Abstract
Background
Focal cerebral arteriopathy includes unifocal or multifocal lesions that are unilateral or bilateral. Large- and/or medium-sized vessels are involved and can be visualized on angiography.
Case report
We report a case of cerebral infarction in a 9-year-old Japanese female who presented with a transient ischemic attack. Steno-occlusion involving the distal part of the internal carotid artery, proximal middle cerebral artery, and anterior cerebral artery was observed. Digital subtraction angiography demonstrated a beaded appearance in the cervical portion of the diseased internal carotid artery. Revascularization surgery was performed 45 days after the onset. A new infarction appeared on the other side of the anterior cerebral artery territory 7 months after the first onset. Antiplatelets and vasodilators were administered, and no progression was observed during 18 months of follow-up. Genetic analysis did not show ring finger protein 213 (RNF213)-related moyamoya disease, and pathological examination revealed no characteristics of fibromuscular dysplasia.
Conclusion
The radiological and genetic features coincided with focal cerebral arteriopathy, which is a distinct entity from fibromuscular dysplasia and RNF213-related moyamoya disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal cerebral arteriopathy (FCA) is described as angiographic stenosis that is seen as unilateral or multifocal stenosis of medium- to large-sized vessels including the terminal portion of the internal carotid artery (ICA), proximal portion of the anterior cerebral artery (ACA), and the middle cerebral artery (MCA) [1, 3]. Any radiographically specific diagnosis is classified into this type of arteriopathy, including for example, arterial dissection, moyamoya disease (MMD), and fibromuscular dysplasia (FMD) [3, 6].
FCA is also recognized as a leading cause of childhood arterial ischemic stroke and may lead to frequent recurrent strokes and a poor neurological outcome [4, 14]. Recently, Je et al. presented a patient cohort that was suspected to have this nonspecific condition. Patients were from Korea where MMD is significantly prevalent among children [18]. This report indicated that some children with a diagnosis of MMD in East Asia might be misdiagnosed and actually have FCA. To our knowledge, the present report is the first to describe a Japanese pediatric patient suspected of having this type of arteriopathy and, therefore, also the first to describe a genetic and pathologic investigation of such a patient.
Case report
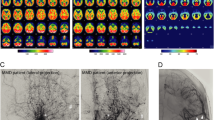
A 9-year-old Japanese female developed dysarthria and right mild hemiparesis and presented with frequent transient ischemic attacks with severe headaches after the onset. A cerebral infarction in the left caudate head and basal ganglia was found on diffusion-weighted imaging (Fig. 1a). Magnetic resonance angiography (MRA) revealed occlusion of the distal part of the left ICA. The proximal parts of the ACA and MCA were also occluded in the absence of new ischemic lesions (Fig. 1b). Digital subtraction angiography (DSA) showed no obvious basal moyamoya collaterals through the lenticulostriate pathway. A beaded appearance in the cervical portion of the left ICA was confirmed on DSA (Fig. 1c). Single-photon emission computed tomography about 3 weeks after the onset indicated decreased cerebral blood flow in the left MCA territory in the resting state and lower cerebrovascular reserve with an acetazolamide challenge. Therefore, superficial temporal artery (STA)-MCA bypass and indirect bypass were performed. The patient was discharged and prescribed aspirin (50 mg daily).
Diffusion-weighted imaging (DWI) showed a cerebral infarction in the left caudate head and basal ganglia (a). MR angiography (MRA) revealed occlusion of the distal part of the left internal carotid artery (ICA). The proximal parts of the left anterior cerebral artery and middle cerebral artery (MCA) were also occluded (b). Digital subtraction angiography (left common carotid angiogram, anterior-posterior view) showed a beaded appearance in the cervical portion of the left ICA (arrow in c). DWI showed a new infarction in the right A1 perforator territory 7 months after the first onset (d). MRA revealed right mid-A1 focal narrowing without ICA or MCA stenosis (arrow in e). The pathological diagnosis from the left superficial temporal artery specimen obtained during revascularization surgery was a normal vascular structure without features of fibromuscular dysplasia (H & E, bar = 200 μm, f)
A new infarction in the A1 perforator territory appeared along with severe headaches at 7 months after the first onset (Fig. 1d). The right mid-A1 showed focal narrowing without ICA or MCA stenosis (Fig. 1e). Another oral antiplatelet (cilostazol) was added, and a Rho kinase inhibitor (fasudil hydrochloride) was administered intravenously as a vasodilator. After this interim progression, the patient became clinically stable on only aspirin, and follow-up imaging at 18 months demonstrated stabilized narrowing in the same portion without major recanalization.
The pathological diagnosis from the STA specimen obtained during revascularization surgery was a normal vascular structure without features of FMD (Fig. 1f). After obtaining a written informed consent, genetic analysis was performed using blood samples which revealed that the patient did not carry p.R4810K polymorphism of ring finger protein 213 (RNF213) [7].
Discussion
This is the first case report that pathologically and genetically differentiated FCA from FM D and RNF213-related MMD. FCA is a leading cause of childhood arterial ischemic stroke, especially in European countries [1, 3].
Guidelines for the diagnosis of MMD do not define the unilateral or bilateral form of stenosis/occlusion in the terminal portion of the ICA [13]. Using this radiological diagnostic procedure, the current case may have met the criteria for a definitive MMD diagnosis. However, basal moyamoya vessels did not develop, and diagnosis remained obscure.
In this case, an initial DSA showed nonspecific vessel irregularity and steno-occlusion in the unilateral anterior circulation without moyamoya collaterals. Although the patient suffered from severe headache at onset, specific angiographic features such as intimal flap or double lumen were not obtained. A beaded appearance in the cervical portion of the left ICA was suspected of type 1 FMD. However, CT angiography on systemic arteries revealed no abnormality, and there was no pathological evidence of FMD in the STA specimen. Furthermore, systemic inflammatory and autoimmune/collagen diseases were ruled out by different kinds of serologic examinations. The patient was healthy and had no clinical history of varicella zoster infection preceding 12 months. Other infectious agents were not detected in the serum sample. Cerebrospinal fluid markers did not demonstrate any infectious and inflammatory responses and other pathophysiological evidences. Given these facts, a definite diagnosis was not confirmed. Finally, we provided a diagnosis of FCA for the patient as a diagnosis of exclusion [9].
In accordance with the Childhood Arterial Ischemic Stroke Classification and Diagnostic Evaluation (CASCADE) criteria [2], unilateral focal cerebral arteriopathy of childhood (FCA) comprised steno-occlusion in the anterior circulation with or without collaterals. If this case had some collaterals, the CASCADE criteria gave a diagnosis as some types of possible MMD or some patients with progressive primary angiitis of the central nervous system of childhood. Without collaterals, the CASCADE criteria would suggest any type of MMD but transient cerebral arteriopathy, post-varicella arteriopathy, and large-vessel childhood primary angiitis of the central nervous system.
Eventually, we have presented that this case met the criteria for FCA which basically affected the unilateral intracranial main arteries. However, interestingly, this case had an interim progression presenting focal narrowing in the contralateral mid-A1 at 7 months after the first onset. This rare phenomenon without ICA or MCA stenosis has not been reported, usually seems not to be observed in the FCA. Given the facts, to be exact, it remains undetermined whether this case should be assigned to unilateral focal cerebral arteriopathy of childhood (FCA) or bilateral cerebral arteriopathy of childhood in the CASCADE criteria. Finally, we have evaluated that this case essentially fell into FCA considering the first attack as a main event in the clinical course. Rapid progression invading the contralateral ICA or MCA might appear in this case; therefore, a careful course observation should be essential.
According to the result of the CBF study obtained about 3 weeks after the onset, we performed STA-MCA bypass and indirect bypass as combined revascularization surgery usually adopted for moyamoya cases. MRA demonstrated a good bypass patency in the early postoperative period (Fig. 2 arrowhead in a). Despite preoperative symptoms induced by cerebral hypoperfusion disappearing immediately after the surgery, a sparse effect was found in the MRA undertaken 6 months after the surgery (Fig. 2 arrowhead in b). We expected a favorable result of revascularization observed in moyamoya cases; however, it was not achieved radiographically. As shown in Fig. 2 (arrow in b), a faint recanalization of the occluded MCA occurred unexpectedly without basal moyamoya collaterals in this case. These clinical courses were usually not seen in moyamoya cases, strongly suspected as the other pathophysiology, such as FCA.
Effect of combined revascularization surgery (superficial temporal artery (STA)-middle cerebral artery (MCA) bypass and indirect bypass) for this case. MR angiography (MRA) revealed patent STA-MCA bypass filling the left MCA in the early postoperative period (day 4, arrowhead in a). On the other hand, direct and indirect bypass development was not observed in MRA about 6 month after the surgery (arrowhead in b). A faint recanalization of the left MCA was confirmed (arrow in b)
RNF213 was identified as a susceptibility gene for MMD, with about 90% of familial MMD cases and 70% of sporadic cases showing an RNF213 polymorphism, p.R4810K [7]. On the other hand, Miyawaki et al. reported that four of eight patients (50%) with unilateral MMD were RNF213 p.R4810K heterozygotes [11]. In the current case, RNF213 genotyping revealed wild-type RNF213, and thus, the diagnosis may not be RNF213-related MMD. On the other hand, Mineharu et al. presented an adult unilateral MMD case with rapid contralateral progression over 1 year in which the patient carried an RNF213 p.R4810K [8]. Thus, information from RNF213 p.R4810K genotyping suggests the possibility of rapid progression of steno-occlusive changes in the affected and unaffected arteries [7, 8]. Present diagnostic criteria using only radiological findings (DSA or MRI/MRA) cannot resolve this problem, and thus, the criteria should be updated with genetic information in the future.
Angiographic beading in the cervical portion of the diseased ICA was uniquely found in this case. This feature is by far the most common angiographic finding in type 1 FMD patients [12]. FMD is a type of arteriopathy that is not atheromatous or inflammatory and has an unknown etiology. Previous reports described that FMD can be diagnosed histopathologically using a biopsy specimen of the distal STA [5, 6]. Thickened medial fibroplasias displacing muscle cells, disruption of the internal elastic lamina, and smooth muscle are observed in FMD specimens [5, 6]. Our present STA specimen showed no characteristics of FMD, and furthermore, this case showed no abnormalities in the other lesion except for a diseased ICA. Thus, our patient was not diagnosed with FMD. On the other hand, Je et al. reported that the arterial beading found in unilateral intracranial arteriopathy indicates reversible disease [18]. They suggested that beading may be associated with arterial wall inflammation and that this condition would have reversible vasoconstriction, based on prior reports [3, 14].
Alisha et al. experienced a case series of ten patients with FCA in a single center in the USA over 9 years. The initial angiographic appearances of these ten cases were similar, including nonspecific steno-occlusion in the anterior circulation that was characterized as FCA. The final diagnoses of six patients (60%) were changed during the follow-up period due to the high, 40%, recurrence rate of stroke [16]. Thus, the absence of a standardized diagnostic regimen and treatment protocol for FCA can lead to a worse prognosis [4, 17]. High-resolution vessel wall imaging on MRI will clarify the vessel wall pathologies and improve diagnostic accuracy for these patients [15]. However, RNF213 genotyping might be contributory to predict an early-onset and severe form of MMD [10]; careful attention should be required even in case of RNF213 wild type at the thought of a FCA case. Hence, a serial radiological follow-up is obviously warranted to catch a treatment opportunity, and a suitable treatment protocol should be established in responding to each pathophysiology of pediatric stroke including FCA.
References
Amlie-Lefond C, Bernard TJ, Sebire G, Friedman NR, Heyer GL, Lerner NB, DeVeber G, Fullerton HJ (2009) Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation 119:1417–1423
Bernard TJ, Manco-Johnson MJ, Lo W, MacKay MT, Ganesan V, DeVeber G, Goldenberg NA, Armstrong-Wells J, Dowling MM, Roach ES, Tripputi M, Fullerton HJ, Furie KL, Benseler SM, Jordan LC, Kirton A, Ichord R (2012) Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke 43:371–377
Braun KP, Bulder MM, Chabrier S, Kirkham FJ, Uiterwaal CS, Tardieu M, Sebire G (2009) The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain 132:544–557
Fullerton HJ, Wintermark M, Hills NK, Dowling MM, Tan M, Rafay MF, Elkind MS, Barkovich AJ, de Veber GA (2016) Risk of recurrent arterial ischemic stroke in childhood: a prospective international study. Stroke 47:53–59
Kimura H, Hosoda K, Hara Y, Kohmura E (2008) A very unusual case of fibromuscular dysplasia with multiple aneurysms of the vertebral artery and posterior inferior cerebellar artery. J Neurosurg 109:1108–1112
Kirton A, Crone M, Benseler S, Mineyko A, Armstrong D, Wade A, Sebire G, Crous-Tsanaclis AM, de Veber G (2013) Fibromuscular dysplasia and childhood stroke. Brain 136:1846–1856
Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, Hashikata H, Matsuura N, Yamazaki S, Toyoda A, Kikuta K, Takagi Y, Harada KH, Fujiyama A, Herzig R, Krischek B, Zou L, Kim JE, Kitakaze M, Miyamoto S, Nagata K, Hashimoto N, Koizumi A (2011) Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One 6:e22542
Mineharu Y, Takagi Y, Takahashi JC, Hashikata H, Liu W, Hitomi T, Kobayashi H, Koizumi A, Miyamoto S (2013) Rapid progression of unilateral moyamoya disease in a patient with a family history and an RNF213 risk variant. Cerebrovasc Dis 36:155–157
Mineyko A, Kirton A (2013) Mechanisms of pediatric cerebral arteriopathy: an inflammatory debate. Pediatr Neurol 48:14–23
Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, Tsurusaki Y, Doi H, Sakai H, Saitsu H, Shimojima K, Yamamoto T, Higurashi M, Kawahara N, Kawauchi H, Nagasaka K, Okamoto N, Mori T, Koyano S, Kuroiwa Y, Taguri M, Morita S, Matsubara Y, Kure S, Matsumoto N (2012) Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology 78:803–810
Miyawaki S, Imai H, Shimizu M, Yagi S, Ono H, Mukasa A, Nakatomi H, Shimizu T, Saito N (2013) Genetic variant RNF213 c.14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke 44:2894–2897
Osborn AG, Anderson RE (1977) Angiographic spectrum of cervical and intracranial fibromuscular dysplasia. Stroke 8:617–626
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52:245–266
Strater R, Becker S, von Eckardstein A, Heinecke A, Gutsche S, Junker R, Kurnik K, Schobess R, Nowak-Gottl U (2002) Prospective assessment of risk factors for recurrent stroke during childhood—a 5-year follow-up study. Lancet 360:1540–1545
Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, Butany J, Wasserman BA, Johnstone DM, Silver FL, Mikulis DJ (2009) Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 72:627–634
Tolani AT, Yeom KW, Elbers J (2015) Focal cerebral arteriopathy: the face with many names. Pediatr Neurol 53:247–252
Wintermark M, Hills NK, de Veber GA, Barkovich AJ, Elkind MS, Sear K, Zhu G, Leiva-Salinas C, Hou Q, Dowling MM, Bernard TJ, Friedman NR, Ichord RN, Fullerton HJ (2014) Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the vascular effects of infection in pediatric stroke study. Stroke 45:3597–3605
Yeon JY, Shin HJ, Seol HJ, Kim JS, Hong SC (2014) Unilateral intracranial arteriopathy in pediatric stroke: course, outcome, and prediction of reversible arteriopathy. Stroke 45:1173–1176
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of financial or material support were gained for conducting this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from the participant included in this case report.
Rights and permissions
About this article
Cite this article
Araki, Y., Takagi, Y., Mineharu, Y. et al. Rapid contralateral progression of focal cerebral arteriopathy distinguished from RNF213-related moyamoya disease and fibromuscular dysplasia. Childs Nerv Syst 33, 1405–1409 (2017). https://doi.org/10.1007/s00381-017-3451-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3451-9