Abstract
Osteoarthritis (OA) is the most common rheumatic pathology accounting for much of worldwide disability. OA is related to aging and may affect the structure of any joint tissue. The early stage accurate diagnosis of OA and the ability to monitor the efficacy of putative disease-modifying drugs remain among the essential unmet medical needs for this disease. Despite the prevalence of OA, the diagnostic methods currently available are limited and lack sensitivity. Furthermore, there is currently no effective therapy capable of slowing or reversing the pathological changes that occur in the joint during the disease process. Therefore, the discovery and application of novel, noninvasive, specific biochemical markers remain a priority. This chapter will focus on the current OA protein markers and the value of proteomics for the discovery and validation of useful candidates for early diagnosis and drug discovery.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Key Facts About Osteoarthritis (OA)
-
OA is a slowly progressive disease that can affect the structure of all joint tissues.
-
OA is a major cause of pain and chronic disability in the elderly.
-
To date, OA lacks effective therapies. Current therapeutic strategies are limited to symptomatic relief, but are not able to slow or reverse joint alterations.
-
The diagnosis of OA relies on the description of symptoms, such as pain or stiffness, and radiography, which is not sensitive enough to detect small changes.
-
The complex pathogenesis and the heterogeneity of the clinical manifestations and progression of OA have hindered development of specific and sensitive tools for its management and therapy.
-
Novel specific and sensitive biomarkers are required to prevail over the medical needs of OA. Although several molecules have been assayed, to date, none is approved for use in clinical routines.
Key Facts About Proteomics
-
Proteomics is a science focusing on the large-scale analysis of proteins, their abundance, interactions, and modifications.
-
The set of proteins produced by a living organism is termed its proteome.
-
Proteomes differ between organisms, tissues, and cell types and change continuously with time, environmental conditions, pathologies, or drug treatment.
-
The use of proteomics is a valuable technology for the discovery of protein markers of disease.
-
Techniques employed in proteomics approaches include gel electrophoresis, liquid chromatography, mass spectrometry, protein microarrays, and bioinformatics.
Key Facts About Mass Spectrometry (MS)
-
MS is an analytical technique capable of characterizing a large variety of chemical species in pure and complex mixtures through measurement of their mass.
-
MS is the most commonly employed technology for protein characterization in proteomics.
-
In MS for proteomics, protein fragments are ionized and sorted to obtain their mass-to-charge (m/z) ratios.
-
The ionization modes most commonly employed in proteomics are electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI).
-
The mass analyzers most commonly employed in proteomics are time of flight (TOF), quadrupole, ion traps, and Orbitrap or Fourier transform (FT).
Definition of Words and Terms
- Articular cartilage:
-
Hyaline tissue found on joint surfaces, covering the articular end of the bones. It is a highly structured avascular and aneural tissue, composed of an extracellular matrix in which the cells (chondrotytes) are embedded.
- Biomarker:
-
Substance or feature used as an indicator of a biological state.
- Mass spectrometry:
-
Analytical technology capable of identifying and quantifying molecules (peptides, lipids, metabolites and other small molecules) in simple and complex mixtures by measuring the mass-to-charge ratio of their ions.
- Osteoarthritis:
-
A disorder involving movable joints characterized by cell stress and extracellular matrix degradation initiated by micro- and macro-injury that activates maladaptive repair responses including proinflammatory pathways of innate immunity.
- Proteomics:
-
An area of biology focused on the large-scale analysis of proteins (Proteome), their abundance, modifications, and interactions.
- Synovial fluid:
-
Viscous fluid found in the cavities of synovial joints, responsible of lubricating these joints and reducing friction between the cartilages that cover the articular ends of the bones.
- Synovium:
-
A specialized connective tissue localized at the surface of capsules of synovial joints. It is composed of two layers (intima and subintima).
Osteoarthritis
Osteoarthritis (OA), the most frequently diagnosed arthropathy, is a common, slowly progressive condition that may affect the structure of any joint and is a major cause of pain and chronic disability in the elderly. A definition for OA recently proposed by OARSI (Osteoarthitis Research Society International) is “a disorder involving movable joints characterized by cell stress and extracellular matrix degradation initiated by micro- and macro-injury that activates maladaptive repair responses including proinflammatory pathways of innate immunity” (Kraus et al. 2015). Prevalence studies show that OA usually develops from the age of 45 and increases with age. It affects more than 10% of the population, and most people over 65 exhibit OA pathology. OA is the leading cause of permanent work incapacity and one of the most common reasons for visiting a primary care physician.
OA is a very complex disease that has a multifactorial etiology. Some clearly identified risk factors include aging, obesity, genetic factors (Valdes et al. 2006), and mechanical injuries (Lohmander et al. 2004; Roos 2005). The complexity of processes underlying OA pathogenesis and the diversity of its clinical presentation hamper the development of tools sensitive and specific enough for precise diagnosis and monitoring. In most individuals, OA is characterized by an initial clinically silent phase, followed by radiographically detectable extensive deterioration of cartilage and structural joint changes. To date, the diagnosis of OA relies on the patient’s subjective description of pain or stiffness symptoms and on radiographic criteria, such as joint space width. Unfortunately, these diagnostic tools lack sufficient sensitivity for detection of small changes and do not provide accurate information about disease progression. The lack of accurate and sensitive monitoring methods is especially critical in the case of OA because the development of new drugs or therapeutic strategies is hindered. Existing therapies for OA are limited to pain alleviation and have no effect in slowing or halting disease progression. Therefore, there is great interest in the discovery and validation of novel OA disease markers, both to enable early diagnosis and monitoring of joint destruction and to facilitate the development of OA-modifying therapies.
The Challenge of Finding Biomarkers for OA
Biological markers are needed to understand and characterize disease types, status, progression, and response to therapy; they must possess proven validity, reproducibility, and predictive value. By the time that OA patients manifest symptoms of the disease, cartilage degradation and other joint alterations have progressed. Therefore, markers specific for pathological joint turnover that can be screened for in advance of symptom development would be most useful. This is a difficult mission because cartilage degradation is not consistent during OA disease evolution, being characterized by intermittent periods of progressive cartilage destruction and remission. Moreover, highly sensitive methods are required to identify biological markers because the release of specific proteins or the appearance of neoepitopes during periods of cartilage degradation is slow, and because of their dilution in biological fluids.
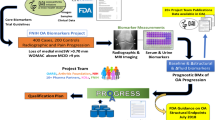
Strong candidate biomarkers for OA should be relevant to processes occurring in the joint and to clinical endpoints, including structural damage, pain, dysfunction, or joint replacement. These biomarkers would permit screening for early diagnosis, thus enabling the selection of procedures designed to slow disease progression (Ruiz-Romero et al. 2015) before there are clinical symptoms or imaging evidence of the development of the disease (Fig. 1). This early diagnosis would facilitate the discovery of new drugs and efficacy monitoring, thus providing information concerning their success in pharmacological trials. Although both images (x-rays or MRI) and biochemical molecules can be considered to be markers of OA, this chapter will focus on protein biomarkers and the utility that proteomic technologies have for generating novel biomarker candidates that may prove useful for early diagnosis, prognosis, and drug efficacy studies.
The need for molecular biomarkers to improve the diagnosis of OA. Molecular biomarkers need to facilitate disease diagnosis at early stages of the process, in which there are no clinical symptoms or imaging features (Adapted from Ruiz-Romero et al. 2015)
“Classical” Protein Biomarkers of OA
Optimal OA biomarker candidates are molecules or molecular fragments present in the cartilage, bone, or synovium of the joint. To date, several proteins directly or indirectly involved in cartilage degradation, or proteins synthesized in an attempt to repair cartilage , have been tested in clinical trials for their use as putative biomarkers of OA (Table 1). Many of these proteins shown in Table 1 are associated with the metabolism of type II collagen in cartilage or type I collagen in subchondral bone, or the metabolism of aggrecan in cartilage.
Because type II collagen (COL2) is relatively specific to and highly abundant in the articular cartilage extracellular matrix, several studies have examined this protein and its fragments (Olsen et al. 2007). One widely used biomarker for COL2 degradation is a fragment from the C-telopeptide region (CTX-II), which can be measured in serum or urine; urinary levels of this fragment are strongly associated with radiographic subtypes of OA (Valdes et al. 2014; van Spil et al. 2013). A sequence of the triple helix of COL2 and its nitrosylated form (Coll2-1 and Coll2-1NO2) has been recognized by two recently developed different assays and suggested for use as markers for response to viscosupplementation (McAlindon et al. 2014). COL2 degradation is also indicated by other molecules, including Helix-II, C2C, and urinary TIINE (type II collagen neoepitope). Finally, measuring COL2 synthesis has proven indicative of OA. Determination of COL2 propeptides, such as PIIANP showed increased levels in incipient knee OA compared to healthy controls, while patients in later stages of OA had lowered values (Rousseau et al. 2004). This finding suggests that there is a cartilage repair mechanism in the early development of OA that is not detectable in advanced stages.
Other noncollagenous proteins have been examined as possible markers for cartilage turnover which are as follows: cartilage oligomeric matrix protein (COMP) (Hoch et al. 2011); cartilage glycoprotein 39 (YKL-40) (Huang and Wu 2009); proteoglycans, such as keratan sulfate (KS) and chondroitin sulfate (CS); and enzymes involved in the breakdown and turnover of collagens, such as matrix metalloproteinases (MMPs), whose activity and inhibition is controlled by a variety of tissue inhibitors (TIMPs), proinflammatory cytokines, and growth factors (Raynauld et al. 2011).
Traditional OA biomarker studies have been impacted by problems created by the use of small sample sizes and case–control designs using cases recruited from secondary care facilities. In recent years, several studies have addressed these limitations by using larger cohorts. These studies have included the analysis of urinary CTX-II, serum COMP, serum MMP-degraded type II collagen (sC2M), and serum hyaluronic acid (sHA), among others (Aslam et al. 2014; Bos et al. 2013; Valdes et al. 2014; van Spil et al. 2013). These studies reported various associations between the altered presence of these molecules in urine or serum of patients and OA and suggested their possible value as predictors or to measure disease activity.
In spite of the promising results obtained with these proteins, no single biomarker has yet been introduced clinically. Because biochemical markers of joint tissue turnover would have important roles in clinical rheumatology, a classification of biomarker utility was proposed some years ago by the Osteoarthritis Biomarkers Network. This classification, designated as BIPED, focused on the description of the potential uses of a marker to qualify it for a specific clinical use, including burden of disease, investigative, prognostic, and efficacy of intervention and diagnosis (Bauer et al. 2006) (Table 2).
Because most studies performed for biomarker qualification in OA revealed a large overlap in single marker levels between OA patients and controls, they are insufficiently sensitive to be useful as diagnostic tools when used independently (Henrotin et al. 2007). These authors pointed out the utility of analyzing entire panels of available biomarkers as putative diagnostic tests. Accordingly, other investigators analyzing the association between individual biochemical markers and radiographic features established that this association was improved when selected biochemical markers were combined into a single factor (Davis et al. 2007). Overall, these findings suggest, as found with diagnostic biomarkers, that combinations of biomarkers may be more sensitive than individual measurements for reflecting structural damage in OA patients. Large-scale analyses, allowing for the simultaneous analysis of multiple molecules, are valuable tools for discovery and validation of biomarkers that would facilitate more OA biomarker candidates to achieve the qualification phase (Blanco 2014).
Value of Proteomics in OA Biomarker Research
As previously mentioned, the lack of a complete understanding of the complex etiology and pathogenesis of OA has hindered the identification of molecules that might serve as disease process markers, thus contributing to the difficulties of early diagnosis and evaluation of drug efficacy. In the search for novel biomarkers of OA, large-scale “omics” analyses have become vital research tools for biomarker discovery, and several approaches using this technology have been implemented (Ruiz-Romero and Blanco 2010). In contrast to nucleic acid-based expression studies, the recently emerged proteomic approaches have the advantage of studying actual functional molecules. Because possible disconnections between genes and protein expression levels are eliminated by proteomics approaches, this technology is a powerful method for the discovery of potential novel biomarkers.
Proteomic research utilizes the isolation of proteins from biological samples and their separation, identification, and quantification, usually by mass spectrometry (MS) (Fig. 2). This profound characterization of protein mixtures facilitates the understanding of complex biological systems and the determination of relationships among proteins, including function and protein-protein interactions. The nontargeted global approaches used in proteomics make it possible to monitor changes in abundance and structural modifications of proteins, as well as to establish putative associations of proteins with disease and treatments. During the last decade, several “shotgun” proteomics studies have been performed to increase knowledge of the pathogenesis of OA and to facilitate the search for novel protein biomarkers. Most studies on the pathogenesis of OA have used joint tissues and cells and their secreted fractions (Ruiz-Romero and Blanco 2009). On the other hand, analyses with the goal of discovering novel biomarkers have primarily made use of more accessible biological fluids and samples derived from them. A summary of the studies performed to date to search for OA biomarkers in such biological fluids as serum and synovial fluid is shown in Tables 3 and 4.
Workflow of proteomic approaches for biomarker discovery in osteoarthritis. Proteomic analyses involve three independent steps: obtaining a protein extract, separation of its proteins (carried out using either gel electrophoresis- or liquid chromatography-based techniques) and identification/quantification of the proteins, generally by mass spectrometry (Ruiz-Romero and Blanco 2010). 2-DE two-dimensional gel electrophoresis, LC liquid chromatography, MS mass spectrometry
There are two general approaches to finding proteomic biomarkers: target-specific and global/nondirected. The target-specific approach uses antibodies to screen for specific proteins through enzyme-linked immunosorbent assays (ELISAs), antibody arrays, or western blot analyses. Although these techniques generally can only survey a few proteins simultaneously, and therefore are not ideal for discovering biomarkers, recent advances in the field of MS, which include selected/multiple reaction monitoring assays and protein arrays, are more suitable for detecting biomarkers. Immunoaffinity LC-MS/MS for detection of serum amyloid A-derived peptides in rheumatic SFs (Yavin et al. 2000), collagen type II neoepitope peptides in urine (Nemirovskiy et al. 2007), and endogenous aggrecan fragments in both SF and urine (Dufield et al. 2010) have been developed as targeted approaches to finding proteomic biomarkers. Additionally, a targeted nucleic acid programmable 80-protein array (NAPPA) was recently established to more completely inventory the autoantibody profile OA (Henjes et al. 2014).
In contrast, relatively unbiased, high-throughput screens using global/nondirected approaches may be better suited than target-specific approaches for biomarker discovery. Nondirected approaches encompass those that profile unidentified proteins and those that profile identified proteins (Ruiz-Romero and Blanco 2010). Protein profiling of unidentified proteins is usually accomplished through matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF) mass spectrometry (MS), or surface-enhanced laser desorption/ionization (SELDI)-TOF-MS. These techniques have the advantage of speed, which makes them attractive tools for clinical screening. The detection of serum biomarkers associated with RA was the first peptide profiling strategy (de Seny et al. 2005, 2008). In a subsequent study specifically focused on OA, four differential MS peaks occurring among OA, RA, and control serum samples were identified by these authors (de Seny et al. 2011). Because of their high throughput, similar approaches using ion exchange chromatography magnetic beads to reduce sample complexity prior to MALDI-TOF analysis have been frequently used. This approach recently enabled the identification of potentially prognostic markers for knee OA (Takinami et al. 2013) (Table 3).
However, because peptide profiling approaches do not usually allow differential MS peak identification, validation of findings by other means is difficult and information on the biological significance of the findings is lacking. Therefore, many proteomic strategies based on protein fragmentation, identification by tandem MS, and subsequent data analysis have been developed for generating profiles of identified proteins. In OA research, quantitative proteomic profiling of sera from patients with different grades of OA and from healthy controls first removed the most abundant proteins (albumin, immunoglobulins and others) from the sample, thus enriching the levels of the less abundant proteins in the serum fraction. To identify a panel of proteins whose abundance was associated with OA, differential labeling was then performed, followed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) analysis (Fernandez-Puente et al. 2011).
Studying the synovial fluid (SF) proteome has proven highly advantageous for OA research. The progression of OA is a long process and inconsistent in development with the slow release of specific proteins or the appearance of neoepitopes. Therefore, the use of SF proteomic technologies is advantageous in the quest for biochemical markers of OA because of dilution in serum and other biological fluids (Table 4). SF is derived directly from the disease site, thus this compartment has great potential to contain OA biomarkers, which directly reflect joint cavity alterations in the composition of SF due to injury or disease and should better correlate with disease severity and progression (Hui et al. 2012). In SF samples from OA and RA patients, and in those from healthy subjects, LC-MS/MS tools have been used to identify several proteins (Gobezie et al. 2007; Kamphorst et al. 2007). A proteomic comparison of SF from OA and RA patients has identified two panels of SF proteins characteristic of each disease (Mateos et al. 2012). A more recently study identified at least 575 proteins in SF, 135 of which were differentially abundant in OA compared to RA (Balakrishnan et al. 2014). Putative SF biomarkers for psoriatic arthritis were documented using OA samples as the control group (Cretu et al. 2014). Finally, other techniques not based on LC-MS/MS have been performed on SF. Peaks associated with OA were identified in peptide profiling studies using SELDI (Han et al. 2012) and weak cation exchange magnetic beads with subsequent MALDI-MS profiling (Pan et al. 2012). Furthermore, two-dimensional electrophoresis (2-DE) strategies coupled to MS identification have also been employed to screen for differentially expressed proteins in SF. Using this approach, samples from early OA, late OA, and healthy controls were evaluated in a study using differential in-gel electrophoresis (DIGE) (Ritter et al. 2013).
Potential Applications of OA Biomarkers to Disease Prognosis and Other Diseases or Conditions
In view of the results obtained in clinical trials to date for targeted screening of proteins possibly associated with OA, C-terminal telopeptide of collagen type II (CTX-II) and serum cartilage oligomeric protein (COMP) appear to be the most promising biomarker candidates. Unfortunately, none of the candidates thus far analyzed has proven to be discriminative enough to: (i) differentiate between individual OA patients and controls (diagnostic) or between patients with different disease severities (burden of disease); (ii) predict prognosis in individuals with or without the disease (prognostic); or (iii) perform sufficiently consistently to function as a surrogate outcome in clinical trials (efficacy of intervention). In spite of the intense efforts over the last decade, to date, there is no sufficiently validated or qualified biochemical marker acceptable for systematic use in diagnostic or monitoring tests for OA (Lotz et al. 2013).
This conclusion emphasizes the need to analyze entire panels of available biomarkers as putative diagnostic tests. The limited multiplex capacity of classical ELISA-based strategies has increased costs and hampered the simultaneous evaluation of biomarker panels in large cohorts. Therefore, the capacity of proteomics technologies to perform multiplexed analysis of proteins is very advantageous in the study of the complex disease of OA, for which no single molecule is currently the gold standard. In an effort to facilitate the movement of candidates from the discovery phase into clinical application, after 2 years of basic research, proteomics technologies have matured sufficiently that their use in clinical practice appears practical and useful (Aebersold et al. 2013). Targeted proteomics strategies, either based on MS, such as selected/multiple reaction monitoring assays (Ritter et al. 2014), or antibodies, such as multiplex bead array assays (Henjes et al. 2014), are increasingly applicable for biomarker verification in OA and other pathological conditions. However, standardization and quality control of these procedures must be established to ensure that proteomics assays are validated for use as in vitro diagnostic tests to assure the analytical validity of the test procedure and outcome.
Summary Points
-
This chapter focuses on protein biomarkers for osteoarthritis (OA), the most prevalent rheumatic disease.
-
To date, no single protein has been qualified to be an OA biomarker in clinical applications.
-
Results obtained in clinical trials show that the power of combining marker candidates increases their association with disease.
-
Proteomics is a powerful technology for large-scale monitoring of changes in protein abundance or structure and for establishing their potential association with disease or treatments.
-
In the last decade, using shotgun proteomics technologies, a number of proteins have emerged as possible biomarkers for OA.
-
Further efforts are required to qualify biomarker candidates for use in the management of OA.
Abbreviations
- 2-DE:
-
Two-dimensional gel electrophoresis
- COL2:
-
Type II collagen
- COMP:
-
Cartilage oligomeric matrix protein
- CS:
-
Chondroitin sulfate
- CTX-II:
-
C-telopeptide region of COL2
- DIGE:
-
Differential in-gel electrophoresis
- HA:
-
Hyaluronic acid
- HC:
-
Healthy controls
- iTRAQ:
-
Isobaric tag for relative quantitation
- K/L:
-
Kellgren and Lawrence scale
- KS:
-
Keratan sulfate
- LC:
-
Liquid chromatography
- MALDI-TOF/TOF:
-
Matrix-assisted laser desorption/ionization-time of flight
- MB:
-
Magnetic beads
- MMP:
-
Matrix metalloproteinases
- MS:
-
Mass spectrometry
- NAPPA:
-
Nucleic acid programmable protein array
- OA:
-
Osteoarthritis
- OARSI:
-
Osteoarthritis Research Society International
- PAGE:
-
Polyacrylamide gel electrophoresis
- PsA:
-
Psoriatic arthritis
- RA:
-
Rheumatoid arthritis
- SELDI:
-
Surface-enhanced laser desorption/ionization
- SF:
-
Synovial fluid
- SRM:
-
Selected reaction monitoring
- TIMP:
-
Tissue inhibitor of matrix metalloproteinase
- UF:
-
Ultrafiltration
- YKL-40:
-
Cartilage glycoprotein 39
References
Aebersold R, Bader GD, Edwards AM, et al. The biology/disease-driven human proteome project (B/D-HPP): enabling protein research for the life sciences community. J Proteome Res. 2013;12(1):23–7.
Aslam I, Perjar I, Shi XA, et al. Associations between biomarkers of joint metabolism, hand osteoarthritis, and hand pain and function: the Johnston County Osteoarthritis Project. J Rheumatol. 2014;41(5):938–44.
Balakrishnan L, Bhattacharjee M, Ahmad S, et al. Differential proteomic analysis of synovial fluid from rheumatoid arthritis and osteoarthritis patients. Clin Proteomics. 2014;11(1):1.
Bauer DC, Hunter DJ, Abramson SB, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14(8):723–7.
Blanco FJ. Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthritis Cartilage. 2014;22(12):2025–32.
Bos SD, Beekman M, Maier AB, et al. Metabolic health in families enriched for longevity is associated with low prevalence of hand osteoarthritis and influences OA biomarker profiles. Ann Rheum Dis. 2013;72(10):1669–74.
Chaganti RK, Kelman A, Lui L, et al. Change in serum measurements of cartilage oligomeric matrix protein and association with the development and worsening of radiographic hip osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):566–71.
Cretu D, Prassas I, Saraon P, et al. Identification of psoriatic arthritis mediators in synovial fluid by quantitative mass spectrometry. Clin Proteomics. 2014;11(1):27.
Davis CR, Karl J, Granell R, et al. Can biochemical markers serve as surrogates for imaging in knee osteoarthritis? Arthritis Rheum. 2007;56(12):4038–47.
de Seny D, Fillet M, Meuwis MA, et al. Discovery of new rheumatoid arthritis biomarkers using the surface-enhanced laser desorption/ionization time-of-flight mass spectrometry ProteinChip approach. Arthritis Rheum. 2005;52(12):3801–12.
de Seny D, Fillet M, Ribbens C, et al. Monomeric calgranulins measured by SELDI-TOF mass spectrometry and calprotectin measured by ELISA as biomarkers in arthritis. Clin Chem. 2008;54:1066.
de Seny D, Sharif M, Fillet M, et al. Discovery and biochemical characterisation of four novel biomarkers for osteoarthritis. Ann Rheum Dis. 2011;70(6):1144–52.
Drynda S, Ringel B, Kekow M, et al. Proteome analysis reveals disease-associated marker proteins to differentiate RA patients from other inflammatory joint diseases with the potential to monitor anti-TNFalpha therapy. Pathol Res Pract. 2004;200(2):165–71.
Dufield DR, Nemirovskiy OV, Jennings MG, Tortorella MD, Malfait AM, Mathews WR. An immunoaffinity liquid chromatography-tandem mass spectrometry assay for detection of endogenous aggrecan fragments in biological fluids: use as a biomarker for aggrecanase activity and cartilage degradation. Anal Biochem. 2010;406(2):113–23.
Fernandes FA, Pucinelli ML, da Silva NP, Feldman D. Serum cartilage oligomeric matrix protein (COMP) levels in knee osteoarthritis in a Brazilian population: clinical and radiological correlation. Scand J Rheumatol. 2007;36(3):211–5.
Fernandez-Costa C, Calamia V, Fernandez-Puente P, Capelo-Martinez J-L, Ruiz-Romero C, Blanco FJ. Sequential depletion of human serum for the search of osteoarthritis biomarkers. Proteome Sci. 2012;10:55.
Fernandez-Puente P, Mateos J, Fernandez-Costa C, et al. Identification of a panel of novel serum osteoarthritis biomarkers. J Proteome Res. 2011;10(11):5095–101.
Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43(5):953–68.
Gobezie R, Kho A, Krastins B, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9(2):R36.
Han MY, Dai JJ, Zhang Y, et al. Identification of osteoarthritis biomarkers by proteomic analysis of synovial fluid. J Int Med Res. 2012;40(6):2243–50.
Henjes F, Lourido L, Ruiz-Romero C, et al. Analysis of autoantibody profiles in osteoarthritis using comprehensive protein array concepts. J Proteome Res. 2014;13(11):5218–29.
Henrotin Y, Addison S, Kraus V, Deberg M. Type II collagen markers in osteoarthritis: what do they indicate? Curr Opin Rheumatol. 2007;19(5):444–50.
Hoch JM, Mattacola CG, Medina McKeon JM, Howard JS, Lattermann C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;19(12):1396–404.
Huang K, Wu LD. YKL-40: a potential biomarker for osteoarthritis. J Int Med Res. 2009;37(1):18–24.
Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;4(1):15–37.
Kamphorst J, van der Heijden R, DeGroot J, et al. Profiling of endogenous peptides in human synovial fluid by NanoLC-MS: method validation and peptide identification. J Proteome Res. 2007;6(11):4388–96.
Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage. 2015;23(8):1233–41.
Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–52.
Lotz M, Martel-Pelletier J, Christiansen C, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72(11):1756–63.
Mateos J, Lourido L, Fernández-Puente P, et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDI TOF/TOF. J Proteomics. 2012;75(10):2869–78.
Mazieres B, Hucher M, Zaim M, Garnero P. Effect of chondroitin sulphate in symptomatic knee osteoarthritis: a multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2007;66(5):639–45.
McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88.
Nemirovskiy OV, Dufield DR, Sunyer T, Aggarwal P, Welsch DJ, Mathews WR. Discovery and development of a type II collagen neoepitope (TIINE) biomarker for matrix metalloproteinase activity: from in vitro to in vivo. Anal Biochem. 2007;361(1):93–101.
Olsen AK, Sondergaard BC, Byrjalsen I, et al. Anabolic and catabolic function of chondrocyte ex vivo is reflected by the metabolic processing of type II collagen. Osteoarthritis Cartilage. 2007;15(3):335–42.
Pan X, Huang L, Chen J, Dai Y, Chen X. Analysis of synovial fluid in knee joint of osteoarthritis: 5 proteome patterns of joint inflammation based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Int Orthop. 2012;36(1):57–64.
Raynauld JP, Martel-Pelletier J, Haraoui B, et al. Risk factors predictive of joint replacement in a 2-year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis. 2011;70(8):1382–8.
Reijman M, Hazes JM, Bierma-Zeinstra SM, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50(8):2471–8.
Ritter SY, Subbaiah R, Bebek G, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65(4):981–92.
Ritter SY, Collins J, Krastins B, et al. Mass spectrometry assays of plasma biomarkers to predict radiographic progression of knee osteoarthritis. Arthritis Res Ther. 2014;16(5):456.
Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17(2):195–200.
Rousseau JC, Zhu Y, Miossec P, et al. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2004;12(6):440–7.
Ruiz-Romero C, Blanco FJ. The role of proteomics in osteoarthritis pathogenesis research. Curr Drug Targets. 2009;10(6):543–56.
Ruiz-Romero C, Blanco FJ. Proteomics role in the search for improved diagnosis, prognosis and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):500–9.
Ruiz-Romero C, Fernandez-Puente P, Calamia V, Blanco FJ. Lessons from the proteomic study of osteoarthritis. Expert Rev Proteomics. 2015;12(4):433–43.
Sinz A, Bantscheff M, Mikkat S, et al. Mass spectrometric proteome analyses of synovial fluids and plasmas from patients suffering from rheumatoid arthritis and comparison to reactive arthritis or osteoarthritis. Electrophoresis. 2002;23(19):3445–56.
Takinami Y, Yoshimatsu S, Uchiumi T, et al. Identification of potential prognostic markers for knee osteoarthritis by serum proteomic analysis. Biomark Insights. 2013;8:85–95.
Uchida T, Fukawa A, Uchida M, Fujita K, Saito K. Application of a novel protein biochip technology for detection and identification of rheumatoid arthritis biomarkers in synovial fluid. J Proteome Res. 2002;1(6):495–9.
Valdes AM, Van Oene M, Hart DJ, et al. Reproducible genetic associations between candidate genes and clinical knee osteoarthritis in men and women. Arthritis Rheum. 2006;54(2):533–9.
Valdes AM, Meulenbelt I, Chassaing E, et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage. 2014;22(5):683–9.
van Spil WE, Drossaers-Bakker KW, Lafeber FP. Associations of CTX-II with biochemical markers of bone turnover raise questions on its tissue origin: data from CHECK, a cohort study of early osteoarthritis. Ann Rheum Dis. 2013;72(1):29–36.
Yavin EJ, Preciado-Patt L, Rosen O, et al. Serum amyloid A-derived peptides, present in human rheumatic synovial fluids, induce the secretion of interferon-gamma by human CD(4)(+) T-lymphocytes. FEBS Lett. 2000;472(2–3):259–62.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Ruiz-Romero, C., Fernández-Puente, P., Blanco, F.J. (2017). Biomarkers in Osteoarthritis: Value of Proteomics. In: Patel, V., Preedy, V. (eds) Biomarkers in Bone Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7693-7_44
Download citation
DOI: https://doi.org/10.1007/978-94-007-7693-7_44
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7692-0
Online ISBN: 978-94-007-7693-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences