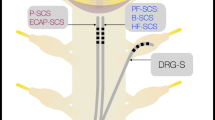
Abstract
Spinal cord stimulation (SCS) is a well-established treatment for chronic pain. Despite substantial research effort and significant technical advances, clinical success rates have remained stagnant for half a century. Only through increased understanding of the neural mechanisms that drive SCS efficacy will the field improve outcomes for the large patient population suffering from chronic pain. This work seeks to summarize current knowledge and research directions related to the biophysics and mechanisms behind modern SCS therapy.
Similar content being viewed by others
Abbreviations
- ACC:
-
Anterior cingulate cortex
- BDNF:
-
Brain-derived neurotrophic factor
- CFA:
-
Complete Freud’s adjuvant
- CRPS:
-
Complex regional pain syndrome
- CSF:
-
Cerebrospinal fluid
- DC:
-
Dorsal columns
- DCN:
-
Dorsal column nuclei
- DDH:
-
Deep dorsal horn
- DH:
-
Dorsal horn
- DR:
-
Dorsal roots
- DRG:
-
Dorsal root ganglion
- DRN:
-
Dorsal raphe nucleus
- ECAP:
-
Evoked compound action potential
- EEG:
-
Electroencephalogram
- EPSC:
-
Excitatory postsynaptic current
- FBSS:
-
Failed back surgery syndrome
- FEM:
-
Finite element method
- fMRI:
-
Functional magnetic resonance imaging
- GABA:
-
Gamma-aminobutyric acid
- IPSC:
-
Inhibitory postsynaptic current
- KHF:
-
Kilohertz frequency
- LC:
-
Locus coeruleus
- LPB:
-
Lateral parabrachial nucleus
- mIPSC:
-
Miniature inhibitory postsynaptic current
- MT:
-
Motor threshold
- NS:
-
Nociceptive specific
- PAG:
-
Periaqueductal gray matter
- PKCγ:
-
γ variant of protein kinase C
- RCT:
-
Randomized controlled trial
- RVLM:
-
Rostral ventrolateral medial nucleus
- SCS:
-
Spinal cord stimulation
- SDH:
-
Superficial dorsal horn
- VPL:
-
Ventral posterolateral nucleus of the thalamus
- WDR:
-
Wide dynamic range
References
Nahin, R.L.: Estimates of pain prevalence and severity in adults: United States, 2012. J. Pain. 16(8), 769–780 (2015)
Meyr, A.J., Saffran, B.: The pathophysiology of the chronic pain cycle. Clin. Podiatr. Med. Surg. 25(3), 327–346 (2008)
Elliott, A.M., Smith, B.H., Penny, K.I., Cairns Smith, W., Alastair Chambers, W.: The epidemiology of chronic pain in the community. Lancet. 354(9186), 1248–1252 (1999)
Gaskin, D.J., Richard, P.: The economic costs of pain in the United States. J. Pain. 13(8), 715–724 (2012)
Perl, E.R.: Ideas about pain, a historical view. Nat. Rev. Neurosci. 8, 71–80 (2007)
Rosenblum, A., Marsch, L.A., Joseph, H., Portenoy, R.K.: Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp. Clin. Pychopharmacol. 16(5), 405–416 (2008)
Vowles, K.E., McEntee, M.L., Julnes, P.S., Frohe, T., Ney, J.P., van der Goes, D.N.: Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 156(4), 569–576 (2015)
WONDER, C.: Overdose Rates Involving Opioids, by Type, United States, 2000–2017. Atlanta, GA, US Department of Health and Human Services, CDC/NCHS (2018)
Rosenblatt, R.A., Catlin, M.: Opioids for chronic pain: first do no harm. Ann. Fam. Med. 10(4), 300–301 (2012)
Zhang, T.C., Janik, J.J., Grill, W.M.: Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res. 1569, 19–31 (2014)
North, R.B., Kidd, D.H., Zahurak, M., James, C.S., Long, D.M.: Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery. 32(3), 384–395 (1993)
Linderoth, B., Foreman, R.D.: Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 20(6), 525–533 (2017)
Wall, P.D.: Presynaptic control of impulses at the first central synapse in the cutaneous pathway. Prog. Brain Res. 12, 92–118 (1964)
Melzack, R., Wall, P.D.: Pain mechanisms: a new theory. Science. 150(3699), 971–979 (1965)
Wall, P.D., Sweet, W.H.: Temporary abolition of pain in man. Science. 155(3758), 108–109 (1967)
Shealy, C.N., Taslitz, N., Mortimer, J.T., Becker, D.P.: Electrical inhibition of pain: experimental evaluation. Anesth. Analg. 46(3), 299–306 (1967)
Shealy, C.N., Mortimer, J.T., Reswick, J.B.: Electrical inhibition of pain by stimulation of the dorsal columns. Anesth. Analg. 46(4), 489–491 (1967)
Shealy, C.N., Mortimer, J.T., Hagfors, N.R.: Dorsal column electroanalgesia. J. Neurosurg. 32, 560–564 (1970)
Deer, T.R., Mali, J.M.: History of Neurostimulation Atlas of Implantable Therapies for Pain Management, 2nd edn, p. 4. Springer, New York (2016)
FDA: P130022, Nevro Senza Spinal Cord Stimulation (SCS) System. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130022A.pdf (2015)
FDA: P010032, Genesis Neurostimulation (IPG) System. https://www.accessdata.fda.gov/cdrh_docs/pdf/P010032A.pdf (2001)
FDA: P030017 PRECISION Spinal Cord Stimulation (SCS) System. https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030017A.pdf (2004)
Song, J.J., Popescu, A., Bell, R.L.: Present and Potential Use of Spinal Cord Stimulation to Control Chronic Pain. Pain Physician. 17, 235–246 (2014)
Deer, T., Slavin, K.V., Amirdelfan, K., North, R.B., Burton, A.W., Yearwood, T.L., Tavel, E., Staats, P., Falowski, S., Pope, J., Justiz, R., Fabi, A.Y., Taghva, A., Paicius, R., Houden, T., Wilson, D.: Success Using Neuromodulation With BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 21(1), 56–66 (2018)
Kapural, L., Yu, C., Doust, M.W., Gliner, B.E., Vallejo, R., Sitzman, T., Amirdelfan, K., Morgan, D.M., Brown, L.L., Yearwood, T.L., Bundshcu, R., Burton, A.W., Yang, T., Benyamin, R., Burgher, A.H.: Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 123(4), 851–860 (2015)
Al-Kaisy, A., Van Buyten, J.-P., Smet, I., Palmisani, S., Pang, D., Smith, T.: Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 15, 347–354 (2014)
North, J.M., Hong, K.J., Cho, P.Y.: Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia-based stimulation: results of a prospective randomized controlled trial. Neuromodulation. 19(7), 731–737 (2016)
Veizi, E., Hayek, S.M., North, J., Brent Chafin, T., Yearwood, T.L., Raso, L., Frey, R., Cairns, K., Berg, A., Brendel, J., Haider, N., McCarty, M., Vucetic, H., Sherman, A., Chen, L., Mekel-Bobrov, N.: Spinal Cord Stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA study. Pain Med. 18(8), 1534–1548 (2017)
Sdrulla, A.D., Guan, Y., Raja, S.N.: Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 18(8), 1048–1067 (2018)
Kumar, K., Taylor, R., Jacques, L., Eldabe, S., Meglio, M., Molet, J., Thomson, S., O’Callaghan, J., Eisenberg, E., Milbouw, G., Buchser, E., Fortini, G., Richardson, J., North, R.: Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 132, 179–188 (2007)
Thomson, S.J., Tavakkolizadeh, M., Love-Jones, S., Patel, N.K., Gu, J.W., Bains, A., Doan, Q., Moffitt, M.: Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial (in eng). Neuromodulation. 21(1), 67–76 (2018)
Taylor, R.S., Desai, M.J., Rigoard, P., Taylor, R.J.: Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract. 14(6), 489–505 (2014)
de Vos, C.C., Meier, K., Zaalberg, P.B., Nijhuis, H.J., Duyvendak, W., Vesper, J., Enggaard, T.P., Lenders, M.W.: Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 155(11), 2426–2431 (2014)
Crosby, N.D., Janik, J.J., Grill, W.M.: Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation (in eng). J. Neurophysiol. 117(1), 136–147 (2017)
Song, Z., Viisanen, H., Meyerson, B.A., Pertovaara, A., Linderoth, B.: Efficacy of kilohertz-frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation. 17(3), 226–235 (2014)
Todd, A.J.: Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp. Physiol. 87(2), 245–249 (2002)
Olson, W., Abdus-Saboor, I., Cui, L., Burdge, J., Raabe, T., Ma, M., Luo, W.: Sparse genetic tracing reveals regionally specific functional organization of mammalian nociceptors (in eng). elife. 6, e29507 (2017)
Duan, B., Cheng, L., Ma, Q.: Spinal circuits transmitting mechanical pain and itch. Neurosci. Bull. 34(1), 186–193 (2018)
Seal, R.P.: Illuminating the gap: neuronal cross-talk within sensory ganglia and persistent pain. Neuron. 91(5), 950–951 (2016)
Wang, H., Zylka, M.J.: Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J. Neurosci. 29(42), 13202–13209 (2009)
Uta, D., Furue, H., Pickering, A.E., Rashid, M.H., Mizuguchi-Takase, H., Katafuchi, T., Imoto, K., Yoshimura, M.: TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur. J. Neurosci. 31(11), 1960–1973 (2010)
Braz, J., Solorzano, C., Wang, X., Basbaum, A.I.: Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 82(3), 522–536 (2014)
Hee Jung, C., Basbaum, A.I.: Arborization of single axons of the spinal dorsolateral funiculus to the contralateral superficial dorsal horn. Brain Res. 477(1), 344–349 (1989)
Light, A.R.: The spinal terminations of single, physiologically characterized axons originating in the pontomedullary raphe of the cat (in eng). J. Comp. Neurol. 234(4), 536–548 (1985)
Jones, S.L., Light, A.R.: Termination patterns of serotoninergic medullary raphespinal fibers in the rat lumbar spinal cord: an anterograde immunohistochemical study. J. Comp. Neurol. 297(2), 267–282 (1990)
Deer, T.R., Pope, J.E.: Dorsal root ganglion stimulation approval by the Food and Drug Administration: advice on evolving the process. Expert. Rev. Neurother. 16(10), 1123–1125 (2016)
Krames, E.S.: The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 18, 24–32 (2015)
Niu, J., Ding, L., Li, J.J., Kim, H., Liu, J., Li, H., Moberly, A., Badea, T.C., Duncan, I.D., Son, Y.J., Scherer, S.S., Luo, W.: Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J. Neurosci. 33(45), 17691–17709 (2013)
Smith, K.J., Bennett, B.J.: Topographic and quantitative description of rat dorsal column fibres arising from the lumbar dorsal roots. J. Anat. 153, 203–215 (1987)
Mendell, L.M., Wall, P.D.: Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 206(4979), 97–99 (1965)
Todd, A.J.: Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 11(12), 823–836 (2010)
Simone, D.A., Sorkin, L.S., Oh, U., Chung, J.M., Owens, C., LaMotte, R.H., Willis, W.D.: Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J. Neurophysiol. 66(1), 228–246 (1991)
Grudt, T.J., Perl, E.R.: Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J. Physiol. 540(1), 189–207 (2002)
Todd, A.J.: Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol. Pain. 13, 1–19 (2017)
Yasaka, T., Tiong, S.Y., Hughes, D.I., Riddell, J.S., Todd, A.J.: Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 151(2), 475–488 (2010)
Cui, L., Miao, X., Liang, L., Abdus-Saboor, I., Olson, W., Fleming, M.S., Ma, M., Tao, Y.X., Luo, W.: Identification of early RET+ deep dorsal spinal cord interneurons in gating pain. Neuron. 91(5), 1137–1153 (2016)
Duan, B., Cheng, L., Bourane, S., Britz, O., Padilla, C., Garcia-Campmany, L., Krashes, M., Knowlton, W., Velasquez, T., Ren, X., Ross, S.E., Lowell, B.B., Wang, Y., Goulding, M., Ma, Q.: Identification of spinal circuits transmitting and gating mechanical pain. Cell. 159(6), 1417–1432 (2014)
Abraira, V.E., Kuehn, E.D., Chirila, A.M., Springel, M.W., Toliver, A.A., Zimmerman, A.L., Orefice, L.L., Boyle, K.A., Bai, L., Song, B.J., Bashista, K.A., O’Neill, T.G., Zhuo, J., Tsan, C., Hoynoski, J., Rutlin, M., Kus, L., Niederkofler, V., Watanabe, M., Dymecki, S.M., Nelson, S.B., Heintz, N., Hughes, D.I., Ginty, D.D.: The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 168, 1–16 (2017)
Kramer, P.R., Strand, J., Stinson, C., Bellinger, L.L., Kinchington, P.R., Yee, M.B., Umorin, M., Peng, Y.B.: Role for the ventral posterior medial/posterior lateral thalamus and anterior cingulate cortex in affective/motivation pain induced by varicella zoster virus (in eng). Front. Integr. Neurosci. 11, 27–27 (2017)
Polgár, E., Wright, L.L., Todd, A.J.: A quantitative study of brainstem projections from lamina I neurons in the cervical and lumbar enlargement of the rat (in eng). Brain Res. 1308(5), 58–67 (2010)
Bushnell, M.C., Ceko, M., Low, L.A.: Cognitive and emotional control of pain and its disruption in chronic pain (in eng). Nat. Rev. Neurosci. 14(7), 502–511 (2013)
Hudson, P.M., Lumb, B.M.: Neurones in the midbrain periaqueductal grey send collateral projections to nucleus raphe magnus and the rostral ventrolateral medulla in the rat. Brain Res. 733(1), 138–141 (1996)
Atkinson, L., Sundaraj, S.R., Brooker, C., O’Callaghan, J., Teddy, P., Salmon, J., Semple, T., Majedi, P.M.: Recommendations for patient selection in spinal cord stimulation. J. Clin. Neurosci. 18(10), 1295–1302 (2011)
Moffitt, M.A., Lee, D.C., Bradley, K.: Spinal cord stimulation: engineering approaches to clinical and physiological challenges. In: Implantable Neural Prostheses 1, pp. 155–194. Springer, New York (2009)
De Carolis, G., Paroli, M., Tollapi, L., Doust, M.W., Burgher, A.H., Yu, C., Yang, T., Morgan, D.M., Amirdelfan, K., Kapural, L., Sitzman, B.T., Bundschu, R., Vallejo, R., Benyamin, R.M., Yearwood, T.L., Gliner, B.E., Powell, A.A., Bradley, K.: Paresthesia-independence: an assessment of technical factors related to 10 kHz paresthesia-free spinal cord stimulation (in eng). Pain Physician. 20(4), 331–341 (2017)
Abstracts from the 10th World Congress of the International Neuromodulation Society: spine. Neuromodulation. 14(5), 444–484, 2011
Haider, N., Ligham, D., Quave, B., Harum, K.E., Garcia, E.A., Gilmore, C.A., Miller, N., Moore, G.A., Bains, A., Lechleiter, K., Jain, R.: Spinal cord stimulation (SCS) trial outcomes after conversion to a multiple waveform SCS system (in eng). Neuromodulation. 21(5), 504–507 (2018)
Hegarty, D.: Spinal cord stimulation: the clinical application of new technology. Anesthesiol. Res. Pract. 2012, 5 (2012). Art. no. 375691
Miller, J.P., Eldabe, S., Buchser, E., Johanek, L.M., Guan, Y., Linderoth, B.: Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 19(4), 373–384 (2016)
Zhang, T.C., Janik, J.J., Grill, W.M.: Modeling effects of spinal cord stimulation on wide-dynamic range dorsal horn neurons: influence of stimulation frequency and GABAergic inhibition (in eng). J. Neurophysiol. 112(3), 552–567 (2014)
Zhang, T.C., Janik, J.J., Peters, R.V., Chen, G., Ji, R.R., Grill, W.M.: Spinal sensory projection neuron responses to spinal cord stimulation are mediated by circuits beyond gate control. J. Neurophysiol. 114(1), 284–300 (2015)
Wille, F., Breel, J.S., Bakker, E.W., Hollmann, M.W.: Altering conventional to high density spinal cord stimulation: an energy dose-response relationship in neuropathic pain therapy (in eng). Neuromodulation. 20(1), 71–80 (2017)
Cassar, I.R., Titus, N.D., Grill, W.M.: An improved genetic algorithm for designing optimal temporal patterns of neural stimulation (in eng). J. Neural Eng. 14(6), 066013 (2017)
Babu, R., Hazzard, M.A., Huang, K.T., Ugiliweneza, B., Patil, C.G., Boakye, M., Lad, S.P.: Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: a comparative analysis of complications, reoperation rates, and health-care costs. Neuromodulation. 16(5), 418–426 (2013)
Howell, B., Lad, S.P., Grill, W.M.: Evaluation of intradural stimulation efficiency and selectivity in a computational model of spinal cord stimulation. PLoS One. 9(12), 1–25 (2014)
Holsheimer, J., Buitenweg, J.R.: Review: bioelectrical mechanisms in spinal cord stimulation. Neuromodulation. 18(3), 161–170 (2015)
Villavicencio, A.T., Leveque, J.C., Rubin, L., Bulsara, K., Gorecki, J.P.: Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 46(2), 399–406 (2000)
North, R.B., Kidd, D.H., Petrucci, L., Dorsi, M.J.: Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: part II–clinical outcomes. Neurosurgery. 57(5), 990–996 (2005)
Eldabe, S., Buchser, E., Duarte, R.V.: Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 17(2), 325–336 (2016)
Gazelka, H.M., Freeman, E.D., Hooten, W.M., Eldrige, J.S., Hoelzer, B.C., Mauck, W.D., Moeschler, S.M., Pingree, M.J., Rho, R.H., Lamer, T.J.: Incidence of clinically significant percutaneous spinal cord stimulator lead migration. Neuromodulation. 18(2), 123–125 (2015)
Moffitt, M., Peterson, D.K.L.: System and method for maintaining a distribution of currents in an electrode array using independent voltage sources. US 20100023070A1 (2011)
Barolat, G., Zeme, S., Ketcik, B.: Multifactorial analysis of epidural spinal cord stimulation. Stereotact. Funct. Neurosurg. 56(2), 77–103 (1991)
Takazawa, T., Choudhury, P., Tong, C.K., Conway, C.M., Scherrer, G., Flood, P.D., Mukai, J., MacDermott, A.B.: Inhibition mediated by glycinergic and gabaergic receptors on excitatory neurons in mouse superficial dorsal horn is location-specific but modified by inflammation. J. Neurosci. 37(9), 2336–2348 (2017)
Deer, T.R., Krames, E., Mekhail, N., Pope, J., Leong, M., Stanton-Hicks, M., Golovac, S., Kapural, L., Alo, K., Anderson, J., Foreman, R.D., Caraway, D., Narouze, S., Linderoth, B., Buvanendran, A., Feler, C., Poree, L., Lynch, P., McJunkin, T., Swing, T., Staats, P., Liem, L., Williams, K.: The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation. 17(6), 599–615 (2014)
Ross, E., Abejon, D.: Improving patient experience with spinal cord stimulation: implications of position-related changes in neurostimulation (in eng). Neuromodulation. 17(Suppl 1), 36–41 (2014)
Kallewaard, J.W., Koy, J., Rigoard, P., Abejon, D., Gatzinsky, K.: Adaptive spinal cord stimulation: the results from testing RestoreSensor usability and satisfaction (TRUST) survey. Presented at the The 10th World Congress of the International Neuromodulation Society, London, 2011
Russo, M., Brooker, C., Cousins, M.J., Taylor, N., Boesel, T., Sullivan, R., Holford, L., Hanson, E., Gmel, G.E., Shariati, N.H., Poree, L., Parker, J.: Sustained long-term outcomes with closed-loop spinal cord stimulation: 12-month results of the prospective, multicenter, open-label avalon study. Neurosurgery. 87, E485–E495 (2020)
Mekhail, N.: Closed-loop neurostimulation: is it needed and does it improve outcomes? In: NANS Annual Meeting, Las Vegas, 2019
Mekhail, N., Levy, R.M., Deer, T.R., Kapural, L., Li, S., Amirdelfan, K., Hunter, C.W., Rosen, S.M., Costandi, S.J., Falowski, S.M., Burgher, A.H., Pope, J.E., Gilmore, C.A., Qureshi, F.A., Staats, P.S., Scowcroft, J., Carlson, J., Kim, C.K., Yang, M.I., Stauss, T., Poree, L., Brounstein, D., Gorman, R., Gmel, G.E., Hanson, E., Karantonis, D.M., Khurram, A., Kiefer, D., Leitner, A., Mugan, D., Obradovic, M., Parker, J., Single, P., Soliday, N.: Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 19(2), 123–134 (2020)
Jensen, M.P., Brownstone, R.M.: Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. Eur. J. Pain. 23(4), 652–659 (2019)
Struijk, J.J., Holsheimer, J., Boom, H.B.K.: Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans. Biomed. Eng. 40(7), 632–639 (1993)
Pelot, N.A., Thio, B.J., Grill, W.M.: Modeling current sources for neural stimulation in COMSOL. Front. Comput. Neurosci. 12, 40 (2018)
McIntyre, C.C., Grill, W.M.: Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 88, 1592–1604 (2002)
Chakraborty, D., Truong, D.Q., Bikson, M., Kaphzan, H.: Neuromodulation of axon terminals. Cereb. Cortex. 28(8), 2786–2794 (2018)
Gustafsson, B., Jankowska, E.: Direct and indirect activation of nerve cells by electrical pulses appplied extracellularly. J. Physiol. 258, 33–61 (1976)
Amir, R., Michaelis, M., Devor, M.: Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J. Neurosci. 19(19), 8589–8596 (1999)
Koopmeiners, A.S., Mueller, S., Kramer, J., Hogan, Q.H.: Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation. 16, 304–311 (2013)
Deer, T., Masone, R.J.: Selection of spinal cord stimulation candidates for the treatment of chronic pain. Pain Med. 9(S1), S82–S92 (2008)
Deer, T.R., Jain, S., Hunter, C., Chakravarthy, K.: Neurostimulation for intractable chronic pain. Brain Sci. 9(2) (2019)
Pope, J.E., Deer, T.R., Falowski, S., Provenzano, D., Hanes, M., Hayek, S.M., Amrani, J., Carlson, J., Skaribas, I., Parchuri, K., McRoberts, W.P., Bolash, R., Haider, N., Hamza, M., Amirdelfan, K., Graham, S., Hunter, C., Lee, E., Li, S., Yang, M., Campos, L., Costandi, S., Levy, R., Mekhail, N.: Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 20(6), 543–552 (2017)
Wall, P.D., Gutnick, M.: Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp. Neurol. 43(3), 580–593 (1974)
Sdrulla, A.D., Xu, Q., He, S.Q., Tiwari, V., Yang, F., Zhang, C., Shu, B., Shechter, R., Raja, S.N., Wang, Y., Dong, X., Guan, Y.: Electrical stimulation of low-threshold afferent fibers induces a prolonged synaptic depression in lamina II dorsal horn neurons to high-threshold afferent inputs in mice. Pain. 156(6), 1008–1017 (2015)
Weiner, R., Reed, K.L.: Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 2(3), 217–221 (1999)
Rattay, F., Aberham, M.: Modeling axon membranes for functional electrical stimulation. IEEE Trans. Biomed. Eng. 40(12), 1201–1209 (1993)
Rattay, F.: The basic mechanism for the electrical stimulation of the nervous system. Neuroscience. 89(2), 335–346 (1999)
Aberra, A., Wang, B., Grill, W.M., Peterchev, A.V.: Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. bioRxiv. (2018)
Wang, B., Aberra, A.S., Grill, W.M., Peterchev, A.V.: Modified cable equation incorporating transverse polarization of neuronal membranes for accurate coupling of electric fields. J. Neural Eng. 15(2), 026003 (2018)
Clark, B., Häusser, M.: Neural coding: hybrid analog and digital signalling in axons. Curr. Biol. 16(15), R585–R588 (2006)
Dubner, R., Kenshalo Jr., D.R., Maixner, W., Oliveras, J.-L.: The correlation of monkey medullary dorsal horn neuronal activity and the perceived intensity of noxious heat stimuli. J. Neurophysiol. 62(2), 450–456 (1989)
O’Neill, J., Sikandar, S., McMahon, S.B., Dickenson, A.H.: Human psychophysics and rodent spinal neurones exhibit peripheral and central mechanisms of inflammatory pain in the UVB and UVB heat rekindling models. J. Physiol. 593(17), 4029–4042 (2015)
Guan, Y.: Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr. Pain Headache Rep. 16(3), 217–225 (2012)
Linderoth, B., Foreman, R.D., Meyerson, B.A.: Mechanisms of action of spinal cord stimulation. In: Textbook of Stereotactic and Functional Neurosurgery, pp. 2331–2348. McGrawHill, New York (2009)
Beck, S., Hallett, M.: Surround inhibition in the motor system. Exp. Brain Res. 210(2), 165–172 (2011)
Blakemore, C., Carpenter, R.H.S., Georgeson, M.A.: Lateral inhibition between orientation detectors in the human visual system. Nature. 228, 37–39 (1970)
Hillman, P., Wall, P.D.: Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp. Brain Res. 9(4), 284–306 (1969)
Kato, G., Kosugi, M., Mizuno, M., Strassman, A.M.: Separate inhibitory and excitatory components underlying receptive field organization in superficial medullary dorsal horn neurons. J. Neurosci. 31(47), 17300–17305 (2011)
Luz, L.L., Szucs, P., Pinho, R., Safronov, B.V.: Monosynaptic excitatory inputs to spinal lamina I anterolateral-tract-projecting neurons from neighbouring lamina I neurons (in eng). J. Physiol. 588(Pt 22), 4489–4505 (2010)
Menétrey, D., Giesler, G.J., Besson, J.M.: An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp. Brain Res. 27, 15–33 (1977)
Kato, G., Kawasaki, Y., Koga, K., Uta, D., Kosugi, M., Yasaka, T., Yoshimura, M., Ji, R.R., Strassman, A.M.: Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J. Neurosci. 29(16), 5088–5099 (2009)
Hylden, J.L.K., Nahim, R.L., Traub, R.J., Dubner, R.: Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 37, 229–243 (1989)
Bains, A., McDonald, M., Lin, S.: Longitudinal study of pain area changes and paresthesia coverage in neuropathic pain patients. In: 2018 World Congress on Regional Anesthsia & Pain Medicine, New York, 2015
Ruscheweyh, R., Sandkühler, J.: Epileptiform activity in rat spinal dorsal horn in vitro has common features with neuropathic pain. Pain. 105(1), 327–338 (2003)
Schoffnegger, D., Ruscheweyh, R., Sandkuhler, J.: Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain. 135(3), 300–310 (2008)
Sandkuhler, J.: The role of inhibition in the generation and amplification of pain. In: Castro-Lopes, J. (ed.) Current Topics in Pain: 12th World Congress on Pain, 1st edn. IASP Press, Seattle (2009)
Lu, Y., Dong, H., Gao, Y., Gong, Y., Ren, Y., Gu, N., Zhou, S., Xia, N., Sun, Y.Y., Ji, R.R., Xiong, L.: A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest. 123(9), 4050–4062 (2013)
Miraucourt, L.S., Dallel, R., Voisin, D.L.: Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS One. 2(11), e1116 (2007)
Petitjean, H., Pawlowski, S.A., Fraine, S.L., Sharif, B., Hamad, D., Fatima, T., Berg, J., Brown, C.M., Jan, L.Y., Ribeiro-da-Silva, A., Braz, J.M., Basbaum, A.I., Sharif-Naeini, R.: Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep. 13(6), 1246–1257 (2015)
Malmberg, A.B., Chen, C., Susumu, T., Basbaum, A.I.: Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 278, 279–283 (1997)
Guo, D., Hu, J.: Spinal presynaptic inhibition in pain control. Neuroscience. 283, 95–106 (2014)
Takkala, P., Zhu, Y., Prescott, S.A.: Combined changes in chloride regulation and neuronal excitability enable primary afferent depolarization to elicit spiking without compromising its inhibitory effects. PLoS Comput. Biol. 12(11), e1005215 (2016)
Leitner, J., Westerholz, S., Heinke, B., Forsthuber, L., Wunderbaldinger, G., Jager, T., Gruber-Schoffnegger, D., Braun, K., Sandkuhler, J.: Impaired excitatory drive to spinal GABAergic neurons of neuropathic mice. PLoS One. 8(8), e73370 (2013)
Moore, K.A., Kohno, T., Karchewski, L.A., Scholz, J., Baba, H., Woolf, C.J.: Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 22(15), 6724–6731 (2002)
Coull, J.A.M., Boudreau, D., Bachand, K., Prescott, S.A., Nault, F., Sík, A., De Koninck, P., De Koninck, Y.: Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 424, 938–942 (2003)
Imlach, W.L., Bhola, R.F., Mohammadi, S.A., Christie, M.J.: Glycinergic dysfunction in a subpopulation of dorsal horn interneurons in a rat model of neuropathic pain. Sci. Rep. 6, 37104 (2016)
Lavertu, G., Côté, S.L., De Koninck, Y.: Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain. 137(3), 724–738 (2014)
Ratte, S., Prescott, S.A.: Afferent hyperexcitability in neuropathic pain and the inconvenient truth about its degeneracy. Curr. Opin. Neurobiol. 36, 31–37 (2016)
Holsheimer, J., Barolat, G., Struijk, J.J., He, J.: Significance of the spinal cord position in spinal cord stimulation. In: Advances in Stereotactic and Functional Neurosurgery 11, pp. 119–124. Springer Vienna, Vienna (1995)
Holsheimer, J., Wesselink, W.A.: Optimum electrode geometry for spinal cord stimulation: the narrow bipole and tripole. Med. Biol. Eng. Comput. 35(5), 493–497 (1997)
Holsheimer, J.: Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation. 5(1), 25–31 (2002)
Oakley, J.C., Espinosa, F., Bothe, H., McKean, J., Allen, P., Burchiel, K.J., Quartey, G., Spincemaille, G., Nuttin, B., Gielen, F., King, G., Holsheimer, J.: Transverse tripolar spinal cord stimulatino: results of an international multicenter study. Neuromodulation. 9(3), 192–203 (2006)
Slavin, K.F., Burchiel, K.J., Anderson, V.C., Cooke, B.: Efficacy of transverse tripolar stimulation for relief of chronic low back pain. Stereotact. Funct. Neurosurg. 73(1-4), 126–130 (1999)
Radwani, H., Lopez-Gonzalez, M.J., Cattaert, D., Roca-Lapirot, O., Dobremez, E., Bouali-Benazzouz, R., Eiriksdottir, E., Langel, U., Favereaux, A., Errami, M., Landry, M., Fossat, P.: Cav1.2 and Cav1.3 L-type calcium channels independently control short- and long-term sensitization to pain. J. Physiol. 594(22), 6607–6626 (2016)
Li, J., Simone, D.A., Larson, A.A.: Windup leads to characteristics of central sensitization. Pain. 79(1), 75–82 (1999)
Prescott, S.A., De Koninck, Y.: Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization (in eng). J. Neurosci. 25(19), 4743–4754 (2005)
Prescott, S.A., Sejnowski, T.J., De Koninck, Y.: Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: towards a biophysical basis for neuropathic pain (in eng). Mol. Pain. 2, 32 (2006)
Crosby, N.D., Weisshaar, C.L., Smith, J.R., Zeeman, M.E., Goodman-Keiser, M.D., Winkelstein, B.A.: Burst and tonic spinal cord stimulation differentially activate GABAergic mechanisms to attenuate pain in a rat model of cervical radiculopathy (in eng). I.E.E.E. Trans. Biomed. Eng. 62(6), 1604–1613 (2015)
Cregg, R., Momin, A., Rugiero, F., Wood, J.N., Zhao, J.: Pain channelopathies (in eng). J. Physiol. 588(Pt 11), 1897–1904 (2010)
Balachandar, A., Prescott, S.A.: Origin of heterogeneous spiking patterns from continuously distributed ion channel densities: a computational study in spinal dorsal horn neurons. J. Physiol. 596(9), 1681–1697 (2018)
Ruscheweyh, R., Sandkühler, J.: Lamina-specific membrane and discharge properties of rat spinal dorsal horn neuronesin vitro. J. Physiol. 541(1), 231–244 (2002)
Melnick, I.V., Santos, S.F., Safronov, B.V.: Mechanism of spike frequency adaptation in substantia gelatinosa neurones of rat. J. Physiol. 559(2), 383–395 (2004)
Melnick, I.V., Santos, S.F., Szokol, K., Szucs, P., Safronov, B.V.: Ionic basis of tonic firing in spinal substantia gelatinosa neurons of rat. J. Neurophysiol. 91(2), 646–655 (2004)
Bellinger, S.C., Miyazawa, G., Steinmetz, P.N.: Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study (in eng). J. Neural Eng. 5(3), 263–274 (2008)
Aguiar, P., Sousa, M., Lima, D.: NMDA channels together with L-type calcium currents and calcium-activated nonspecific cationic currents are sufficient to generate windup in WDR neurons. J. Neurophysiol. 104(2), 1155–1166 (2010)
Parker, J.L., Karantonis, D.M., Single, P.S., Obradovic, M., Cousins, M.J.: Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 153(3), 593–601 (2012)
Parker, J.L., Karantonis, D.M., Single, P.S., Obradovic, M., Laird, J., Gorman, R.B., Ladd, L.A., Cousins, M.J.: Electrically evoked compound action potentials recorded from the sheep spinal cord. Neuromodulation. 16(4), 295–303 (2013)
Qin, C., Lehew, R.T., Khan, K.A., Wienecke, G.M., Foreman, R.D.: Spinal cord stimulation modulates intraspinal colorectal visceroreceptive transmission in rats. Neurosci. Res. 58(1), 58–66 (2007)
Cui, J.-G., O’Connot, W.T., Understedt, U., Linderoth, B., Meyerson, B.A.: Spinal cord stimulation attenuates augmented dorsal horn release of excitatoryamino acids in mononeuropathy via a GABAergic mechanism. Pain. 73, 87–95 (1997)
Ultenius, C., Song, Z., Lin, P., Meyerson, B.A., Linderoth, B.: Spinal GABAergic mechanisms in the effects of spinal cord stimulation in a rodent model of neuropathic pain: is GABA synthesis involved? Neuromodulation. 16(2), 114–120 (2013)
Lu, Y., Perl, E.R.: Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J. Physiol. 582(1), 127–136 (2007)
Barchini, J., Tchachaghian, S., Shamaa, F., Jabbur, S.J., Meyerson, B.A., Song, Z., Linderoth, B., Saade, N.E.: Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 215, 196–208 (2012)
Saade, N.E., Barchini, J., Tchachaghian, S., Chamaa, F., Jabbur, S.J., Song, Z., Meyerson, B.A., Linderoth, B.: The role of the dorsolateral funiculi in the pain relieving effect of spinal cord stimulation: a study in a rat model of neuropathic pain. Exp. Brain Res. 233(4), 1041–1052 (2015)
Yakhnitsa, V., Linderoth, B., Myerson, B.: Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 79, 223–233 (1999)
Schuh-Hofer, S., Fischer, J., Unterberg, A., Treede, R.-D., Ahmadi, R.: Spinal cord stimulation modulates descending pain inhibition and temporal summation of pricking pain in patients with neuropathic pain. Acta Neurochir. 160(12), 2509–2519 (2018)
Demartini, L., Terranova, G., Innamorato, M.A., Dario, A., Sofia, M., Angelini, C., Duse, G., Costantini, A., Leoni, M.L.G.: Comparison of tonic vs. burst spinal cord stimulation during trial period (in eng). Neuromodulation. 22(3), 327–332 (2019)
De Ridder, D., Vanneste, S., Plazier, M., van der Loo, E., Menovsky, T.: Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery. 66(5), 986–990 (2010)
De Ridder, D., Vanneste, S.: Burst and tonic spinal cord stimulation: different and common brain mechanisms (in eng). Neuromodulation. 19(1), 47–59 (2016)
Shechter, R., Yang, F., Xu, Q., Cheong, Y.-K., He, S.-Q., Sdrulla, A., Carteret, A.F., Wacnik, P.W., Dong, X., Meyer, R.A., Raja, S.N., Guan, Y.: Conventional and kilohertz-frequency spinal cord stimulation produces intensity- and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology. 119(2), 422–432 (2013)
Muhammad, S., Roeske, S., Chaudhry, S.R., Kinfe, T.M.: Burst or high-frequency (10 kHz) spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: one year comparative data (in eng). Neuromodulation. 20(7), 661–667 (2017)
Chakravarthy, K., Richter, H., Christo, P.J., Williams, K., Guan, Y.: Spinal cord stimulation for treating chronic pain: reviewing preclinical and clinical data on paresthesia-free high-frequency therapy. Neuromodulation. 21(1), 10–18 (2018)
Van Den Honert, C., Mortimer, J.T.: A technique for collision block of peripheral nerve: frequency dependence. IEEE Trans. Biomed. Eng. 28(5), 379–382 (1981)
Kilgore, K.L., Bhadra, N.: Nerve conduction block utilising high-frequency alternating current. Med. Biol. Eng. Comput. 42, 394–406 (2004)
Kilgore, K.L., Bhadra, N.: Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation. 17(3), 242–254 (2014)
Lempka, S.F., McIntyre, C.C., Kilgore, K.L., Machado, A.G.: Computational analysis of kilohertz frequency spinal cord stimulation for chronic pain management (in eng). Anesthesiology. 122(6), 1362–1376 (2015)
Arle, J.E., Mei, L., Carlson, K.W., Shils, J.L.: High-frequency stimulation of dorsal column axons: potential underlying mechanism of paresthesia-free neuropathic pain relief (in eng). Neuromodulation. 19(4), 385–397 (2016)
Pelot, N.A., Behrend, C.E., Grill, W.M.: On the parameters used in finite element modeling of compound peripheral nerves (in eng). J. Neural Eng. 16(1), 016007 (2019)
Sivanesan, E., Maher, D.P., Raja, S.N., Linderoth, B., Guan, Y.: Supraspinal mechanisms of spinal cord stimulation for modulation of pain: five decades of research and prospects for the future. Anesthesiology. 130(4), 651–665 (2019)
Puskar, Z., Polgár, E., Todd, A.J.: A population of large lamina I projection neurons with selective inhibitory input in rat spinal cord (in eng). Neuroscience. 102(1), 167–176 (2001)
Finch, P., Price, L., Drummond, P.: High-frequency (10 kHz) electrical stimulation of peripheral nerves for treating chronic pain: a double-blind trial of presence vs absence of stimulation (in eng). Neuromodulation. (2018)
Zannou, A.L., Khadka, N., Truong, D.Q., Zhang, T., Esteller, R., Hershey, B., Bikson, M.: Temperature increases by kilohertz frequency spinal cord stimulation (in eng). Brain Stimul. 12(1), 62–72 (2019)
Vallejo, R., Bradley, K., Kapural, L.: Spinal cord stimulation in chronic pain: mode of action (in eng). Spine (Phila Pa 1976). 42(Suppl 14), S53–S60 (2017)
North, R.B., Ewend, M.G., Lawton, M.T., Piantadosi, S.: Spinal cord stimulation for chronic, intractable pain: superiority of “multi-channel” devices. Pain. 44(2), 119–130 (1991)
Bocci, T., De Carolis, G., Paroli, M., Barloscio, D., Parenti, L., Tollapi, L., Valeriani, M., Sartucci, F.: Neurophysiological comparison among tonic, high frequency, and burst spinal cord stimulation: novel insights into spinal and brain mechanisms of action (in eng). Neuromodulation. 21(5), 480–488 (2018)
Tang, R., Martinez, M., Goodman-Keiser, M., Farber, J.P., Qin, C., Foreman, R.D.: Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model (in eng). Neuromodulation. 17(2), 143–151 (2014)
Kriek, N., Groeneweg, J.G., Stronks, D.L., de Ridder, D., Huygen, F.J.: Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: A multicentre, double-blind, randomized and placebo-controlled crossover trial (in eng). Eur. J. Pain. 21(3), 507–519 (2017)
Törk, I.: Anatomy of the serotonergic system (in eng). Ann. N. Y. Acad. Sci. 600, 9–34; discussion 34–35 (1990)
Aicher, S.A., Hermes, S.M., Whittier, K.L., Hegarty, D.M.: Descending projections from the rostral ventromedial medulla (RVM) to trigeminal and spinal dorsal horns are morphologically and neurochemically distinct (in eng). J. Chem. Neuroanat. 43(2), 103–111 (2012)
Bruinstroop, E., Cano, G., Vanderhorst, V.G.J.M., Cavalcante, J.C., Wirth, J., Sena-Esteves, M., Saper, C.B.: Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats (in eng). J. Comp. Neurol. 520(9), 1985–2001 (2012)
Millan, M.J.: Descending control of pain (in eng). Prog. Neurobiol. 66(6), 355–474 (2002)
Ridet, J.L., Rajaofetra, N., Teilhac, J.R., Geffard, M., Privat, A.: Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions (in eng). Neuroscience. 52(1), 143–157 (1993)
Song, Z., Ansah, O.B., Meyerson, B.A., Pertovaara, A., Linderoth, B.: The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain (in eng). Neuroscience. 247, 134–144 (2013)
Song, Z., Ansah, O.B., Meyerson, B.A., Pertovaara, A., Linderoth, B.: Exploration of supraspinal mechanisms in effects of spinal cord stimulation: role of the locus coeruleus (in eng). Neuroscience. 253, 426–434 (2013)
North, R.B., Ewend, M.G., Lawton, M.T., Kidd, D.H., Piantadosi, S.: Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 28(5), 692–699 (1991)
North, R.B., Kidd, D.H., Piantadosi, S.: Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir. 64, 106–108 (1995)
Manca, A., Kumar, K., Taylor, R.S., Jacques, L., Eldabe, S., Meglio, M., Molet, J., Thomson, S., O’Callaghan, J., Eisenberg, E., Milbouw, G., Buchser, E., Fortini, G., Richardson, J., Taylor, R.J., Goeree, R., Sculpher, M.J.: Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur. J. Pain. 12(8), 1047–1158 (2008)
Son, B.-C., Kim, D.-R., Lee, S.-W., Chough, C.-K.: Factors associated with the success of trial spinal cord stimulation in patients with chronic pain from failed back surgery syndrome (in eng). J. Korean Neurosurg. Soc. 54(6), 501–506 (2013)
Sun, L., Tai, L., Qiu, Q., Mitchell, R., Fleetwood-Walker, S., Joosten, E.A., Cheung, C.W.: Endocannabinoid activation of CB1 receptors contributes to long-lasting reversal of neuropathic pain by repetitive spinal cord stimulation. Eur. J. Pain. 21(5), 804–814 (2017)
Tilley, D.M., Vallejo, R., Kelley, C.A., Benyamin, R., Cedeno, D.L.: A continuous spinal cord stimulation model attenuates pain-related behavior in vivo following induction of a peripheral nerve injury. Neuromodulation. 18(3), 171–176 (2015)
Decosterd, I., Woolf, C.J.: Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 87, 149–158 (2000)
Tal, M., Eliav, E.: Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 64(3), 511–518 (1996)
Jensen, T.S., Finnerup, N.B.: Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 13(9), 924–935 (2014)
Foreman, R.D., Linderoth, B.: Chapter five – Neural mechanisms of spinal cord stimulation. In: Clement, H., Elena, M. (eds.) International Review of Neurobiology, vol. 107, pp. 87–119. Academic (2012)
Hansson, P.: Difficulties in stratifying neuropathic pain by mechanisms. Eur. J. Pain. 7(4), 353–357 (2003)
Bradman, M.J., Ferrini, F., Salio, C., Merighi, A.: Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes-Weinstein monofilaments: Towards a rational method. J. Neurosci. Methods. 255, 92–103 (2015)
Baba, H., Ji, R.R., Kohno, T., Moore, K.A., Ataka, T., Wakai, A., Okamoto, M., Woolf, C.J.: Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 24(3), 818–830 (2003)
Fan, R.J., Shyu, B.C., Hsiao, S.: Analysis of nocifensive behavior induced in rats by C02 laser pulse stimulation. Physiol. Behav. 57(6), 1131–1137 (1995)
Sufka, K.J.: Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 58(3), 355–366 (1994)
Martucci, K.T., Mackey, S.C.: Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology. 128(6), 1241–1254 (2018)
Ceko, M., Bushnell, M.C., Fitzcharles, M.A., Schweinhardt, P.: Fibromyalgia interacts with age to change the brain. Neuroimage Clin. 3, 249–260 (2013)
Zatorre, R.J., Fields, R.D., Johansen-Berg, H.: Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15(4), 528–536 (2012)
Bentley, L.D., Duarte, R.V., Furlong, P.L., Ashford, R.L., Raphael, J.H.: Brain activity modifications following spinal cord stimulation for chronic neuropathic pain: A systematic review. Eur. J. Pain. 20(4), 499–511 (2016)
Koyama, S., Xia, J., Leblanc, B.W., Gu, J.W., Saab, C.Y.: Sub-paresthesia spinal cord stimulation reverses thermal hyperalgesia and modulates low frequency EEG in a rat model of neuropathic pain. Sci. Rep. 8(1), 7181 (2018)
Deogaonkar, M., Sharma, M., Oluigbo, C., Nielson, D.M., Yang, X., Vera-Portocarrero, L., Molnar, G.F., Abduljalil, A., Sederberg, P.B., Knopp, M., Rezai, A.R.: Spinal Cord Stimulation (SCS) and Functional Magnetic Resonance Imaging (fMRI): modulation of cortical connectivity with therapeutic SCS. Neuromodulation. 19(2), 142–153 (2016)
Chahine, M., O’Leary, M.E.: Regulatory role of voltage-gated Na channel β subunits in sensory neurons (in eng). Front. Pharmacol. 2, 70–70 (2011)
Traub, R.D., Wong, R.K., Miles, R., Michelson, H.: A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J. Neurophysiol. 66(2), 635–650 (1991)
Risson, E.G., Serpa, A.P., Berger, J.J., Koerbel, R.F.H., Koerbel, A.: Spinal cord stimulation in the treatment of complex regional pain syndrome type 1: Is trial truly required? Clin. Neurol. Neurosurg. 171, 156–162 (2018)
Simopoulos, T., Sharma, S., Aner, M., Gill, J.S.: A temporary vs. permanent anchored percutaneous lead trial of spinal cord stimulation: a comparison of patient outcomes and adverse events. Neuromodulation. 21(5), 508–512 (2018)
Oakley, J.C., Krames, E.S., Stamatos, J., Foster, A.M.: Successful long-term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation. 11(1), 66–53 (2008)
Eldabe, S.: Trialing and SCS: does trialing improve outcomes. In: NANS Annual Meeting, Las Vegas, 2019
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Titus, N.D., Gilbert, J.E., Grill, W.M. (2021). Biophysics and Mechanisms of Spinal Cord Stimulation for Chronic Pain. In: Thakor, N.V. (eds) Handbook of Neuroengineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-2848-4_99-2
Download citation
DOI: https://doi.org/10.1007/978-981-15-2848-4_99-2
Received:
Accepted:
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2848-4
Online ISBN: 978-981-15-2848-4
eBook Packages: Springer Reference EngineeringReference Module Computer Science and Engineering