Abstract
Aims
In Brazil N fertilization of sugarcane (Saccharum spp.) is low compared to most other countries. 15N-aided studies and the occurrence of many N2-fixing bacteria associated with cane plants suggest significant contributions from biological N2 fixation (BNF). The objective of this study was to evaluate BNF contributions to nine cane varieties under field conditions using N balance and 15N natural abundance techniques.
Methods
The field experiment was planted near Rio de Janeiro in 1989, replanted in 1999 and harvested 13 times until 2004. Soil total N was evaluated at planting and again in 2004. Samples of cane leaves and weeds for the evaluation of 15N natural abundance were taken in 2000, 2003 and 2004.
Results
N accumulation of the commercial cane varieties and a variety of Saccharum spontaneum were persistently high and N balances (60 to 107 kg N ha−1 yr−1) significantly (p < 0.05) positive. The δ15N of leaf samples were lower than any of the weed reference plants and data obtained from a greenhouse study indicated that this was not due to the cane plants tapping into soil of lower 15N abundance at greater depth.
Conclusion
The results indicate that the Brazilian varieties of sugarcane were able to obtain at least 40 kg N ha−1 yr−1 from BNF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Brazilian Pro-Álcool program to produce ethanol as a vehicle fuel from sugarcane started in 1975. At that time the area under this crop was just less than 2 million ha (Mha) and the mean national yield was 47 Mg millable cane ha−1 (MAPA 2007). The area and yield increased steadily for the first 10–12 years of the program reaching over 4 Mha in 1987 with a mean yield of 62 Mg ha−1. At this time world crude oil prices remained low and direct subsidies for the program were phased out. Over the next 15 years the area under sugarcane only increased by about 1 Mha but in 2001 a law was passed making it obligatory to add between 20% and 24% of anhydrous ethanol to all gasoline (Federal Law No. 10,203 of 22nd February). In 2002 the area passed the 5 Mha mark and the mean national yields exceeded 70 Mg ha−1 (Boddey et al. 2008). With the commercialization of the first Flex-Fuel motors which can run on any mixture of ethanol or gasoline, and the rise in international crude oil prices, the area under cane grew more rapidly to almost 10 Mha today and mean yields have increased to almost 80 Mg ha−1 (IBGE-SIDRA 2011).
Despite the very significant increase in yields since the inception of the Pro-Álcool program, an exceptional aspect of the Brazilian cane crop is that the N fertilizer inputs remain low compared to other regions of the world which have similar yields. As N fertilizer manufacture has a high fossil fuel (generally natural gas) input, this enhances the mitigation of greenhouse gas emissions derived from the use of this biofuel (Boddey et al. 2008). In countries such as India, the USA, Colombia and Australia, N fertilizer is usually added at between 150 and 200 kg N ha−1 yr−1 while in Brazil mean applications are between 60 and 70 kg N ha−1 yr−1.
Boddey et al. (2003) calculated that for a crop with yield of 70 Mg ha−1 (mean national yield at that time) approximately 100 kg of N ha−1 are exported from the field either as harvested cane or lost during burning. As only 60 to 70 kg N ha−1 yr−1 are added as fertilizer this means that there is a deficit of 30 to 40 kg N ha−1 yr−1 even assuming that there is no N loss from the system as ammonia volatilisation, denitrification, leaching or erosion. Long-term decreases in yield have not been observed and this constitutes support for the idea that there may be an input of biological N2 fixation (BNF) to this crop (Boddey et al. 2003). Basanta et al. (2003) conducted a N balance study in the São Paulo production region (Piracicaba) and estimated that over three crops (plant crop and two ratoons) an annual mean 103.6 kg N ha−1 were exported to the mill as cane and 83.5 kg of N was lost on burning. In this experiment N fertilizer was only added at planting (63 kg N ha−1) such that the net N export for the three crops was 163 kg N ha−1 yr−1. Despite this the third ratoon crop, 36 months after planting, accumulated 155 kg N ha−1, again indicative of a considerable input from BNF or other external sources.
Further evidence for BNF is derived from microbiological studies. In early studies N2-fixing bacteria of the genera Beijerinkia were found to be associated with soils in cane fields (Dobereiner 1961). Subsequently high numbers of Gluconacetobacter diazotrophicus (Li and Macrae 1992; Reis et al. 1994; dos Reis et al. 2000) and other diazotrophs of the genera Herbaspirillum and Burkholderia (amongst others) have been found in the interior of cane roots, stems and even leaves (James 2000; Boddey et al. 2003; Perin et al. 2006a; Castro-González et al. 2011).
To quantify the contribution of BNF to different Brazilian cane varieties the team at Embrapa Agrobiologia has used the 15N isotope dilution technique combined with short-term N balances on plants grown in large (60 L) pots or a 120 m2 concrete tank filled with 15N-labelled soil (Lima et al. 1987; Urquiaga et al. 1992). In these studies the cane varieties showing the lowest 15N enrichment also showed higher N accumulation and N balance in the soil/plant system, which was powerful evidence for a contribution of BNF to the former varieties. A field sampling conducted by Boddey et al. (2001) in 11 different plantations in four States of Brazil showed that at all but one site weed plants were significantly higher in 15N natural abundance, suggesting input of BNF from 0% to 70% of shoot N. A long-term study (16 years) by the same team performed on one cane variety (CB 45-3) on a plantation in Pernambuco State, NE Brazil demonstrated that there was a positive N balance in the treatments not receiving N fertilizer, again indicating significant contributions of BNF to the cane crop (Resende et al. 2006).
However, in studies conducted in Australia (Biggs et al. 2002) and South Africa (Hoefsloot et al. 2005) the 15N abundance of non-N2-fixing reference plants was not found to be higher than in sugarcane and the authors concluded that BNF inputs were insignificant.
While these more recent studies have utilized the 15N natural abundance technique to evaluate possible inputs of N2 fixation to sugarcane, this technique has not been used concurrently with a long-term N balance study. The objective of this present study was to estimate the contribution of BNF to nine cane varieties planted in the field by investigating the long-term N balance of the crop/soil system and from the 15N abundance of the cane varieties at the last five annual harvests.
Material and methods
Site and experimental layout
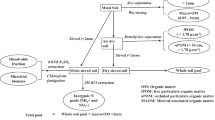
The experiment was planted on the field station of Embrapa Agrobiologia in Seropédica, Rio de Janeiro State (22°49′S, 43°39′W) and was not irrigated. The soil (Itaguaí series—sand, silt and clay, 32, 26 and 42%, respectively) at this site is classified as an Acrisol (FAO) or Typic Hapladult (USDA, Soil Taxonomy). Results of soil analyses (0–15 cm depth) were: pH (water), 5.4, exchangeable Al3+, Ca2+ and Mg2+ 0.0, 1.4 and 2.2 cmolc dm−3, respectively; exchangeable K, 22 mg dm−3; available (Mehlich 1) P, 4.9 mg dm−3 and total N 0.57 g kg−1 EMBRAPA (1997). Nine varieties of sugarcane (Table 1) were planted as setts (stem pieces) in May of 1989 in plots of five rows, 5 m in length, and spaced 1.1 m between rows. The experiment was laid out in a randomized complete block design with four replicates. Just prior to planting the experiment the area was fertilized with 125 kg K and 66 kg P ha−1 as potassium chloride and single superphosphate, respectively, and 50 kg ha−1 of fritted trace elements (FTE) type BR12 (http://www.nutriplant.com.br/). Each year after harvest 66 kg P and 132 kg K and 50 kg ha−1 of fritted trace elements were added in the same forms as at planting but no N fertilizer added was at planting or at any later time.
After 9 years of growth (eight harvests), in May 1999, the area was ploughed and the experiment was re-planted (2nd cycle) with the same varieties in the same plots.
Harvests
The first plant crop of each cycle was harvested after 18 months of growth (standard practice in Brazil) in December 1990 and December 2000, for the two cycles respectively. Thenceforth the ratoon crops were harvested every 12 months in all three crop cycles.
At each harvest the aerial tissue of the plants from the central three rows was separated into fresh stems, senescent leaves (trash) and flag leaves (green leaves left after decapitating the stems). Subsequently all above-ground biomass was removed from the whole area of each plot. The fresh weight of each fraction was determined using a platform balance and subsamples were taken to determine dry weight content in a forced air oven (65°C for >72 h). For the harvests during the second cycle (2000 to 2004) bulked samples of the third-emergent leaf of 20 cane plants from each plot were also taken for analysis of 15N abundance. Previous work of Boddey et al. (2001) indicated that the 15N abundance of the third-emergent leaf would closely correspond to that of the above-ground biomass.
In 2000, 2003 and 2004 whole shoots of non-leguminous weeds growing in the plots were collected to serve as non-N2-fixing reference plants for the application of the 15N natural abundance technique. For each weed species a composite sample of all aerial tissue was taken from the whole experiment.
All samples were ground with a Wiley mill to <1 mm and aliquots for analysis for total N and 15N natural abundance were further ground to a fine powder using a roller mill similar to that described by Arnold and Schepers (2004).
Soil sampling
At the original planting of the experiment in May 1989 soil samples were taken from three points in each plot with an auger at the depth intervals 0–15, 15–30, 30–45 and 45–60 cm. The samples were bulked for the same depth interval in each plot. The sampling depth was restricted to 60 cm because between 60 and 70 cm depth there was a hard clay pan, through which almost no roots were observed to penetrate. Simultaneously in four plots of each block trenches were excavated to permit the evaluation of soil bulk density using bevelled rings (length 7.4, diameter 5 cm = 145 cm3), four bulked samples for each depth in each trench. After the final harvest of the second cycle (December 2004) the sampling and determination of soil density were repeated exactly as before.
All augered soil samples were air dried, passed through a 2 mm sieve removing all visible root material. Subsamples were then taken for fine grinding using the roller grinder described by Arnold and Schepers (2004). The bulk density samples were dried at 110°C for subsequent weighing and determination of bulk density.
Analyses of plant material and soil
Finely ground samples (200 mg aliquots) of the third-emergent leaves of the cane and the shoot tissue of the weeds were analysed for total N using the semi-micro Kjeldahl procedure as described by Urquiaga et al. (1992) and aliquots containing between 20 and 40 μg N were analysed for 15N abundance using an automated continuous-flow isotope-ratio mass spectrometer (Finnigan MAT, Bremen, Germany) in the “John M Day Stable Isotope Laboratory” at Embrapa Agrobiologia (Ramos et al. 2001).
The ground soil samples were analysed for total N using the same semi-micro Kjeldahl procedure on 1 g aliquots.
Determination of mineral N precipitated in rainfall
Commercial rainfall collectors (20 cm diameter) were installed at five sites within a distance of 2 km from the field experiment in 2008. Rainfall water samples were collected within 24 h of each precipitation event from August 2008 to May 2010. The volume was measured to estimate rainfall and then a sub-sample of each sample was stored at −15 to −20°C.
The samples were thawed and the concentrations of ammonium and nitrate estimated using the salicylate method of Kempers and Zweers (1986) for ammonium and using ultraviolet adsorption for the determination of nitrate concentration (Norman et al. 1985). For the determination of nitrate the technique used is based on the linear relationship between NO −3 concentration and the difference in absorbance at 210 and at 270 nm wavelengths. The sensitivity of both techniques was better than 0.05 μg N ml−1.
Investigation of variation in δ15N of plant-available N with depth
In order to examine the change in 15N natural abundance of the plant-available N with depth in the soil in this experiment, the procedure of Ledgard et al. (1984) was adopted. Soil samples were taken in October 2003 from each of three blocks at depth intervals of 0–10, 10–20, 20–30, 30–40, 40–50, 50–60 and 60–75 cm and all samples were planted to four weed species. The experiment consisted of 4 weed species × 7 depth intervals and three replicates (representing the three replicate blocks of the field experiment) arranged in a completely randomized design and planted in the greenhouse at Embrapa Agrobiologia. Each pot was filled with 400 g soil, and all were fertilized with 44 mg P, 83 mg K kg soil−1 as single super phosphate and potassium chloride, respectively, and 50 mg of FTE kg soil−1. Each pot was planted to eight seeds of each of the weed species, Commelina erecta (slender dayflower), Eleusine indica (Indian goosegrass), Sorghum bicolor (grain sorghum) and Panicum miliaceum (proso millet). After 1 week excess plants were removed to leave four plants per pot. The shoot tissue of these plants was harvested after 75 days of growth when all were showing signs of severe N deficiency. The plant material was dried, weighed, ground and analysed for total N and 15N abundance as described above for the samples of sugarcane.
In order to visualise the rooting depth of the different weed species and the sugarcane varieties, pits were opened in 2009 in plots of the continuation of Experiment 2 around the roots of these plants. The profile wall was washed with a spray to show the clean roots. The profiles of roots of the weeds Emilia sonchifolia and Commelina erecta and the sugarcane variety CB 47–89 are shown in the supplementary information (Fig. S1.).
Calculations
N balance of the soil/plant system
The N balance for each sugarcane variety was calculated from the sum of the total N stock in the soil (0–60 cm) at the final harvest for each plot plus the total N accumulated by the cane variety over the 13 harvests minus the initial total N stock in the soil to the same depth at planting. The mass of N in each depth interval was calculated from the N concentration and the bulk density of the soil. The total N stock was calculated by correcting the mass of soil in the profile at the final sampling to that at the first sampling as described by Ellert and Bettany (1995).
15N abundance of plant-available N with depth
The shoot tissue of the weed reference plants grown in the soil samples taken from different depths was analyzed for total N and 15N abundance as described above. To simulate a deep rooting plant which removed all plant-available N to a depth of 75 cm the weighted mean 15N abundance of reference plant (rp) was calculated using the following equation:
where δ15Nrp and NTrp are the 15N abundance and total N accumulated by the weed species grown in the soil from each depth interval.
Statistical analyses
The statistical procedures followed for all plant parameters (cane yield), DM, N total, δ15N) were standard analysis of variance using the software SAEG 9.1 of the Federal University Viçosa, MG, (Brazil). Normality and homogeneity of variance of errors were analysed using the Lilliefors and Cochran & Bartley tests, respectively. The required conditions were fulfilled in all cases. The analysis of variance was then performed with the application of the F test. Differences between means were separated by the use of the Tukey HSD test at p = 0.05.
The Student “t” test was used to compare the four replicates of the initial and final soil N stocks, and the N balance, for each variety.
Results
First cycle—1989 to 1999
The commercial SP varieties all yielded over 80 Mg ha−1 of fresh stems at the first harvest (plant crop) (Table 1). Plant crops in Brazilian practice are almost always harvested after 18 months which allows the plants to establish well and, as was observed in this case, the varieties obtain their highest yield during the cycle. Krakatau (S. spontaneum) is a vigorously growing species but produces only thin canes with little sugar. Likewise Chunee (S. barberi) is not a productive cane, and like Krakatau is a component of the interspecific commercial hybrids.
The first ratoon harvest in 1991 was lost when the whole experiment caught fire shortly before harvest time. The stem yields over the eight harvests of the commercial varieties did not differ significantly, ranging from 57 to 77 Mg ha−1, although the variety SP 70-1143 was amongst the best varieties in every year after 1992, and presented the highest yield in four of the remaining 6 years.
The total N accumulation (stem, flag leaves and senescent leaves) of these varieties followed approximately the trend for cane yield, and again the commercial variety SP 70-1143 showed the best performance (Table 2). To better visualise the variation with time and rainfall, the annual mean yields and N accumulation of all the commercial varieties are presented in comparison with Krakatau and Chunee (Fig. 1). There was no clear relationship with the total rainfall in periods between harvest and DM yield or N accumulation.
Annual mean cane stem yield (a) and total N accumulation of shoot biomass (b) of seven commercial varieties of sugar cane (Saccharum spp.), of the S. spontaneum variety, Krakatau, and the S. barberi variety Chunee during the first cycle of growth 1989 to 1999 in the long-term experiment. Values are means of four replicates
The S. spontaneum variety, Krakatau, showed lower cane yield than the commercial varieties at all harvests, but the accumulated N was higher at all harvests owing principally to the high DM content of the stems and the high N concentration in the flag leaves and stems. Overall mean (1989–1998) cane yield of Chunee (S. barberi) was significantly lower than any of the commercial varieties and Krakatau, and also lower in accumulated N than Krakatau and all but two of the commercial varieties (Table 2).
While the P, K and micronutrient levels in the soil were adjusted each year with the addition of 66 kg P and 132 kg K and 50 kg ha−1 of fritted trace elements (FTE BR 12), no N fertilizer of any kind was added to the plots and all plant material was removed. Despite this, after the first ratoon crop (1992) there was no tendency for yields, or N accumulation, of the commercial varieties or Krakatau to decline with time (Fig. 1).
Second cycle—2000 to 2004
The varieties SP 70-1143 and SP 79-2312 showed cane yields equivalent to over 100 Mg ha−1 for both the plant–crop and the first ratoon (Table 3) and maintained yields over 67 Mg ha−1 until 2003. Similarly DM yield and N accumulation of the commercial varieties remained high through to 2003, but in the final year of the study 2004 fresh cane and DM yield and N accumulation decreased very appreciably and we have no explanation for this (Tables 3, 4 and 5).
In order to visualise the contribution of the stems, senescent leaves and flag leaves to the total N accumulation, the mean values for all eight commercial varieties and Krakatau and Chunee are shown in Fig. 2. For the commercial varieties mean proportions of N accumulation in the stem, senescent leaves and flag leaves were 31.5, 30.8 and 37.7%, respectively.
Total N accumulation by the stems, flag leaves and senescent leaves of seven commercial varieties of sugar cane (Saccharum spp.), of the S. spontaneum variety, Krakatau and the S. barberi variety Chunee during the second cycle of growth 1999 to 2004 in the long-term experiment. Values are means of four replicates
The mean total stock of N in the soil to a depth of 60 cm at planting of the experiment (1989) was 4,211 kg N ha−1 and at the end of the second cycle (2004) 4,070 kg N ha−1 (Table 6), this difference (141 kg N ha−1) being non-significant (p = 0.22). During this period the above-ground N accumulation of the commercial varieties averaged a total of 1,344 kg N ha−1, and Krakatau and Chuee, 1,896 and 901 kg N ha−1, respectively. The total N balances in the soil/cane crop system for the whole study were calculated from the total N accumulated by each cane variety at the 13 harvests plus the total N present in the soil (0–60 cm) at the end of the study in the respective plots, minus the total soil N at planting in 1989. The total N in the crop/soil system was significantly higher (p = 0.05) than that initially in the soil (0–60 cm) in 1989 for the six commercial varieties and Krakatau, but this difference was not significant for Chunee (p = 0.32). If these positive N balances are considered as due to inputs from BNF, then mean BNF inputs for the commercial varieties ranged from 70 (CB 47-89) to 124 kg N ha−1 harvest−1 (SP70-1143) for 11 annual harvests and two plant crops harvested after 18 months.
At the harvest of the plant crop (2000) of the second cycle the 15N abundance of the leaf samples of the different sugar varieties ranged from +5.29‰ (Krakatau) to +7.66‰ (CB 45-3) (Table 7). The weed samples (used as non-N2-fixing reference plants) taken at this time showed values of 15N abundance of between +5.4 and +6.2‰, approximately the same as, or slightly lower than, the leaf samples from the cane varieties. It was not possible to compare statistically the δ15N values of the cane leaves and the weeds, as the weed samples were bulked for the whole experiment. However, these results suggest that there was little or no input of N2 fixation to the cane varieties at this harvest.
There were significant differences in 15N natural abundance of the cane leaf samples amongst cane varieties at all ratoon harvests in the second cycle but no variety showed consistently lower or higher δ15N than the others across all five harvests (Table 7). At the first three harvests of the ratoon crops (2001 to 2003) the 15N abundance of leaf samples of all the cane varieties decreased to between +2.6 and 4.8‰, and at the harvest of the fourth ratoon to between +1.9 and +3.2‰. Samples of weed plants were not taken in 2001 and 2002. While no statistical comparison can be made, all weed reference species sampled at the last two harvests (2003 and 2004) showed δ15N values between +4.3 and +5.7‰, which suggested that the cane varieties were obtaining considerable inputs of N from BNF.
N input in rainfall
For all five sites a full set of samples was collected for the period March 2009 to February 2010. The total rainfall during this period measured at the field site was 1,159 mm which agrees well with the total given for this period, 1,208 mm, by the Seropédica INMETRO (National Institute of Meteorology) station (22.758 °S, 43.685 °E) which is also within 2 km of the long-term experiment. Ammonium concentrations varied from traces (<0.05 μg N ml−1) to 0.96 μg N ml−1 and nitrate from traces to 0.87 μg N ml−1. The higher concentrations were recorded in the drier season of the year (May to August 2009) associated with moderate (<25 mm) to low rainfall events. The monthly totals of rainfall and NH +4 plus NO −3 -N for the site of the long-term experiment are displayed in Fig. 3. The annual total at this site amounted to 5.42 kg N ha−1. The total N precipitated as ammonium and nitrate at the other four sites in the same 12-month period ranged from 4.15 to 5.19 kg N ha−1.
Rooting depth and 15N abundance of plant-available N
The images of the roots of two weed species (Emilia sonchifolia and Commelina erecta) and one sugar cane variety (CB 47-89) shown in Fig. S1 (Supplementary information) show that the roots of the weeds are restricted to the top 20 cm of soil but the roots of the sugarcane exceeded 50 cm in depth. Hence, an alternative explanation for the lower values of δ15N of the sugarcane varieties is that the cane varieties are deeper rooting than the weed reference plants and that available N at greater depth was lower in 15N abundance. This hypothesis was tested using the technique of Ledgard et al. (1984). Soil samples were taken at seven depth intervals, fertilized with P, K and micronutrients and planted to three weed species and sorghum. After 75 days of growth when all plants were showing symptoms of severe N deficiency, all shoot material was harvested and analyzed for total N and 15N abundance. Weeds/sorghum growing in the top 10 cm of soil showed 15N abundance values of between +5.2 and +6.4‰, while at greater depths to 60 cm the δ15N of the weeds increased significantly (Table 8). The sum of N extracted by these plants from the soil taken from all seven depth intervals was significantly higher for the species C. erecta, E. indica and S. bicolor than for P. miliaceum. However, the differences between the 15N abundance of weed plants grown in soil from the same depth interval were in no case greater than 1.5‰ and the weighted means for all depth intervals of the weeds were not significantly different between species (p = 0.27). As was to be expected the amount of soil N available to the weeds decreased with depth such that the soil from 50 to 75 cm depth yielded per kg soil less than 20% of the N supplied by the soil taken from the 0–10 cm depth interval (Fig. 4).
Mean N accumulation and 15N abundance of sorghum (Sorghum bicolor) three weed species: Commelina erecta (slender dayflower), Eleusine indica (Indian goosegrass) and Panicum miliaceum (proso millet) grown in the greenhouse in pots of soil taken from seven depth intervals in the long-term sugarcane experiment. Values are means of four replicates. Error bars indicate standard errors of the means
The weighted mean value of the 15N abundance of all of the weed species grown in soil samples from all depth intervals was +7.13‰. It was assumed that this value of 15N abundance represented that of the N extracted from the soil by the sugarcane varieties and the proportion of N derived from BNF (%Ndfa) by each cane variety at all four ratoon harvests of the second cycle (2001 to 2004) was calculated (Table 9).
Discussion
There are many reports of the occurrence of different N2-fixing bacteria associated with sugar cane, including species of Beijerinckia, Azospirillum, Azotobacter, Bacillus, Derxia, Enterobacter and Erwinia isolated from the root surface or rhizosphere (See review of Boddey et al. 2003). More recently, species such as Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae, H. rubrisubalbicans, and Burkolderia silvatlantica (Boddey et al. 2003; James 2000; Perin et al. 2006b) have been isolated from within cane plant tissues, including roots, stems and leaves. Silva-Froufe et al. (2009) reported numbers of viable cells of the species Herbaspirillum seropedicae and Gluconacetobacter diazotrophicus, evaluated using the ELISA technique, to be between 105 and 106 cells g fresh weight in shoot tissues and between 107 and 108 in roots of the cane variety SP 70-1143 taken from the long-term experiment reported in this publication. While the presence of large populations of N2-fixing bacteria within the plant tissue cannot be regarded as proof of a significant input of N from BNF, it is an essential pre-requisite.
The results of this study showed that Brazilian commercial varieties of sugarcane grown continuously over a 15-year period in an Acrisol of low total N content (<0.6 g N kg−1) showed sustainable yields until the 14th year. One year’s results (1991) were lost owing to an accidental fire and, discounting this year, mean N accumulation of these varieties ranged from 84 to 118 kg N ha−1 yr−1. The variety S. spontaneum (Krakatau) accumulated more N than the commercial varieties (135 kg N ha−1 yr−1) and the S. barberi variety, Chunee, only 64 kg N ha−1 yr−1.
The soil (0–60 cm) showed no significant change in N content over the 15 year period and the N balance (all crop N + final soil N – initial soil N) for all cane varieties with the exception of Chunee, was significantly positive (Table 6). These results are similar to those obtained by Urquiaga et al. (1992) where sugar cane varieties were grown in a concrete tank containing 15N-labelled soil for a 3-year period. In that study Krakatau also accumulated more N than the commercial varieties and Chunee less. Using the N accumulation and N balance data, the estimates of the mean annual contribution of BNF to the commercial varieties of sugar cane ranged from 65 to 115 kg N ha−1 and the Krakatau and Chunee, 135 and 24 kg N ha−1, respectively.
The problems associated with the utilisation of N balances to quantify BNF inputs to graminaceous crops were thoroughly discussed many years ago in the review of Boddey (1987). If the crop/soil system is losing N by leaching or in gaseous forms the estimates of BNF inputs are underestimated. Virtually the only reason that BNF inputs would be overestimated would be if there were significant inputs of N in rainfall or another atmospheric source. The site of the experiment is some 70 km west of the city of Rio de Janeiro and prevailing winds are from the south and east so that atmospheric N pollution from industrial or urban sources is unlikely. For the last 3 years, levels of NH +4 and NO −3 in rainfall at this site and four others within 2 km were monitored and amounted to between 4.15 and 5.42 kg N ha−1 yr−1. Data for the offshore island of Ilha Grande, 70 km SE of Seropédica and the National Park of Itataia (100 km NE), both nature reserves, showed similar or lower inputs of total mineral N of between 3.3 and 5.4 kg N ha−1 yr−1 (de Mello and de Almeida 2004; de Souza et al. 2006). Surprisingly in the Piracicaba region in the State of São Paulo, which is surrounded by cane factories and a great deal of light industry, total annual precipitation of NH +4 + NO −3 in rainfall was estimated to be approximately 6 kg N ha−1 yr−1, and the authors speculated that including dry deposition total inputs could be approximately 9 kg ha−1 (Krusche et al. 2003). Our data suggest that rainfall N inputs would not exceed 6 kg N ha−1 and that the quantities are far too low to account for the observed positive N balances of over 60 kg N ha−1 yr−1.
A field N-balance study was conducted by our group in the NE State of Pernambuco over a 16 year period (Resende et al. 2006). The effect of pre-harvest burning, N fertiliser and vinasse (distillery waste) additions on the yield, N balance and soil fertility of just one sugarcane variety (CB 45-3) were studied. In the treatments where N fertiliser was not added, the N balance (soil sampled to a depth of 0–60 cm) was positive, suggesting an annual input of BNF of up to 58 kg N ha−1 in the plots where no N fertilizer was added. There do not appear to be any other N balance studies on sugarcane conducted in the field, but Walsh et al. (2006) found only negative N balances after 165 days for sugarcane plants inoculated with Gluconacetobacter diazotrophicus grown in a greenhouse. In this study there was doubt whether the inoculated G. diazotrophicus successfully infected the plants.
Unlike the management adopted for our field study, under standard plantation practice not all of the aerial biomass is removed from the plots at harvest. Over 40% of the Brazilian cane crop is now harvested without burning so that all trash is conserved. In this case less than one third of the N is removed from the field at harvest. Where cane is still burned before harvest almost all N in the trash is lost and approximately half of the N in the flag leaves. Basanta et al. (2003) estimated that 73% of trash + flag leaf N was lost on burning, and using the data acquired in this N balance study this would mean that only 24% of the N in the aerial biomass would be recycled when the crop is burned before harvest.
The values of 15N abundance of the leaf samples collected at the first (plant crop) harvest of the second crop cycle (2000–2004) were similar to those of the weed reference plants suggesting that there were no significant inputs of BNF to any cane variety (Table 7). At the subsequent harvests of the ratoon crops the 15N abundance of the cane varieties were almost all lower than that of the weed species at the harvests of 2000, 2003 and 2004, which showed a mean of approximately +5.0‰. Using these data the estimates for mean contribution of N from BNF to the commercial varieties ranged from 23 %to 44% and for the Krakatau and Chunee from 38% to 62% and 24% to 41%, respectively. Boddey et al. (2001) sampled leaves of all stages of maturity and node and internodes of stems and found that the third emergent leaf (that used in this study and commonly used for “crop logging”) had a 15N abundance of +4.1‰ compared to +3.9‰ for the whole plant. Biggs et al. (2002) also concluded that the 15N abundance of the third emergent leaf represented closely that of the whole sugarcane shoot. These results justify the practice of using leaf samples for 15N abundance sampling in this present study.
The higher N accumulation and N balance over the whole experiment and the lower 15N abundance for the last three ratoon crops of the Krakatau compared to the commercial varieties and the Chunee indicate that this S. spontaneum variety was able to obtain a greater proportion and quantity of N from BNF, which confirms the results obtained by Urquiaga et al. (1992). Chunee accumulated less N than any of the commercial varieties and less than half that of Krakatau. It was only this variety that did not show a statistically significant positive N balance. These data suggest that Chunee was able to obtain little N from BNF, but the 15N abundance of this was similar to that of the commercial varieties. If Chunee is incapable of obtaining N from BNF the 15N abundance should be close to that of the weed reference plants, which was not the case.
The first study to use 15N natural abundance to evaluate the contribution of BNF to sugarcane was conducted by Yoneyama et al. (1997). The authors sampled leaves of cane plants and neighbouring weeds in different plantations in Brazil, the Philippines and Japan. The 15N abundance of the cane leaves was found to be lower than that of the weeds in 68% of the sites, but there was no statistical treatment of the data. In another field sampling reported by Boddey et al. (2001) in 10 out of 11 sites across four states in Brazil weed samples were shown to be significantly (p < 0.05) higher in 15N abundance than the sugarcane leaves, suggesting that BNF contributions ranged from 0% to 60%Ndfa. However, no study of the rooting depth of the different weeds and sugarcane plants or the changes of 15N abundance of plant-available N with depth was conducted.
The values of 15N abundance of the weeds varied little from 2000 to 2004 and between species, but at the final two harvests (2003 and 2004) the values of 15N abundance of the weeds were all higher than those of all of the cane varieties. From the plant crop onwards the 15N abundance of the cane varieties decreased while that of the weeds remained almost constant. This is consistent with an increased BNF input during the ratoon crops as available N in the soil was depleted. As it was not possible to compare statistically the 15N abundance of the cane leaves and the weeds this evidence is far from conclusive on its own. In contrast Biggs et al. (2002) working in Australia and Hoefsloot et al. (2005) in South Africa found that the values of 15N abundance of non-N2-fixing reference plants were not higher than, and in the case of the South African study were lower than, those of the cane leaves which led both teams to conclude that BNF contributions to cane in their studies were insignificant. Biggs et al. (2002) did find low 15N abundance values in leaves of cane fertilized with between 150 and 250 kg N ha−1, but they showed evidence from their data and from the literature which indicates that high fertilizer N additions can lower 15N abundance, and this lower δ15N was not due to BNF inputs.
Hoefsloot et al. (2005) did not manage to confirm the presence of N2-fixing bacteria in the sugarcane variety they studied, and Biggs et al. (2002) did not study the microflora associated with Australian cane varieties. However, Gluconacetobacter diazotrophicus has been successfully isolated from sugarcane in Australia, India and Mexico (Li and Macrae 1992; Muthukumarasamy et al. 1999; Muñoz-Rojos and Caballero-Mellado 2003)
The investigation of the change in 15N abundance of plant-available N with depth showed that δ15N increased with depth from a mean of +5.8‰ in the surface 10 cm to +7.7 to +8.2‰ from 10 to 50 cm and approximately +7.4‰ in soil taken from 50 to 75 cm depth. There were no significant differences in N accumulation or 15N abundance amongst species of weeds and sorghum grown in soil taken from different depths which indicates that all four species accumulated N from very similar sources. Three of the species were graminaceous and the other a dicot (Commelina erecta), C. erecta, E. indica and S. bicolor extracted between 64% and 118% more N from the soil (total of all depths) than Panicum miliaceum, but despite these differences appeared to tap into the same available N (Table 8). The 15N abundance of the weeds taken from the plots showed mean δ15N of between +4.96 and +5.67‰, very similar to the plants grown in the surface 10 cm of the soil. If the sugarcane roots extracted N from greater depth in the soil as the photographs of the roots suggest, then the N accumulated by the cane plants derived from soil should be higher than that accumulated by the weed species and the weighted mean 15N enrichment of +7.1‰ derived from the pot experiment (Table 8) may be a more suitable value to use as reference. In that case the estimates of N derived from BNF (%Ndfa) by the commercial varieties range from 29% to 68%, the Krakatau from 53% to 74% and the Chunee from 43% to 56%.
The total N contribution from BNF calculated using these estimates of the proportion of %Ndfa derived from the δ15N data from the last four harvests, and the total N data from the whole 15-year study, suggest that annual inputs of BNF ranged from 40 to 64 kg N ha−1 for the commercial varieties and 83 and 33 kg N ha−1 for Krakatau and Chunee, respectively (Table 9).
Giller and Merckx (2003) suggested that the N balance technique could be regarded as the “ultimate test” for contributions of BNF to crops in experiments of over 10 years such as this present study. There are several pathways of N loss including gaseous losses (ammonia volatilization and denitrification), leaching, run off and soil erosion which, as these authors pointed out are difficult to estimate. However, failure to account for these losses will lead to an under-estimation of possible BNF inputs. N deposition in rainfall was quantified in this study, and although dry deposition was not estimated for lack of a suitable technique, the consensus seems to be that it is less than wet deposition, especially in a wet climate. Giller and Merckx (2003) also suggest that inputs of BNF are difficult to quantify, by which we assume they mean other than by the crop under study. Limited inputs of non-symbiotic N2 fixation have been registered, but contributions of the magnitude to account for the positive balances observed in our study, (>40 kg N ha−1 yr−1) are very improbable unless there was an obvious surface layer of cyanobacteria, as there seems to be no available C source for any heterotrophic free-living bacteria (Roper and Ladha 1995; Giller 2001). We find no evidence for lateral flow/run-on or any possible source of N to enrich such water. As Giller and Merckx (2003) point out, the most reliable and convincing technique involves the exposure of plants to 15N2 enriched atmospheres. The difficulty here is the large size of the plants and the sophisticated infra-structure required to control CO2 concentrations and temperature amongst other environmental factors (Eskew et al. 1981).
The positive N balance associated with the Krakatau variety was significantly higher than that associated with Chunee and three of the commercial cane varieties (SP 70-1143, SP 79-2312 and SP 71-6163), and the 15N natural abundance of the Krakatau was significantly lower than the Chunee and cv. SP 6163 (Tables 6 and 7). In this case the evidence from the δ15N data reinforced that from the N balance. However, although the Chunee accumulated significantly less N and showed much lower positive N balance than the commercial varieties, the estimate of the proportion of N derived from BNF by Chunee using the δ15N data did not differ from those for the commercial varieties.
Conclusions
Despite the disparity between the estimates of BNF inputs from the two different techniques, the consistently high and sustainable N accumulation by the crop, the highly positive N balance and the lower 15N abundance of the cane varieties compared to the weed reference plants constitute together strong evidence that the commercial cane varieties and the S. spontaneum variety, Krakatau, obtained over 40 kg N ha−1 yr−1 from plant-associated biological N2 fixation. This study and earlier ones performed in Brazil (Lima et al. 1987; Urquiaga et al. 1992; Boddey et al. 2001; Resende et al. 2006) all indicate that Brazilian sugarcane varieties are able to obtain agronomically significant contributions of N from plant-associated N2-fixing bacteria, probably of endophytic habit. While several species of these same N2-fixing endophytes have been found in sugar cane in other countries, no studies other than those conducted in Brazil have showed any evidence that the crops could obtain any significant N contribution of BNF. In the rhizobium/legume symbioses, the N2-fixing association is reproducible using one bacterial strain in one legume variety. The diazotroph/sugarcane association would appear to be far more complex, perhaps consisting of a consortium of bacteria, both N2-fixing and not, which may establish within the plant (Fischer et al. 2011; Thaweenut et al. 2011). Additional evidence for this hypothesis comes from the fact that the most successful inoculant of N2-fixing bacteria that has been developed to date for sugar cane in Brazil consists of five species of diazotrophs (de Oliveira et al. 2006; 2009). For this reason studies aimed towards an understanding of how the Brazilian cane varieties have greater success in establishing associations with N2-fixing diazotrophs will probably have to be realized on plants originating from the field and the application of a combination of recently-developed techniques of molecular biology, such as real-time PCR, may be the key to success.
References
Arnold SL, Schepers JS (2004) A simple roller-mill grinding procedure for plant and soil samples. Comm Soil Sci Plant Anal 35:537–545
Basanta MV, Dourado-Neto D, Reichardt K, Bacchi OOS, Oliveira JCM, Trivellin PCO, Timm LC, Tominaga TT, Correchel V, Cássaro FAM, Pires LF, de Macedo JR (2003) Management effects on nitrogen recovery in a sugarcane crop grown in Brazil. Geoderma 116:235–248
Biggs IM, Stewart GR, Wilson JR, Critchley C (2002) 15N natural abundance studies in Australian commercial sugarcane. Plant Soil 238:21–30
Boddey RM (1987) Methods for quantification of nitrogen fixation associated with gramineae. CRC Crit Rev Plant Sci 6:209–266
Boddey RM, Polidoro JC, Resende AS, Alves BJR, Urquiaga S (2001) Use of the 15N natural abundance technique for the quantification of the contribution of N2 fixation to sugar cane and other grasses. Aust J Plant Physiol 28:889–895
Boddey RM, Urquiaga S, Alves BJR, Reis VM (2003) Endophytic nitrogen fixation in sugar cane: present knowledge and future applications. Plant Soil 252:139–149
Boddey RM, de B Soares LH, Alves BJR, Urquiaga S (2008) Bio-ethanol production in Brazil. Chapter 13. In: Pimentel D (ed) Biofuels, solar and wind as renewable energy systems: benefits and risks. Springer, New York, pp 321–356
Castro-González R, Martinez-Aguilar L, Ramirez-Trujillo A, Estrada-de los Santos P (2011) High diversity of culturable Burkholderia species associated with sugarcane. Plant Soil. doi:10.1007/s11104-011-0768-0
de Mello WZ, de Almeida MD (2004) Rainwater chemistry at the summit and southern flank of the Itatiaia massif, Southeastern Brazil. Environ Pollut 129:63–68
de Oliveira ALM, Canuto EDL, Urquiaga S, Reis VM, Baldani JI (2006) Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil 284:23–32
de Oliveira ALM, Stoffels M, Schmid M, Reis VM, Baldani JI, Hartmann A (2009) Colonization of sugarcane plantlets by mixed inoculations with diazotrophic bacteria. Eur J Soil Biol 45:106–113
de Souza PA, de Mello WZ, Maldonado J (2006) Composição química da chuva e aporte atmosférico na Ilha Grande, RJ. Quím Nova 29:471–476
Dobereiner J (1961) Nitrogen-fixing bacteria of the genus Beijerinckia Derx in the rhizosphere of sugar cane. Plant Soil 15:211–216
dos Reis FB Jr, Reis VM, Urquiaga S, Dobereiner J (2000) Influence of nitrogen fertilisation on the population of diazotrophic bacteria Herbaspirillum spp. and Acetobacter diazotrophicus in sugarcane (Saccharum spp.). Plant Soil 219:153–159
Ellert BH, Bettany JR (1995) Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can J Soil Sci 75:529–538
EMBRAPA (1997) Centro Nacional de Pesquisa de Solos. Manual de métodos de análises de solo. Rio de Janeiro
Eskew DL, Eaglesham ARJ, App AA (1981) Heterotrophic 15N2 fixation and distribution of newly fixed nitrogen in a rice-flooded soil system. Plant Physiol 68:48–52
Fischer D, Pfitzner B, Schmid M, Simões-Araújo JL, Reis VM, Pereira W, Ormeño-Orrillo E, Hai B, Hofmann A, Schloter M, Martinez-Romero E, Baldani JI, Hartmann A (2011) Molecular characterisation of the diazotrophic bacterial community in uninoculated and inoculated field-grown sugarcane (Saccharum sp.). Plant Soil (in press doi:10.1007/s11104-011-0812-0)
Giller KE (2001) Nitrogen fixation in tropical cropping systems, 2nd edn. CAB International, Wallingford, p 423
Giller KE, Merckx R (2003) Exploring the boundaries of N2-fixation in cereals and grasses: a hypothetical and experimental framework. Symbiosis 35:3–17
Hoefsloot G, Termorshuizen AJ, Watt DA, Cramer MD (2005) Biological nitrogen fixation is not a major contributor to the nitrogen demand of a commercially grown South African sugarcane cultivar. Plant Soil 277:85–96
IBGE-SIDRA (2011) Instituto Brasileiro de Geografia e Estatística, sistema IBGE de Recuperação Automática. Available on-line at http://www.sidra.ibge.gov.br/bda/prevsaf/default.asp, accessed 8 February, 2011
James EK (2000) Nitrogen fixation in endophytic and associative symbiosis. Field Crops Res 65:197–209
Kempers AJ, Zweers A (1986) Ammonium determination in soil extracts by the salicylate method. Comm Soil Sci Plant Anal 17:715–723
Krusche AV, de Camargo PB, Cerri CEP, Ballester MV, Lara LBLS, Victoria RL, Martinelli LA (2003) Acid rain and nitrogen deposition in a sub-tropical watershed (Piracicaba): ecosystem consequences. Environ Pollut 121:389–399
Ledgard SF, Freney JR, Simpson JR (1984) Variations in natural enrichment of 15N in the profiles of some Australian pasture soils. Aust J Soil Res 22:155–164
Li RP, Macrae IC (1992) Specific identification and enumeration of Acetobacter diazotrophicus in sugarcane. Soil Biol Biochem 24:413–419
Lima E, Boddey RM, Dobereiner J (1987) Quantification of biological nitrogen fixation associated with sugar cane using a 15N aided nitrogen balance. Soil Biol Biochem 19:165–170
MAPA (2007) Balanço Nacional da Cana de Açúcar e Agroenergia. Ministério da Agricultura, Pecuária e Abastecimento, Brasília
Muñoz-Rojos J, Caballero-Mellado J (2003) Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb Ecol 46:454–464
Muthukumarasamy R, Revathi G, Lakshminarasimhan C (1999) Influence of N fertilisation on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp. from Indian sugarcane varieties. Biol Fertil Soils 29:157–164
Norman RJ, Edberg JC, Stucki JW (1985) Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci Soc Am J 49:1182–1185
Perin L, Martínez-Aguilar L, Castro-González R, Estrada-de los Santos P, Cabellos-Avelar T, Guedes HV, Reis VM, Caballero-Mellado J (2006a) Diazotrophic Burkholderia species associated with field-grown maize and sugarcane. Appl Environ Microbiol 72:3103–3110
Perin L, Martínez-Aguilar L, Paredes-Valdez G, Baldani JI, de Los E, Santos P, Reis VM, Caballero-Mellado J (2006b) Burkholderia silvatlantica sp. nov., a diazotrophic bacterium associated with sugar cane and maize. Int J Syst Evol Microbiol 56:1931–1937
Ramos MG, Villatoro MAA, Urquiaga S, Alves BJR, Boddey RM (2001) Quantification of the contribution of biological nitrogen fixation to tropical green manure crops and the residual benefit to a subsequent maize crop using 15N-isotope techniques. J Biotechnol 91:105–115
Reis VM, Olivares FL, Dobereiner J (1994) Improved methodology for isolation and identification of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbiol Biotechnol 10:101–104
Resende AS, Xavier RP, de Oliveira OC, Urquiaga S, Alves BJR, Boddey RM (2006) Long-term effects of pre-harvest burning and nitrogen and vinasse applications on yield of sugar cane and soil carbon and nitrogen stocks on a plantation in Pernambuco, N.E. Brazil. Plant Soil 281:339–351
Roper MM, Ladha JK (1995) Biological N-2 fixation by heterotrophic and phototrophic bacteria in association with straw. Plant Soil 174:211–224
Silva-Froufe LG, Boddey RM, Reis VM (2009) Quantification of natural populations of Gluconacetobacter diazotrophicus and Herbaspirillum spp.in sugar cane (Saccharum spp.) using different polyclonal antibodies. Braz J Microbiol 40:866–878
Thaweenut N, Hachisuka Y, Ando S, Yanagisawa S, Yoneyama T (2011) Two seasons’ study on nifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane (Saccharum spp. hybrids): expression of nifH genes similar to those of rhizobia. Plant Soil 338:435–449
Urquiaga S, Cruz KHS, Boddey RM (1992) Contribution of nitrogen fixation to sugar cane: nitrogen-15 and nitrogen-balance estimates. Soil Sci Soc Am J 56:105–114
Walsh KB, Brown SM, Harrison R (2006) Can a N2-fixing Gluconacetobacter diazotrophicus association with sugarcane be achieved. Aust J Agric Res 57:235–241
Yoneyama T, Muraoka T, Kim TH, Dacanay EV, Nakanishi Y (1997) The natural 15N abundance of sugarcane and neighbouring plants in Brazil, the Philippines and Miyako (Japan). Plant Soil 189:239–244
Acknowledgements
The authors express their gratitude to Altiberto M. Baêta and Roberto G. de Souza for the total N and 15N abundance analyses, respectively. The author RPX gratefully acknowledges National Research Council (CNPq) for a PhD Research Fellowship and RFM, RBB, NS and JML for undergraduate fellowships. The authors SU, BJRA and RMB gratefully acknowledge “productivity” fellowships from CNPq and from the program Cientista de Nosso Estado of the Rio State Research Foundation (FAPERJ). The work was funded by Embrapa, CNPq, FAPERJ and the Universidade Federal Rural do Rio de Janeiro.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Root profiles of: A commercial sugarcane variety CB 47-89, B Emilia sonchifolia (lilac tasselflower), and C Commelina erecta (slender day flower), growing in the experimental plots of the long-term sugarcane experiment. (PDF 3219 kb)
Rights and permissions
About this article
Cite this article
Urquiaga, S., Xavier, R.P., de Morais, R.F. et al. Evidence from field nitrogen balance and 15N natural abundance data for the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant Soil 356, 5–21 (2012). https://doi.org/10.1007/s11104-011-1016-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1016-3