Abstract
Recent evidences reported that bone is a metabolically active tissue that undergoes continuous remodeling that realizes through the activity of osteoclasts and osteoblasts. The family of bone-related proteins includes several active proteins, i.e., osteopontin (OPN), osteoprotegerin (OPG), osteonectin (OSN), osteocalcin (OCN), sclerostin, and RANKL/RANK system that regulate bone formation, matrix reposition, and remodeling. More evidences indicate that bone-related proteins are involved in extra bone mineralization, calcification at ectopic sites, and they might play a pivotal role in atherosclerosis, plaque formation, vascular remodeling and integrity, neovascularization, and malignancy. This review is dedicated to the discussion of controversial role of the bone-related proteins among patients with cardiovascular disease and a predictive value of bone-related proteins as biomarker at risk stratification.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Bone-related proteins
- Osteopontin
- Osteoprotegerine
- Osteonectin
- Osteocalcin
- Cardiovascular diseases
- Age-related diseases
- Metabolic comorbidities
Key Facts
See Tables 1, 2, and 3 and Figs. 1 and 2.
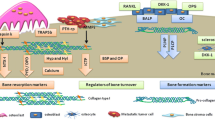
Key facts of initiation of vascular calcification. Figure shows that initiation and supporting of vascular calcification are tightly regulated pathological process, which involves wide-spectrum cells, including bone environmental cells and extracellular matrix, apoptotic bodies, antigen-presenting cells, etc. Abbreviations: OPN – osteopontine; OPG – osteoprotegerine; RANK - receptor activator of nuclear factor-kB; RANKL – RANK ligand; OSN – osteonectine; OCN – osteocalcin
- Biomarker:
-
Biomarker is defined as objectively measured indicator of several faces of biological or pathological processes, pharmacologic responses, therapeutic interventions that may have diagnostic and predictive values to determine these markers as potent surrogate endpoint indicators.
- Bone remodeling:
-
Bone remodeling in normal state is defined as dynamic well-balanced process associated with bone formation and bone resorbtion that is under control of multifactorial molecular mechanisms
- Bone-related proteins:
-
Bone-related proteins are secreted proteins produced by wide spectrum of cells (dendritic cells, macrophages, osteoblasts/osteoclasts, adipocytes, etc.) and realize their direct effect toward formation, modeling, remodeling of bone extracellular matrix.
- Osteoblast-like cells:
-
Osteoblast-like cells are defined as cell with osteogenic phenotype originated from smooth muscle cells, pericytes, or stem cells.
- Surrogate endpoint biomarker:
-
Surrogate endpoint biomarker is defined as indicator of clear clinical endpoints in target patient populations only.
- Vascular calcification:
-
Vascular calcification is a consequence of closely regulated pathological processes that culminate in organized extracellular matrix deposition of calcium and phosphate produced by osteoblast-like cells.
Introduction
Bone-related proteins are referred as family members of matricellular proteins that are the main components of the extracellular matrix which are highly expressed in the bone developing, vascular remodeling, and tissue regeneration (Alford and Hankenson 2006). Members of this protein class serve as biological mediators of cell function by interacting directly with cells or by modulating the activity of growth factors, proteases, and other extracellular matrix proteins (Hruska et al. 2005; Obert et al. 2009; Wright et al. 2009). Within past decade substantial progress has been made in our understanding of the molecular mechanisms by which these proteins regulate bone mineralization, vascular integrity, and remodeling (Johnsen and Beuschlein 2010). Bone-related proteins are multifunctional growth factors that are activated in response to a hypoxic bone microenvironment stimulates the transcription of multiple genes (David et al. 2009; Kassem and Marie 2011). They contribute bone development and remodeling, as well as extra bone tissue calcification, vascular integrity and remodeling, atherosclerosis and plaque formation, angiogenesis and neovascularization (Drager et al. 2015; Hauschka et al. 1989). Moreover, bone-related proteins are intricately prone regulation by hypoxia signaling system, hormones, electrolyte and mineral changes, inflammation, and they might involve in coupling angiogenesis and osteogenesis during bone development and repair (Drager et al. 2015). In this review controversial role of the bone-related proteins among patients with cardiovascular disease and a predictive value of bone-related proteins as biomarker of vascular remodeling with possible predictive value are discussed.
Biological Role of Bone-Related Proteins
It has been previously reported that the bone-related proteins include osteopontin (OPN), osteoprotegerin (OPG), osteonectin (OSN), osteocalcin (OCN), sclerostin, and RANKL/RANK system (Hofbauer et al. 2000). The most common biological function of bone-related proteins is the control of bone mineralization processes (Okamura et al. 2011). Although the innate pathophysiological mechanisms of bone remodeling balance are not fully defined, bone-related proteins are considered turnover factors directly regulating bone formation and resorption via mediating effects of co-regulators, such as inflammatory cytokines, homocysteine, oxidized lipids, sex steroids, vitamin D, vitamin K, and others (Yasuda et al. 1998). Therefore, they are involved in multiple level controls for extra-bone mineralization at ectopic sites, i.e., vascular wall, valvular leaflets, kidney, gall, tendons, and muscles. The bone-related proteins are expressed in wide spectrum of cells (antigen presenting cells, preosteoblasts/osteoblasts, osteocytes, chondrocytes, fibroblasts, endothelial cells, smooth muscle cells, epithelial cells) as well as skeletal muscles, mammary glands, and several organs (inner ear, brain, placenta, and kidney) (Zimmermann and Ritchie 2015).
All bone-related proteins realize their direct (regulation of biological mineralization) and indirect (tissue remodeling and regulation immunity) biological effects via surface-expressed receptors that are presented as CD44 and various types of integrins (avb1, avb3, avb5, avb6, a4b1, a5b1, a8b1, a9b1) (Lund et al. 2013). Recent investigations have been shown that bone-related proteins may play a pivotal role in atherosclerosis, cardiovascular diseases, chronic rheumatic diseases, multiple sclerosis, inflammation bowel diseases, autoimmune disorders, and cancer and malignancy (Wright et al. 2009).
Osteopontin
Osteopontin (OPN, secreted phosphoprotein 1 -SPP 1, 44 kDa bone phosphoprotein, sialoprotein 1, 2ar, uropontin, and early T-lymphocyte activation-1 [Eta-1]) is a secreted low-molecular (41–75 kDa) matricellular protein. OPN is defined as integrin-binding ligand (N-linked glycoprotein) that is involved in several physiological and pathological processes. OPN belongs to SPARC (secreted protein acidic and rich in cysteine) family and demonstrates prominent roles in cell proliferation, migration, differentiation, apoptosis, adhesion, angiogenesis, tissue repair, and regulation of extracellular matrix remodeling. There are evidences regarding the pivotal role of OPN in carcinogenesis and metastasis (Nagaraju et al. 2014).
OPN is encoding by a single copy gene but exists in various isoforms (OPNa, OPNb, and OPNc) as a result of alternative splicing, alternative translation, and different posttranslational modifications (Sarosiek et al. 2015). Despite functional role of OPN isoforms in systemic inflammation is essential to understanding, overexpression of OPNa, OPNb, and OPNc isoforms was not found in similar clinical settings (Coombes and Syn 2014). In fact, presence of OPNc isoform associates well with diabetes mellitus and obesity (Sarosiek et al. 2015). The role of OPNa, OPNb, and OPNc in vascular remodeling is under recognized. OPN interacts with several integrins via two domains: Arg159-Gly-Asp161 (RGD) sequence binding to α(v)-containing integrins, and Ser162-Val-Val-Tyr-Gly-Leu-Arg168 (SLAYGLR) sequence binding to α(4) β(1), α(4) β(7) and α(9) β(1) integrins (Ito et al. 2009). This interaction plays a pivotal role in regulating migration, survival, and accumulation of macrophage and other types of antigen presenting cells. Indeed, OPN may induce the transcription of interleukin (IL)-6 and reduced tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and IL-10 (Sarosiek et al. 2015). Therefore, after translocation into nucleus OPN may interact with p85α regulatory subunit of the signaling kinase PI(3)K and Bcl-6 that leads to protection of Bcl-6 from ubiquitin-dependent proteasome degradation and inducing apoptosis (Leavenworth et al. 2015). Overall, OPN is considered a mediator regulating the extracellular matrix modeling and interactions between cells through growth factor signaling pathway, cell adhesion, migration, and proliferation.
Osteoprotegerin
Osteoprotegerin (OPG) is a member of the tumor necrosis factor receptor superfamily and is a soluble secreted protein produced by osteoblasts, osteogenic stromal stem cells, and activated mononuclears (Simonet et al. 1997). OPG acts as a decoy receptor for RANKL and thus inhibits osteoclastogenesis. The main biological role of OPG is protection of the skeleton from excessive bone resorbtion and protection of tissue injury beyond bone (Liu and Zhang 2015). These effects realize by binding OPG with specific ligand named RANKL (receptor activator of nuclear factor-kB ligand) that leads to prevention of interacting OPG with RANK (Boyce and Xing 2007a). An imbalance in the RANKL/RANK/OPG axis, with decreased OPG and/or increased RANKL, is associated with diseases that favor bone loss, including osteoporosis (Warren et al. 2015). Therefore, recent studies showed that OPG has been identified as candidate mediators for paracrine signaling in cell metabolism and extracellular matrix regulation but have also been shown to modulate dendritic cells and activated T cells, as well as to promote B-cell maturation and antibody response, which suggests a role in both innate and adaptive immunity (Ueland et al. 2005; Yndestad et al. 2002).
Osteonectin
Osteonectin (OSN, BM-40) is secreted extracellular matrix glycoprotein that belongs to SPARC family (secreted protein acidic and rich in cysteine) and expressed in active remodeling in the skeleton and other tissues (Ribeiro et al. 2014). The main biological effect of ONC is considered a regulator of fibrosis and increased extracellular matrix deposition (Dhore et al. 2001). Overall, OSN supports osteoblastogenesis and is prone over expression on surface of osteoblasts in response of effect of proinflammatory cytokines, sex hormones, vitamin D3, and several growth factors. Therefore, OSN together with myostatin, insulin-like growth factor I, irisin, and osteocalcin may be associated with the interactions between muscle tissues and bone metabolism through the commitment of myoprogenitor cells to the osteoblast lineage (Kawao and Kaji 2015).
Osteocalcin
Osteocalcin (OCN, bone γ-carboxyglutamic acid-containing protein, bone Gla-protein) is a small (49 amino acid residues) osteoblast-specific non-collagenous protein that is specially synthesized and secreted by osteoblast and osteocyte (Baron and Kneissel 2013). Synthesis of OCN is under control of and is regulated by 1α,25-dihydroxy-Vitamin D3 (Wei and Karsenty 2015). OCN is secreted by bone osteoblasts in response to stimulation of osteoblastic differentiation and osteocytic maturation (Fukumoto and Martin 2009). The most of OCN is found in bone matrix and only a small amount is in circulation (Garnero et al. 1992). The main biological role of OCN is proosteoblastic effect or bone-building function (Shao et al. 2015). This effect is realized through the appropriate OCN receptor (GPRC6A) (Zhang et al. 2015). Therefore, OCN regulate glucose homeostasis, fertile function, the fat cells, and male gonad endocrine activity and be regulated by insulin and the neural system (Shao et al. 2015; Wei and Karsenty 2015). There are evidences that plasma OCN is inversely related to fat mass and plasma glucose (Kindblom et al. 2009) and that leptin may effect on OCN carboxylation through the hypothalamus (Reinehr and Roth 2010). Finally, OCN is considered an effector switched dysmetabolic responces and prevented vascular calcification (Chen and Yang 2015). Notwithstanding, OCN is a well-known regulator of body energy metabolism, it still remained unclear as to how OCN might modulate extra bone mineralization and vascular function.
Sclerostin
Sclerostin (SOST) is low molecular signal secreted cystine-knot protein that is widely expressed on the surface of osteocytes and plays essential roles in bone formation, modeling, remodeling, and homeostasis (Shao et al. 2015). SOST acts a negative regulator of bone growth through inhibiting the canonical Wnt signaling cascade by binding to and blocking the Wnt co-receptor LRP5/6. Thus, in contrast of OPG, which specifically inhibits osteoclastogenesis, SOST and Dickkopf-related protein 1 (DKK1) exerting their inhibitory effects on osteoblastogenesis (Morena et al. 2015). Therefore, SOST may play a key role in vascular and tissue calcification (Morena et al. 2015; Shao et al. 2015). Moreover, recent studies have been shown that SOST is linked to bone physiology and cardiovascular disease through the Wnt/β-catenin signaling pathway (Shao et al. 2015).
RANK/RANK Ligand System
The interaction between RANKL (receptor activator of nuclear factor-kB ligand) and its receptor RANK (receptor activator of nuclear factor-kB) is essential for the differentiation and bone resorbing capacity of the osteoclastes, as well as controlling mineralization process that is suitable for several physiological and pathological states. RANKL is a type II homotrimeric transmembrane protein that is expressed as a membrane-bound and a secreted protein, which is derived from the membrane form as a result of either proteolytic cleavage or alternative splicing (Boyce and Xing 2007b). Serum RANK/RANKL have been identified as candidate mediators for paracrine signaling in cell metabolism and extracellular matrix regulation but have also been shown to modulate dendritic cells and activated T cells, as well as to promote B-cell maturation and antibody response, which suggests a role in both innate and adaptive immunity (Anderson et al. 1997; Yndestad et al. 2002; Ueland et al. 2005). There are various mutated RANKL proteins that abolish binding to OPG while preserving recognition of RANK (Warren et al. 2015). Interestingly, the physiological RANKL/RANK interaction is not optimized for maximal signaling and function, perhaps reflecting the need to maintain receptor specificity within the tumor necrosis factor (TNF) superfamily. Therefore, integrin β3, V-ATPase, CAII, CTSK, TNF receptor-associated factor (TRAP), matrix metalloproteinase (MMP)-9, parathyroid hormone, and hormonally active form of vitamin D3, 1α,25-(OH)2D3, have been identified as essential regulators of RANK/RANKL system activity (Gu et al. 2015; Silva and Bilezikian 2015).
Basic Principles of Biological Control for Mineralization Processes
According to the contemporary insight, bone mineralization is a regulated process induced by complex molecular mechanisms (Kassem and Marie 2011). In vivo bone formation requires recruitment and replication of mesenchymal precursors of osteoblasts, differentiation into preosteoblasts, osteoblasts, and mature osteoblasts ultimately result in the accumulation and mineralization of the extracellular matrix (Raouf and Seth 2000). Thus, bone development is accomplished by a cascade of biological processes that may include differentiation of pluripotential tissue, angiogenesis, osteogenesis, mineralization, and remodeling (Rachmiel and Leiser 2014). The interactions between osteoblasts and osteoclasts that is considered as pivotal mechanism of bone formation closely affects other cells present within the bone microenvironment, i.e., stem cells, endothelial progenitor, and particularly vascular endothelial cells, fibroblasts, as well as extracellular matrix. The complex communications between all components of the network in bone undergo gene regulation. There are evidences regarding bone synthesis and turnover is under control of genes regulated numerous cytokines (transforming growth factor-β, bone morphogenetic proteins, insulin-like growth factor-1, and fibroblast growth factor-2) and bone-related extracellular and matrix proteins (osteonectin, osteopontin, and osteoprotegerin).
The main inductors of mineralization are inflammation oxidative stress, sex steroids, high level of phosphate, parathyroid hormone and its fragments, and system of specific regulators, i.e., osteocalcin, bone-morphogenic proteins, and vitamin D. Vitamin K-dependent Gla-rich protein (GRP) together with fetuin-A- matrix Gla protein are a main calcification inhibitory system that prevents of calcium-induced signaling pathways and directly inhibits mineral binding, crystal formation, and resident cell maturation in vascular wall (Viegas et al. 2015). This system is able to inhibit vascular wall calcification and osteochondrogenic differentiation through α-smooth muscle actin upregulation and OPN downregulation (Hruska et al. 2005). Indeed, extracellular vesicles released from normal vascular smooth muscle cells are loaded with GRP, matrix Gla protein, and fetuin-A. In pathology state, extracellular vesicles released from vascular smooth muscle cells appear to increase calcium loading and GRP and matrix Gla protein depletion (Viegas et al. 2015). The key facts regarding bone-related proteins’ effects are in the Table 1. Finally, bone mineralization is well balanced and regulated process supported by several mutual controlled molecular mechanisms. Bone-related proteins are considered an innate physiological switching involved in the positive and negative regulation of bone formation and they might mediate extra bone calcification in the pathology state.
Biology of Ectopic Vascular Calcification
On contrary to bone mineralization, vascular calcification is a pathological process, occurring in response to dysregulated/inappropriate environmental cues (Shroff and Shanahan 2007; Drüeke 2005). It results of imbalance between calcification inhibitors and promoters, which act at the systemic and the local level (Reynolds et al. 2004). Therefore, this imbalance leads probably to phenotypic change of smooth muscle cells towards osteoblast-like calcifying smooth muscle cells, which mediate organized extracellular matrix deposition in the vascular wall (Drüeke 2005; Johnson et al. 2006). Figure 1 shows consequently mechanisms that directly and indirectly lead to ectopic vascular wall calcification. Overall there is a hypothesis that ectopic vascular calcifications could be mediated by pathophysiological mechanisms underlying bone biomineralization affected residence cells allocated in vascular wall. Indeed, calcified vascular tissue expresses bone-related proteins, bone specific transcription factors, and bone morphogenetic proteins (BMPs), which contribute in osteogenesis in bone (Bostrom et al. 1993; Dhore et al. 2001). However, the origin of calcifying cells that directly promote vascular tissue mineralization is still unknown. Vascular smooth muscle cells may differentiate into calcifying osteoblast-like cells which via several molecular mechanisms regulate biomineralization in nature manner. Overexpression of extracellular matrix and biomineralization genes relevant for bone formation are sufficiently modulated by calcifying vascular smooth muscle cells (Morhayim et al. 2015; Alves et al. 2014). Moreover, these genes constitute the strongest link between residence cells and pathological vascular remodeling phenotype associated with calcification of vascular wall (Persy and D’Haese 2009). Finally, ectopic artery mineralization is frequently accompanied by decreased bone mineral density or disturbed bone turnover (Shroff and Shanahan 2007). Interestingly, type 2 diabetes mellitus (T2DM) associates with increased fracture risk despite the fact that T2DM patients have higher bone mineral density as compared to non-diabetic individuals. Therefore, there are evidences that T2DM might contribute to decreased bone formation through interference of advanced glycosylation end-products with osteoblast development, function and attachment to collagen matrix, increased levels of osteocyte-derived sclerostin, and hyperglycemia-induced suppression of osteogenic differentiation of marrow-derived progenitor cells diverting osteoblastic precursor cells (Meier et al. 2015). Overall T2DM-dependend inflammatory process contributing to bone demineralization may lead fracture risk, but extra bone mineralization activity might increase. All these mediate augmented vascular calcification and promote atherosclerosis in T2DM patients. However, these are still unclear, whether is inversely related relationship between processes of bone and vascular calcification that appear to be inversely related (Yamamoto 2015). Further examinations are needed to improve understanding of the precise mechanism in this area.
There are evidences regarding an activation of resident pericytes in the vascular wall that may contribute vascular calcification (Yamamoto 2015). Indeed, pericytes that are discussed as mesenchymal progenitor cells have the powerful potential to develop into osteoblasts and chondrocytes in situ under influence of inflammatory cytokines, oxidated lipids, free active radicals, turbulent blood flow, high pressure, shear stress, and growth factors contributed in angiogenesis and neovascualrization (Evrard et al. 2015). Interestingly, neoangiogenesis that is suitable for atherosclerosis malignancy may facilitate migration of pericytes and thereby induce vascular wall mineralization (Stegen et al. 2015).
Bone-Related Proteins in Atherosclerosis and Vascular Remodeling
Vascular calcification frequently appears in arterial wall due to atherosclerosis, inflammation, and worsening of calcium homeostasis. Vascular calcification demonstrates increased prevalence in cardiovascular and chronic kidney disease, atherosclerosis, and dyslipidemia (Evrard et al. 2015). Recent investigations have shown that vascular calcification is a complex sophisticated pathological process affecting promoters and inhibitors of calcification, resembling skeletal metabolism, and regulated by resident cells, intermediates, hormones, cytokines, and active peptides (Viegas et al. 2015). Therefore, atherosclerosis, low-intense inflammation, stretch-induce arterial wall hypertrophy, and dyslipidemia are considered main factors that contribute in endothelial dysfunction and directly relate to clinical outcomes among subjects belong to general and selective populations. Key facts of regulation of vascular wall calcification are in Table 2.
The key point of the beginning of vascular calcification is formation of osteogenic phenotype of target cells, i.e., pericytes, vascular smooth muscle cells, and probably stem cells. Osteogenic differentiation of target cells including vascular smooth muscle cells is characterized by the expression of bone-related molecules including bone morphogenetic protein (BMP) -2, Msx2, OPG, and OPN, which are produced by osteoblasts and chondrocytes. Osteogenic transforming target cells produce hydroxyapatite, which includes calcium deposition in extra bone sites including vascular wall, valvular leaflets, etc. Finally, calcium deposits of atherosclerotic plaque and vascular wall, which appear to be identical to fully formed lamellar bone, may worse mechanical and structural properties of vessel and lead to vascular complications. Key Facts of initiation of vascular calcification are in Fig. 2.
Recent data have linked RANKL and OPG to cardiovascular disease, including CHF, immunity, vascular calcification, osteoporosis, and bone remodeling (Loncar et al. 2010; Kearns et al. 2008; Leistner et al. 2012). Low-intense inflammation is being discussed as a powerful trigger of vascular remodeling and calcification realized through bone-related protein pathway. Recent clinical studies have shown that coronary atherosclerosis associates with a significant increase of OPG and with a trend towards a decrease of soluble RANKL and RANKL/OPG Ratio (Motovska et al. 2015). Therefore, C-reactive protein (CRP) that overexpressed in atherosclerosis, dyslipidemia, and other dysmetabolic states (diabetes mellitus, abdominal obesity, metabolic syndrome) and cardiovascular diseases (ischemic heart disease, heart failure, hypertension, etc.) may stimulate RANKL production in monocytes. The RANKL-stimulated expression of wide spectrum of transcription factors, i.e., TRAF6, p38 mitogen-activated protein kinases (MAPKs), JNK IkB-α, and NF-kB p65 DNA, triggers overproduction of bone-related proteins. Overall RANKL/RANK/OPG system and its downstream signaling pathway are closely controlled via inflammatory cytokine (TNF alpha, interleukin (IL) 1β, IL-6, IL-10, IL-21, and IL-23) productions (Doumouchtsis et al. 2007). These effects may consider an important mechanism of endogenous protection from tissue injury. Indeed, recent animal studies have shown that OPG protects large arteries from medial calcification (El Hadj et al. 2008). Overall, OPG is discussed as a vascular protector (Hofbauer and Schoppet 2004) and a surrogate marker of early coronary vascular calcification in patients with known asymptomatic coronary artery disease, dysmetabolic disease (Berezin and Kremzer 2013).
OSN has found to cause myocardial hypertrophy; increase fibrillar collagen content; stimulate cell signaling, adhesion, survival, proliferation, and migration in several cell types; mediate calcification of the vascular wall, coagulation, and endothelial dysfunction (McCurdy et al. 2010). Recent animal studies have revealed that increased circulating OSN associates with higher incidence of mortality following myocardial infarction due to increased rates of rupture and new heart failure over the first 14 days after MI that associate with left ventricular dysfunction and increased mortality in short- and long-term period (Schellings et al. 2009).
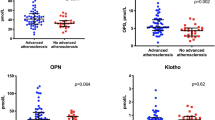
OPN is reported a surrogate marker of atherosclerotic lesions, especially in calcified plaques, and is linked to the progression of coronary artery disease. Moreover, OPN is powerful biomarker of asymptomatic coronary artery disease (Mohamadpour et al. 2015) and vascular calcification in patients with chronic kidney disease (Gluba-Brzózka et al. 2014) with possible predictive value. OPN has a renal clearance and demonstrates a close interaction with glomerular filtration rate. Recent studies have shown that OPN and renal failure were the independent risk factors for coronary heart disease (Chen et al. 2014). There are evidences that OPN relates a systemic inflammatory activation and endothelial dysfunction that is considered a marker of negative clinical outcomes in cardiovascular disease.
Finally, circulating level of bone-related proteins associates with vascular wall calcification, target organ damage including lowered kidney function, plaque formation, and endothelial dysfunction. Key facts of dual role of bone-related proteins in cardiovascular diseases are in Table 3. However, the predictive role of these biological markers is not fully understood and requires more investigations.
The Role of Bone-Related Proteins in Age-Related Diseases
Bone has evolved to provide structural support to organisms, and therefore its mechanical properties are vital physiologically (Zimmermann and Ritchie 2015). Bone remodeling is age dependently regulated and changes dramatically during the course of development. Progressive accumulation of reactive oxygen species and hypoxia have been suspected to be the leading cause of many inflammatory and degenerative diseases, as well as an important factor underlying many effects of aging (Chen et al. 2015; Drager et al. 2015). However, the role of bone-related proteins and its co-regulators in age-related diseases is still under discussion and appears to be very controversial.
Osteopontin (OPN) and vascular endothelial growth factor (VEGF) are characterized by a convergence in function for regulating cell motility and angiogenesis, the response to hypoxia, and apoptosis (Gong et al. 2015; Ramchandani and Weber 2015). OPN and VEGF may co-express in age-related settings (Almeida 2012). In vascular diseases, these two cytokines mediate remodeling but may also perpetuate inflammation and narrowing of the arteries (Rodríguez et al. 2015). OPN and VEGF are elevated and contribute to vascularization in age-related manner (Ramchandani and Weber 2015). Indeed, cyclic stretch as a main mechanical forces influencing vascular smooth muscle cells in vasculature may initiate stimulation of NADPH oxidase isoform 1 (Nox1)-derived ROS via MEF2B, leading to endothelial dysfunction via a switch from a contractile to a synthetic phenotype (Rodríguez et al. 2015). This process is upregulated by OPN and downregulated by calponin1 and smoothelin B. Thus, OPN-dependent pathway of vascular dysfunction bases on MEF2B-Nox1-ROS upregulation under pathological stretch conditions is suitable for hypertension. Indeed, stretch-induced Nox1 activation decreases actin fiber density and augments matrix metalloproteinase-9 activity, vascular smooth muscle cells migration (Rodríguez et al. 2015). All these findings may have a pivotal role for explanation of age-related hypertension.
There are controversial data about age-related increase of OSN. However, the diagnostic and predictive role of this fact is not clear and requires more studies.
Predictive Value of Bone-Related Proteins in Patients After Stroke
The prognostic relevance of biomarkers related to atherosclerotic plaque calcification, i.e., OPN, OPG, and RANKL, was determined in several investigations. Interestingly that serum OPN may be a useful biomarker of atherosclerosis and vascular calcification. Importantly, note that there was determined a positive association between circulating OPN and the presence of CAD but not to the extent of coronary atherosclerosis (Mohamadpour et al. 2015). Therefore, serum levels of OPN, but not OPG and RANKL, peaked at day 7 after acute ischemic stroke and predicted worse neurological scores independently of age, gender, hypertension, and thrombolytic procedures (Carbone et al. 2015). Whether serial measurements of OPN, OPG, and RANKL are necessary for risk stratification of the patients after stroke is not clear.
Predictive Value of Bone-Related Proteins in Heart Failure
Notwithstanding, bone-related proteins are considered a surrogate biomarker of vascular calcification in atherosclerosis, dyslipidemia, diabetes, obesity, etc., the role of sRANKL/OPG complex in maintenance of reparative repair potency among CHF persons shows to be very intriguing while clinical data are limited. There are data OPG/RANKL/RANK system that contributes to cardiac remodeling and left ventricular dilation after acute myocardial infarction in acute phase of cardiac failure as well as in chronic phase of heart failure development, while not so profoundly (Ueland et al. 2004). It is reported that serum RANKL was a significant determinant of NT-pro-BNP independent of age, BMI, and creatinine clearance in CHF subjects (Loncar et al. 2010). There is interrelationship between OPG and serum RANKL concentrations in patients with advanced atherosclerosis in relation to medical history, risk factors, and medication intake (Giaginis et al. 2012). On the one hand, sRANKL/OPG complex contributes different stages in ischemic CHF development, whereas the clinical implication of RANKL seems uncertain (Bjerre et al. 2014). On the other hand, the independent predictive value was determined for OPG only (Montagnana et al. 2013). Finally, OPG is suggested to be a modulator rather than a marker of extracellular remodeling that may play critical role in CHF pathogenesis by neutralizing the effect of receptor activator of nuclear factor-kappa B ligand on differentiation and activation of wide spectrum cells, including circulating endothelial progenitor cells. The imbalance between free fraction of RANKL, calculated as sRANKL/OPG ratio, and circulating OPG may be responsible for the homeostatic mechanism of differentiation and apoptosis of endothelial progenitor cells (Berezin and Kremzer 2014). This effect may mediate overproduction of reactive oxygen species and oxidized lipoproteins through OPG-related activation of NOX-2 and NOX-4 and triggered phosphorylation of ERK-1/2 and p38 MAPK. All this mechanisms are suitable for ischemic CHF development. Results of our study showed that components of sRANKL/OPG complex were increased in CHF patients when compared with none-CHF persons with stable CAD as well as with healthy volunteers. Therefore, decreased circulating EPCs -related CHF in CAD subjects were also found. However, sRANKL/OPG ratio when compared with other components of cytokines-induced bone-related proteins RANKL and OPG in ischemic CHF patients was not only significantly associated with parameters of neuroendocrine activation such as NT-pro-BNP and hs-CRP but it closely effected on EPCs with proangiogenic phenotypes. It has been suggested that sRANKL/OPG complex affected reparative face of the pathogenesis of ischemic CHF through modulating count of circulating endothelial progenitor cells. Because OPG may stimulate differentiation of the endothelial progenitor cells and positively regulate their count in circulation, it has suggested that free fraction of serum RANKL, calculated as serum RANKL/OPG ratio, consider powerful predictor for depletion of CD14 + CD309+ EPCs and CD14 + CD309 + Tie2+ endothelial progenitor cells in CHF patients. Probably, this effect may have a prognostic value for subjects with CHF. Overall the role of OPG as independent predictor of CHF development and progression requires detail investigations.
OPN has been demonstrated to be upregulated in left ventricular hypertrophy, dilated cardiomyopathy, and diabetic cardiomyopathy, and it is discussed as a possible predictor of heart failure development and heart failure-related clinical outcomes (Lok et al. 2015). Osteopontin independently predicted all-cause mortality and acutely CHF-related rehospitalization after 1 and 5 years. Compared with NT-proBNP, osteopontin was of superior prognostic value, specifically in CHF patients and for the prognostic outcome of acutely CHF-related rehospitalization (Behnes et al. 2013).
It is found that the serum OSN in patients with CHF predominantly reflected a positive pro-inflammatory response and alterations in protein metabolism that leads to biomechanical stress. However, the roles of OSN in the CHF have not been defined. Finally, further studies are needed to elucidate the potential role of bone-related proteins in the complex pathogenesis of CHF
Potential Applications to Prognosis, Other Diseases or Conditions
Although there are not sufficient evidences that the clinical correlations of circulating levels of bone-related proteins in subjects with documented cardiovascular diseases might have predictive value, it has been suggested that exaggerated level of OPG and probably OPN and OSN would confer a better prognosis in CAD patients, especially those who underwent revascularization procedures or have acute/acutely decompensated heart failure. By now there are evidences regarding an association of circulating bone-related proteins predominantly OPG and OPN with vascular calcification. Therefore, the negative effect of these proteins on progression of age-related diseases has been reported. Currently the continued monitoring for OPG/RANKL, OPN, and OSN levels is not recommended, but patient from vulnerable populations at high cardiovascular risk, probably, may have some benefit in prediction of clinical outcomes based on serial assessment of circulating bone-related proteins (Table 3).
Summary Points
-
Bone-related proteins are multifunctional growth factors that are involved in extra bone mineralization, calcification at ectopic sites, and they might play a pivotal role in atherosclerosis, plaque formation, vascular remodeling and integrity, neovascularization, and malignancy.
-
Bone-related proteins include osteopontin, osteoprotegerin, osteonectin, osteocalcin, sclerostin, and RANKL/RANK system.
-
Bone-related proteins are intricately prone to regulation by hypoxia signaling system, hormones, electrolyte and mineral changes, inflammation, and they might involve in coupling angiogenesis and osteogenesis during bone development and repair.
-
On contrary to bone mineralization, vascular calcification is a pathological process, occurring in response to dysregulated/inappropriate environmental cues as results of imbalance between calcification inhibitors and promoters.
-
Ectopic vascular calcifications could be mediated by pathophysiological mechanisms underlying bone biomineralization affected residence cells allocated in vascular wall.
-
The main calcifying cells that directly promote vascular tissue mineralization are vascular smooth muscle cells, stem cells, and pericytes.
-
Vascular calcification demonstrates increased prevalence in cardiovascular and chronic kidney disease, atherosclerosis, and dyslipidemia.
-
Elevated circulation levels of OPG, OPN, and probably OSN are considered a surrogate marker of vascular calcification, lowered kidney function, asymptomatic atherosclerosis and coronary artery disease, and severity of heart failure with possible high predictive value.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CHF:
-
Chronic heart failure
- CRP:
-
C-reactive protein
- MACE:
-
Major adverse cardiac events
- MI:
-
Myocardial infarction
- OCN:
-
Osteocalcin
- OPG:
-
Osteoprotegerin
- OPN:
-
Osteopontin
- OSN:
-
Osteonectin
- RANKL:
-
Receptor activator of nuclear factor-kB ligand
References
Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38(6):749–57.
Almeida M. Aging mechanisms in bone. Bonekey Rep. 2012;1:1.
Alves RD, Eijken M, van de Peppel J, van Leeuwen JP. Calcifying vascular smooth muscle cells and osteoblasts: independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genomics. 2014;15:965.
Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
Behnes M, Brueckmann M, Lang S, et al. Diagnostic and prognostic value of osteopontin in patients with acute congestive heart failure. Eur J Heart Fail. 2013;15(12):1390–400.
Berezin AE, Kremzer AA. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis. 2013;229(2):475–81.
Berezin AE, Kremzer AA. Relationship between serum RANKL/osteoprotegerin complex and endothelial progenitor cells in ischemic chronic heart failure. J Cardiol Ther. 2014;1(8):189–95.
Bjerre M, Munk K, Sloth AD, et al. High osteoprotegerin levels predict MACCE in STEMI patients, but are not associated with myocardial salvage. Scand Cardiovasc J. 2014;48(4):209–15.
Bostrom K, Watson KE, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9.
Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007a;9 Suppl 1:S1.
Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007b;5(3):98–104.
Carbone F, Vuilleumier N, Burger F, et al. Serum OPN levels are up regulated and predict disability after an ischemic stroke. Eur J Clin Invest. 2015. doi:10.1111/eci.12446. [Epub ahead of print].
Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab. 2015. [Epub ahead of print].
Chen J, Lu Y, Huang D, et al. Relationship of osteopontin and renal function with severity of coronary artery lesions. Int J Clin Exp Med. 2014;7(4):1122–7.
Chen JR, Lazarenko OP, Blackburn ML et al. p47phox/Nox2-dependent ROS signaling inhibits early bone development in mice but protects against skeletal aging. J Biol Chem. 2015. pii: jbc.M114.633461. [Epub ahead of print].
Coombes JD, Syn WK. Differential osteopontin functions: the role of osteopontin isoforms. Hepatology. 2014. doi:10.1002/hep.27555. [Epub ahead of print].
David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20(3):203–12.
Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003.
Doumouchtsis KK, Kostakis AI, Doumouchtsis SK, et al. sRANKL/osteoprotegerin complex and biochemical markers in a cohort of male and female hemodialysis patients. J Endocrinol Invest. 2007;30(9):762–6.
Drager J, Harvey EJ, Barralet J. Hypoxia signalling manipulation for bone regeneration. Expert Rev Mol Med. 2015;17:e6.
Drüeke TB. Pathophysiological aspects of vascular calcification in chronic renal failure. Nefrologia. 2005;25 Suppl 2:96–9.
El Hadj Othmane T, Speer G, Fekete B, et al. Osteoprotegerin: regulator, protector and marker. Orv Hetil. 2008;149(42):1971–80.
Evrard S, Delanaye P, Kamel S, SFBC/SN joined working group on vascular calcifications, et al. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. 2015;438:401–14.
Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab. 2009;20(5):230–6.
Garnero P, Grimaux M, Demiaux B, et al. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992;7(12):1389–98.
Giaginis C, Papadopouli A, Zira A, et al. Correlation of plasma osteoprotegerin (OPG) and receptor activator of the nuclear factor kB ligand (RANKL) levels with clinical risk factors in patients with advanced carotid atherosclerosis. Med Sci Monit. 2012;18(10):CR597–604.
Gluba-Brzózka A, Michalska-Kasiczak M, Franczyk-Skóra B, et al. Markers of increased cardiovascular risk in patients with chronic kidney disease. Lipids Health Dis. 2014;13:135.
Gong X, Tong Q, Chen Z, et al. Microvascular density and vascular endothelial growth factor and osteopontin expression during the implantation window in a controlled ovarian hyperstimulation rat model. Exp Ther Med. 2015;9(3):773–9.
Gu J, Tong XS, Chen GH, et al. Effects of 1α,25-(OH)2D3 on the formation and activity of osteoclasts in RAW264.7 cells. J Steroid Biochem Mol Biol. 2015. pii: S0960-0760(15)00100-4. doi:10.1016/j.jsbmb.2015.04.003. [Epub ahead of print].
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047.
Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5.
Hofbauer LC, Khosla S, Dunstan CR, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12.
Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97(2):105–14.
Ito K, Kon S, Nakayama Y, et al. The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix Biol. 2009;28(1):11–9.
Johnsen IK, Beuschlein F. Role of bone morphogenetic proteins in adrenal physiology and disease. J Mol Endocrinol. 2010;44(4):203–11.
Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99(10):1044–59.
Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10:191–7.
Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem. 2015;116(5):687–95.
Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29(2):155–92.
Kindblom JM, Ohlsson C, Ljunggren O, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24(5):785–91.
Leavenworth JW, Verbinnen B, Yin J, et al. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol. 2015;16(1):96–106.
Leistner DM, Seeger FH, Fischer A, et al. Elevated levels of the mediator of catabolic bone remodeling RANKL in the bone marrow environment link chronic heart failure with osteoporosis. Circ Heart Fail. 2012;5(6):769–77.
Liu W, Zhang X. Receptor activator of nuclear factor-kB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (Review). Mol Med Rep. 2015;11(5):3212–8.
Lok SI, Nous FM, van Kuik J, et al. Myocardial fibrosis and pro-fibrotic markers in end-stage heart failure patients during continuous-flow left ventricular assist device support. Eur J Cardiothorac Surg. 2015. pii: ezu539. [Epub ahead of print].
Loncar G, Bozic B, Cvorovic V, et al. Relationship between RANKL and neuroendocrine activation in elderly males with heart failure. Endocrine. 2010;37(1):148–56.
Lund SA, Wilson CL, Raines EW, et al. Osteopontin mediates macrophage chemotaxis via α(4) and α(9) integrins and survival via the α(4) integrin. J Cell Biochem. 2013;114(5):1194–202.
McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J Mol Cell Cardiol. 2010;48(3):544–9.
Meier C, Schwartz AV, Egger A, Lecka-Czernik B. Effects of diabetes drugs on the skeleton. Bone. 2015. pii: S8756-3282(15)00139-8. doi:10.1016/j.bone.2015.04.026. [Epub ahead of print].
Mohamadpour AH, Abdolrahmani L, Mirzaei H, et al. Serum osteopontin concentrations in relation to coronary artery disease. Arch Med Res. 2015;46(2):112–7.
Montagnana M, Lippi G, Danese E, Guidi GC. The role of osteoprotegerin in cardiovascular disease. Ann Med. 2013;45(3):254–64.
Morena M, Jaussent I, Dupuy AM, et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications. Nephrol Dial Transplant. 2015;30(8):1345–56
Morhayim J, van de Peppel J, Demmers JA, et al. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J. 2015;29(1):274–85.
Motovska Z, Vichova T, Doktorova M, Labos M, Maly M, Widimsky P. Serum Dickkopf-1 signaling and calcium deposition in aortic valve are significantly related to the presence of concomitant coronary atherosclerosis in patients with symptomatic calcified aortic stenosis. J Transl Med. 2015;13(1):63.
Nagaraju GP, Dontula R, El-Rayes BF, Lakka SS. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35(5):967–73.
Obert L, Lepage D, Gindraux F, Garbuio P. Bone morphogenetic proteins in soft-tissue reconstruction. Injury. 2009;40 Suppl 3:S17–20.
Okamura H, Yoshida K, Ochiai K, Haneji T. Reduction of protein phosphatase 2A Cα enhances bone formation and osteoblast differentiation through the expression of bone-specific transcription factor Osterix. Bone. 2011;49(3):368–75.
Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15(9):405–16.
Rachmiel A, Leiser Y. The molecular and cellular events that take place during craniofacial distraction osteogenesis. Plast Reconstr Surg Glob Open. 2014;2(1):e98.
Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor: implications for cancer. Biochim Biophys Acta. 2015;1855(2):202–22.
Raouf A, Seth A. Ets transcription factors and targets in osteogenesis. Oncogene. 2000;19(55):6455–63.
Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obesity. 2010;34(5):852–8.
Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67.
Ribeiro N, Sousa SR, Brekken RA, Monteiro FJ. Role of SPARC in bone remodeling and cancer-related bone metastasis. J Cell Biochem. 2014;115(1):17–26.
Rodríguez AI, Csányi G, Ranayhossaini DJ, et al. MEF2B-Nox1 signaling is critical for stretch-induced phenotypic modulation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2015;35(2):430–8.
Sarosiek K, Jones E, Chipitsyna G, et al. Osteopontin (OPN) isoforms, diabetes, obesity, and cancer; what is one got to do with the other? A new role for OPN. J Gastrointest Surg. 2015;19(4):639–50.
Schellings MW, Vanhoutte D, Swinnen M, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–23.
Shao J, Wang Z, Yang T, et al. Bone regulates glucose metabolism as an endocrine organ through osteocalcin. Int J Endocrinol. 2015;2015:967673.
Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial. 2007;20(2):103–9.
Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50.
Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19.
Stegen S, van Gastel N, Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19–27.
Ueland T, Jemtland R, Godang K, et al. The prognostic value of osteoprotegerin in patients with acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–6.
Ueland T, Yndestad A, Øie E, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111(19):2461–8.
Viegas CS, Rafael MS, Enriquez JL, et al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol. 2015;35(2):399–408.
Warren JT, Zou W, Decker CE, et al. Correlating RANK ligand/RANK binding kinetics with osteoclast formation and function. J Cell Biochem. 2015. doi:10.1002/jcb.25191. [Epub ahead of print].
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Curr Osteoporos Rep. 2015;13(3):180–5
Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009;2(1):56–64.
Yamamoto M. Vascular calcification – pathological mechanism and clinical application -.vascular calcification as a clinical manifestation of bone-vascular axis. Clin Calcium. 2015;25(5):655–60.
Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602.
Yndestad A, Kristian DJ, Geir EH, et al. Increased gene expression of tumor necrosis factor superfamily ligands in peripheral blood mononuclear cells during chronic heart failure. Cardiovasc Res. 2002;54:175–82.
Zhang Q, Riddle RC, Clemens TL. Bone and the regulation of global energy balance. J Intern Med. 2015. doi:10.1111/joim.12348. [Epub ahead of print].
Zimmermann EA, Ritchie RO. Bone as a structural material. Adv Healthc Mater. 2015. doi:10.1002/adhm.201500070. [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Berezin, A.E. (2017). Bone-Related Proteins as Markers in Vascular Remodeling. In: Patel, V., Preedy, V. (eds) Biomarkers in Bone Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7693-7_4
Download citation
DOI: https://doi.org/10.1007/978-94-007-7693-7_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7692-0
Online ISBN: 978-94-007-7693-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences