals. Int J Toxicol 26:279–288
Balandrin MF, Klocke JA, Wurtele ES, Bollinger WH (1985) Natural plant chemicals: sources of industrial and medicinal materials. Science 228:1154–1160
Devappa RK, Maes J, Makkar HPS, Greyt WD, Becker K (2009) Isolation of phorbol esters from Jatropha curcas oil and quality of produced biodiesel. 2nd International Congress on Biodiesel: the science and the technologies, Munich, Germany
Makkar HPS, Maes J, De Greyt W, Becker K (2009) Removal and degradation of phorbol esters during pre-treatment and transesterification of Jatropha curcas oil. J Am Oil Chem Soc 86:173–181
AOCS (1990) Official methods and recommended practices of the American Oil Chemists Society, 4th edn. AOCS Press, Champaign
Makkar HPS, Siddhuraju P, Becker K (2007) A laboratory manual on quantification of plant secondary metabolites. Humana press, New Jersey, p 130
Tate RE, Watts KC, Allen CAW, Wilkie KI (2006) The viscosities of three biodiesel fuels at temperatures up to 300 °C. Fuel 85:1005–1010
Mittelbach M (1996) Diesel fuel derived from vegetable oils, VI: specifications and quality control of biodiesel. Bioresour Technol 56:7–11
Mittelbach M (2000) Chemische und motortechnische Untersuchungen der Ursachen der Einspritzpumpenverklebung bei Biodieselbetrieb; Bund-Bundesländer-kooperations-projekt
Meher LC, Sagar DV, Naik SN (2004) Technical aspects of biodiesel production by transesterification: a review. Renew Sust Energy Rev 3:1–21
ASTM (American Society for Testing and Materials) (2008) Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels, ASTM D6751-09. In: ASTM Annual Book of Standards. American Society for Testing and Materials, West Conshohocken. http://www.astm.org)
European Committee for Standardization (CEN) (2008) Automotive fuels fatty acid methyl esters (FAME) for diesel engines requirement methods EN 14214:2008. European Committee for Standardization (CEN), Brussels
Devappa RK, Makkar HPS, Becker K (2009) Fate of Jatropha curcas phorbol esters in soil. 13th Annual green chemistry and engineering conference, Washington DC. (available at: http://acs.confex.com/acs/green09/webprogram/Paper69396.html)
Acknowledgments
The authors are grateful to the Bundesministerium für Bildung und Forschung (BMBF), Berlin, Germany for financial assistance. The technical assistance of Mr. Hermann Baumgartner is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Devappa, R.K., Maes, J., Makkar, H.P.S. et al. Quality of Biodiesel Prepared from Phorbol Ester Extracted Jatropha curcas Oil. J Am Oil Chem Soc 87, 697–704 (2010). https://doi.org/10.1007/s11746-010-1547-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1547-4