Abstract
Different experiments have estimated that the contribution of biological nitrogen fixation (BNF) is largely variable among sugarcane cultivars. Which bacteria are the most important in sugarcane-associated BNF is unknown. However, Gluconacetobacter diazotrophicus has been suggested as a strong candidate responsible for the BNF observed. In the present study, bacteria-free micropropagated plantlets of five sugarcane cultivars were inoculated with three G. diazotrophicus strains belonging to different genotypes. Bacterial colonization was monitored under different nitrogen fertilization levels and at different stages of plant growth. Analysis of the population dynamics of G. diazotrophicus strains in the different sugarcane varieties showed that the bacterial populations decreased drastically in relation to plant age, regardless of the nitrogen fertilization level, bacterial genotype or sugarcane cultivars. However, the persistence of the three strains was significantly longer in some cultivars (e.g., MEX 57-473) than in others (e.g., MY 55-14). In addition, some strains (e.g., PAl 5T) persisted for longer periods in higher numbers than other strains (e.g., PAl 3) inside plants of all the cultivars tested. Indeed, the study showed that the inoculation of G. diazotrophicus may be beneficial for sugarcane plant growth, but this response is dependent both on the G. diazotrophicus genotype and the sugarcane variety. The most positive response to inoculation was observed with the combination of strain PAl 5T and the variety MEX 57-473. Although the positive effect on sugarcane growth apparently occurred by mechanisms other than nitrogen fixation, the results show the importance of the sugarcane variety for the persistence of the plant–bacteria interaction, and it could explain the different rates of BNF estimated among sugarcane cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been estimated that the contribution of biological nitrogen fixation (BNF) in some sugarcane cultivars may reach up to 70% of total plant nitrogen [5]. However, such BNF is largely variable among sugarcane cultivars [23, 45, 46]. Even though many diazotrophic species have been isolated from both the rhizosphere and inner tissues of sugarcane plants [1, 9, 31, 40], it is still unknown which bacteria are the most important in plant-associated BNF. The endophytic bacterium Gluconacetobacter diazotrophicus has long been proposed as a strong candidate responsible for such N2-fixation observed in sugarcane [4, 9, 18, 42]. It has also been suggested that G. diazotrophicus could promote and improve sugarcane growth through hormonal effects on metabolic processes [14] because of its ability to produce indoleacetic acid (IAA) and gibberellins [3, 13].
In addition to sugarcane plants, G. diazotrophicus has been isolated from inner tissues of sweet potato (Ipomoea batatas), Pennisetum purpureum var. Cameroon [11], Coffea arabica [22], Eleusine coracana [25], and pineapple plants [43]. G. diazotrophicus has been commonly recovered from inner tissues of the sugarcane plant in the range of 101 to 105 cells per gram of fresh weight [12, 14, 36, 37, 38]. Isolation of G. diazotrophicus from sugarcane plants seems to depend on the amount of nitrogen fertilization applied to the crops [13, 28, 36]. In these studies low isolation frequencies or low cell numbers of G. diazotrophicus were found in sugarcane cultivated with high nitrogen fertilization rates, and vice versa. Greenhouse experiments showed that the ability of one strain of G. diazotrophicus to colonize sugarcane plants diminishes when high nitrogen fertilizer doses were applied [14]. Using multilocus enzyme electrophoresis (MLEE) assays to determine the genetic diversity, seven distinct electrophoretic types (ETs) were identified among G. diazotrophicus isolates recovered from sugarcane plants cultivated in Brazil with low nitrogen doses, but only one genotype (designed ET 1) was identified among many isolates recovered from sugarcane plants cultivated in Mexico with high N-fertilization levels. These results suggested that the genetic diversity of this bacterial species, living endophytically in sugarcane, could be diminished by the high rates of nitrogen fertilization used in the sugarcane crops in Mexico [7].
With respect to the factors that influence the endophyte–plant interaction; little is known. This work was carried out with the aim of assessing the influence of bacterial genotype, plant cultivar, and nitrogen fertilization rates on the sugarcane–G. diazotrophicus interaction. The potential of G. diazotrophicus to promote sugarcane growth was also evaluated.
Materials and Methods
Bacterial Strains and Growth Conditions
Different strains of G. diazotrophicus were used for plant inoculation. Each strain represented a different electrophoretic type (ET), recognized as genotype, as described previously [7]. Strains used were UAP 5560 (ET 1), CFNE 550 (ET 2), PAl 5T (ET 3), PSP 22 (ET 4), PAl 3 (ET 5), 1772 (ET 6), and PRC 1 (ET 7). Cells were grown in MESMA liquid medium [14] at 29°C and shaken at 200 rpm for 24 h. The cultures were centrifuged and the pellet washed three times with 10 mM MgSO4 and finally resuspended in the MgSO4 solution at optical density of 0.8 at 450 nm (approximately 2 × 108 bacterial cells per mL).
Sugarcane Cultivars
Micropropagated sterile sugarcane plants of cultivars MY 55-14, MEX 57-473, MEX 62-280, CP 72-2086, and SP 70-1141 were obtained by meristem tissue culture [16]. MS medium [27], supplemented with 10% coconut water and plant hormones (3 mg of 2,4-diclorophenoxyacetic acid and 0.1 mg of kinetin per liter), was used for callus induction. Explants were maintained in MS medium at 28°C in the dark for approximately 2 months for callus propagation. The presence of bacterial contamination of callus was evaluated by plating macerated samples (ratio 1:9 w/v) on different culture media such as Congo Red [39], MacConkey, LB (Luria Bertani), PY (Peptone yeast), acetic LGI [9], and MESMA [14]. Contaminated calli were discarded. Differentiation to plantlets from calli was accomplished by transfer of callus to the basal MS medium without hormones, and then maintained for 70 days with a 16/8 photoperiod (light/dark) provided by cool fluorescent light (50 µmol m−2s−1) at 25–28°C [16]. Differentiated plantlets (after the plantlets had rooted) were separated and maintained for 40 days in the same medium and conditions described above. Plantlets were tested for bacterial contamination as described for callus.
Inoculation, Evaluation of the Colonization of Sugarcane Plants, and Plant Growth Conditions
Micropropagated sterile sugarcane plants are essential for evaluating the endophytic establishment of bacteria as well as to evaluate effects on the plant growth produced by the endophytic bacteria inoculated. In this work these evaluations were carried out with the following experiments.
Experiment 1
Endophytic colonization of sugarcane plants var. MY 55-14 by different G. diazotrophicus strains (PAl 5T, UAP 5560, and PAl 3) was evaluated. These strains were selected considering their ET as well as the predominance of the ET among G. diazotrophicus populations. Strain PAl 5T corresponds to ET-3, which is predominant among G. diazotrophicus populations recovered from sugarcane plants cultivated in Brazil; strain UAP 5560 represents the predominant ET-1 genotype identified among G. diazotrophicus populations collected from sugarcane cultivated in distant geographical regions from different host plants including Ipomoea batatas, Pennisetum purpureum [7], Coffea arabica [22], and Ananas comosus plants [43]; strain PAl 3 represents the ET-5, a genotype rarely identified among isolates of G. diazotrophicus [7]. The variety MY 55-14 is extensively cultivated in Morelos State, Mexico. Micropropagated sterile sugarcane plantlets of this variety were inoculated separately, by immersing the roots in a bacterial suspension for 1 h under sterile conditions, with three strains of G. diazotrophicus. Each inoculated plantlet was transplanted to a 1-L capacity pot containing sterile vermiculite. Plantlets were watered with 200 mL of MS nutrient solution (only mineral salts) supplemented with NH4NO3 as nitrogen source. In this experiment the plants were fertilized with different nitrogen doses (10, 60, 180 mg N/plant). Uninoculated plantlets were included in the experiment with each treatment. The pots were covered with aluminum foil, and the zone where the plants emerged was protected with sterile cotton. The plantlets were maintained in a greenhouse with controlled temperature (26–30°C) and the natural photoperiod corresponding to January through July of 1999. Endophytic bacterial recovery from shoots and roots of inoculated plants was determined at 35, 65, 105, and 170 days postinoculation (dpi). Five replicate plants for each ET and nitrogen level were analyzed. At harvest, plants were removed from the pots, washed with tap water, and disinfected with 70% ethanol for 30 s. Then the plants were rinsed with distilled water and surface sterilized with a 1.5% sodium hypochlorite solution for 20 min. Later, the plants were rinsed six times with sterile distilled water under sterile conditions. Fresh plants were divided into roots and shoots and macerated separately in water in a 1:10 (w/v) proportion. The macerates were serially diluted with sterile water. Three replicates per 10-fold dilution were inoculated in vials containing N-free-semisolid acetic LGI medium [9] and incubated for 8 days at 29°C. Vials with a thick yellow surface pellicle were streaked onto acetic LGI agar plates supplemented with yeast extract (50 mg/L) and incubated at 29°C for 3 days to verify the presence of the inoculated strain. The electrophoretic type and plasmid profile of six colonies recovered on LGIP agar plates, from each nitrogen treatment where G. diazotrophicus was isolated, were further verified both by MLEE assays of 11 metabolic enzymes [7] and by the modified Eckhardt method [17]. The bacterial number was determined by the most probable number (MPN) method using the McCrady tables.
Experiment 2
To evaluate the influence of sugarcane variety on the endophytic and rhizospheric establishment of G. diazotrophicus, five sugarcane varieties (MY 55-14, MEX 57-473, MEX 69-290, CP 72-2086, and SP 70-1141) were evaluated at different sugarcane growth states. Micropropagated sterile sugarcane plantlets of each variety were inoculated separately with the three strains of G. diazotrophicus used in experiment 1. Plantlets were watered with 200 mL of MS nutrient solution containing 10 mg NH4NO3, which was considered a basal level, in order to avoid nitrogen deficiencies of the plants. The plantlets were maintained under the greenhouse conditions described in experiment 1, but during the months of February to August of 2000. Uninoculated control plants were included in all of the experiments. At 35, 70, 105, and 170 dpi five plants of each treatment were removed from the pots under sterile conditions. The vermiculite adhered to the roots (considered as the “rhizosphere” in this work) was resuspended in water in a proportion of 1:10 (w/v). This suspension was vortexed at 3000 rpm for 3 min. The resulting suspension, which was considered to contain bacteria from the rhizosphere, was serially diluted. Endophytic bacteria were recovered as described in experiment 1. The cell numbers of G. diazotrophicus, both rhizospheric and endophytic, and the confirmation of the inoculated strain were determined as described in experiment 1.
Experiment 3
This experiment was carried out to evaluate the potential of G. diazotrophicus to promote sugarcane growth in two varieties. Micropropagated plantlets of the varieties MY 55-14 and MEX 57-473 were inoculated as described in experiment 1. Plantlets of variety MY 55-14 were inoculated separately with seven different strains of G. diazotrophicus and maintained under the greenhouse conditions and during the period described in experiment 1. The leaf numbers, height, and diameter of stems of sugarcane of this variety were measured at 35, 65, 105, and 170 dpi, for both inoculated and noninoculated plants (50 inoculated plants with each strain assayed and 50 control plants). In addition, 10 inoculated plants and 10 uninoculated control plants were used to determine the fresh and dry weight of roots and shoots at 35 and 105 dpi.
Once we identified the variety MEX 57-473 (results from experiment 2) as maintaining G. diazotrophicus strains at higher numbers for longer periods than other sugarcane cultivars tested, plantlets of this variety were inoculated with strains PAl 5T (a good colonizer) and PAl 3 (a poor colonizer). Thereafter, the inoculated plantlets were treated as in experiment 1 but supplemented with 10 mg of nitrogen at 0 and 35 dpi. Plantlets were maintained under similar greenhouse conditions described in experiment 1 during the months of February to April of 2001. Uninoculated plants were included as controls. The fresh and dry weight of roots and shoots of 17 plants as well as the total N content of these plants were evaluated at 35 and 75 dpi. Total N content of plants was evaluated with the semimicro-Kjeldahl method modified for inclusion of nitrates [6].
Data Analysis
All data were analyzed statistically using Student’s t test.
Results
Micropropagated sugarcane plantlets were obtained from callus in a period of about 4 months. All sugarcane plantlets tested were free of bacteria. The inoculation of plantlets by immersion of roots into a bacterial suspension for 1 h was adequate for the endophytic establishment of G. diazotrophicus. All of the G. diazotrophicus strains recovered from sugarcane plants analyzed in the different experiments had the same ET and showed a plasmid profile identical to that of the inoculated strain (data not shown). Electrophoretic type and plasmid profile from strains of G. diazotrophicus inoculated have been previously reported [8, 44]. The endophytic establishment of G. diazotrophicus within stems of sugarcane was confirmed by scanning electron microscopy (data not shown) using stem samples treated as described previously [14]. Apparently, the xylem vessels were the stem tissues colonized by G. diazotrophicus. However, a detailed analysis on the localization of this bacterium was not carried out.
Ability of G. diazotrophicus to Colonize Sugarcane var. MY 55-14 Growing with Different Nitrogen Levels
The ability of three strains of G. diazotrophicus (UAP 5560, PAl 3, and PAl 5T) to colonize sugarcane plants growing with different N levels is shown in Table 1. The three G. diazotrophicus strains were recovered 65 and 160 dpi when low nitrogen levels (10 mg N/plant) were applied, but not when high N-levels (180 mg N/plant) were used (Table 1). Surprisingly, it was observed that the endophytic bacterial number diminished drastically in relation with the age of the plant. This occurred regardless of the G. diazotrophicus strain inoculated or the nitrogen level applied in the experiment. The cell numbers of strain PAl 3 diminished more drastically than those of strains UAP 5560 and PAl 5T after 35 dpi.
Population Dynamics of G. diazotrophicus in Sugarcane Varieties
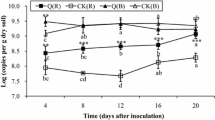
A drastic decrease of G. diazotrophicus populations, with all three strains tested, was observed in the rhizosphere as well as inside the plant tissues from all of the sugarcane varieties tested through plant growth time (Table 2). This behavior was a general feature in the G. diazotrophicus–sugarcane interaction. Population dynamics of G. diazotrophicus was similar in roots and in the rhizosphere, although the bacterial population in the rhizosphere was always higher than inside the roots (Table 2). The presence of G. diazotrophicus inside shoot tissues was not consistent; bacteria were not recovered from aerial parts of some plants even at 35 dpi. During the experiment it was observed that strains UAP 5560 (ET 1) and PAl 5T (ET 3) were always maintained in higher numbers than strain PAl 3 (ET 5). This behavior was observed in all sugarcane varieties tested. Two examples are shown in Fig. 1.
Population dynamics of G. diazotrophicus in sugarcane. Comparative analysis among genotypes of G. diazotrophicus. Values of log of cell number/g fresh weight used for analysis data were from (I) plants of sugarcane variety MEX 57-473 and (II) plants of sugarcane variety MY 55-14. Each point represents the average of 5 values. Points with the same letter within each graph do not differ by Student t-test to P < 0.05.
Higher numbers of G. diazotrophicus cells of three ETs (ET 1, ET 3, ET 5) tested were always found in association with plants of sugarcane var. MEX 57-473, while in sugarcane varieties SP 70-1141 and CP 72-2086 lower bacterial numbers always were detected. Figure 2 shows two examples of this.
Population dynamics of G. diazotrophicus in sugarcane. Comparative analysis among sugarcane cultivars. Values of log of cell number/g fresh weight used for analysis data were from (I) plants inoculated with strain PAl 5T and (II) plants inoculated with strain PAl 3. Each point represents the average of 5 values. Points with the same letter within each graph do not differ by Student t-test to P < 0.05. dpi, days postinoculation.
Evaluation of G. diazotrophicus Inoculation on Sugarcane Plant Growth (var. MY 55-14)
The inoculation of sugarcane var. MY 55-14 plants with seven different strains of G. diazotrophicus showed that only strain PAl 3 slightly increased height and diameter of shoots. These increases were observed even 160 dpi, although the strain was not recovered from aerial tissues. However, the dry weight of inoculated plants was not statistically different from that of control plants (data not shown).
Effect of G. diazotrophicus Inoculation on Growth of Sugarcane var. MEX 57-473
In order to verify that G. diazotrophicus is able to promote sugarcane growth, the strains PAl 5T (a good colonizer) and PAl 3 (a poor colonizer) were evaluated in association with the variety MEX 57-473, which maintain the highest numbers of this bacterium. Positive effects on sugarcane growth were observed with both strains but the most beneficial response was observed with the strain PAl 5T (Tables 3, 4 and Fig. 3). The fresh and dry weights as well as the total nitrogen content from sugarcane plants inoculated with strain PAl 5T were statistically higher than those of control plants at both 35 (Table 3) and 75 dpi (Table 4), with the increases being more evident at 35 dpi. Plants inoculated with the strain PAl 3 showed increases in fresh and dry weight only at 75 dpi (Table 4), slightly lower than those observed in sugarcane plants inoculated with the strain PAl 5T at this time. In contrast, the percent nitrogen content of plants inoculated with the strain PAl 5T was lower at 35 dpi and similar at 75 dpi compared to the nitrogen percentages determined in control plants (Tables 3, 4). Obviously, the increase in total nitrogen content resulted from a significant increase in plant dry weight. Sugarcane plants inoculated with the strain PAl 3 showed percent nitrogen contents similar to those of uninoculated control plants. The endophytic bacterial number of the strains PAl 5T and PAl 3 recovered from sugarcane plants from this experiment (data not shown) was in accordance with results described in Table 2 and Fig. 1-I.
Discussion
In the present work, without causing visible disease symptoms, the infection followed by colonization of sugarcane plantlets by G. diazotrophicus was successful with a single immersion of the plantlet roots in a bacterial suspension. This result confirms the endophytic colonization ability of G. diazotrophicus described in different studies using other inoculation methods [14, 19, 21].
Although the population dynamics of G. diazotrophicus in association with five sugarcane varieties tested was variable, the decrease of bacterial population related to the age of the plant was a general characteristic of this bacterial species. Strains UAP 5560 and PAl 5T (genotypes 1 and 3, respectively) remained associated with all sugarcane cultivars for longer periods than strain PAl 3 (genotype 5). This result shows the different ability of G. diazotrophicus genotypes to endophytically colonize sugarcane, and it could explain the predominance of ET 1 and ET 3 identified among G. diazotrophicus isolates recovered from sugarcane cultivated in fields of Mexico and Brazil, respectively [7], as well as the highest isolation frequency of ET 1 strains recovered from different host plants such as coffee and pineapple [22, 43]. A similar effect on the decrease of the G. diazotrophicus population in the rhizosphere of sugarcane plants was observed. However, the population of G. diazotrophicus in the rhizosphere was always higher than inside the roots, which suggests that under suitable conditions this putatively endophytic bacterium is capable of surviving and proliferating in such an environment. Although little emphasis has been given to the isolation of “endophytic” bacteria out of plants, the natural occurrence of G. diazotrophicus in the rhizosphere of coffee and sugarcane plants has been reported previously [22, 29]. Recent results show that the bacterial population on the root surface may be as important as, if not more important than, the bacterial population within the plant, as was observed with Herbaspirillum seropedicae, another putative endophyte, benefiting rice plant growth [15].
Previously, it was reported that high nitrogen fertilization levels diminished the sugarcane colonization by one strain of G. diazotrophicus [14]. In the present work we observed that such a decrease occurs regardless of the G. diazotrophicus genotype. Recently, Muthukumarasamy et al. [29] reported that G. diazotrophicus form long, pleomorphic, immobile cells in the presence of high concentrations of nitrogen sources, especially ammonium (25 mM NH4NO3), in culture media. These authors suggested that the morphological changes might play a negative role in the survival of G. diazotrophicus in high N-fertilized environments. This possibility cannot be discarded; however, in the present study it was observed that the population of G. diazotrophicus decreases even with a low (10 mg N/plant = 0.35 mM NH4NO3) nitrogen level.
We cannot explain the influence of plant age or nitrogen fertilization level on the population decrease of G. diazotrophicus in the inner tissues of sugarcane plants. However, it is known that changes in tissue water relations [26] and in the concentration of sucrose may occur [10, 24] during sugarcane growth. In addition, changes in enzymatic activities have been observed in sugarcane plants when they are nitrogen fertilized [33]. These physiological and metabolic changes might modify the establishment and even the endophytic permanence of G. diazotrophicus in sugarcane. Moreover, other possibilities could explain the population diminution of G. diazotrophicus. Although this species is considered a nonpathogen [2, 35, 42], it has been reported that G. diazotrophicus elicits a localized host defense response [19]. On this basis, it is conceivable that the endophytic population of G. diazotrophicus decreases, as a result of host plant defense response mechanisms similar to systemic acquired resistance (SAR) induced by pathogens, or induced systemic resistance (ISR) observed with nonpathogenic rhizobacteria [34].
In the present work, the G. diazotrophicus number inside root tissues ranged from 104 to 105 CFU/g fresh weight of plants at 35 dpi in all varieties tested, but at 170 dpi this number decreased to 10 CFU/g fresh weight, or the bacterium was not detected. In contrast, the cell numbers of G. diazotrophicus found in adult sugarcane plants were in the range of 105–107 CFU/g fresh tissue [9, 38]. However, cell numbers of G. diazotrophicus as low as 10 to 102 CFU/g fresh weight of plant have been found in mature sugarcane cultivated in Brazil [36]. These authors suggested that the variation in the bacterial number of G. diazotrophicus was due to changes in environmental factors, mainly rainfall, but in the present work conditions were controlled and the number of G. diazotrophicus cells decreased as well.
The data analysis of population dynamics of G. diazotrophicus in association with sugarcane revealed that variety MEX 57-473 is able to harbor this diazotrophic species in greater populations than the other four sugarcane varieties assessed. This fact shows the significance of sugarcane variety for the persistence of the plant–bacteria interaction, and it could explain the discrepancies in the frequencies and bacterial number of G. diazotrophicus recovered from sugarcane plants analyzed in diverse studies [9, 13, 36, 38], as well as the different rates of BFN estimated among sugarcane cultivars [23, 45, 46]. It is important to mention that G. diazotrophicus has been isolated from sugarcane var. MEX 57-473 cultivated in fields fertilized with 275–300 kg N/ha but not from other varieties fertilized with the same amount of nitrogen [13].
Interestingly, plant growth promotion was observed in var. MEX 57-473, but not in var. MY 55-14, inoculated with G. diazotrophicus strains PAl 5T and PAl 3. The lack of growth promotion of MY 55-14 plants could be due to the drastic diminution of G. diazotrophicus population through plant growth time. Even though the population of strain PAl 3 declined more rapidly than PAl 5T populations in all sugarcane tested through plant growth, the ability of strain PAl 3 to promote the growth of sugarcane variety MEX 57-473 might be explained by the bacterial permanence at the time evaluated (75 dpi) in this variety.
The present work shows the sugarcane growth promotion when there are appropriate interaction between sugarcane variety and G. diazotrophicus genotype. However, the beneficial effect on sugarcane growth observed with variety MEX 57-473 was apparently not due to BNF, since the percent nitrogen content of inoculated plants was statistically similar to or even lower than that in uninoculated plants. This could reflect an effect of dilution of nutrients generally observed when a hormonal effect is involved. Because G. diazotrophicus has been shown to produce plant growth–promoting substances [3, 13], IAA could be the compound responsible for the beneficial effects observed, as suggested by Fuentes-Ramírez et al. [13] and recently by Sevilla et al. [41], as well as by Oliveira et al. [32]. In addition, the consistent decrease of G. diazotrophicus populations observed and the low cell numbers of this bacterium inside sugarcane seems not to be sufficient to sustain the BNF process required by the plant. In fact, it has been argued that the endophytic bacterial number appears trivial when it is compared with the Rhizobium–legume association where high bacterial numbers are required in the nodule for sustaining BNF [20]. However, Sevilla et al. [41] with an 15N2 incorporation experiment, reported that G. diazotrophicus strain PAl 5T was capable of fixing N2 inside sugarcane plants var. SP 70-1143. Although these authors did not show evidence that strain PAl 5T was responsible of such an activity inside sugarcane, because they did not eliminate the rhizosphere or root surface bacterial populations, the possibility that other growth-promoting factors might be responsible for the enhancement of sugarcane growth was not excluded. Differences in sugarcane variety might explain the discrepancies between the results of the present study and those of Sevilla et al. [41]. Recently, data on expression of sugarcane genes induced by inoculation with G. diazotrophicus have suggested that the plant might be actively involved in the establishment of this bacterium [30].
Although the inoculation of sugarcane with G. diazotrophicus may promote plant growth, it will be necessary to search for the best G. diazotrophicus genotype–sugarcane variety interaction to obtain consistent responses that contribute to sugarcane growth enhancement.
References
CA Asis Jr M Kubota VK Chebotar H Ohta Y Arima K Nishiyama KI Tsuchiya S Akao (2000) ArticleTitleEndophytic bacterial population in Philippine sugarcane cultivars and isolation of nitrogen-fixing strains. Microbes Environ 15 209–216 Occurrence Handle10.1264/jsme2.2000.209
JI Baldani L Caruso VLD Baldani SR Got J Döbereiner (1997) ArticleTitleRecent advances in BNF with non-legume plants. Soil Biol Biochem 29 911–922 Occurrence Handle10.1016/S0038-0717(96)00218-0 Occurrence Handle1:CAS:528:DyaK2sXks1ajt7s%3D
F Bastian A Cohen P Piccoli V Luna R Baraldi R Bottini (1998) ArticleTitleProduction of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Reg 24 7–11 Occurrence Handle10.1023/A:1005964031159 Occurrence Handle1:CAS:528:DyaK1cXhs1yns7o%3D
RM Boddey OC De Oliveira S Urquiaga VM Reis FL De Olivares VLD Baldani J Döbereiner (1995) ArticleTitleBiological nitrogen fixation associated with sugarcane and rice: contributions and prospects for improvement. Plant Soil 174 195–209 Occurrence Handle1:CAS:528:DyaK2MXotFalsLw%3D
RM Boddey S Urquiaga V Reis J Döbereiner (1991) ArticleTitleBiological nitrogen fixation associated with sugarcane. Plant Soil 137 111–117
JM Bremmer (1965) Total nitrogen. CA Black (Eds) Methods of Soil Analysis, Part 2, Agronomy 9. American Society of Agronomy Madison, WI 1149–1178
J Caballero-Mellado LE Fuentes-Ramírez VM Reis E Martínez-Romero (1995) ArticleTitleGenetic structure of Acetobacter diazotrophicus populations and identification of a new genetically distant group. Appl Environ Microbiol 61 3008–3013
J Caballero-Mellado E Martínez-Romero (1994) ArticleTitleLimited genetic diversity in the endophytic sugarcane bacterium Acetobacter diazotrophicus. Appl Environ Microbiol 60 1532–1537 Occurrence Handle1:CAS:528:DyaK2cXjtF2iur0%3D
VA Cavalcante J Döbereiner (1988) ArticleTitleA new acid-tolerant nitrogen fixing bacterium associated with sugarcane. Plant Soil 108 23–31
RS Chaughule SS Ranade NC Shah (2000) ArticleTitleMagnetic resonance imaging (MRI) of Saccharum officinarum L. (sugarcane) during its growth for sucrose content. Indian J Exp Biol 38 1062–1065
J Döbereiner VM Reis MA Paula FL Olivares (1993) ArticleTitleEndophytes diazotrophs in sugarcane, cereals and tuber plants. Curr Plant Sci Biotechnol Agric 17 671–676
Z Dong MJ Canny ME McCully MR Roboredo CF Cavadilla E Ortega R Rodés (1994) ArticleTitleA nitrogen-fixing endophyte of sugarcane stems. A new role for the apoplast. Plant Physiol 105 1139–1147 Occurrence Handle1:CAS:528:DyaK2cXlsFegu78%3D Occurrence Handle12232271
LE Fuentes-Ramírez T Jiménez-Salgado IR Abarca-Ocampo J Caballero-Mellado (1993) ArticleTitle Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil 154 145–150
LE Fuentes-Ramírez J Caballero-Mellado J Sepúlveda E Martinez-Romero (1999) ArticleTitleColonization of sugarcane by Acetobacter diazotrophicus is inhibited by high N-fertilization. FEMS Microbiol Ecol 29 117–128
P Gyaneshwar EK James PM Reddy JK Ladha (2002) ArticleTitle Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium-tolerant rice varieties. New Phytol 154 131–145 Occurrence Handle10.1046/j.1469-8137.2002.00371.x Occurrence Handle1:CAS:528:DC%2BD38Xjs1eisrs%3D
DJ Heinz GWP Mee (1969) ArticleTitlePlant differentiation from callus tissue of Saccharum species. Crop Sci 9 316–318
MF Hynes NF McGregor (1990) ArticleTitleTwo plasmids other than the nodulation plasmid are necessary for formation of nitrogen fixing nodules by Rhizobium leguminosarum. Mol Microbiol 4 567–574 Occurrence Handle1:CAS:528:DyaK3cXlt1OhsL0%3D Occurrence Handle2161988
EK James (2000) ArticleTitleNitrogen fixation in endophytic and associative symbiosis. Field Crops Res 65 197–209 Occurrence Handle10.1016/S0378-4290(99)00087-8
EK James FL Olivares ALM De Oliveira FB Dos Reis Jr LG Da Silva VM Reis (2001) ArticleTitleFurther observations on the interaction between sugarcane and Gluconacetobacter diazotrophicus under laboratory and greenhouse conditions. J Exp Bot 52 747–760 Occurrence Handle1:CAS:528:DC%2BD3MXltVaqu7k%3D Occurrence Handle11413211
EK James P Gyaneshwar WL Barraquio N Mathan JK Ladha (2000) Endophytic diazotrophs associated with rice. JK Ladha PM Reddy (Eds) The Quest for Nitrogen Fixation in Rice Los Baños Laguna, Philippines 119–140
EK James VM Reis FL Olivares JI Baldani J Döbereiner (1994) ArticleTitleInfection of sugarcane by the nitrogen-fixing bacterium Acetobacter diazotrophicus. J Exp Bot 45 757–766 Occurrence Handle1:CAS:528:DyaK2cXmtVChtbw%3D
T Jimenez-Salgado LE Fuentes-Ramirez A Tapia-Hernandez MA Mascarua-Esparza E Martinez-Romero J Caballero-Mellado (1997) ArticleTitle Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl Environ Microbiol 63 3676–3683
E Lima RM Boddey J Döbereiner (1987) ArticleTitleQuantification of biological nitrogen fixation associated with sugarcane using a 15N aided nitrogen balance. Soil Biol Biochem 19 165–170 Occurrence Handle10.1016/0038-0717(87)90077-0 Occurrence Handle1:CAS:528:DyaL2sXktlylsbg%3D
SE Lingle (1999) ArticleTitleSugar metabolism during growth and development in sugarcane internodes. Crop Sci 39 480–486 Occurrence Handle1:CAS:528:DyaK1MXisFaisro%3D
P Loganathan R Sunlta AK Parlda S Nair (1999) ArticleTitleIsolation and characterization of two genetically distant groups of Acetobacter diazotrophicus from a new host plant Eleusine coracana L. J Appl Microbiol 87 167–172
PH Moore DJ Cosgrove (1991) ArticleTitleDevelopmental changes in cell and tissue water relation parameters in storage parenchyma of sugarcane. Plant Physiol 96 794–801 Occurrence Handle1:CAS:528:DyaK3MXltlajt7c%3D Occurrence Handle11538006
T Murashige F Skoog (1962) ArticleTitleA revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15 473 Occurrence Handle1:CAS:528:DyaF3sXksFKm
R Muthukumarasamy G Revathi C Lakshminarasimhan (1999) ArticleTitleInfluence of N fertilization on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp. from Indian sugarcane varieties. Biol Fertil Soils 29 157–164 Occurrence Handle10.1007/s003740050539 Occurrence Handle1:CAS:528:DyaK1MXisleksLo%3D
R Muthukumarasamy G Revathi P Loganathan (2002) ArticleTitleEffect of inorganic N on the population, in vitro colonization and morphology of Acetobacter diazotrophicus (syn. Gluconacetobacter diazotrophicus). Plant Soil 243 91–102 Occurrence Handle10.1023/A:1019963928947 Occurrence Handle1:CAS:528:DC%2BD38XmsFWmu78%3D
EM Nogueira F Vinagre HP Masuda C Vargas VL Muniz de Pádua FR Da Silva RV Dos Santos JI Baldani PCG Ferreira AS Hemerly (2001) ArticleTitleExpression of sugarcane genes induced by inoculation with Gluconacetobacter diazotrophicus and Herbaspirillum rubrisubalbicans. Gen Mol Biol 24 199–206 Occurrence Handle1:CAS:528:DC%2BD38Xks1Sqsro%3D
FL Olivares VLD Baldani VM Reis JI Baldani J Döbereiner (1996) ArticleTitleOccurrence of the endophytic diazotrophs Herbaspirillum spp. in roots, stems and leaves predominantly of gramineae. Biol Fertil Soils 21 197–200 Occurrence Handle10.1007/s003740050049
ALM Oliveira S Urquiaga J Döbereiner JI Baldani (2002) ArticleTitleThe effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242 205–215 Occurrence Handle10.1023/A:1016249704336 Occurrence Handle1:CAS:528:DC%2BD38XltFKisb8%3D
AI Pelaez R De Armas Urquiza M-H Valadier ML Champigny (1994) ArticleTitleShort-term effect of nitrate on carbon metabolism of two sugarcane cultivars differing in their biomass production. Phytochemistry 36 819–833 Occurrence Handle10.1016/S0031-9422(00)90444-8
CM Pieterse LC van Loon (1999) ArticleTitleSalicylic acid-independent plant defense pathways. Trends Plant Sci 4 52–58 Occurrence Handle10234273
B Reinhold-Hurek T Hurek (1998) ArticleTitleLife in grasses: diazotrophic endophytes. Trends Microbiol 6 139–144 Occurrence Handle1:STN:280:DyaK1c3kvVOnsA%3D%3D Occurrence Handle9587190
FB Reis dos Jr VM Reis S Urquiaga J Döbereiner (2000) ArticleTitleInfluence of nitrogen fertilisation on the population of diazotrophic bacteria Herbaspirillum spp. and Acetobacter diazotrophicus in sugar cane (Saccharum spp.). Plant Soil 219 153–159 Occurrence Handle10.1023/A:1004732500983
VM Reis FL Olivares ALM De Oliveira FB Dos Reis Jr JI Baldani J Döbereiner (1999) ArticleTitleTechnical approaches to inoculate micropropagated sugarcane plants with Acetobacter diazotrophicus. Plant Soil 206 205–211 Occurrence Handle10.1023/A:1004436611397
VM Reis FL Olivares J Döbereiner (1994) ArticleTitleImproved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbiol Biotechnol 10 401–405
E Rodríguez-Cáceres (1982) ArticleTitleImproved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44 990–991
AP Ruschel (1981) Associative N2-fixation by sugarcane. PB Vose AP Ruschel (Eds) Associative N2-Fixation, vol 2, CRC Press Boca Raton, FL 81–90
M Sevilla RH Burris N Gunapala C Kennedy (2001) ArticleTitleComparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif− mutant strains. Mol Plant-Microb Interact 14 358–366 Occurrence Handle1:CAS:528:DC%2BD3MXhsVKgsb8%3D
M Sevilla C Kennedy (2000) Genetic Analysis of nitrogen fixation and plant-growth stimulating properties of Acetobacter diazotrophicus, an endophyte of sugarcane. EW Triplett (Eds) Prokaryotic Nitrogen Fixation: A Model System for the Analysis of a Biological Process. Horizon Scientific Press Wymondham, UK 737–760
A Tapia-Hernández MR Bustillos-Cristales T Jiménez-Salgado J Caballero-Mellado LE Fuentes-Ramírez (2000) ArticleTitleNatural endophytic occurrence of Acetobacter diazotrophicus in pineapple plants. Microb Ecol 39 49–55
KRS Teixeira R Galler C Kennedy JI Baldani (1994) Plasmid contents and nif genes detection in Acetobacter diazotrophicus strains. NA Hegazi M Fayes . Monib (Eds) Nitrogen Fixation with Non-legumes The American University in Cairo Press 273–281
S Urquiaga KHS Cruz RM Boddey (1992) ArticleTitleContribution of nitrogen fixation to sugarcane: Nitrogen-15 and nitrogen-balance estimates. Soil Sci Soc Am J 56 105–114
T Yoneyama T Muraoka TH Kim EV Dacanay Y Nakanishi (1997) ArticleTitleThe natural 15N abundance of sugarcane and neighbouring plants in Brazil, the Philippines and Miyako (Japan). Plant Soil 189 239–244 Occurrence Handle10.1023/A:1004288008199 Occurrence Handle1:CAS:528:DyaK2sXksVyksLw%3D
Acknowledgements
We are indebted to Dr. Michael Dunn for constructive English corrections, and Dr. Maria Valdés for valuable opinions on the work. Jesús Muñoz-Rojas was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT)-México and by Dirección General de Estudios de Posgrado (DGEP)-UNAM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz-Rojas, J., Caballero-Mellado, J. Population Dynamics of Gluconacetobacter diazotrophicus in Sugarcane Cultivars and Its Effect on Plant Growth . Microb Ecol 46, 454–464 (2003). https://doi.org/10.1007/s00248-003-0110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-003-0110-3