Abstract
Extracellular vesicles (EV) released by various types of cells are detectable in body fluids such as blood and urine. Both clinical and animal studies showed that circulating EVs were elevated in response to many types of liver diseases, such as hepatocellular carcinoma (HCC), nonalcoholic fatty liver disease, acute liver failure, liver cirrhosis, hepatic ischemia-reperfusion (I/R) injury, and drug-induced liver injury. The increase in subgroups of EVs and certain EV molecules was found to be of diagnostic and prognostic value for some liver diseases such as HCC and liver cirrhosis. Some microRNA species were solely increased in serum EVs but not the whole serum under disease states, indicating that circulating EVs may provide more sensitive liver biomarkers. Blood EVs from patients appeared to play a detrimental role contributing disease progression. Blocking blood EV elevation by pharmacological approaches afforded protection against liver damage. Circulating EVs are emerging as a rich source of new biomarkers for liver diseases.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Extracellular vesicles
- Biomarkers
- Hepatocellular carcinoma
- Nonalcoholic fatty liver disease
- Acute liver failure
- Liver cirrhosis
- Hepatic ischemia-reperfusion (I/R) injury
- Drug-induced liver injury
Key Facts of Extracellular Vesicles
-
Extracellular vesicles (EVs) were first described in 1946 as “dust” produced by platelets in normal plasma. EVs were considered as cellular waste for many years.
-
The 2013 Nobel Prize in Physiology or Medicine was awarded to three scientists who study vesicles including EVs.
-
A professional organization on EVs studies, the International Society for Extracellular Vesicles (ISEV), was established in 2011. ISEV launched its official journal, Journal of Extracellular Vesicles, in 2012.
-
EVs are produced by almost all cells and circulate in the body. EVs can be isolated and detected in all types of body fluids.
-
EVs are of different sizes and forms. EVs contain RNAs, proteins, and lipids.
-
When liver diseases occur, both EVs produced by liver cells and non-liver cells are changed. These changes can be detected in the blood and urine, and the pattern of changes may help determine the severity of liver disorders.
-
The changes in blood EVs can be prevented by pharmacological approaches, and this intervention is beneficial to liver diseases.
Definitions of Words and Terms
- Acetaminophen overdose:
-
Acetaminophen is active ingredient of many pain killers such as Tylenol. Though acetaminophen is a very safe drug, it can cause serious liver injury when taken too much. Acetaminophen overdose is a main reason for acute liver failure.
- Acute liver failure (ALF):
-
ALF refers to the very rapid loss of liver functions that causes mental problems and bleeding disorders. ALF is life threatening and often requires liver transplantation.
- Biomarkers:
-
A biomarker usually refers to a molecule that can be quantified to reflect the occurrence and/or progress of specific diseases. An ideal biomarker is expected to be both sensitive and specific.
- Extracellular vesicles (EVs):
-
EVs are small particles released by all types of cells. The size of EVs ranges from 50 to 5,000 nm in diameter. EVs are composed of proteins, lipids, and nucleic acids. EVs circulate in the blood playing an important role in cell-to-cell communications.
- Hepatic ischemia-reperfusion (I/R) injury:
-
Hepatic I/R injury usually occurs during liver surgery. When the blood flow through the liver is reduced, the liver will be damaged due to lack of oxygen and other nutrients. However, such damage will be made worse when the blood flow was restored to normal.
- Hepatocellular carcinoma (HCC):
-
HCC is a type of cancer that originally occurs in the liver. HCC is actually the most common form of liver cancer. Hepatitis B or C patients are more likely to develop HCC.
- Hepatocytes:
-
Hepatocytes are the main type of cells in the liver. Hepatocytes perform the main functions of the liver.
- Liver cirrhosis:
-
Liver cirrhosis refers to the serious scarring of liver tissues. Liver scars do not work as normal liver cells and therefore liver functions are compromised in the long run. Those drinking too much alcohol and those with hepatitis B or C are more likely to have liver cirrhosis.
- MicroRNAs:
-
MicroRNAs are a type of short RNAs with about 20 nucleotides. MicroRNAs regulate the expression of many genes. MicroRNAs are very stable and therefore are considered as good disease biomarkers.
- Nonalcoholic fatty liver disease (NAFLD):
-
NAFLD refers to the buildup of fat in liver cells in those who do not drink alcohol. NAFLD is a common liver disorder, and the main cause of NAFLD is obesity. The liver cells do not have inflammation or damage in NAFLD patients.
- Nonalcoholic steatohepatitis (NASH):
-
NASH is a liver disorder caused by the accumulation of too much fat that leads to inflammation and damage in the liver. NASH occurs in people who do not drink alcohol.
Introduction
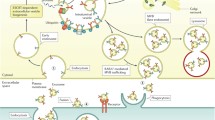
Extracellular vesicles (EVs), first discovered in 1946, generally refer to membrane-enclosed vesicles secreted by essentially all types of cells including prokaryotic cells. The secretion of EVs appears to be highly conserved in evolution (Yanez-Mo et al. 2015). Both healthy and abnormal cells produce EVs in a constant manner, though the number, morphology, and constituents of EV are changing with the environmental conditions. EVs were found to be cup shaped or round shaped or of irregular morphology. As with other biological entities, EVs are composed of proteins, nucleic acids, and lipids. At least 41,860 proteins, 7,540 RNAs, and 1,116 lipid molecules have been confidently identified in EVs (Keerthikumar et al. 2016). However, the function of EV molecules remains largely unknown. As of April 26, 2016, the PubMed contained 9,212 abstracts associated with EVs. However, only 295 abstracts were related to both EVs and the “liver.” This indicates that the study of liver EVs is still at the very early stage. It should be pointed out that EVs is the preferred term for studies involving different types of secreted vesicles including exosomes, microvesicles/microparticles/ectosomes, and apoptotic bodies (Gould and Raposo 2013). The main characteristics of these subgroups of EVs are summarized in Table 1. All these subgroups of EVs in the circulation have been studied in liver diseases for the discovery of new biomarkers.
EVs secreted by hepatocytes have been extensively characterized using systems biology approaches such as proteomics and genomics. The majority of genes and proteins identified in hepatocyte EVs were similar to those in EVs from other cell types, though some molecules appeared to be liver EV specific (Royo et al. 2013; Rodriguez-Suarez et al. 2014; Conde-Vancells et al. 2008). Figure 1 shows two typical protein markers for EVs isolated from primary cultured hepatocytes. This raised the possibility that liver EVs released into the circulation may serve as a potential source for highly specific biomarkers of liver diseases. Lack of specificity, and to a lesser extent sensitivity, is a key drawback of many standard liver biomarkers such as those for drug-induced liver injury (DILI) (Shi et al. 2010). Indeed, circulating EVs have been shown to be not only a potential source for more specific liver biomarkers but also an appealing target for therapeutic interventions.
When liver disease occurred, not only the morphology but also the components of hepatocyte EVs were changed. These altered hepatocyte EVs together with EVs of non-hepatocyte origin were released into the circulation and can be used as new liver biomarkers. Though EVs are detectable in various types of body fluids, only these in the blood and urine have been explored as liver biomarkers. Findings regarding circulating EVs as DILI biomarkers have been summarized in a recent review (Yang et al. 2014) and therefore will not be covered in this chapter.
Hepatocellular Carcinoma (HCC)
HCC is the most common form of liver cancer, and the most studied area on circulating EVs as liver biomarkers is HCC. This is not unexpected in that EVs from cancer cells have distinct constituents and circulating tumor EVs have been extensively characterized for discovering new cancer biomarkers. Though circulating EVs as cancer biomarkers were recognized as early as in the 1970s (Taylor and Shah 2015), the first study on blood EVs in HCC patients was not published until 2008 (Brodsky et al. 2008). It was found that the number of total blood EVs was increased by fivefold in HCC/HepC (HepC, hepatitis C infection) patients as compared with control subjects, and such elevation was relatively small, that is, about threefold, in HepC only patients. Though the total number of blood EVs did not directly reflect tumor size, the level of blood EVs from hepatocytes and endothelial cells, but not these from apoptotic cells, correlated well with tumor size (Brodsky et al. 2008). Interestingly, circulating EVs were first increased and then decreased after surgery. It appeared that the number of circulating EVs was associated with HCC clinical outcome, as a patient who later died had persistent increases of EVs prior to and after liver transplantation, and patients whose blood EV level returned to normal showed little clinical complications after liver transplantation. However, the small sample size (n = 8) made it difficult to draw a statistically meaningful conclusion (Brodsky et al. 2008). A more recent study confirmed and expanded some of these early findings. Using blood samples from 55 HCC patients, it was demonstrated that serum EV levels were significant increased and the changes correlated well with tumor size prior to treatment, and surgery treatment remarkably reduced EV numbers in the blood (Wang et al. 2013). Notably, serum EV levels showed diagnostic value in differentiating HCC stages (Wang et al. 2013).
As the number and concentration of blood EVs cannot be accurately quantified without extensive experience, measuring specific molecules in EVs, particularly the stable ones such as microRNAs, would likely provide more reliable and reproducible results. A recent study examined microRNA-21 levels in serum EVs from HCC patients (Wang et al. 2014). The rationale for selecting microRNA-21 was that it is a highly expressed microRNA species in numerous cancer cells. It was found that microRNA-21 in serum EVs was increased by over 16-fold in HCC patients as compared with healthy subjects. In contrast, serum EV microRNA-21 was increased by about fourfold in patients with hepatitis B infection. However, the microRNA-21 in whole serum was nearly unchanged, indicating that microRNA-21 was enriched in EVs (Wang et al. 2014). This observation demonstrates the unique advantage of using blood EVs as compared to whole blood. The additional value of microRNA-21 in EVs was that its level showed good correlation to HCC stages and the development of cirrhosis (Wang et al. 2014).
To identify more candidate biomarkers for HCC, serum EV microRNAs were detected in a more comprehensive manner. Specially, ten microRNAs that were dysregulated in HCC tissues were measured in serum EVs from HCC patients. As compared to patients with chronic hepatitis B, four EV microRNAs including miR-18a, miR-221, miR-222, and miR-22 were increased in HCC patients, and four EV microRNAs including miR-101, miR-106b, miR-122, and miR-195 were decreased, and the remaining two microRNAs miR-21 and miR-93 showed no difference (Sohn et al. 2015). In line with a previous report, the tested serum microRNAs showed no difference among disease groups, indicating that microRNAs enriched in EV are more sensitive in detecting HCC. A significant drawback of this study is that there were no samples from healthy subjects.
Recently, blood EV microRNAs from HCC patients were comprehensively analyzed using microRNA arrays. It was found that one microRNA, miR-1246, was remarkably increased and one microRNA, hsa-miR-718, was significantly decreased in serum EVs from HCC patients with recurrence as compared with those having no recurrence (Sugimachi et al. 2015). The level of hsa-miR-718, but not miR-1246, showed good correlation to tumor size, histological differentiation, and recurrence-free survival rate (Sugimachi et al. 2015).
Perturbation of circulating EVs was also observed in animal models for HCC. In rats treated with diethylnitrosamine, a well-established HCC model, two serum EV microRNAs, miRNA-10b and miRNA-21, were increased at later HCC stages. Combining the expressional levels of serum EV microRNAs and circulating microRNAs showed strong predictive value in the development of HCC in rats (Liu et al. 2015).
In clinical studies mentioned above, HCC was often associated with chronic hepatitis B or C infection. In most cases, an elevation of blood EVs was also observed in chronic hepatitis B or C patients, but the extent of changes was different.
A significant challenge in using circulating EV microRNAs as HCC biomarkers is that no standard normalization method is currently available. A recent study showed that when serum EV miR-21 level was normalized to a group of microRNAs including miR-221, miR-191, miR-181a, and miR-26a, its expression was higher in chronic hepatitis B patients than in HCC patients. However, such difference disappeared when miR-181c or U6 (CCG-1) was used as normalizers (Li et al. 2015). Regardless of the method for data normalization, serum EV miR-21 levels were significantly higher in HCC patients than in healthy subjects, though the difference between HCC patients and hepatitis B patients was no longer statistically different when some normalizers were used (Li et al. 2015).
In the abovementioned studies, blood EVs and EV microRNAs consistently outperformed traditional biomarkers such as alpha-fetoprotein (AFP) in HCC detection. Circulating EVs have been demonstrated to be a rich source for novel biomarkers of HCC. To date, only microRNAs in blood EV have been studied in HCC patients. Other molecules, such as proteins and long noncoding RNAs that have been studied in the whole blood for HCC detection, await further characterization regarding their expression profiles in circulating EVs (Table 2).
Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
NAFLD is the most common form of chronic liver diseases. NASH is the more severe form of NAFLD. Circulating EVs as biomarkers for NAFLD and NASH have studied using both animal models and clinical samples (Table 3).
In mouse models of NAFLD, blood EVs were found to be significantly increased, and the alterations appeared to be time dependent and correlated well with the histopathological features of disease severity (Povero et al. 2014). Further characterization of the increased blood EVs showed that the liver-enriched microRNAs miR-122 and miR-192 were remarkably increased, which was accompanied by a notable decrease of these two microRNAs in the liver tissue, indicating that at least part of blood EVs were produced by the liver. This is further confirmed by the significant increase of hepatocyte-specific protein asialoglycoprotein receptor (ASGPR1) in the blood EVs from NAFLD mice. Extensive analysis of blood EVs by proteomics approach showed that 25 EV proteins were increased in NAFLD mice and can be used to help the diagnosis of NAFLD (Povero et al. 2014).
In a mouse model of NASH, the concentration of serum EVs was increased by twofold, and some EV-associated proteins, such as the hepatocyte-specific enzyme CYP2E1, were also upregulated in the circulating EVs (Hirsova et al. 2016). The overproduced circulating EVs appeared to be functional, as EVs from both NASH patients and NASH mice activated macrophages causing enhanced cytokine production (Hirsova et al. 2016). Interestingly, when EV overproduction in the circulation was prevented by fasudil, a chemical inhibitor of rho-associated, coiled-coil-containing protein kinase 1 (ROCK1), the macrophage-mediated liver inflammation was reduced (Hirsova et al. 2016). Though increased circulating EVs seemed detrimental to liver functions, the responsible molecules and pathways have not been identified. Nevertheless, circulating EVs do have values in not only helping the diagnosis of NASH but also monitoring the therapeutic responses of anti-NASH medications.
In a clinical study with 67 NAFLD or NASH patients and 44 healthy subjects, it was found that blood EVs were significantly increased and blood EVs levels correlated well with the gold standard criteria for the severity of inflammation and apoptosis in NAFLD and NASH. This study also noted a slight difference between EVs from plasma and serum (Kornek et al. 2012).
Acute Liver Failure (ALF) and Acute Liver Injury
ALF is a relatively rare liver condition with a high mortality, and liver transplantation is often needed for treatment. In a recent prospective study involving 50 patients with ALF, plasma EVs were found to be increased by nearly 20-fold in ALF patients as compared with healthy subjects. The plasma level of a subgroup of EVs with the size of 280–640 nm was found to be predictive of patient outcome (alive or dead) (Stravitz et al. 2013). In this study, the etiology of ALF-included acetaminophen overdose, hepatitis B infection, autoimmune hepatitis, and mushroom poisoning (Stravitz et al. 2013) indicates that circulating EVs can be used to detect ALF caused by various reasons. In smaller study involving 10 patients who developed acute liver injury, plasma EVs were also found to be increased, though the fold change was relatively small, that is, two to three folds (Schmelzle et al. 2013).
Liver Cirrhosis
Liver cirrhosis i s a severe condition of scarring of the liver and loss of liver function occurring at the terminal stages of chronic liver disease. In a relatively large clinical study with 91 liver cirrhosis patients and 30 healthy subjects, it was found that blood EVs were significantly elevated regardless of the cause of liver cirrhosis, and a subpopulation of these EVs was shown to be from hepatocytes. The elevation of hepatocyte-derived EVs in the blood was associated with the severity of cirrhosis and systemic inflammation, indicating the circulating EVs may serve as new biomarkers for the grading of liver cirrhosis. Interestingly, women appeared to have a slightly higher level of certain blood EVs as compared to men (Rautou et al. 2012). The blood EVs from liver cirrhosis patients may contribute to the pathogenesis of liver cirrhosis, as these EVs caused vascular hyporeactivity and decreased arterial blood pressure which may contribute to arterial vasodilation associated with portal hypertension (Rautou et al. 2012). Targeting these detrimental EVs may provide therapeutic benefits, but this has not been examined in details with liver cirrhosis patients.
Hepatic Ischemia-Reperfusion (I/R) Injury
Hepatic I/R injury refers to the heightened hypoxic cellular damage following the restoration of liver blood flow during surgical procedures. Changes in circulating EVs for I/R injury detection have only been explored in animal models. In a mouse model of hepatic I/R injury, plasma EVs were found to be significantly increased. Subpopulation analysis showed that EVs from platelets and neutrophils were elevated at the acute injury stage while those from endothelial were increased at a later stage, indicating the former may serve as injury biomarkers and the latter may better reflect liver regeneration (Freeman et al. 2014).
Another mouse study found that blood EVs began to increase 15–30 min after postischemic reperfusion when serum alanine aminotransferase (ALT) was unchanged, and the elevation persisted and reached the peak level at 24 h (Teoh et al. 2014). The origin of elevated blood EVs was dependent on time course of liver injury. At 30 min after reperfusion, the blood EVs were mainly from endothelial cells, platelets, and neutrophils, while at 2 h and later, the blood EVs appeared to be mainly from Kupffer cells/macrophages (Teoh et al. 2014). These circulating EVs after I/R injury were able to trigger inflammatory response causing further liver injury. Interestingly, blocking the overproduction of circulating EVs by intravenous injection of diannexin, a synthetic human recombinant homodimer of annexin V, afforded significant protection against hepatic I/R injury in mice (Teoh et al. 2014). The clinical value of using circulating EVs for detecting hepatic I/R injury awaits further investigation.
Potential Applications to Prognosis, Other Diseases, or Conditions
The magnitude of changes in circulating EVs under different liver disease conditions was relatively small as compared to serum ALT. Therefore they may not be highly sensitive in detecting liver diseases. However, blood EVs appeared to be more specific and more informative than traditional liver biomarkers. Evidence is emerging that blood EVs of certain origins and their selective constituents such as microRNAs have the potential to predict HCC stages and recurrence (Brodsky et al. 2008; Liu et al. 2015; Sohn et al. 2015; Sugimachi et al. 2015; Wang et al. 2013, 2014), and plasma EVs of certain sizes may help predict if the ALF patients will die or survive (Stravitz et al. 2013). As for NAFLD and NASH, the blood EVs were predictive of disease severity (Kornek et al. 2012). However, the lack of standardized methods for EV isolation and data normalization makes it difficult to compare and evaluate the result from different groups. The methods for isolating circulating EVs are evolving with time. The reproducibility of published data needs to be confirmed using more patient samples, as a common drawback of existing data is that only a limited number of patients, that is, usually less than 100, were examined. The likely reason is that EV isolation from blood requires expensive instruments such as an ultracentrifuge and the whole process is rather time-consuming. The characterization of isolated blood EVs is also a challenging task. Improvement in EV isolation method is urgently needed for the wide application of circulating EV-based liver biomarkers.
Accumulating data suggest that circulating EVs play a functional role in mediating the pathogenesis of various liver diseases (Lemoinne et al. 2014). Therapeutic interventions very likely will affect the blood EV profiles. Interestingly, intrasplenic administration of EVs from certain cell types afforded protection against acute liver injury induced by chemicals, possible via the activation of regenerative process (Tan et al. 2014). This should be taken into consideration when circulating EVs are to be used for prognosis of liver diseases.
In addition to liver disorders, blood EVs have been shown to be disrupted under numerous disease conditions, particularly various types of cancers. A comprehensive study comparing the alterations of circulating EVs under different disease states is not available, making it difficult to assess the specificity of blood EVs as liver disease biomarkers. Nevertheless, organ-specific molecules in circulating EVs may hold the promise of serving as disease-specific biomarkers.
Summary Points
-
This chapter focuses on circulating extracellular vesicles (EVs) as biomarkers for various liver diseases.
-
EVs are produced by various cell types and are released in the blood and urine.
-
The number, morphology, and constituents of EVs are altered when liver diseases occur.
-
Circulating EVs are elevated in patients or animals with hepatocellular carcinoma (HCC), nonalcoholic fatty liver disease (NAFLD), acute liver failure (ALF) and acute liver injury, liver cirrhosis, hepatic ischemia-reperfusion (I/R) injury, and drug-induced liver injury (DILI).
-
Blood EVs originated from hepatocytes have the potential to predict liver disease stages and outcome (alive or dead).
-
Certain proteins and microRNAs in blood EVs may serve as new biomarkers for liver diseases.
-
Methods for the isolation of circulating EVs need to be improved and standardized.
Abbreviations
- ALF:
-
Acute liver failure
- ALT:
-
Alanine aminotransferase
- AFP:
-
Alpha-fetoprotein
- ASGPR1:
-
Asialoglycoprotein receptor
- DILI:
-
Drug-induced liver injury
- EV:
-
Extracellular vesicles
- HepB:
-
Hepatitis B
- HepC:
-
Hepatitis C
- HCC:
-
Hepatocellular carcinoma
- I/R:
-
Ischemia-reperfusion
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- ROCK1:
-
Rho-associated, coiled-coil-containing protein kinase 1
References
Andaloussi ELA, Mager I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57.
Brodsky SV, Facciuto ME, Heydt D, et al. Dynamics of circulating microparticles in liver transplant patients. J Gastrointestin Liver Dis. 2008;17:261–88.
Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–66.
Freeman CM, Quillin 3rd RC, Wilson GC, et al. Characterization of microparticles after hepatic ischemia-reperfusion injury. PLoS One. 2014;9:e97945.
Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2:201389.
Hirsova P, Ibrahim SH, Krishnan A, et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterolog. 2016;150:956–67.
Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428:688–92.
Kornek M, Lynch M, Mehta SH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–58.
Lemoinne S, Thabut D, Housset C, et al. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol. 2014;11:350–61.
Li Y, Zhang L, Liu F, et al. Identification of endogenous controls for analyzing serum exosomal miRNA in patients with hepatitis B or hepatocellular carcinoma. Dis Markers. 2015;2015:893594.
Liu WH, Ren LN, Wang X, et al. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin Oncol. 2015;141:1767–78.
Povero D, Eguchi A, Li H, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651.
Rautou PE, Bresson J, Sainte-Marie Y, et al. Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology. 2012;143:166–76.
Rodriguez-Suarez E, Gonzalez E, Hughes C, et al. Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. J Proteomics. 2014;103:227–40.
Royo F, Schlangen K, Palomo L, et al. Transcriptome of extracellular vesicles released by hepatocytes. PLoS One. 2013;8:e68693.
Schmelzle M, Splith K, Andersen LW, et al. Increased plasma levels of microparticles expressing CD39 and CD133 in acute liver injury. Transplantation. 2013;95:63–9.
Shi Q, Hong H, Senior J, et al. Biomarkers for drug-induced liver injury. Expert Rev Gastroenterol Hepatol. 2010;4:225–34.
Sohn W, Kim J, Kang SH, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184.
Stravitz RT, Bowling R, Bradford RL, et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58:304–13.
Sugimachi K, Matsumura T, Hirata H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112:532–8.
Tan CY, Lai RC, Wong W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76.
Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10.
Teoh NC, Ajamieh H, Wong HJ, et al. Microparticles mediate hepatic ischemia-reperfusion injury and are the targets of Diannexin (ASP8597). PLoS One. 2014;9:e104376.
van der Pol E, Boing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705.
Wang W, Li H, Zhou Y, et al. Peripheral blood microvesicles are potential biomarkers for hepatocellular carcinoma. Cancer Biomark. 2013;13:351–7.
Wang H, Hou L, Li A, et al. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894.
Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Yang X, Weng Z, Mendrick DL, et al. Circulating extracellular vesicles as a potential source of new biomarkers of drug-induced liver injury. Toxicol Lett. 2014;225:401–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Disclaimer: The information in these materials is not a formal dissemination of information by FDA and does not represent agency position or policy.
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht (outside the USA)
About this entry
Cite this entry
Shi, Q. (2017). Circulating Extracellular Vesicles as Liver Biomarkers. In: Patel, V., Preedy, V. (eds) Biomarkers in Liver Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7675-3_38
Download citation
DOI: https://doi.org/10.1007/978-94-007-7675-3_38
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7674-6
Online ISBN: 978-94-007-7675-3
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences